Abstract
Clinical recommendations limit menopausal hormone therapy to a few years, yet the impact of a shorter treatment duration on cardiovascular health is unknown. We hypothesized that both short- and long-term estradiol (E2) treatment exerts positive and lasting effects on blood pressure, vascular reactivity, and renal health. This study was designed to mimic midlife menopause, followed by E2 treatment, that either followed or exceeded the current clinical recommendations. Female Long-Evans retired breeders were ovariectomized (OVX) at 11 mo of age and randomized into three groups: 80-day (80d) vehicle (Veh>Veh), 40-day (40d) E2 + 40d vehicle (E2>Veh), and 80d E2 (E2>E2). In comparison to Veh>Veh, both the E2>Veh and E2>E2 groups had lower systolic blood pressure and enhanced mesenteric relaxation in response to estrogen receptor-α stimulation. Despite the reduced blood pressure, E2>E2 induced renal and cardiac hypertrophy, reduced glomerular filtration, and increased proteinuria. Interestingly, kidneys from E2>Veh rats had significantly fewer tubular casts than both of the other groups. In conclusion, long-term E2 lowered blood pressure but exerted detrimental effects on kidney health in midlife OVX Long-Evans rats, whereas short-term E2 lowered blood pressure and reduced renal damage. These findings highlight that the duration of hormone therapy may be an important factor for renal health in aging postmenopausal women.
Keywords: estrogen, menopause, Long-Evans rat, blood pressure, renal, vascular reactivity
premenopausal women have a lower incidence of hypertension compared with age-matched men, whereas women >65 yr are more likely than men to be hypertensive (28). Decades of patient data provide evidence that estradiol (E2) therapy reduces cardiovascular risk (13), suggesting that hormone therapy may provide benefits beyond the relief of menopausal symptoms. However, in 2002, the Women’s Health Initiative (WHI) showed that patients on conjugated equine estrogens alone or with combined medroxyprogesterone acetate had an increased risk for coronary heart disease, stroke, and breast cancer (33). As a result of the WHI findings, clinical recommendations were modified to limit postmenopausal hormone therapy to a few years (3), causing a drastic decrease in hormone therapy prescriptions (10, 19). However, the impact of this shorter duration of treatment on long-term cardiovascular consequences is not known.
The Long-Evans strain mimics the sexual dimorphism of blood pressure (BP) in the patient population, with higher BP in males compared with aged-matched females (11, 31). Sex hormones influence this parameter in multiple animal models (34), including female Long-Evans rats, which display ovariectomized (OVX)-induced increases in BP (8, 11). Prior studies showed that 40-day (40d) E2 treatment initiated immediately after midlife OVX in Long-Evans rats exerts positive effects on cognition well beyond the duration of treatment (32). Since hypertension exacerbates cognitive dysfunction via decreases in gray matter volume, white matter integrity, and cerebrovascular function (9, 40), the effect of E2 on cognition may partially be attributed to improvements in cardiovascular health. Moreover, the cardiovascular implications of limiting hormone therapy for the treatment of menopausal symptoms to only a few years is unknown. Whether short-term E2 therapy alters the cardiovascular system, or whether a longer duration of E2 is necessary, remains to be determined.
The goal of the present study was to compare the impact of short- and long-term E2 treatment on cardiovascular health. Similar to all rodents, female Long-Evans rats begin the transition from regular to irregular estrus cycling around 11 mo of age and reach persistent estrus by 14 mo (11). In contrast to undetectable circulating E2 during human menopause, reproductive senescence in rodents is characterized by sustained plasma E2 levels (21). Therefore, all animals in the present study underwent OVX at 11 mo to reliably and unequivocally reduce circulating levels of endogenous E2. Animals were randomized into three treatment regimens following midlife OVX: 1) vehicle control; 2) short-term (40d) E2 to mimic the current guideline of 2–3 yr treatment, followed by 40d of vehicle; and 3) long-term [80-day (80d)] E2 to mimic hormone therapy extending past the current guidelines. Cardiovascular health was assessed by measuring BP, vascular reactivity, and renal health. We hypothesized that both short- and long-term E2 treatment initiated after midlife OVX would exert positive and lasting effects on BP, vascular reactivity, and renal health.
METHODS
Animals.
All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (1996) and approved and monitored by the Tulane University Institutional Animal Care and Use Committee. Animals were group housed in a temperature-controlled Association for Assessment and Accreditation of Laboratory Animal Care International-accredited vivarium under a 12:12-h light-dark cycle with ad libitum access to food and water. Animals were maintained on Teklad Global Soy Protein-Free Extruded Rodent Diet 2020X diet from Harlan Laboratories during the entire study. Female retired breeder Long-Evans rats were received from Harlan Laboratories (Indianapolis, IN) at 11 mo of age. All animals were OVX at 11.5–12 mo of age and then randomized to three treatment groups (n = 10 per group). Treatments were administered via a subcutaneous 5-mm silastic capsule (0.58-in. inner diameter and 0.077-in. outer diameter; Dow Corning, Midland, MI) containing either 25% 17β-E2 (Sigma-Aldrich, St. Louis, MO) diluted with cholesterol, or 100% cholesterol vehicle (4, 37). One capsule was inserted at the time of OVX, and, after 40d, the original capsule was replaced by a new capsule for the remaining 40d. The first treatment group received only vehicle capsules (Veh>Veh), the second group received 40d of E2 and then 40d of vehicle (E2>Veh), and the third group received only E2 capsules (E2>E2). The efficacy of implants was confirmed by vaginal smears collected before 40d and 80d time points, along with uterine weights and serum E2 levels (Calbiotech, Spring Valley, CA).
Blood pressure measurements.
Systolic BP was measured via tail-cuff plethysmography. After a 1-wk recovery from OVX, animals were placed in restrainers for biweekly BP measurements (1 day for acclimation and 1 day for collection). Animals were warmed in a 37°C incubator for 15–20 min before seven cycles of cuff inflation. Only readings with clear arterial pulsation were included in the pressure average. At the end of the 80d treatment, nonfasting blood glucose was measured with a glucometer, and plasma ANG II was measured by radioimmunoassay, as previously described (36). Wet weights of the uterus, kidney, and left ventricle were obtained and normalized to body weight.
Urinary markers.
Animals were placed in metabolic cages to collect urine at the 40d and 80d time points. All urine samples were centrifuged to remove particulate matter and frozen at −80°C until assayed. Urinary protein concentration was determined via Bradford assay (Bio-Rad, Hercules, CA) with bovine serum albumin as the standard and is expressed as milligrams of protein per day. Serum and urine creatinine were measured using the Jaffe method (39). Creatinine values were measured at 492 nm with known concentrations of creatinine as assay standards. The estimated glomerular filtration rate (GFR) in milliliters per minute was calculated as previously described (24).
Vascular reactivity.
Animals were decapitated, and the mesenteric arcade was isolated and equilibrated on a wire myograph, as previously described (23). Vascular contractility to increasing concentrations of phenylephrine (PE) (10−7 to 10−3 M; MP Biomedicals, Santa Ana, CA) and prostaglandin F2α (PGF2α; 10−8 to 10−4.5 M; Tocris, Bristol, UK) were normalized to the maximum contraction induced by potassium chloride (KCl; 80 mM). After repeated 5-min washings, mesenteric vessels were preconstricted with PE (10−5 M), and relaxation to increasing concentrations of sodium nitroprusside (SNP; 10−10 to 10−5 M) and acetylcholine (ACh; 10−10 to 10−5 M) were expressed as the percentage of the initial PE contraction. To assess the contribution of estrogen receptors (ER) in mediating the vasodilatory response to E2, we used E2 for nonselective ER activation and {4,4′,4′′-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol} (PPT; Tocris) for selective activation of ER-α. Vessels were preconstricted with PE, and the relaxation to 10−5 M E2 or PPT was normalized to the initial PE contraction.
Immunohistochemistry.
Kidneys were formalin-fixed overnight and paraffin-embedded, and 4-µm sections were mounted onto slides. Renal morphology was assessed using Richard-Allan Scientific Gomori Trichrome Blue (Thermo Fisher Scientific, Waltham, MA) and NovaUltra Periodic Acid-Schiff Stain Kit (IHC World, Woodstock, MD), according to the manufacturer's directions. Glomerulosclerosis was scored in a blinded manner, as previously described (25). Tissue sections were quantified as the percent of pixels with positive straining over total pixels.
Statistical analysis.
All data are presented as means ± SE. BP between-treatment comparisons were analyzed using one-way ANOVA, and within-treatment comparisons used repeated-measures ANOVA. All other data were analyzed using one-way or two-way ANOVA, followed by Tukey’s multiple-comparisons test. Differences were considered statistically significant when P < 0.05. Analyses were performed using Prism Version 6.0 software (GraphPad Software, La Jolla, CA).
RESULTS
E2 opposes OVX-induced increases in BP.
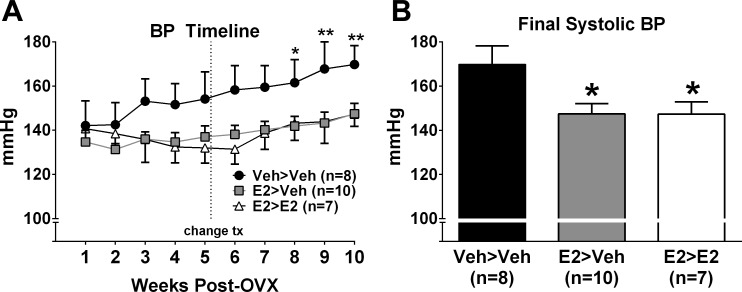
Systolic BP was not different between treatment groups at baseline (Veh>Veh: 142 ± 11 mmHg, n = 8; E2>Veh: 135 ± 5 mmHg, n = 10; E2>E2: 141 ± 5 mmHg, n = 7; P = 0.75). The Veh>Veh group displayed an incremental increase in BP that became significant from baseline by week 8 (Fig. 1A). In contrast, BP in the E2>Veh and E2>E2 groups did not increase from baseline. Final systolic BP was significantly lower in both E2>Veh and E2>E2 groups vs. Veh>Veh (Veh>Veh: 170 ± 9 mmHg; E2>Veh: 148 ± 5 mmHg; E2>E2: 148 ± 6 mmHg; P < 0.05) (Fig. 1B).
Fig. 1.
A: systolic BP was significantly increased over time only in the Veh>Veh group. *P < 0.05, **P < 0.01 vs. week 1. B: final systolic BP was significantly greater in Veh>Veh compared with all other groups. *P < 0.05. Values are means ± SE.
Long-term E2 increases tissue hypertrophy.
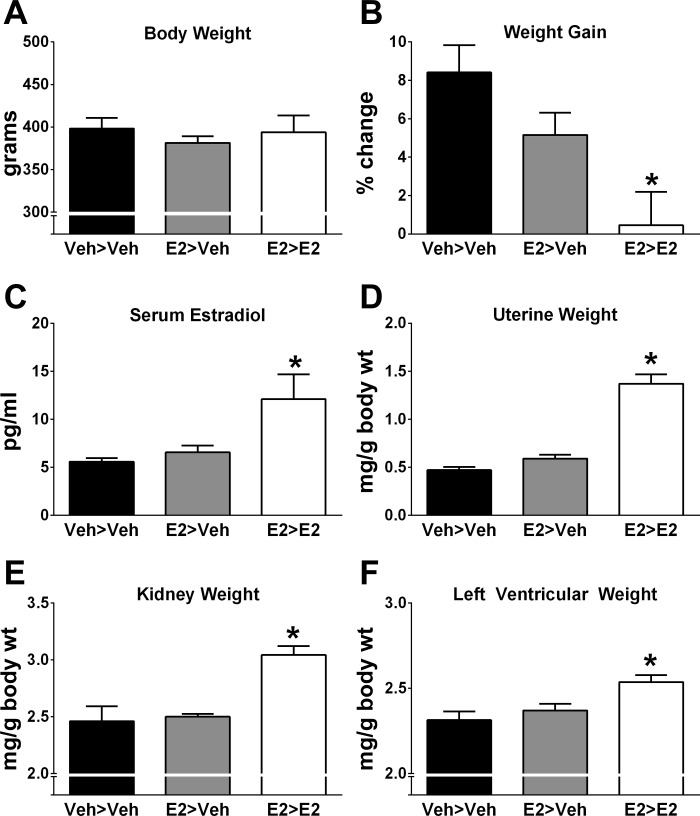
Body weight at the time of OVX was comparable between treatment groups (Veh>Veh: 368 ± 13 g; E2>Veh: 363 ± 7 g; E2>E2: 378 ± 9 g, n = 7–10 per group; P = 0.54). At the end of the study, body weight was not significantly different between groups (Fig. 2A). However, when body weight was expressed as percent change from baseline, the E2>E2 group gained significantly less weight compared with the Veh>Veh group (P < 0.005; Fig. 2B). Success of E2 treatment and OVX was confirmed by serum E2 levels, which were significantly greater in E2>E2 rats (Fig. 2C) compared with animals that did not receive E2 on days 40–80. Uterine weight was measured to further confirm the effectiveness of E2 treatment during days 40–80 and was significantly greater in the E2>E2 group (P < 0.0001) compared with Veh>Veh and E2>Veh (Fig. 2D). Despite having a lower BP, renal and left ventricular wet weights were significantly greater in the E2>E2 group (Fig. 2, E and F). There were no alterations in circulating ANG II between treatment groups, but E2>E2 rats had significantly lower nonfasting blood glucose levels compared with Veh>Veh and E2>Veh groups (Table 1).
Fig. 2.
Raw body weight was not different between treatment groups (P = 0.61; A), but percent change in body weight over baseline was significantly lower in the E2>E2 group (B). Serum estradiol levels (C) and uterine (D), kidney (E), and heart weights (F) were significantly greater in the E2>E2 group. Values are means ± SE; N = 7–10. *P < 0.05 vs. Veh>Veh.
Table 1.
Indexes of renal function and damage in vehicle- and E2-treated OVX-female Long-Evans rats
| Veh>Veh | E2>Veh | E2>E2 | One-way ANOVA, P Value | |
|---|---|---|---|---|
| Nonfasting glucose, mg/dl | 147 ± 7 | 133 ± 8 | 118 ± 5* | <0.01 |
| Plasma ANG II, fmol/ml | 18.3 ± 4.4 | 20.2 ± 2.1 | 16.3 ± 3.7 | 0.31 |
| Bowman’s space, µm2 | 2.29 ± 0.04 | 2.08 ± 0.18 | 1.98 ± 0.23 | 0.49 |
| Renal cortical fibrosis, %affected | 19.3 ± 5.4 | 15.2 ± 2.4 | 13.0 ± 1.0 | 0.40 |
| Glomerulosclerosis index | 1.76 ± 0.12 | 1.79 ± 0.13 | 1.83 ± 0.15 | 0.94 |
Values are means ± SE; N = 5–10 rats.
Significant difference from Veh<Veh.
E2 enhances vessel reactivity to ER-α.
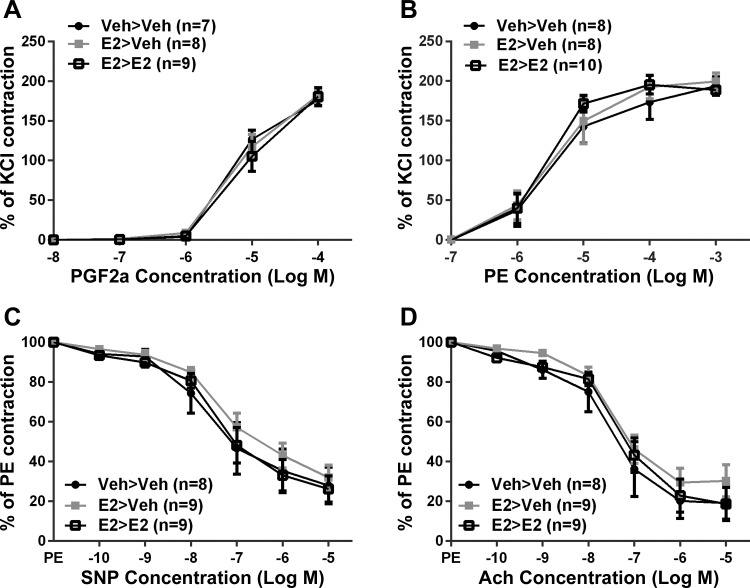
In isolated mesenteric arteries, vasoconstriction to PGF2α (Fig. 3A) and PE (Fig. 3B) was concentration-dependent with a maximum contraction of 180 ± 5% for PGF2α and 199 ± 6% for PE when normalized to the KCl contraction. There were no significant differences between all treatment groups to KCl, PGF2α, or PE-induced contraction. Endothelium-independent relaxation was measured using SNP, and endothelium-dependent relaxation was measured using ACh. The vasodilatory response reached maximum relaxation at 29 ± 4% for SNP (Fig. 3C) and 23 ± 5% for ACh (Fig. 3D) when vessels were preconstricted with PE. There were no significant differences between all treatment groups for SNP- or ACh-induced relaxation.
Fig. 3.
Mesenteric artery responses to prostaglandin F2α (PGF2α; A), phenylephrine (PE; B), sodium nitroprusside (SNP; C), and acetylcholine (D) were not different between treatment groups. Values are means ± SE; N = 7–10. P > 0.05.
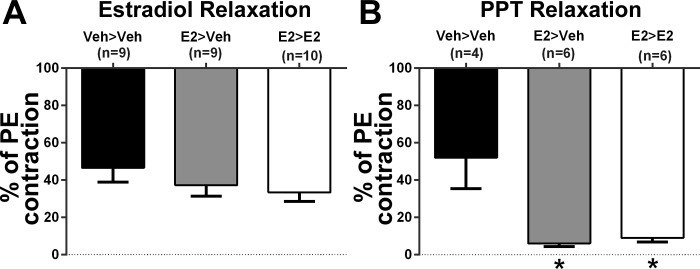
Maximum relaxation to nonselective ER activation with E2 was 39 ± 4% and was not significantly different between treatment groups (Fig. 4A). Selective ER-α activation with the selective agonist PPT was significantly greater in both E2>Veh and E2>E2 treated groups (8 ± 1%) compare with Veh>Veh (52 ± 17%; Fig. 4B). There was not a significant difference in the PPT response between E2>Veh and E2>E2.
Fig. 4.
A: mesenteric artery responses to estradiol (E2) were not different between treatment groups (P = 0.31). B: both E2>Veh and E2>E2 groups had significantly greater relaxation to the estrogen receptor-α agonist PPT compared with Veh>Veh. Values are means ± SE. *P < 0.005 vs. Veh<Veh.
Long-term E2 increases indexes of renal damage.
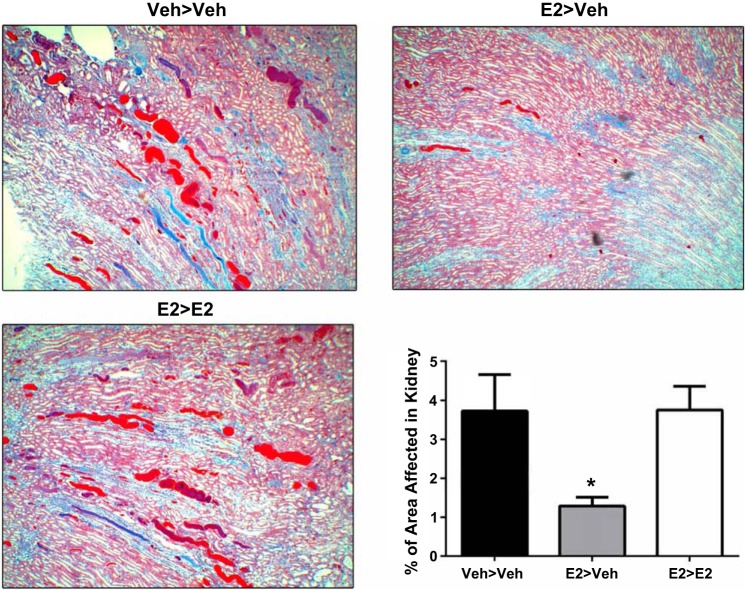
Since renal hypertrophy was increased in the E2>E2 group, despite a lower BP, additional experiments were performed to assess kidney damage. Urine was collected using metabolic cages at the 40d and 80d time points. There was a trend for E2 to increase proteinuria at 40d, but a significant increase was found only after 80d of E2 (Fig. 5A). While creatinine excretion was similar between all groups at the end of the study (Fig. 5B), E2>E2 rats had a significant increase in serum creatinine (Fig. 5C) and a decline in estimated GFR (P = 0.009) compared with Veh>Veh and E2>Veh (Fig. 5D). Renal pathology found no significant differences in glomerulosclerosis, Bowman’s space, or interstitial fibrosis between treatment groups (Table 1). Interestingly, renal tubular cast formation in the renal outer medulla was significantly reduced in E2>Veh compared with Veh>Veh and E2>E2 (Fig. 6).
Fig. 5.
A: proteinuria was significantly increased in the E2>E2 group at the end of the study. *P < 0.05 vs. Veh>Veh and E2>Veh. B: urine creatinine was not altered by treatment (P = 0.38). C: serum creatinine was significantly greater in E2>E2 rats. *P < 0.05 vs. all other groups. D: estimated GFR was significantly reduced by E2>E2 treatment. *P < 0.05 vs. all other groups. Values are means ± SE.
Fig. 6.
Tubular casts were significantly reduced in the kidneys of E2>Veh rats. Values are means ± SE; N = 9. *P < 0.05 vs. all other groups.
DISCUSSION
The primary novel finding of the present study was that, after midlife OVX, both short- and long-term E2 maintained a lower BP, but long-term E2 induced renal damage. This study demonstrates that the benefits of E2 on the cardiovascular system in midlife OVX Long-Evans rats may be organ dependent. While both short- and long-term E2 prevented BP elevation and enhanced vascular relaxation to ER-α, long-term E2 decreased GFR and increased proteinuria and renal tubular casts. Overall, our present findings indicate that the duration of E2 treatment is critical for determining the relative benefits and risks of postmenopausal hormone therapy.
In the present study, both short- and long-term E2 attenuated OVX-induced increases in BP. Our findings support previous work in young animals, including the mRen2 female rat (7), the Dahl salt-sensitive rat (15), and the ANG II-infused mouse (42), however, data from older animals are less clear. In 60-wk-old mRen2 rats that underwent OVX at 15 wk, estrogen has no effect on BP, even when challenged with a high-salt diet (43). Conversely, BP in 12-mo-old Dahl salt-sensitive rats is higher in animals OVX at 2 mo compared with rats treated with E2 or those that are ovary intact (14). Strain and timing of OVX may also be a factor, as OVX at 18 mo of Fischer 344 × Brown Norway rats induces a significant increase in BP (41), but OVX at either 12 wk or 24 mo does not impact BP in spontaneously hypertensive rats (18). Our results support previous work in the Long-Evans strain showing that midlife OVX increases BP, whereas E2 treatment blunts this hypertensive effect (8). Moreover, because the impact of surgical menopause on BP in the midlife Long-Evans rat mirrors postmenopausal hypertension in women, this strain is an excellent model for studying the impact of hormone treatment on cardiovascular health.
In the present study, the beneficial effects of short- and long-term E2 on BP were not associated with alterations in the response to vasoconstrictors (PGF2α, PE) or vasodilators (SNP, ACh). Since estrogen induces vasodilation through both stimulation of endothelial nitric oxide and direct effects on vascular smooth muscle (22), we also assessed the vasodilatory response to ER stimulation. No significant differences were found in the response to nonselective ER activation, but vessels from both E2 treatment groups had an enhanced response to the ER-α agonist PPT. Similarly, aortic vasodilation to PPT in 11- to 15-wk-old female Sprague-Dawley rats is impaired following OVX (5), while hippocampal ER-α expression is preserved using the same treatment paradigm as in the present study (32). Specific receptor subtypes may be altered by aging and hormones, despite no change in the global response, revealing opposing actions of ERs in the vasculature. Furthermore, the enhanced PPT response in both E2>Veh and E2>E2 vessels suggests that even short-term E2 treatment preserved the vasodilatory function of ER-α.
The kidneys play an important role in long-term BP regulation, and defects in renal function exacerbate cardiovascular complications. In the present study, short-term E2 treatment induced renoprotection, as evidenced by a decrease in renal tubular casts, while long-term E2 negatively impacted the kidney. Assessments of renal oxidative stress, nitric oxide status, inflammation, proliferation, and epithelial-mesenchymal transition did not reveal a mechanism for the observed renal damage (data not shown). In other animal studies, E2 is protective against some forms of renal injury (16, 17, 20), but not others (30). Few studies utilize older animals, but estrogen is protective against the development of renal disease in 12-mo-old Dahl salt-sensitive rats (26). Clinically, renal disease progresses more slowly in women compared with men (6, 29), until the protective benefits of estrogen are disrupted or impaired through kidney dialysis or natural causes, such as menopause (12). However, the impact of postmenopausal E2 therapy in renal health is unclear, as studies report both positive (1, 35) and negative (2, 27) consequences.
In our study, the renal damage induced by long-term E2 was associated with decreased GFR and elevated serum creatinine. Since these changes occurred in the absence of overt structural damage, we speculate that the decline in GFR was due to changes in hemodynamics. Interestingly, renal pathology revealed a predominance of tubular casts in the vehicle control and long-term E2 group that was not present in the short-term E2 group. We also found that long-term E2 increased proteinuria independent of BP. The proteinuria detected after 80d of E2, and not at 40d, suggests that treatment duration is an important factor. This effect is not exclusive to females, since E2 treatment in castrated 14-mo-old male Otsuka-Long-Evans-Tokushima-Fatty rats increased proteinuria independent of changes in BP (38). Similar to our results, postmenopausal patients prescribed estrogen-only therapies have a significant decline in GFR compared with nonusers (2), and menopausal hormone therapy for more than 5 yr is associated with an increased risk of microalbuminuria independent of BP (27). Our results highlight a need to further investigate how aging and chronic noncyclical E2 dosing affects the estrogenic response in the kidney.
It is unknown how present treatments using E2 combined with a synthetic progesterone or the use of selective or cyclic E2 administration would influence our study outcomes. However, our present findings suggest that the duration of hormone therapy may be an important factor for renal health in aging postmenopausal women, and provide support that hormone therapy in postmenopausal women with a decline in renal function could further exacerbate their condition.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute (Grant 4R00-HL-103974 to S. H. Lindsey) and the American Heart Association (Grant 16POST27600001 to M. A. Zimmerman). Services provided by Hypertension Center Core facility were supported by the National Institute of General Medical Sciences (Grant CoBRE P30-GM-103337).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.A.Z., E.H.T., S.N.K., J.L.D., and S.H.L. performed experiments; M.A.Z., D.D.H., B.M., and S.H.L. analyzed data; M.A.Z., B.M., and S.H.L. interpreted results of experiments; M.A.Z., D.D.H., and S.H.L. prepared figures; M.A.Z. and S.H.L. drafted manuscript; M.A.Z., D.D.H., E.H.T., S.N.K., J.L.D., B.M., E.M.G., J.M.D., and S.H.L. edited and revised manuscript; M.A.Z., D.D.H., E.H.T., J.L.D., B.M., E.M.G., J.M.D., and S.H.L. approved final version of manuscript.
REFERENCES
- 1.Agarwal M, Selvan V, Freedman BI, Liu Y, Wagenknecht LE. The relationship between albuminuria and hormone therapy in postmenopausal women. Am J Kidney Dis 45: 1019–1025, 2005. doi: 10.1053/j.ajkd.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed SB, Culleton BF, Tonelli M, Klarenbach SW, Macrae JM, Zhang J, Hemmelgarn BR; Alberta Kidney Disease Network . Oral estrogen therapy in postmenopausal women is associated with loss of kidney function. Kidney Int 74: 370–376, 2008. doi: 10.1038/ki.2008.205. [DOI] [PubMed] [Google Scholar]
- 3.[Anon]. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol 123: 202–216, 2014. doi: 10.1097/01.AOG.0000441353.20693.78. [DOI] [PubMed] [Google Scholar]
- 4.Bohacek J, Daniel JM. The beneficial effects of estradiol on attentional processes are dependent on timing of treatment initiation following ovariectomy in middle-aged rats. Psychoneuroendocrinology 35: 694–705, 2010. doi: 10.1016/j.psyneuen.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Bolego C, Cignarella A, Sanvito P, Pelosi V, Pellegatta F, Puglisi L, Pinna C. The acute estrogenic dilation of rat aorta is mediated solely by selective estrogen receptor-alpha agonists and is abolished by estrogen deprivation. J Pharmacol Exp Ther 313: 1203–1208, 2005. doi: 10.1124/jpet.104.082867. [DOI] [PubMed] [Google Scholar]
- 6.Carrero JJ. Gender differences in chronic kidney disease: underpinnings and therapeutic implications. Kidney Blood Press Res 33: 383–392, 2010. doi: 10.1159/000320389. [DOI] [PubMed] [Google Scholar]
- 7.Chappell MC, Gallagher PE, Averill DB, Ferrario CM, Brosnihan KB. Estrogen or the AT1 antagonist olmesartan reverses the development of profound hypertension in the congenic mRen2. Lewis rat. Hypertension 42: 781–786, 2003. doi: 10.1161/01.HYP.0000085210.66399.A3. [DOI] [PubMed] [Google Scholar]
- 8.Clark JT, Chakraborty-Chatterjee M, Hamblin M, Wyss JM, Fentie IH. Estrogen depletion differentially affects blood pressure depending on age in Long-Evans rats. Endocrine 25: 173–186, 2004. doi: 10.1385/ENDO:25:2:173. [DOI] [PubMed] [Google Scholar]
- 9.de Leeuw FE, de Groot JC, Oudkerk M, Witteman JC, Hofman A, van Gijn J, Breteler MM. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain 125: 765–772, 2002. doi: 10.1093/brain/125.4.765. [DOI] [PubMed] [Google Scholar]
- 10.Ettinger B, Wang SM, Leslie RS, Patel BV, Boulware MJ, Mann ME, McBride M. Evolution of postmenopausal hormone therapy between 2002 and 2009. Menopause 19: 610–615, 2012. doi: 10.1097/gme.0b013e31823a3e5d. [DOI] [PubMed] [Google Scholar]
- 11.Fentie IH, Greenwood MM, Wyss JM, Clark JT. Age-related decreases in gonadal hormones in Long-Evans rats: relationship to rise in arterial pressure. Endocrine 25: 15–22, 2004. doi: 10.1385/ENDO:25:1:15. [DOI] [PubMed] [Google Scholar]
- 12.Gluhovschi G, Gluhovschi A, Anastasiu D, Petrica L, Gluhovschi C, Velciov S. Chronic kidney disease and the involvement of estrogen hormones in its pathogenesis and progression. Rom J Intern Med 50: 135–144, 2012. [PubMed] [Google Scholar]
- 13.Grady D, Rubin SM, Petitti DB, Fox CS, Black D, Ettinger B, Ernster VL, Cummings SR. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med 117: 1016–1037, 1992. doi: 10.7326/0003-4819-117-12-1016. [DOI] [PubMed] [Google Scholar]
- 14.Hinojosa-Laborde C, Craig T, Zheng W, Ji H, Haywood JR, Sandberg K. Ovariectomy augments hypertension in aging female Dahl salt-sensitive rats. Hypertension 44: 405–409, 2004. doi: 10.1161/01.HYP.0000142893.08655.96. [DOI] [PubMed] [Google Scholar]
- 15.Hinojosa-Laborde C, Lange DL, Haywood JR. Role of female sex hormones in the development and reversal of dahl hypertension. Hypertension 35: 484–489, 2000. doi: 10.1161/01.HYP.35.1.484. [DOI] [PubMed] [Google Scholar]
- 16.Hutchens MP, Fujiyoshi T, Komers R, Herson PS, Anderson S. Estrogen protects renal endothelial barrier function from ischemia-reperfusion in vitro and in vivo. Am J Physiol Renal Physiol 303: F377–F385, 2012. doi: 10.1152/ajprenal.00354.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikeda M, Swide T, Vayl A, Lahm T, Anderson S, Hutchens MP. Estrogen administered after cardiac arrest and cardiopulmonary resuscitation ameliorates acute kidney injury in a sex- and age-specific manner. Crit Care 19: 332, 2015. doi: 10.1186/s13054-015-1049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jazbutyte V, Hu K, Kruchten P, Bey E, Maier SK, Fritzemeier KH, Prelle K, Hegele-Hartung C, Hartmann RW, Neyses L, Ertl G, Pelzer T. Aging reduces the efficacy of estrogen substitution to attenuate cardiac hypertrophy in female spontaneously hypertensive rats. Hypertension 48: 579–586, 2006. doi: 10.1161/01.HYP.0000240053.48517.c7. [DOI] [PubMed] [Google Scholar]
- 19.Jewett PI, Gangnon RE, Trentham-Dietz A, Sprague BL. Trends of postmenopausal estrogen plus progestin prevalence in the United States between 1970 and 2010. Obstet Gynecol 124: 727–733, 2014. doi: 10.1097/AOG.0000000000000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji H, Menini S, Mok K, Zheng W, Pesce C, Kim J, Mulroney S, Sandberg K. Gonadal steroid regulation of renal injury in renal wrap hypertension. Am J Physiol Renal Physiol 288: F513–F520, 2005. doi: 10.1152/ajprenal.00032.2004. [DOI] [PubMed] [Google Scholar]
- 21.LaPolt PS, Lu JKH. 53 - Factors influencing the onset of female reproductive senescence. In: Functional Neurobiology of Aging, edited by Hof PR, Mobbs CV. San Diego, CA: Academic, 2001, p. 761–768. doi: 10.1016/B978-012351830-9/50055-X. [DOI] [Google Scholar]
- 22.Lindsey SH, Carver KA, Prossnitz ER, Chappell MC. Vasodilation in response to the GPR30 agonist G-1 is not different from estradiol in the mRen2.Lewis female rat. J Cardiovasc Pharmacol 57: 598–603, 2011. doi: 10.1097/FJC.0b013e3182135f1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindsey SH, Liu L, Chappell MC. Vasodilation by GPER in mesenteric arteries involves both endothelial nitric oxide and smooth muscle cAMP signaling. Steroids 81: 99–102, 2014. doi: 10.1016/j.steroids.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsey SH, Yamaleyeva LM, Brosnihan KB, Gallagher PE, Chappell MC. Estrogen receptor GPR30 reduces oxidative stress and proteinuria in the salt-sensitive female mRen2.Lewis rat. Hypertension 58: 665–671, 2011. doi: 10.1161/HYPERTENSIONAHA.111.175174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maric C, Sandberg K, Hinojosa-Laborde C. Glomerulosclerosis and tubulointerstitial fibrosis are attenuated with 17beta-estradiol in the aging Dahl salt sensitive rat. J Am Soc Nephrol 15: 1546–1556, 2004. doi: 10.1097/01.ASN.0000128219.65330.EA. [DOI] [PubMed] [Google Scholar]
- 26.Maric C, Xu Q, Sandberg K, Hinojosa-Laborde C. Age-related renal disease in female Dahl salt-sensitive rats is attenuated with 17 beta-estradiol supplementation by modulating nitric oxide synthase expression. Gend Med 5: 147–159, 2008. doi: 10.1016/j.genm.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monster TB, Janssen WM, de Jong PE, de Jong-van den Berg LT; Prevention of Renal and Vascular End Stage Disease Study Group . Oral contraceptive use and hormone replacement therapy are associated with microalbuminuria. Arch Intern Med 161: 2000–2005, 2001. doi: 10.1001/archinte.161.16.2000. [DOI] [PubMed] [Google Scholar]
- 28.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; Writing Group Members; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 133: e38–e360, 2016. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 29.Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol 11: 319–329, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Oestreicher EM, Guo C, Seely EW, Kikuchi T, Martinez-Vasquez D, Jonasson L, Yao T, Burr D, Mayoral S, Roubsanthisuk W, Ricchiuti V, Adler GK. Estradiol increases proteinuria and angiotensin II type 1 receptor in kidneys of rats receiving L-NAME and angiotensin II. Kidney Int 70: 1759–1768, 2006. doi: 10.1038/sj.ki.5001897. [DOI] [PubMed] [Google Scholar]
- 31.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension 37: 1199–1208, 2001. doi: 10.1161/01.HYP.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 32.Rodgers SP, Bohacek J, Daniel JM. Transient estradiol exposure during middle age in ovariectomized rats exerts lasting effects on cognitive function and the hippocampus. Endocrinology 151: 1194–1203, 2010. doi: 10.1210/en.2009-1245. [DOI] [PubMed] [Google Scholar]
- 33.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J; Writing Group for the Women’s Health Initiative Investigators . Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 288: 321–333, 2002. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 34.Sandberg K, Ji H. Sex differences in primary hypertension. Biol Sex Differ 3: 7, 2012. doi: 10.1186/2042-6410-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schopick EL, Fisher ND, Lin J, Forman JP, Curhan GC. Post-menopausal hormone use and albuminuria. Nephrol Dial Transplant 24: 3739–3744, 2009. doi: 10.1093/ndt/gfp321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shao W, Seth DM, Navar LG. Augmentation of endogenous intrarenal angiotensin II levels in Val5-ANG II-infused rats. Am J Physiol Renal Physiol 296: F1067–F1071, 2009. doi: 10.1152/ajprenal.90596.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ström JO, Theodorsson A, Ingberg E, Isaksson IM, Theodorsson E. Ovariectomy and 17β-estradiol replacement in rats and mice: a visual demonstration. J Vis Exp 64: e4013, 2012. 10.3791/4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomiyoshi Y, Sakemi T, Aoki S, Miyazono M. Different effects of castration and estrogen administration on glomerular injury in spontaneously hyperglycemic Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Nephron 92: 860–867, 2002. doi: 10.1159/000065442. [DOI] [PubMed] [Google Scholar]
- 39.Toora BD, Rajagopal G. Measurement of creatinine by Jaffe’s reaction–determination of concentration of sodium hydroxide required for maximum color development in standard, urine and protein free filtrate of serum. Indian J Exp Biol 40: 352–354, 2002. [PubMed] [Google Scholar]
- 40.Vuorinen M, Damangir S, Niskanen E, Miralbell J, Rusanen M, Spulber G, Soininen H, Kivipelto M, Solomon A. Coronary heart disease and cortical thickness, gray matter and white matter lesion volumes on MRI. PLoS One 9: e109250, 2014. doi: 10.1371/journal.pone.0109250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H, da Silva J, Alencar A, Zapata-Sudo G, Lin MR, Sun X, Ahmad S, Ferrario CM, Groban L. Mast cell inhibition attenuates cardiac remodeling and diastolic dysfunction in middle-aged, ovariectomized Fischer344xBrown Norway rats. J Cardiovasc Pharmacol 68: 49–57, 2016. doi: 10.1097/FJC.0000000000000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xue B, Pamidimukkala J, Hay M. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am J Physiol Heart Circ Physiol 288: H2177–H2184, 2005. doi: 10.1152/ajpheart.00969.2004. [DOI] [PubMed] [Google Scholar]
- 43.Yamaleyeva LM, Pendergrass KD, Pirro NT, Gallagher PE, Groban L, Chappell MC. Ovariectomy is protective against renal injury in the high-salt-fed older mRen2. Lewis rat. Am J Physiol Heart Circ Physiol 293: H2064–H2071, 2007. doi: 10.1152/ajpheart.00427.2007. [DOI] [PubMed] [Google Scholar]