Abstract
We have characterized the expression and secretion of the acute kidney injury (AKI) biomarkers insulin-like growth factor binding protein 7 (IGFBP7) and tissue inhibitor of metalloproteinases-2 (TIMP-2) in human kidney epithelial cells in primary cell culture and tissue. We established cell culture model systems of primary kidney cells of proximal and distal tubule origin and observed that both proteins are indeed expressed and secreted in both tubule cell types in vitro. However, TIMP-2 is both expressed and secreted preferentially by cells of distal tubule origin, while IGFBP7 is equally expressed across tubule cell types yet preferentially secreted by cells of proximal tubule origin. In human kidney tissue, strong staining of IGFBP7 was seen in the luminal brush-border region of a subset of proximal tubule cells, and TIMP-2 stained intracellularly in distal tubules. Additionally, while some tubular colocalization of both biomarkers was identified with the injury markers kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin, both biomarkers could also be seen alone, suggesting the possibility for differential mechanistic and/or temporal profiles of regulation of these early AKI biomarkers from known markers of injury. Last, an in vitro model of ischemia-reperfusion demonstrated enhancement of secretion of both markers early after reperfusion. This work provides a rationale for further investigation of these markers for their potential role in the pathogenesis of acute kidney injury.
Keywords: acute kidney injury, biomarkers, IGFBP7, and TIMP-2
two novel biomarkers, insulin-like growth factor binding protein 7 (IGFBP7) and tissue inhibitor of metalloproteinases-2 (TIMP-2), were recently discovered for the identification of moderate to severe acute kidney injury (AKI) in critically ill patients (9, 26). From these and additional studies, a clinical immunoassay for detection of [TIMP-2]·[IGFBP7], the NephroCheck test (Astute Medical), received US Food and Drug Administration approval for AKI risk assessment in critically ill patients. Additionally, these biomarkers have shown to be sensitive, specific, and highly predictive early biomarkers for AKI in adults after surgeries (21, 36), and in children after cardiac surgery (37). In addition to predicting AKI, these biomarkers have been associated with long-term outcomes after AKI (28), predicting renal recovery (18), and prediction of dialysis or recovery after kidney transplantation (19). Last, remote ischemic preconditioning increased TIMP-2 and IGFBP7 preoperatively, reduced these biomarker levels in the postoperative period, and ultimately reduced the occurrence of AKI after cardiac surgery in high-risk patients, supporting the hypothesis that IGFBP7 and TIMP-2 may serve as “alarm” signals that may be protective in some conditions (74).
Expression of various TIMP family members has been reported in kidney cells, including glomerular mesangial and epithelial cells, tubulointerstitial cells, and cystic cells in culture (4, 13, 34, 50, 57, 71, 73). Expression has also been reported in proximal tubule cells, but primarily only in response to insults, mitogens, or second messengers (14, 20, 41, 45, 62, 63). There is a dearth of information regarding IGFBP7 in the kidney, and what is available is contradictory, showing expression in the glomerulus and alternatively in distal and proximal tubule cells (17, 35, 42, 65, 72).
While there are variable reports about TIMP family member expression in the kidney, very little is known regarding IGFBP7 expression, and there is currently no clear molecular evidence in the kidney for either molecule to explain their value as biomarkers for AKI. Therefore, we initiated this study to clarify their expression in the human kidney, to examine how the expression of these molecules relates to the expression of markers of kidney injury, and to determine whether we could identify modulation of expression and/or secretion of these biomarkers by cellular insult.
MATERIALS AND METHODS
Tissue procurement and processing.
Whole adult human kidneys were obtained from the Center for Organ Recovery and Education (CORE, Pittsburgh, PA) through a protocol approved by the University of Pittsburgh Committee for Oversight of Research and Clinical Training Involving Decedents. Samples obtained were kidneys from donation-after-cardiac-death or brain-dead donors that were not accepted for transplant. Before receipt, samples were recovered and packaged for transplant by perfusion in HTK or UW (SPS-1) and packing in ice. Upon receipt, kidneys were maintained in a sterile environment on ice, decapsulated, and processed as follows. 1) For cryosectioning and microscopic analysis of whole human tissue, (5–10 mm) kidney transverse sections were fixed in 4% paraformaldehyde (PFA; Electron Microscopy Sciences, Hatfield, PA) in PBS (Fisher Scientific, Pittsburgh, PA) overnight at 4°C, followed by 3 × 15-min washes in cold PBS, quenching in cold 200 mM NH4Cl2 (Fisher), in PBS for 15 min, followed by 3 × 15-min washes with cold PBS, and storage in PBS 0.02% NaN3 (Fisher) at 4°C. 2) For tissue total protein and RNA/DNA analysis, the cortex and medulla from 5- to 10-mm transverse sections were separated, sectioned into ~0.5-mm cubes, and snap frozen in liquid nitrogen and stored at −150°C. Tissue samples for RNA analysis were incubated at 4°C in RNALater (Fisher) overnight before removal of excess RNALater and snap freezing. 3) For preparation of tissue for cell culture, the remaining cortex and medulla were processed separately on ice. Tissue was diced by razor blade mincing into pieces as small as possible (0.1–1 mm), then digested for 1 h at 37°C in HBSS (Life Technologies, Grand Island, NY) containing collagenase IV (200 U/ml), DNAse (100 U/ml), MgCl2 (200 mM), and CaCl2 (200 mM) at a ratio of 1.5 ml digestion solution/g tissue for 1 h. with intermittent mixing. The resultant slurry was forced through a 250-µm sieve (Gilson, Lewis Center, OH) and washed with HBSS to remove undigested tissue. The filtrate from the 250-µm sieve was passed through a 180-µm sieve to isolate glomeruli. After extensive washing of the retentate on the sieve with HBSS from a squirt bottle (no less than 50 ml/sample), the retentate (which by microscopic evaluation routinely consisted of pure intact glomeruli and very little to no tubule fragments) was fractionated and frozen for future analysis or placed into culture for study. Samples from this fraction are referred to as “GLOM.” The filtrate from the 180-µm sieve (FT), which consisted of tubule fragments and individual cells, with little to no glomeruli, was fractionated and frozen for future use or placed into culture for expansion and immunoaffinity isolation. A total of six separate subject samples were used in this study. The number of samples assessed in each experiment is noted in results.
Culture of dissociated cells for propagation.
All reagents for cell culture were from Life Technologies unless mentioned otherwise. Dissociation fractions from the process above were propagated by culture in DMEM/F12 with the addition of 5% FBS, insulin, transferrin, selenium, glutamax, and penicillin-streptomycin in 150-cm2 flasks coated with 5 μg/cm2 rat tail collagen-1 at 37°C, 5% CO2. Resultant heterogeneous primary cell cultures were fed every 3 days until confluent. The GLOM fraction was plated directly for experimentation, and the FT fraction was subjected to immunoaffinity isolation and plating for experimentation in different media as described below.
Immunoaffinity isolation of primary cells of proximal and distal tubule origin.
Confluent cell cultures (FT from passages 1–3) were trypsinized using TrypLE at 37°C for no more than 10 min, counted with a hemocytometer, and subjected to immunoaffinity isolation via the Dynal Pan-Mouse IgG magnetic bead system exactly according to the manufacturer’s instructions (Fisher). For isolation of cells of proximal tubule origin, an antibody directed against aminopeptidase N (APN; BD Biosciences, San Jose, CA) was used, and to isolate cells of distal origin, an antibody directed against MUC-1 (CD227; BD Biosciences) was used. Cell isolation was performed using 10 µg of antibody and 25 µl of Dynabead preparation per 107 total cells/ml. After release from the Dynabeads, isolated cells were plated for further propagation or directly for experimentation in the media mentioned above.
Culture of cells for experimentation.
HK2 (ATCC, Manassas, VA) and isolated primary cells were plated onto 12- or 24-mm rat tail collagen-1-coated Transwells at ~5.5 × 104 cells/cm2 to be confluent at plating. The following day, the media was exchanged to the hormonally defined, serum-free media DMEM + F12, 20 ng/ml EGF, 40 ng/ml hydrocortisone (Sigma-Aldrich, St. Louis, MO), 4 pg/ml triiodothyronine (Sigma), penicillin-streptomycin, and glutamax. The cells were cultured for at least 6 days with daily feeding before experimentation.
Immunoblotting for characterization and analysis of IGFBP7 and TIMP-2.
For characterization and expression of IGFBP7 and TIMP-2, confluent monolayers of cells were lysed in PBSTDS lysis buffer (10 mM Na2HPO4, 150 mM NaCl, 1% Triton X-100, 0.5% Na deoxycholate, 0.1% SDS, 1 mM EDTA, 1 mM NaF, 0.02% NaN3, pH 7.4) containing protease and phosphatase inhibitors (Thermo Fisher Scientific, Waltham, MA). Total protein concentration of all lysates was determined using the Bio-Rad DC protein assay (Bio-Rad, Hercules, CA). For immunoblot analysis of secreted protein, conditioned media was spun out and mixed with Laemmli sample buffer, aliquoted, and frozen at −20°C or used directly. In this study, conditioned media refers to experimental media (see paragraph above) that was exposed to cell culture for the time periods indicated in each figure. The control for these studies was unconditioned media, which refers to the same media that was not exposed to cells for any period of time. Equivalent amounts of lysate or media were subjected to SDS-PAGE and electroblotting using 4–20% Tris-glycine gels (Life Technologies) using the Laemmli system. The antibodies used for immunoblotting were directed against IGFBP7 (1:5,000, Abcam, Cambridge, MA), TIMP-2 (1:2,000, Cell Signaling Technology, Danvers, MA), E-cadherin (E-CAD; 1:20,000, BD Biosciences), sodium/hydrogen exchanger 3 (1:500, Thermo Scientific, Rockford, IL), Na+-K+-ATPase (1:1,000, alpha 1 subunit, Abcam), γ-glutamyl transpeptidase [GGT; 1:20,000, Santa Cruz Biotechnology (SCB), Dallas, TX], vacuolar H+-ATPase (E subunit, 1:500, Sigma-Aldrich), aquaporin-1 (AQP1; 1:250, SCB), GAPDH (1:10,000, SCB), or β-actin (1:40,000, Sigma); the secondary antibodies were donkey anti-rabbit/mouse/sheep/goat/chicken HRP (1:20,000–100,000, Jackson ImmunoResearch, West Grove, PA). Staining was visualized using the Pierce ECL Western blotting substrate (Thermo Scientific).
For all experimentation, immunoaffinity isolated cells from passages 2–6 were used, and each passage was characterized for consistency. If any passage demonstrated evidence of significant differentiation or dedifferentiation by morphological change assessed by light microscopy and/or gain or loss of appropriate marker expression, it was discarded and not used for experimentation.
Preparation of cells and tissue for immunofluorescence and confocal microscopy.
Wedges from the PFA-fixed kidney sections were infused with 30% sucrose in PBS 0.02% NaN3 (Fisher) by soaking overnight, followed by embedding and freezing in OCT (Fisher Healthcare, Houston, TX) at −20°C. Cryosections (10 µm) cut at −20°C using a permanent blade (C. L. Sturkey, Lebanon, PA) in a Microm HM 505N cryostat were placed onto permafrost slides (Fisher), and stored at −20°C until use. For imaging of cryostat tissue sections, OCT was removed by 3 × 10-min immersions in PBS, and sections were permeabilized in PBS 0.5% Triton X-100 for 15 min at room temperature, blocked for 30 min in PBS 5% nonfat dry milk (Bio-Rad), washed two times briefly with PBS, and incubated in primary antibody in PBS 2% BSA (Sigma) overnight at 4°C. Samples were then washed 3 × 5 min with PBS 2% BSA, incubated in secondary antibody in PBS 2% BSA, covered in Fluoro-Gel II + 4′-6-diamidino-2-phenylindole (DAPI; EMS), and sealed with coverslips. For the zonula occludens (ZO)-1 staining of cells cultured on Transwells, the cells were fixed with PBS 2% PFA and processed as with the tissue sections. The primary antibodies and concentrations used for immunofluorescence are as follows: IGFBP7 (1:400, Abcam), TIMP-2 (1:200, SCB), aminopeptidase N (1:200, CD13, BD Biosciences), neprilysin (1:200, CD 10 BD Biosciences), AQP-1 (1:50, SCB), MUC-1 (1:200, CD227, BD Biosciences), Tamm-Horsfall glycoprotein (THG; 1:100, uromodulin, R&D Systems, Minneapolis, MN), E-CAD, 1:200, BD Biosciences), kidney injury molecule 1 (KIM-1; 1:200, R&D Systems), NGAL (1:200, R&D Systems), and ZO-1 (1:200, Invitrogen, Camarillo, CA). The secondary antibodies used for immunofluorescence in this study were Alexa Fluor 488 and 594 conjugated (1:100, Jackson ImmunoResearch). Samples were imaged using an Olympus Fluoview 1000 confocal microscope with a ×40 oil-immersion objective. For DAPI imaging, a 405 laser at 0.1–1% power was used. For Alexa Fluor 488, a 488-nm multiline argon laser at 2–5% power was used, and for Alexa Fluor 594, a 543-nm helium/neon laser at 7–25% power was used. For each image, the PMT voltage was between 600 and 650, and the gain was 1. For the z-scan images of Fig. 1, z-spacing was chosen to be 0.45 µm, equating to a sampling rate of 2. Negative controls were secondary antibody only, and were imaged at the highest laser power, voltage, and gain used in each figure.
Fig. 1.
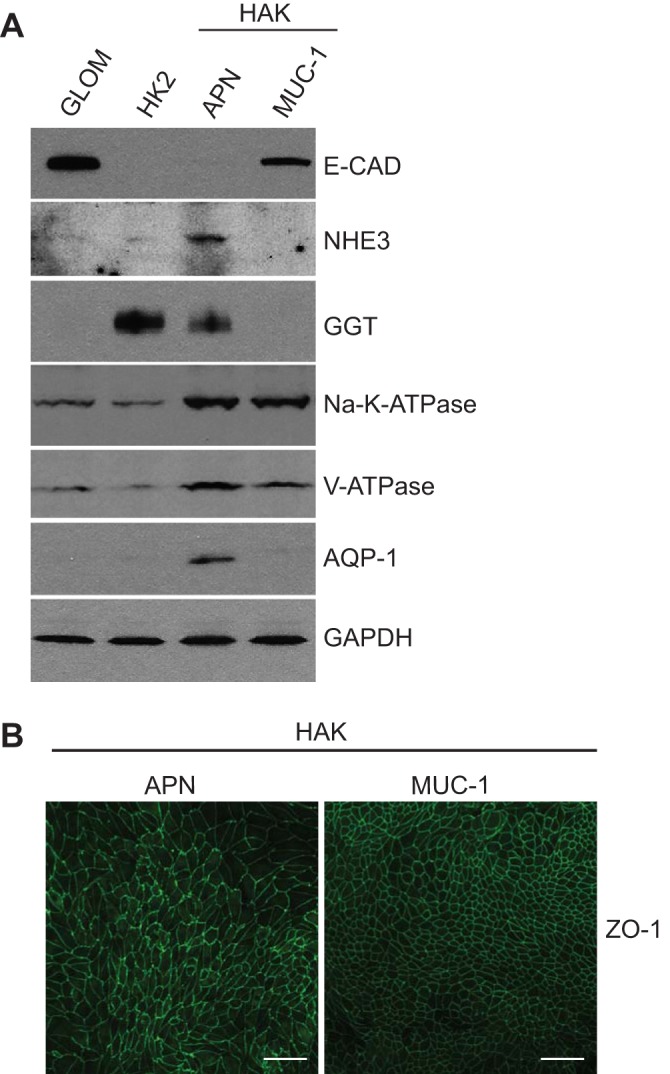
Characterization of immunoaffinity isolated primary human kidney epithelial cells of proximal and distal tubule origin. Heterogeneous pools of cultured human kidney epithelial cells were immunoaffinity isolated using antibodies directed against the proximal tubule marker aminopeptidase N (APN) or the distal tubule marker Muc-1 (MUC-1). A: representative immunoblot characterization of lysates from isolated human adult kidney (HAK) cells of proximal origin (APN), and distal origin (MUC-1), compared with cells from glomeruli isolated by retention on an 180-µm sieve (GLOM), and HK2 cells (HK2). Lysates were assessed for expression of the proximal tubule markers sodium/hydrogen exchanger 3 (NHE3), γ-glutamyl-transpeptidase (GGT), and aquaporin-1 (AQP1), the distal tubule marker E-cadherin (E-CAD), as well as the ion transporters Na+-K+-ATPase (Na-K-ATPase) and the vacuolar H+-ATPase (V-ATPase). GAPDH was used for protein loading. B: HAK-APN and HAK-MUC-1 cells were cultured for at least 6 days on Transwell permeable supports, fixed with 2% paraformaldehyde, and subjected to immunofluorescence with the antibody against zonula occludens (ZO-1) for the identification of epithelial monolayer development. Scale bar = 50 µm.
Oxygen-nutrient deprivation.
Cells were subjected to oxygen-nutrient depravation by culture in deoxygenated HBSS (GIBCO) in a 0% O2 environment for 24 h and compared with cells cultured in oxygenated HBSS or the hormonally defined serum-free media mentioned above under regular culture conditions. After 24 h of deprivation or control conditions, fractions of the conditioned media were prepared for immunoblot analysis as described above, and the remaining media was removed and replaced with regular culture media for 6 or 24 h. At the end of each time point, conditioned media was again prepared for immunoblot analysis as described above, and cells were washed and lysed as above.
Image management and statistical analysis.
Immunoblot and confocal micrographs were prepared in Photoshop CS5, and figures were constructed in Illustrator CS5.1 (Adobe Systems, San Jose, CA). All images were minimally processed, adjustments were applied equally across each image, and no adjustment was made that resulted in data loss. For immunoblot graphing and statistics, films were imaged and quantitated using ImageJ, and the t-test function in GraphPad5 (Graph Pad Software, La Jolla, CA) was used. For quantitation of signals in conditioned media, signals were normalized to the protein concentration from the correlated lysate. For quantitation of signals from lysate, signals were normalized to the signal from GAPDH or β-actin control staining.
RESULTS
Characterization of immunoaffinity-isolated primary human kidney epithelial cell cultures of proximal and distal tubule origin.
We have established cell culture model systems of primary human adult kidney (HAK) tubule epithelial cells of proximal and distal tubule origin from six separate subjects (HAK 3, 4, 10, 11, 14, and 15). In this study, proximal tubule origin refers to the tubule segments from the glomeruli to the bottom of the loop of Henle, and include the S1, 2, and 3 segments of the proximal convoluted and straight tubules, and the thin descending thin limb (DTL) of the loop of Henle. Similarly, distal tubule origin refers to tubule segments from the bottom of the loop of Henle to the collecting duct and includes the thick ascending limb (TAL), distal convoluted tubule (DCT), and the cortical collecting duct (CCD). The cortex of human kidneys was enzymatically and mechanically dissociated, and heterogeneous pools of viable cells were cultured to generate stocks. These heterogeneous cell populations were subjected to immunoaffinity isolation to generate separate cell cultures that were highly enriched for cells of proximal or distal tubule origin. Cells of proximal tubule origin were isolated using an antibody directed against APN, which is expressed across the proximal nephron and is a marker that has been used previously for immunoaffinity isolation of proximal tubule cells from human tissue (6, 67, 68). Cells of distal tubule origin were isolated using an antibody against the sialomucin MUC-1, which is expressed in the TAL, DCT, and CCD (5, 10, 43). We routinely isolated only 10–15% of cells from the total heterogeneous pool with either antibody, and the isolated cells were able to be passaged five to seven times before loss of proliferative ability. Cells were characterized at every passage and not used if evidence of trans-differentiation was found.
For characterization, lysates from these cells were compared by immunoblotting with lysates from the human proximal tubule epithelial cell line HK2 (ATCC) and cells from glomeruli isolated mechanically during tissue processing (GLOM; Fig. 1A). All cell types were tested for expression of the proximal markers sodium/hydrogen exchanger 3 (NHE3), GGT, and AQP1, and for the distal tubule marker E-CAD. NHE3 is expressed in the S1 and S2 segments of the proximal tubule, and in the TAL of some mammalian species (2, 8). GGT-1 is expressed in the latter portion of the proximal convoluted tubule and in the proximal straight tubule (11, 15, 32, 52). AQP1 is expressed in the S2 and S3 segments of the proximal tubule and the DTL (39, 55). E-CAD is expressed in Bowman’s capsule of the glomerulus, is not expressed in the proximal tubule, but is also expressed in the TAL, DCT, and CCD of the distal nephron (33, 40, 47). All three proximal tubule markers are detected in the HAK-APN cells, but the distal nephron marker E-CAD was not detected (Fig. 1A). Conversely, NHE3, GGT, and AQP1 were not detected in the HAK-MUC-1 cells, and E-CAD was detected, demonstrating that our systems do indeed isolate and separate cells of proximal and distal tubule origin. Additionally, both the alpha 1 subunit of the Na+-K+-ATPase (Na-K-ATPase), and the E subunit of the vacuolar H+-ATPase (V-ATPase), which are expressed throughout the nephron (1, 12, 23, 27, 53, 56, 64, 70), were detected in both proximal and distal tubule cell isolates, additionally demonstrating that our in vitro systems isolate and preserve cells that retain expression of multiple transport proteins. While both Na-K-ATPase and V-ATPase are not epithelial cell specific, immunoaffinity isolation of these cell populations with kidney tubule epithelial cell-specific antibodies strongly suggest that these blots represent epithelial cell expression. These data are supported by data (see Fig. 3) that demonstrate APN and MUC-1 staining only in tubule epithelial cells in vivo.
Fig. 3.
Tubule-specific expression of IGFBP7 and TIMP-2 in primary human kidney cortical tissue. Paraformaldehyde-fixed human kidney sections were subjected to double-label immunofluorescent staining for IGFBP7 and TIMP2 vs. the markers used for immunoaffinity isolation, APN and the sialomucin Muc-1 (MUC-1). Single-stain micrographs are shown along with a 3-color micrograph (MERGE+DAPI) to identify localization and nuclei. A: comparison of IGFBP7 staining (green) to the proximal tubule marker APN (red). The long arrows show an example of tubule colocalization of brush-border staining of IGFBP7 with APN, and the short arrows show examples of tubules stained with APN alone. B: comparison of IGFBP7 staining (green) to the distal tubule marker MUC-1 (red). Short arrows show examples of tubules stained with MUC-1 alone, and the arrowheads show an example of tubule staining of IGFBP7 alone. C: comparison of TIMP-2 staining (green) to the proximal tubule marker APN (red). Short arrows show an example of a tubule stained with APN alone, and the arrowheads show tubule staining of TIMP-2 alone. D: comparison of TIMP-2 staining (green) to the distal tubule marker MUC-1 (red). The long arrows show an example of tubule colocalization of TIMP-2 with MUC-1, and the arrowheads show tubule staining of TIMP-2 alone. E: representative staining of IGFBP7 and TIMP2 (green) in glomeruli. Glomeruli are identified by brackets, and TIMP2 staining was present in glomeruli (long arrows), while IGFBP7 staining was very low (short arrows) compared with IGFBP7 positive tubules (arrowheads). The representative secondary only control (E; SECONDARY ONLY) was imaged at the highest laser power, gain, and offset used for all images. Scale bar = 50 µm in all images.
Immunofluorescence analysis of cells cultured on Transwell permeable supports demonstrated that both HAK-APN and HAK-MUC-1 cells were capable of forming epithelial monolayers, as evidenced by uniform staining of the tight junction marker ZO-1 (Fig. 1B). The HAK-APN cells formed monolayers with larger cells and rough tight junction morphology, and the HAK-MUC-1 cells formed monolayers with small, tightly packed cells and a smooth tight junction morphology. The results shown are from HAK3; similar results were obtained from all samples used in this study.
Primary human kidney tubule epithelial cells of proximal and distal tubule origin express and secrete IGFBP7 and TIMP-2 differentially.
By immunoblot analysis, we identified that both IGFBP7 and TIMP-2 are expressed in immunoaffinit-isolated cells of proximal and distal tubule origin in culture (Fig. 2A). IGFBP7 was expressed at the highest level in both HAK-APN and HAK-MUC-1 cells compared with GLOM and HK2 cells. TIMP-2 demonstrated a different expression pattern, where GLOM, HK2, and HAK-MUC-1 cells expressed the most, but expression in HAK-APN cells was very low. Results shown are from HAK3; similar results were obtained from three other samples.
Fig. 2.

Expression and secretion of insulin-like growth factor binding protein 7 (IGFBP7) and tissue inhibitor of metalloproteinases-2 (TIMP-2) from immunoaffinity isolated primary human kidney epithelial cells of proximal and distal tubule origin. A: representative immunoblot analysis of IGFBP7, TIMP-2, and β-actin (loading control) expression in lysates from HAK isolated primary cells of proximal origin (HAK-APN) and distal origin (HAK-MUC-1), compared with cells from glomeruli isolated by retention on a 180-µm sieve (GLOM) and HK2 cells (HK2). B: representative immunoblot analysis of IGFBP7 and TIMP-2 secretion in conditioned media (24 h) from HAK-APN and HAK-MUC-1 cells compared with conditioned media from GLOM and HK2 cells and unconditioned media (UM). C: IGFBP7 and TIMP-2 densitometry values from 4 genetically separate HAK samples were adjusted to lysate protein concentrations and plotted normalized to the HAK-APN levels for both proteins.
We assessed secretion of these proteins in culture by immunoblot analysis of conditioned media (Fig. 2B). Indeed, both IGFBP7 and TIMP-2 were constitutively secreted by cultured primary human kidney epithelial cells, as evidenced by their presence in media conditioned by the cells through 24 h of exposure (GLOM, HK2, and HAK-APN/MUC-1) but not in unconditioned media that was never exposed to cells (UM). We identified a striking difference in the levels of secretion of both proteins between the HAK-APN and HAK-MUC-1 cells. TIMP-2 secretion mimicked its expression pattern, with high secretion from GLOM, HK2, and HAK-MUC-1 cells, and low secretion from HAK-APN cells. Interestingly, while IGFBP7 secretion mimicked its expression in GLOM and HK2 cells, its secretion from HAK-APN cells was much greater than from HAK-MUC-1 cells, despite a relatively equivalent expression pattern across the tubule cell types. Results shown in Fig. 2B are from HAK4. Quantitative analysis of the results from HAK4 and three additional human isolates identified that HAK-APN cells secreted fivefold more IGFBP7 than HAK-MUC-1 cells (P = 0.004), and HAK-MUC-1 cells secreted fivefold more TIMP-2 than HAK-APN cells (P = 0.0002) (Fig. 2C). These data demonstrate that IGFBP7 and TIMP-2 can be constitutively expressed and secreted by human tubule epithelial cells in culture and that there are clear differential expression and secretion patterns for both markers across cell types.
Expression of IGFBP7 and TIMP-2 in human kidney tissue.
Having seen differential expression and secretion in cell culture, we next examined expression of these proteins in human tissue. IGFBP7 and TIMP-2 staining was compared with the markers used for the in vitro immunoaffinity isolation (APN and MUC-1, Fig. 3), as well as to other markers for proximal and distal tubule epithelial cells (Figs. 4 and 5). For this study, five separate samples were assessed with consistent findings. IGFBP7 staining manifested as both a low level of cytoplasmic staining in most if not all tubules, contrasted by a very bright, luminal brush-border staining in a subset of tubules (Fig. 3, A and B). We reference the latter type when referring to IGFBP7 staining in vivo. TIMP-2 manifested as uniform cytoplasmic staining, very low in some tubules, and bright in others (Fig. 3, C and D). We refer to the bright staining as positive for TIMP-2. Only a fraction of all tubules stained for either marker, confirming that their expression is not a general phenomenon.
Fig. 4.
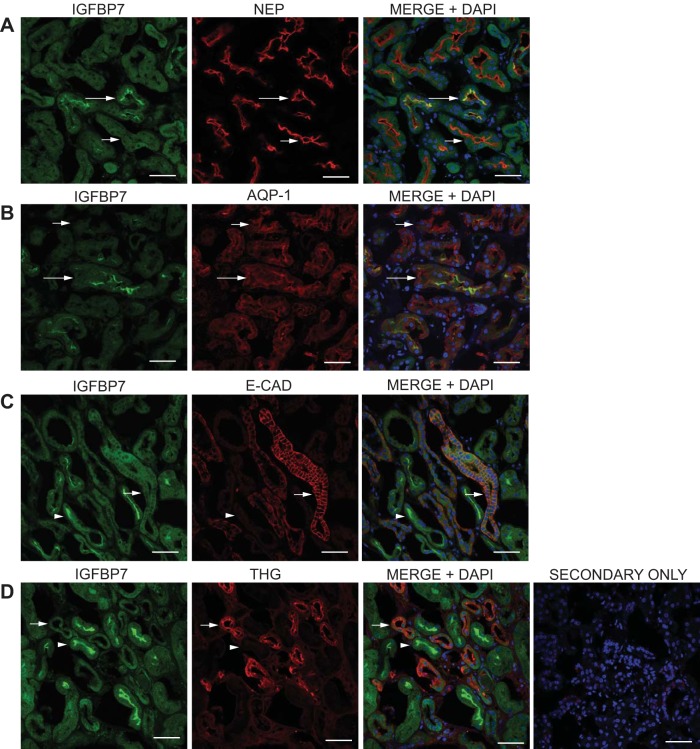
Additional analysis of proximal tubule-specific expression of IGFBP7 in primary human kidney cortical tissue. Kidney sections were prepared, stained, and analyzed as in Fig. 3, using the IGFBP7 antibody compared with antibodies directed against the proximal tubule markers neprilysin (NEP) and AQP1 and the distal tubule markers E-CAD and Tamm-Horsfall glycoprotein (THG). Single-stain micrographs are shown along with a three color micrograph (MERGE+DAPI) to identify localization and nuclei. A and B: IGFBP7 staining (green) was compared with the proximal tubule markers NEP and AQP1 (red). C and D: IGFBP7 staining (green) was compared with the distal tubule markers E-CAD and THG (red). This analysis supports the findings with APN and MUC-1 that the bright, luminal, brush-border staining of IGFBP7 in human tissue samples localizes in tubules of proximal origin and does not localize with tubules of distal origin. The representative secondary only control was imaged the highest laser power, gain, and offset used for all images. Scale bar = 50 µm.
Fig. 5.
Additional analysis of distal tubule-specific expression of TIMP-2 in primary human kidney cortical tissue. Kidney sections were prepared, stained, and analyzed as in Fig. 3, using the TIMP-2 antibody compared with antibodies directed against the proximal tubule markers NEP and AQP1 and the distal tubule markers E-CAD and THG. Single-stain micrographs are shown along with a 3-color micrograph (MERGE+DAPI) to identify localization and nuclei. A and B: TIMP-2 staining (green) was compared with the proximal tubule markers NEP and AQP-1 (red). C and D: TIMP-2 staining (green) was compared with the distal tubule markers E-CAD and THG (red). This analysis supports the findings with APN and MUC-1 that the bright cytoplasmic staining of TIMP-2 in human tissue samples localizes in tubules of distal origin and does not localize with tubules of proximal origin. The representative secondary only control was imaged the highest laser power, gain, and offset used for all images. Scale bar = 50 µm.
Tubular colocalization of IGFBP7 with the proximal tubule marker APN was observed (Fig. 3A, long arrows), but, interestingly, this colocalization was observed in only a small number of APN-stained tubules (Fig. 3A, short arrows). In contrast, when IGFBP7is compared to the distal tubule marker MUC-1, most tubules stained for either IGFBP7 alone (Fig. 3B, arrowheads, or MUC-1 alone (Fig. 3B, short arrows). We did observe a very low level of IGFBP7 staining in the absence of APN staining, and also observed a very low level of partial tubule colocalization of IGFBP7 with MUC-1 (data not shown). Therefore, while some APN-unstained or MUC-1-stained cells may be capable of staining for IGFBP7, it is clearly a minor event.
Comparison of TIMP-2 with the proximal marker APN demonstrated a complete lack of colocalization (Fig. 3C, arrowheads and short arrows, respectively). TIMP-2 did colocalize in tubules with MUC-1 in all micrographs assessed (Fig. 3D, long arrows), yet tubules with TIMP-2 in the absence of MUC-1 could also routinely be identified (Fig. 3D, arrowheads). Evaluation of IGFBP7 and TIMP-2 expression in glomeruli also supported the in vitro findings. TIMP-2 expression in glomeruli was similar to levels of expression in MUC-1-stained tubules (Fig. 3E, TIMP-2/MUC-1 GLOM, long arrows), as was seen in vitro (Fig. 2). Conversely, only a very low level of IGFBP7 staining was identified in glomeruli in contrast to the general cytoplasmic and bright brush-border staining found in APN-stained tubules (Fig. 3E, IGFBP7/APN GLOM, short arrows and arrowheads, respectively).
To further validate the proximal/distal variability of IGFBP7 and TIMP-2 expression in tissue, we compared them to additional markers (Figs. 4 and 5 respectively). As with APN, IGFBP7 exhibited tubule colocalization with the proximal markers neprilysin (NEP), which is expressed in glomeruli and the proximal convoluted tubule (49, 67), and AQP1 (Fig. 4, A and B, long arrows), but only with a small number of tubules that stained for these markers, as many NEP- or AQP1-stained tubules could be seen in the absence of IGFBP7 staining (Fig. 4, A and B, short arrows). Comparing IGFBP7 staining with the distal tubule markers E-CAD and THG, which is expressed in the TAL and the DCT (24, 60, 61), demonstrated a complete lack of colocalization of the bright luminal brush-border staining of IGFBP7 (Fig. 4, C and D, arrowheads) with these distal markers (Fig. 4, C and D, short arrows). The combined data from Figs. 3 and 4 clearly demonstrate that the bright, luminal brush-border staining of IGFBP7 is present in proximal tubules but not distal tubules and suggests that this staining pattern exists throughout the proximal nephron from the proximal convoluted tubule to the DTL.
TIMP-2 staining was completely separate from NEP and AQP1 staining (Fig. 5, A and B, arrowheads vs. short arrows) but exhibited tubule colocalization with E-CAD and THG in all confocal micrographs examined (Fig. 5, C and D, long arrows). A small fraction of TIMP-2-stained tubules could be identified in tubules that were unstained for E-CAD (data not shown), and every micrograph examined showed tubules that were stained for TIMP-2 but not THG (Fig. 5D, arrowheads). These data, in combination with the results from Figs. 1 and 3, suggest that TIMP2 expression spans the distal nephron, across MUC-1-, E-CAD-, and THG-positive and -negative segments.
The combined results from Figs. 1–5 have utilized five separate proximal tubule markers and three separate distal tubule markers, through immunoaffinity isolation and characterization by immunoblotting and immunofluorescence, both in vitro and in vivo. As such, these results clearly define cells of proximal vs. distal tubule origin and provide strong evidence that IGFBP7 expression is consistent across tubule types, yet its secretion is a primarily a proximal tubule phenomena, while TIMP-2 expression and secretion are distal tubule phenomena.
IGFBP7 and TIMP-2 staining vs. staining of the injury markers KIM-1 and neutrophil gelatinase-associated lipocalin-2 in human tissue
KIM-1 is upregulated in tubule epithelial cells in response to injury induced by multiple insults and is primarily known as a proximal tubule injury marker (22, 25, 48). Neutrophil gelatinase-associated lipocalin-2 (NGAL) was originally identified as a neutrophil secondary granule protein and subsequently shown to be an early urine biomarker for renal injury. It has been reported in both proximal tubules (38) and in various locations in the distal nephron (30, 31). Since we received kidneys that were deemed not suitable for transplant, we anticipated that they would have some degree of damage, and thus potentially express these injury markers, allowing the opportunity to characterize IGFBP7 and TIMP-2 expression relative to known markers of injury. Table 1 lists the wedge biopsy reports (when available) of the samples used in this study. In all samples in which reports were available, interstitial fibrosis was present, albeit mild, suggesting that there was likely some degree of tubular injury. Four of the five available reports also list some degree of inflammation or tubular injury, further supporting our anticipation that KIM-1 and/or NGAL expression should be present. KIM-1 staining manifested as clear luminal brush-border staining as well as punctate intracellular staining, and NGAL manifested primarily as punctate intracellular staining.
Table 1.
Wedge biopsy reports of kidneys used in this study
| Sample | Sclerotic Glomeruli | Interstitial Fibrosis | Arterial Nephrosclerosis | Arteriolonephrosclerosis | Glomerular Thrombi | Comments and Other Findings |
|---|---|---|---|---|---|---|
| HAK 3* | NA | NA | NA | NA | NA | NA |
| HAK 4 | 3 of 55 | Mild | Mild | Mild | 0 of 55 | Minimal interstitial inflammation, no thrombi |
| HAK 10 | 18 of 56 | Mild | Mild | Moderate | None | Subcapsular scarring, chronic inflammation |
| HAK 11 | 3 of 78 | Mild | Mild | Mild | None | ** |
| HAK 14 | 19 of 81 | Mild | Moderate | Moderate | None | Mild chronic interstitial, focal, bilateral |
| Focal acute tubular injury | ||||||
| HAK 15 | 25 of 129 | Mild | Moderate | Mild-moderate | None | Occasional tubules with intratubular neutrophils, bilateral |
| Patchy acute tubular injury, mild chronic interstitial inflammation |
HAK, human adult kidney; NA, not applicable. Table lists the complete data from the University of Pittsburgh Medical Center Division of Transplantation and Hepatic Pathology Kidney Biopsy Form. Interstitial fibrosis, arterial nephrosclerosis, and arteriolonephrosclerosis were graded according to Banff 1997 criteria.
No biopsy was performed; kidneys were recovered solely for research.
No additional comments were provided in the “comments and other findings” section of the biopsy report.
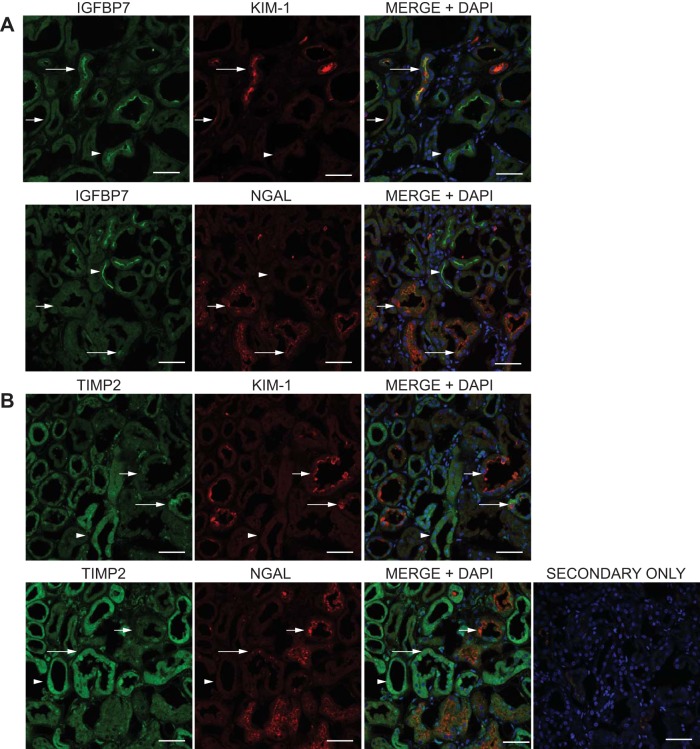
Like IGFBP7 and TIMP-2, individual KIM-1 and NGAL staining were present only in some of the tubules examined. Marker comparison results are demonstrated in Fig. 6 and Table 2. While tubule colocalization of IGFBP7 and KIM-1 could be observed (Fig. 6A long arrows), tubules with IGFBP7 staining only (arrowheads) could routinely be observed, as could tubules with individual cell KIM-1 staining alone (short arrows). In a comparison of IGFBP7 with NGAL, the majority of tubules were IGFBP7 or NGAL alone (arrowheads and short arrows, respectively), and colocalization manifested only as single-cell or partial tubule localization (long arrows). In contrast to IGFBP7, comparison of TIMP-2 with KIM-1 demonstrated that the majority of tubules were TIMP-2 or KIM-1 only (Fig. 6B, arrowheads and short arrows, respectively), and colocalization was infrequent and partial (long arrows, and Table 2). Surprisingly, a similar pattern was observed when TIMP-2 was compared with NGAL, where TIMP-2 or NGAL only tubules predominated (arrowheads and short arrows, respectively), and colocalization was present only in individual cells or portions of the tubule (long arrows), and demonstrated the lowest of all marker combinations assessed (Table 2).
Fig. 6.
Comparison of IGFBP7 and TIMP-2 with kidney injury molecule 1 (KIM-1) and NGAL staining in human kidney tissue. Kidney sections were prepared, stained, and analyzed as in Fig. 3, using the IGFBP7 and TIMP-2 antibodies compared with antibodies directed against the kidney injury markers KIM-1 and NGAL. Single-stain micrographs are shown along with a 3-color micrograph (MERGE+DAPI) to identify localization and nuclei. A: comparison of IGFBP7 staining (green) with KIM-1 and NGAL (red). The long arrows show examples of tubules with tubule colocalization of IGFBP7 with KIM-1 or NGAL, the short arrows show examples of tubules with KIM-1 or NGAL staining only, and the arrowheads demonstrate tubules with brush-border staining of IGFBP7 alone. B: comparison of TIMP-2 staining with KIM-1 and NGAL. The long arrows show examples of tubules with tubule colocalization of TIMP-2 with KIM-1 or NGAL, the short arrows show examples of tubules with KIM-1 or NGAL staining only, and the arrowheads demonstrate tubules with cytoplasmic staining of TIMP-2 alone. The representative secondary only was imaged at the highest laser power, gain, and offset used for all images. Scale bar = 50 µm for all images.
Table 2.
Localization of IGFBP7 and TIMP-2 with KIM-1 and NGAL in primary human tissue samples
| Number of Marker-Positive Tubules | Tubules with IGFBP7 or TIMP2 Only | Tubules with KIM-1 or NGAL Only | Tubules with Marker Tubule Colocalization | |
|---|---|---|---|---|
| IGFBP7 vs. KIM-1 | 366 | 100 (0.27) | 113 (0.31) | 153 (0.42) |
| IGFBP7 vs. NGAL | 620 | 222 (0.36) | 278 (0.45) | 120 (0.19) |
| TIMP2 vs. KIM-1 | 447 | 278 (0.62) | 116 (0.26) | 53 (0.12) |
| TIMP2 vs. NGAL | 455 | 175 (0.39) | 238 (0.52) | 42 (0.09) |
IGFBP7, insulin-like growth factor binding protein 7; TIMP-2, tissue inhibitor of metalloproteinases-2; KIM-1, kidney injury molecule-1; NGAL, neutrophil gelatinase-associated lipocalin. Individual tubules in 30–50 confocal images of markers from 3 to 4 genetically separate samples were counted and graded for marker positivity. The number in parenthesis is the percentage of the presence of that subset from the total number of positive tubules.
Effect of oxygen-nutrient and nutrient deprivation on the expression and secretion of IGFBP7 and TIMP-2 in vitro
Having identified the constitutive expression and secretion characteristics of IGFBP7 and TIMP-2 in our in vitro system, and analyzed their expression in relation to KIM-1 and NGAL in vivo, we assessed whether we could identify modulation of expression and secretion of these molecules by cellular insult. A common in vitro model used in studies of AKI is oxygen-glucose deprivation, used to mimic ischemia-reperfusion injury. We performed oxygen-nutrient and nutrient deprivation alone experiments (so-named because we assessed total nutrient deprivation, not just glucose deprivation), and assessed its effects on IGFBP7 and TIMP-2 expression and secretion in APN and MUC-1 cells from two separate samples, HAK 4 and 11. Cells were subjected to either oxygen-nutrient, or nutrient alone deprivation for 24 h and then “reperfused” by culture in oxygenated regular culture media for 6 and 24 h. Conditioned media from the deprivation period, and conditioned media and lysate from the 6- and 24-h reperfusion periods were assessed by immunoblotting; results are illustrated in Fig. 7. Oxygen-nutrient and nutrient deprivation alone suppressed the constitutive secretion of IGFBP7 and TIMP-2 in both proximal and distal tubule cells compared with nondeprived cells, with the oxygen-nutrient deprivation exerting the greatest effect (Fig. 7A, O and N vs. R). Conversely, restoring oxygen and nutrients to the cells induced a burst of IGFBP7 and TIMP-2 secretion in both oxygen-nutrient and nutrient deprivation cells alone after 6 h (Fig. 7B, CM). This burst of secretion was associated with a concomitant decrease in intracellular protein as evidenced by analysis of cell lysates (Fig. 7B, LYSATE). Of particular interest is that a burst of IGFBP7 secretion was also observed with oxygen-nutrient deprivation in the MUC-1 cells, which constitutively secrete a very low level of this protein (Fig. 7B, MUC-1 and IGFBP7 CM, and Fig. 2). IGFBP7 results were consistent across both samples analyzed, TIMP-2 results were variable at 6 h after restoring oxygen and nutrients, with one sample demonstrating the burst of secretion as shown, and the other demonstrating a relatively mild response. After 24 h of reperfusion, secretion levels of both proteins in both samples assessed normalized across the treatment types, despite a continued reduction in intracellular IGFBP7 in both proximal and distal tubule cells (Fig. 7C).
Fig. 7.
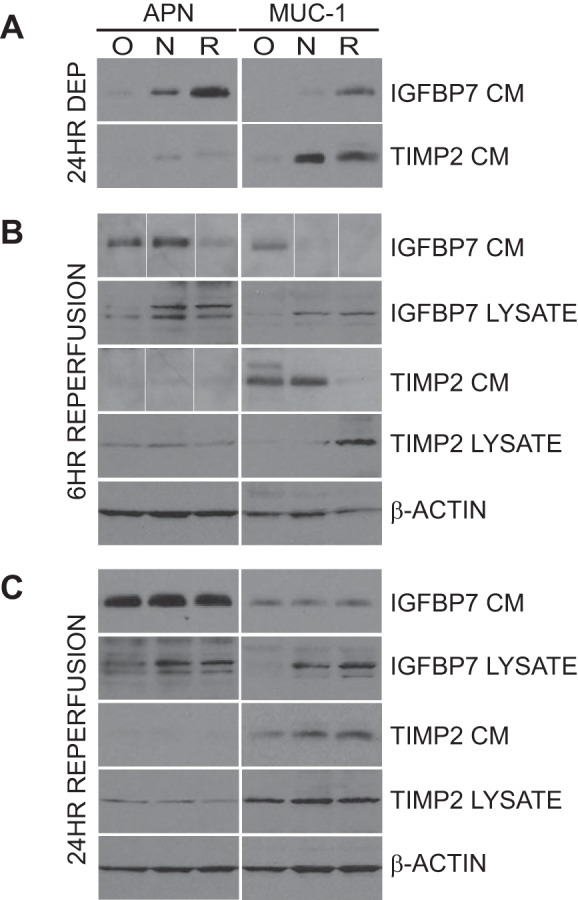
Effect of oxygen-nutrient deprivation and reperfusion on the expression and secretion of IGFBP7 and TIMP2 in cells of proximal and distal tubule origin. Proximal tubule (APN) and distal tubule (MUC-1) cells were prepared as in Fig. 1 and subjected to 24 h of oxygen-nutrient, nutrient deprivation alone, or no deprivation (24HR DEP; O, N, R, respectively) followed by 6 or 24 h of reperfusion by culture in regular culture media (6 HR REPERFUSION and 24 HR REPERFUSION). A: conditioned media (CM) after 24 h of deprivation (24HR DEP) were compared with media from cells cultured under normal culture conditions for the same period to assess the secretion of IGFBP7 and TIMP2. B: all conditions were reperfused in regular culture media for 6 h, and expression (LYSATE) and secretion (CM) of IGFBP7 and TIMP2 were assessed by immunoblot analysis. β-Actin was used as a loading control. The white lines between the lanes in some CM images denote noncontiguous lanes. These blots are composite images with individual lanes reorganized for the sake of presentation. The lanes in each image are compiled from the same blot and same film exposure, and no modification to individual lanes was performed. C: all conditions were reperfused in regular culture media for 24 h, and expression (LYSATE) and secretion (CM) of IGFBP7 and TIMP2 were assessed by immunoblot analysis. β-Actin was used as a loading control. Media and lysate from HAK4 are shown.
DISCUSSION
To our knowledge, this is the first report of cell culture expression and secretion of IGFBP7 and TIMP-2 in human primary kidney epithelial cells of proximal and distal tubule origin, differential expression/secretion in cell culture and in tissue, and effects of ischemic insult on expression and secretion in vitro. While it is possible that these urine AKI biomarkers could increase as a result of increased expression outside the kidney with increased filtration through the glomerulus, our demonstration of direct constitutive expression/secretion, and modulation by ischemic insult in kidney tubule cells in vitro supports the idea that increased levels in the urine during AKI are a result of the kidney’s response to injury or stress.
In cell culture, we identified equivalent expression yet variable secretion of IGFBP7 in proximal vs. distal tubule cells, and we are not aware of any prior work that has demonstrated such phenomena in epithelial cells of any type. In tissue, the IGFBP7 luminal brush-border staining was patchy, and while clearly found only in proximal tubules, was seen only in a fraction of proximal tubule cells. While this may suggest that IGFBP7 can identify a subsection of the normal proximal nephron, it may alternatively identify sections of proximal tubules that are responding to insult and may thus serve as a marker of insult, injury, or alarm. Indeed, this staining pattern is similar to the heterogeneous or patchy tubular cell injury histology of AKI in humans (51). While we cannot directly attribute the IGFBP7 staining in tissue to any response to insult, or to the constitutive HAK-APN cell secretion seen in vitro, this does demonstrate that increased IGFBP7 expression can occur in proximal tubules in vivo. In stark contrast to IGFBP7, our studies demonstrate a preferential constitutive expression and secretion of TIMP-2 in distal tubule cells over proximal tubule cells, both in cell culture and in tissue. While there was tubule colocalization with all distal markers used in tissue, there were also frequent TIMP-2 only tubules, suggesting that TIMP-2 expression may be ubiquitous throughout the distal nephron. Combined, these phenomena suggest differential mechanisms of regulation and function across tubule types. These findings might lend insight into the value of the individual markers regarding different etiologies of AKI, as IGFBP7 alone was found to be superior in surgical patients and TIMP-2 superior in sepsis (26), as well as transplantation (19).
The comparison of these AKI biomarkers with the injury markers KIM-1 and NGAL is also intriguing and informative (Fig. 6 and Table 2), as tubules with either IGFBP7 or TIMP-2 staining alone could routinely be identified. If the bright IGFBP7 staining is a manifestation of effects from insult, these data then suggest that the mechanisms involved in IGFBP7 upregulation are clearly different from those that regulate KIM-1 or NGAL. These data additionally allow for the possibility that IGFBP7 upregulation occurs on a different and potentially earlier temporal course. It is difficult to make inferences regarding any potential injury-induced relationship for TIMP-2 in vivo as no clear differential staining of TIMP-2 within distal tubules was identified, and secretion cannot be assessed in vivo.
Last, the in vitro oxygen-nutrient deprivation studies of Fig. 7 demonstrate that indeed, expression and secretion of both proteins can be modulated by cellular insults. Secretion of both proteins is suppressed during oxygen-nutrient deprivation, yet significantly increased early after restoration of normal cell culture, suggesting a biological role for both in the response to tubule cell stress. Of interest is that nutrient deprivation alone is capable of eliciting these responses, indicating that these biomarkers can be modulated by insults other than ischemia. These results also demonstrate the value of our in vitro model system for the investigation of the molecular basis of AKI.
IGFBP7 has been implicated in multiple biological processes including angiogenesis, tumor suppression, and senescence, mainly as a growth suppressor, and a role in G1 cell-cycle arrest has been implicated (3, 7, 44, 54, 59, 69). TIMP-2 has been implicated positively in kidney pathologies including cancer, glomerular disease, fibrosis, and tubule-interstitial injury, in a matrix metalloproteinase (MMP)-dependent manner (16, 29, 45, 71). However, like IGFBP7, it too has been implicated in G1 cell cycle arrest in various cell types, in an MMP-independent manner (46, 58, 66). As such, we have hypothesized that the increased secretion of IGFBP7 and TIMP-2 in AKI may serve as early “alarm” signals and act in an autocrine or paracrine fashion to induce G1 cell-cycle arrest in kidney tubule epithelial cells to prevent the proliferation of injured cells (26, 37). The identification of expression and secretion in kidney tubule cells, the patchy staining pattern of IGFBP7 in vivo, the variable colocalization with injury markers, and the demonstration of early increased secretion of both markers after cellular injury all lend well to this hypothesis.
In conclusion, we have demonstrated that the AKI urine biomarkers IGFBP7 and TIMP-2 are expressed and secreted in human primary kidney epithelial cells of proximal and distal tubule origin in cell culture and tissue, where IGFBP7 is secreted mostly from proximal tubule cells and TIMP-2 is expressed and secreted mostly by distal tubule cells. We have also identified a variable expression pattern of IGFBP7 within proximal tubules that can be found with and without tubule colocalization of KIM-1 and NGAL. Last, we identified that both proteins are modulated by oxygen-nutrient and nutrient deprivation alone in vitro. These findings are very intriguing and may begin to shed light on the role and mechanisms of these markers in disease. These findings further support the rationale for investigation of these proteins in the kidney and generate interesting hypotheses regarding the potential biological role of these biomarkers in the development and progression of and/or protection/recovery from AKI.
GRANTS
This work was supported in part by intramural funding from the Center for Critical Care Nephrology and a grant from Astute Medical (JAK), as well as P30 DK079307 (Pittsburgh Kidney Research Center) and R01 DK084184 (NPS).
DISCLOSURES
J. A. Kellum has received consulting fees and research funding from Astute Medical. The University of Pittsburgh has sought patent protection for some uses of TIMP-2 and IGFBP7.
AUTHOR CONTRIBUTIONS
D.R.E. conceived and designed research; D.R.E., A.M., S.M., and J.K.V. performed experiments; D.R.E., N.P.-S., X.W., H.G., W.H.H., and J.A.K. analyzed data; D.R.E., N.P.-S., X.W., H.G., W.H.H., and J.A.K. interpreted results of experiments; D.R.E. and W.H.H. prepared figures; D.R.E. drafted manuscript; D.R.E., N.P.-S., A.M., H.G., W.H.H., J.K.V., and J.A.K. edited and revised manuscript; J.A.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge Dr. Kenneth Hallows from the Keck School of Medicine and Dr. Agnieszka Swiatecka-Urban from the University of Pittsburgh Children’s Hospital for expert advice. The authors also thank the Center for Organ Recovery and Education for excellent collaboration.
Present address of N. Pastor-Soler: Div. of Nephrology and Hypertension, USC/UKRO Kidney Research Center, Keck School of Medicine of the University of Southern California, Los Angeles, CA.
REFERENCES
- 1.Al-bataineh MM, Gong F, Marciszyn AL, Myerburg MM, Pastor-Soler NM. Regulation of proximal tubule vacuolar H+-ATPase by PKA and AMP-activated protein kinase. Am J Physiol Renal Physiol 306: F981–F995, 2014. doi: 10.1152/ajprenal.00362.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amemiya M, Loffing J, Lötscher M, Kaissling B, Alpern RJ, Moe OW. Expression of NHE-3 in the apical membrane of rat renal proximal tubule and thick ascending limb. Kidney Int 48: 1206–1215, 1995. doi: 10.1038/ki.1995.404. [DOI] [PubMed] [Google Scholar]
- 3.Amemiya Y, Yang W, Benatar T, Nofech-Mozes S, Yee A, Kahn H, Holloway C, Seth A. Insulin like growth factor binding protein-7 reduces growth of human breast cancer cells and xenografted tumors. Breast Cancer Res Treat 126: 373–384, 2011. doi: 10.1007/s10549-010-0921-0. [DOI] [PubMed] [Google Scholar]
- 4.Asanuma K, Shirato I, Ishidoh K, Kominami E, Tomino Y. Selective modulation of the secretion of proteinases and their inhibitors by growth factors in cultured differentiated podocytes. Kidney Int 62: 822–831, 2002. doi: 10.1046/j.1523-1755.2002.00539.x. [DOI] [PubMed] [Google Scholar]
- 5.Aubert S, Fauquette V, Hémon B, Lepoivre R, Briez N, Bernard D, Van Seuningen I, Leroy X, Perrais M. MUC1, a new hypoxia inducible factor target gene, is an actor in clear renal cell carcinoma tumor progression. Cancer Res 69: 5707–5715, 2009. doi: 10.1158/0008-5472.CAN-08-4905. [DOI] [PubMed] [Google Scholar]
- 6.Baer PC, Nockher WA, Haase W, Scherberich JE. Isolation of proximal and distal tubule cells from human kidney by immunomagnetic separation. Technical note. Kidney Int 52: 1321–1331, 1997. doi: 10.1038/ki.1997.457. [DOI] [PubMed] [Google Scholar]
- 7.Benatar T, Yang W, Amemiya Y, Evdokimova V, Kahn H, Holloway C, Seth A. IGFBP7 reduces breast tumor growth by induction of senescence and apoptosis pathways. Breast Cancer Res Treat 133: 563–573, 2012. doi: 10.1007/s10549-011-1816-4. [DOI] [PubMed] [Google Scholar]
- 8.Biemesderfer D, Pizzonia J, Abu-Alfa A, Exner M, Reilly R, Igarashi P, Aronson PS. NHE3: a Na+/H+ exchanger isoform of renal brush border. Am J Physiol Renal Fluid Electrolyte Physiol 265: F736–F742, 1993. [DOI] [PubMed] [Google Scholar]
- 9.Bihorac A, Chawla LS, Shaw AD, Al-Khafaji A, Davison DL, Demuth GE, Fitzgerald R, Gong MN, Graham DD, Gunnerson K, Heung M, Jortani S, Kleerup E, Koyner JL, Krell K, Letourneau J, Lissauer M, Miner J, Nguyen HB, Ortega LM, Self WH, Sellman R, Shi J, Straseski J, Szalados JE, Wilber ST, Walker MG, Wilson J, Wunderink R, Zimmerman J, Kellum JA. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med 189: 932–939, 2014. doi: 10.1164/rccm.201401-0077OC. [DOI] [PubMed] [Google Scholar]
- 10.Braga VM, Pemberton LF, Duhig T, Gendler SJ. Spatial and temporal expression of an epithelial mucin, Muc-1, during mouse development. Development 115: 427–437, 1992. [DOI] [PubMed] [Google Scholar]
- 11.Brière N, Martel M, Plante G, Petitclerc C. Heterogeneous distribution of alkaline phosphatase and gamma-glutamyl transpeptidase in the mouse nephron. Acta Histochem 74: 103–108, 1984. doi: 10.1016/S0065-1281(84)80035-5. [DOI] [PubMed] [Google Scholar]
- 12.Brown D, Hirsch S, Gluck S. Localization of a proton-pumping ATPase in rat kidney. J Clin Invest 82: 2114–2126, 1988. doi: 10.1172/JCI113833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carome MA, Striker LJ, Peten EP, Moore J, Yang CW, Stetler-Stevenson WG, Striker GE. Human glomeruli express TIMP-1 mRNA and TIMP-2 protein and mRNA. Am J Physiol Renal Fluid Electrolyte Physiol 264: F923–F929, 1993. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Wang J, Zhou F, Wang X, Feng Z. STAT proteins mediate angiotensin II-induced production of TIMP-1 in human proximal tubular epithelial cells. Kidney Int 64: 459–467, 2003. doi: 10.1046/j.1523-1755.2003.00133.x. [DOI] [PubMed] [Google Scholar]
- 15.Curthoys NP, Hughey RP. Characterization and physiological function of rat renal gamma-glutamyltranspeptidase. Enzyme 24: 383–403, 1979. [DOI] [PubMed] [Google Scholar]
- 16.Czech KA, Bennett M, Devarajan P. Distinct metalloproteinase excretion patterns in focal segmental glomerulosclerosis. Pediatr Nephrol 26: 2179–2184, 2011. doi: 10.1007/s00467-011-1897-7. [DOI] [PubMed] [Google Scholar]
- 17.Degeorges A, Wang F, Frierson HF Jr, Seth A, Sikes RA. Distribution of IGFBP-rP1 in normal human tissues. J Histochem Cytochem 48: 747–754, 2000. doi: 10.1177/002215540004800603. [DOI] [PubMed] [Google Scholar]
- 18.Dewitte A, Joannès-Boyau O, Sidobre C, Fleureau C, Bats ML, Derache P, Leuillet S, Ripoche J, Combe C, Ouattara A. Kinetic eGFR and Novel AKI Biomarkers to Predict Renal Recovery. Clin J Am Soc Nephrol 10: 1900–1910, 2015. doi: 10.2215/CJN.12651214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Endre ZH, Pickering JW, Walker RJ, Devarajan P, Edelstein CL, Bonventre JV, Frampton CM, Bennett MR, Ma Q, Sabbisetti VS, Vaidya VS, Walcher AM, Shaw GM, Henderson SJ, Nejat M, Schollum JB, George PM. Improved performance of urinary biomarkers of acute kidney injury in the critically ill by stratification for injury duration and baseline renal function. Kidney Int 79: 1119–1130, 2011. doi: 10.1038/ki.2010.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esposito C, Parrilla B, Cornacchia F, Grosjean F, Mangione F, Serpieri N, Valentino R, Villa L, Arra M, Esposito V, Dal Canton A. The antifibrogenic effect of hepatocyte growth factor (HGF) on renal tubular (HK-2) cells is dependent on cell growth. Growth Factors 27: 173–180, 2009. doi: 10.1080/08977190902834077. [DOI] [PubMed] [Google Scholar]
- 21.Gocze I, Koch M, Renner P, Zeman F, Graf BM, Dahlke MH, Nerlich M, Schlitt HJ, Kellum JA, Bein T. Urinary biomarkers TIMP-2 and IGFBP7 early predict acute kidney injury after major surgery. PLoS One 10: e0120863, 2015. doi: 10.1371/journal.pone.0120863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int 62: 237–244, 2002. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 23.Hennigar RA, Garvin AJ, Hazen-Martin DJ, Schulte BA. Immunohistochemical localization of transport mediators in Wilms’ tumor: comparison with fetal and mature human kidney. Lab Invest 61: 192–201, 1989. [PubMed] [Google Scholar]
- 24.Hoyer JR, Sisson SP, Vernier RL. Tamm-Horsfall glycoprotein: ultrastructural immunoperoxidase localization in rat kidney. Lab Invest 41: 168–173, 1979. [PubMed] [Google Scholar]
- 25.Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem 273: 4135–4142, 1998. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 26.Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, Bihorac A, Birkhahn R, Cely CM, Chawla LS, Davison DL, Feldkamp T, Forni LG, Gong MN, Gunnerson KJ, Haase M, Hackett J, Honore PM, Hoste EA, Joannes-Boyau O, Joannidis M, Kim P, Koyner JL, Laskowitz DT, Lissauer ME, Marx G, McCullough PA, Mullaney S, Ostermann M, Rimmelé T, Shapiro NI, Shaw AD, Shi J, Sprague AM, Vincent JL, Vinsonneau C, Wagner L, Walker MG, Wilkerson RG, Zacharowski K, Kellum JA. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 17: R25, 2013. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kashgarian M, Biemesderfer D, Caplan M, Forbush B III. Monoclonal antibody to Na,K-ATPase: immunocytochemical localization along nephron segments. Kidney Int 28: 899–913, 1985. doi: 10.1038/ki.1985.216. [DOI] [PubMed] [Google Scholar]
- 28.Koyner JL, Shaw AD, Chawla LS, Hoste EA, Bihorac A, Kashani K, Haase M, Shi J, Kellum JA; Sapphire Investigators . Tissue Inhibitor Metalloproteinase-2 (TIMP-2)⋅IGF-Binding Protein-7 (IGFBP7) Levels Are Associated with Adverse Long-Term Outcomes in Patients with AKI. J Am Soc Nephrol 26: 1747–1754, 2015. doi: 10.1681/ASN.2014060556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kugler A, Hemmerlein B, Thelen P, Kallerhoff M, Radzun HJ, Ringert RH. Expression of metalloproteinase 2 and 9 and their inhibitors in renal cell carcinoma. J Urol 160: 1914–1918, 1998. doi: 10.1016/S0022-5347(01)62443-1. [DOI] [PubMed] [Google Scholar]
- 30.Kuwabara T, Mori K, Mukoyama M, Kasahara M, Yokoi H, Saito Y, Yoshioka T, Ogawa Y, Imamaki H, Kusakabe T, Ebihara K, Omata M, Satoh N, Sugawara A, Barasch J, Nakao K. Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Kidney Int 75: 285–294, 2009. doi: 10.1038/ki.2008.499. [DOI] [PubMed] [Google Scholar]
- 31.Langelueddecke C, Roussa E, Fenton RA, Wolff NA, Lee WK, Thévenod F. Lipocalin-2 (24p3/neutrophil gelatinase-associated lipocalin (NGAL)) receptor is expressed in distal nephron and mediates protein endocytosis. J Biol Chem 287: 159–169, 2012. doi: 10.1074/jbc.M111.308296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lebargy F, Bulle F, Siegrist S, Guellaen G, Bernaudin JF. Localization by in situ hybridization of gamma-glutamyl transpeptidase mRNA in the rat kidney using 35S-labeled RNA probes. Lab Invest 62: 731–735, 1990. [PubMed] [Google Scholar]
- 33.Lee SY, Han SM, Kim JE, Chung KY, Han KH. Expression of E-cadherin in pig kidney. J Vet Sci 14: 381–386, 2013. doi: 10.4142/jvs.2013.14.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin J, Knowlden J, Davies M, Williams JD. Identification and independent regulation of human mesangial cell metalloproteinases. Kidney Int 46: 877–885, 1994. doi: 10.1038/ki.1994.345. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto T, Hess S, Kajiyama H, Sakairi T, Saleem MA, Mathieson PW, Nojima Y, Kopp JB. Proteomic analysis identifies insulin-like growth factor-binding protein-related protein-1 as a podocyte product. Am J Physiol Renal Physiol 299: F776–F784, 2010. doi: 10.1152/ajprenal.00597.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meersch M, Schmidt C, Van Aken H, Martens S, Rossaint J, Singbartl K, Görlich D, Kellum JA, Zarbock A. Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS One 9: e93460, 2014. doi: 10.1371/journal.pone.0093460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meersch M, Schmidt C, Van Aken H, Rossaint J, Görlich D, Stege D, Malec E, Januszewska K, Zarbock A. Validation of cell-cycle arrest biomarkers for acute kidney injury after pediatric cardiac surgery. PLoS One 9: e110865, 2014. doi: 10.1371/journal.pone.0110865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14: 2534–2543, 2003. doi: 10.1097/01.ASN.0000088027.54400.C6. [DOI] [PubMed] [Google Scholar]
- 39.Nielsen S, Pallone T, Smith BL, Christensen EI, Agre P, Maunsbach AB. Aquaporin-1 water channels in short and long loop descending thin limbs and in descending vasa recta in rat kidney. Am J Physiol Renal Physiol 268: F1023–F1037, 1995. [DOI] [PubMed] [Google Scholar]
- 40.Nouwen EJ, Dauwe S, van der Biest I, De Broe ME. Stage- and segment-specific expression of cell-adhesion molecules N-CAM, A-CAM, and L-CAM in the kidney. Kidney Int 44: 147–158, 1993. doi: 10.1038/ki.1993.225. [DOI] [PubMed] [Google Scholar]
- 41.Orphanides C, Fine LG, Norman JT. Hypoxia stimulates proximal tubular cell matrix production via a TGF-beta1-independent mechanism. Kidney Int 52: 637–647, 1997. doi: 10.1038/ki.1997.377. [DOI] [PubMed] [Google Scholar]
- 42.Osman I, Bajorin DF, Sun TT, Zhong H, Douglas D, Scattergood J, Zheng R, Han M, Marshall KW, Liew CC. Novel blood biomarkers of human urinary bladder cancer. Clin Cancer Res 12: 3374–3380, 2006. doi: 10.1158/1078-0432.CCR-05-2081. [DOI] [PubMed] [Google Scholar]
- 43.Pastor-Soler NM, Sutton TA, Mang HE, Kinlough CL, Gendler SJ, Madsen CS, Bastacky SI, Ho J, Al-Bataineh MM, Hallows KR, Singh S, Monga SP, Kobayashi H, Haase VH, Hughey RP. Muc1 is protective during kidney ischemia-reperfusion injury. Am J Physiol Renal Physiol 308: F1452–F1462, 2015. doi: 10.1152/ajprenal.00066.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pen A, Moreno MJ, Durocher Y, Deb-Rinker P, Stanimirovic DB. Glioblastoma-secreted factors induce IGFBP7 and angiogenesis by modulating Smad-2-dependent TGF-beta signaling. Oncogene 27: 6834–6844, 2008. doi: 10.1038/onc.2008.287. [DOI] [PubMed] [Google Scholar]
- 45.Phillips AO, Steadman R, Morrisey K, Martin J, Eynstone L, Williams JD. Exposure of human renal proximal tubular cells to glucose leads to accumulation of type IV collagen and fibronectin by decreased degradation. Kidney Int 52: 973–984, 1997. doi: 10.1038/ki.1997.419. [DOI] [PubMed] [Google Scholar]
- 46.Pitiyage GN, Lim KP, Gemenitzidis E, Teh MT, Waseem A, Prime SS, Tilakaratne WM, Fortune F, Parkinson EK. Increased secretion of tissue inhibitors of metalloproteinases 1 and 2 (TIMPs -1 and -2) in fibroblasts are early indicators of oral sub-mucous fibrosis and ageing. J Oral Pathol Med 41: 454–462, 2012. doi: 10.1111/j.1600-0714.2012.01129.x. [DOI] [PubMed] [Google Scholar]
- 47.Prozialeck WC, Lamar PC, Appelt DM. Differential expression of E-cadherin, N-cadherin and beta-catenin in proximal and distal segments of the rat nephron. BMC Physiol 4: 10, 2004. doi: 10.1186/1472-6793-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prozialeck WC, Vaidya VS, Liu J, Waalkes MP, Edwards JR, Lamar PC, Bernard AM, Dumont X, Bonventre JV. Kidney injury molecule-1 is an early biomarker of cadmium nephrotoxicity. Kidney Int 72: 985–993, 2007. doi: 10.1038/sj.ki.5002467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quackenbush EJ, Gougos A, Baumal R, Letarte M. Differential localization within human kidney of five membrane proteins expressed on acute lymphoblastic leukemia cells. J Immunol 136: 118–124, 1986. [PubMed] [Google Scholar]
- 50.Rankin CA, Suzuki K, Itoh Y, Ziemer DM, Grantham JJ, Calvet JP, Nagase H. Matrix metalloproteinases and TIMPS in cultured C57BL/6J-cpk kidney tubules. Kidney Int 50: 835–844, 1996. doi: 10.1038/ki.1996.383. [DOI] [PubMed] [Google Scholar]
- 51.Rosen S, Heyman SN. Difficulties in understanding human “acute tubular necrosis”: limited data and flawed animal models. Kidney Int 60: 1220–1224, 2001. doi: 10.1046/j.1523-1755.2001.00930.x. [DOI] [PubMed] [Google Scholar]
- 52.Ross LL, Barber L, Tate SS, Meister A. Enzymes of the gamma-glutamyl cycle in the ciliary body and lens. Proc Natl Acad Sci USA 70: 2211–2214, 1973. doi: 10.1073/pnas.70.8.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rostgaard J, Møller O. Localization of Na+, K+ -ATPase to the inside of the basolateral cell membranes of epithelial cells of proximal and distal tubules in rabbit kidney. Cell Tissue Res 212: 17–28, 1980. doi: 10.1007/BF00234029. [DOI] [PubMed] [Google Scholar]
- 54.Rupp C, Scherzer M, Rudisch A, Unger C, Haslinger C, Schweifer N, Artaker M, Nivarthi H, Moriggl R, Hengstschläger M, Kerjaschki D, Sommergruber W, Dolznig H, Garin-Chesa P. IGFBP7, a novel tumor stroma marker, with growth-promoting effects in colon cancer through a paracrine tumor-stroma interaction. Oncogene 34: 815–825, 2015. doi: 10.1038/onc.2014.18. [DOI] [PubMed] [Google Scholar]
- 55.Sabolić I, Valenti G, Verbavatz JM, Van Hoek AN, Verkman AS, Ausiello DA, Brown D. Localization of the CHIP28 water channel in rat kidney. Am J Physiol Cell Physiol 263: C1225–C1233, 1992. [DOI] [PubMed] [Google Scholar]
- 56.Scherzer P, Popovtzer MM. Segmental localization of mRNAs encoding Na+-K+-ATPase α1- and β1-subunits in diabetic rat kidneys using RT-PCR. Am J Physiol Renal Physiol 282: F492–F500, 2002. doi: 10.1152/ajprenal.00053.2001. [DOI] [PubMed] [Google Scholar]
- 57.Sekiuchi M, Kudo A, Nakabayashi K, Kanai-Azuma M, Akimoto Y, Kawakami H, Yamada A. Expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of matrix metalloproteinases 2 and 1 in the glomeruli of human glomerular diseases: the results of studies using immunofluorescence, in situ hybridization, and immunoelectron microscopy. Clin Exp Nephrol 16: 863–874, 2012. doi: 10.1007/s10157-012-0633-3. [DOI] [PubMed] [Google Scholar]
- 58.Seo DW, Li H, Qu CK, Oh J, Kim YS, Diaz T, Wei B, Han JW, Stetler-Stevenson WG. Shp-1 mediates the antiproliferative activity of tissue inhibitor of metalloproteinase-2 in human microvascular endothelial cells. J Biol Chem 281: 3711–3721, 2006. doi: 10.1074/jbc.M509932200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Severino V, Alessio N, Farina A, Sandomenico A, Cipollaro M, Peluso G, Galderisi U, Chambery A. Insulin-like growth factor binding proteins 4 and 7 released by senescent cells promote premature senescence in mesenchymal stem cells. Cell Death Dis 4: e911, 2013. doi: 10.1038/cddis.2013.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sikri KL, Foster CL, Bloomfield FJ, Marshall RD. Localization by immunofluorescence and by light- and electron-microscopic immunoperoxidase techniques of Tamm-Horsfall glycoprotein in adult hamster kidney. Biochem J 181: 525–532, 1979. doi: 10.1042/bj1810525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sikri KL, Foster CL, MacHugh N, Marshall RD. Localization of Tamm-Horsfall glycoprotein in the human kidney using immuno-fluorescence and immuno-electron microscopical techniques. J Anat 132: 597–605, 1981. [PMC free article] [PubMed] [Google Scholar]
- 62.Sohn SJ, Kim SY, Kim HS, Chun YJ, Han SY, Kim SH, Moon A. In vitro evaluation of biomarkers for cisplatin-induced nephrotoxicity using HK-2 human kidney epithelial cells. Toxicol Lett 217: 235–242, 2013. doi: 10.1016/j.toxlet.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 63.Stephan JP, Mao W, Filvaroff E, Cai L, Rabkin R, Pan G. Albumin stimulates the accumulation of extracellular matrix in renal tubular epithelial cells. Am J Nephrol 24: 14–19, 2004. doi: 10.1159/000075347. [DOI] [PubMed] [Google Scholar]
- 64.Takada T, Yamamoto A, Omori K, Tashiro Y. Quantitative immunogold localization of Na, K-ATPase along rat nephron. Histochemistry 98: 183–197, 1992. doi: 10.1007/BF00315877. [DOI] [PubMed] [Google Scholar]
- 65.Tanaka T, Kondo S, Iwasa Y, Hiai H, Toyokuni S. Expression of stress-response and cell proliferation genes in renal cell carcinoma induced by oxidative stress. Am J Pathol 156: 2149–2157, 2000. doi: 10.1016/S0002-9440(10)65085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang Y, Huang XR, Lv J, Chung AC, Zhang Y, Chen JZ, Szalai AJ, Xu A, Lan HY. C-reactive protein promotes acute kidney injury by impairing G1/S-dependent tubular epithelium cell regeneration. Clin Sci (Lond) 126: 645–659, 2014. doi: 10.1042/CS20130471. [DOI] [PubMed] [Google Scholar]
- 67.Tauc M, Chatelet F, Verroust P, Vandewalle A, Poujeol P, Ronco P. Characterization of monoclonal antibodies specific for rabbit renal brush-border hydrolases: application to immunohistological localization. J Histochem Cytochem 36: 523–532, 1988. doi: 10.1177/36.5.2895788. [DOI] [PubMed] [Google Scholar]
- 68.Van der Hauwaert C, Savary G, Gnemmi V, Glowacki F, Pottier N, Bouillez A, Maboudou P, Zini L, Leroy X, Cauffiez C, Perrais M, Aubert S. Isolation and characterization of a primary proximal tubular epithelial cell model from human kidney by CD10/CD13 double labeling. PLoS One 8: e66750, 2013. doi: 10.1371/journal.pone.0066750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vizioli MG, Sensi M, Miranda C, Cleris L, Formelli F, Anania MC, Pierotti MA, Greco A. IGFBP7: an oncosuppressor gene in thyroid carcinogenesis. Oncogene 29: 3835–3844, 2010. doi: 10.1038/onc.2010.136. [DOI] [PubMed] [Google Scholar]
- 70.Wagner CA, Finberg KE, Breton S, Marshansky V, Brown D, Geibel JP. Renal vacuolar H+-ATPase. Physiol Rev 84: 1263–1314, 2004. doi: 10.1152/physrev.00045.2003. [DOI] [PubMed] [Google Scholar]
- 71.Wang Z, Famulski K, Lee J, Das SK, Wang X, Halloran P, Oudit GY, Kassiri Z. TIMP2 and TIMP3 have divergent roles in early renal tubulointerstitial injury. Kidney Int 85: 82–93, 2014. doi: 10.1038/ki.2013.225. [DOI] [PubMed] [Google Scholar]
- 72.Watanabe J, Takiyama Y, Honjyo J, Makino Y, Fujita Y, Tateno M, Haneda M. Role of IGFBP7 in Diabetic Nephropathy: TGF-β1 Induces IGFBP7 via Smad2/4 in Human Renal Proximal Tubular Epithelial Cells. PLoS One 11: e0150897, 2016. doi: 10.1371/journal.pone.0150897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang M, Huang H, Li J, Huang W, Wang H. Connective tissue growth factor increases matrix metalloproteinase-2 and suppresses tissue inhibitor of matrix metalloproteinase-2 production by cultured renal interstitial fibroblasts. Wound Repair Regen 15: 817–824, 2007. doi: 10.1111/j.1524-475X.2007.00284.x. [DOI] [PubMed] [Google Scholar]
- 74.Zarbock A, Schmidt C, Van Aken H, Wempe C, Martens S, Zahn PK, Wolf B, Goebel U, Schwer CI, Rosenberger P, Haeberle H, Görlich D, Kellum JA, Meersch M; RenalRIPC Investigators . Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA 313: 2133–2141, 2015. doi: 10.1001/jama.2015.4189. [DOI] [PubMed] [Google Scholar]