Abstract
Stretch activation (SA) is a delayed increase in force that enables high power and efficiency from a cyclically contracting muscle. SA exists in various degrees in almost all muscle types. In Drosophila, the indirect flight muscle (IFM) displays exceptionally high SA force production (FSA), whereas the jump muscle produces only minimal FSA. We previously found that expressing an embryonic (EMB) myosin heavy chain (MHC) isoform in the jump muscle transforms it into a moderately SA muscle type and enables positive cyclical power generation. To investigate whether variation in MHC isoforms is sufficient to produce even higher FSA, we substituted the IFM MHC isoform (IFI) into the jump muscle. Surprisingly, we found that IFI only caused a 1.7-fold increase in FSA, less than half the increase previously observed with EMB, and only at a high Pi concentration, 16 mM. This IFI-induced FSA is much less than what occurs in IFM, relative to isometric tension, and did not enable positive cyclical power generation by the jump muscle. Both isometric tension and FSA of control fibers decreased with increasing Pi concentration. However, for IFI-expressing fibers, only isometric tension decreased. The rate of FSA generation was ~1.5-fold faster for IFI fibers than control fibers, and both rates were Pi dependent. We conclude that MHC isoforms can alter FSA and hence cyclical power generation but that isoforms can only endow a muscle type with moderate FSA. Highly SA muscle types, such as IFM, likely use a different or additional mechanism.
Keywords: stretch activation, Drosophila, indirect flight muscle, jump muscle, inorganic phosphate, myosin
stretch activation (SA) is a delayed force increase in response to a quick stretch of a muscle, which results in greater active tension generation than calcium activation alone (29, 33). Following a rapid stretch of a calcium-activated muscle, a four-phase tension response occurs: an immediate force increase (phase 1), a force decay (phase 2), a secondary force increase (phase 3), and a slow force recovery (phase 4) (Fig. 1A). The phase 3 force increase is delayed in regard to the fiber length change and is defined as SA. SA is an intrinsic mechanical property of almost all muscle types, but it is most prominent and beneficial in muscle types that generate work and power through repetitive cyclical contractions (22). In vertebrate heart muscle, a moderate level of SA augments calcium activation to increase force production during systole, resulting in increased muscle power and efficiency (7, 32, 35, 43). The greatest magnitude of SA is found in asynchronous insect indirect flight muscle (IFM), which enables the muscle to operate without cycling calcium for every contraction-relaxation cycle (31). Instead, asynchronous IFM relies on SA and shortening deactivation (SD) to vary force levels during the shortening and lengthening phases of its oscillatory contraction cycle (27, 29–32). This enables higher contraction frequencies and efficiency by not having to pump calcium with each individual contraction cycle. In contrast, skeletal muscle types generally only have small SA responses; therefore, both activation and relaxation rely almost entirely on intracellular calcium cycling (27, 30, 33).
Fig. 1.
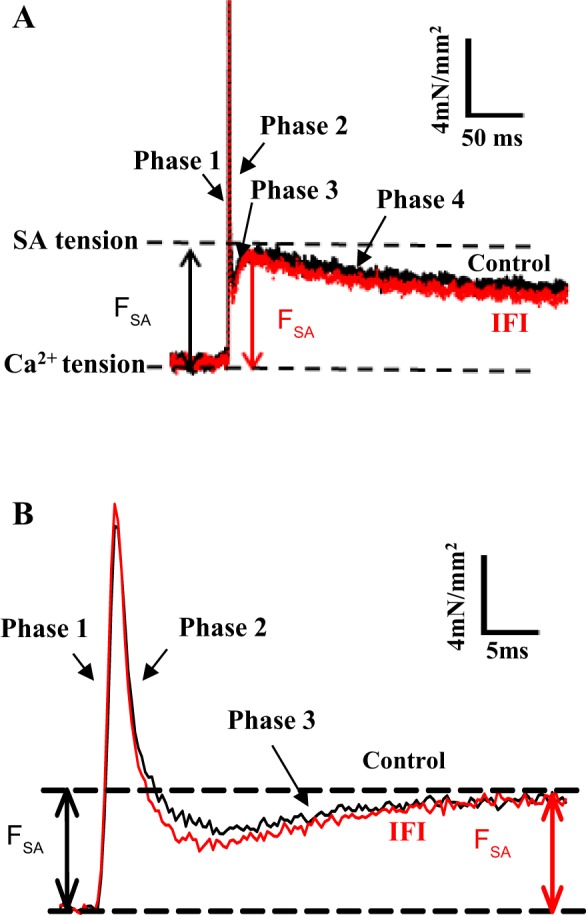
Comparison of IFI and control jump muscle fiber SA responses. A: representative tension traces following a 1% ML step over 0.5 ms from control (black) and IFI (red) fibers at 8 mM Pi. B: tension traces from A on an expanded time axis to show phases 1, 2, and 3 more clearly.
Although the importance of SA to muscle function has been known for many years, the underlying mechanism has yet to be revealed. Several mechanisms have been proposed, most involving a way to transiently increase the number of strongly bound cross bridges (26). Proposed mechanisms include the thick and thin filament lattice matching model (46), the response of connecting filaments, such as titin, to muscle stretch (14, 18), phosphorylation of the myosin regulatory light chain (9, 41), troponin bridges between thick and thin filament displacing tropomyosin (1, 5, 28), and strain-induced alterations in myosin kinetics (40). Myosin isoforms have been shown to change the rate of stretch-activated force generation (phase 3) (2, 15, 34). However, only recently has it been shown that myosin isoforms can also influence the magnitude of force (FSA) generated by the SA response and enable cyclical positive power generation without synchronous calcium cycling (48).
By making use of the unique properties and techniques available with the Drosophila model organism system, we found that myosin isoforms influence FSA (48). In Drosophila, there are two major muscle types in the thorax, IFMs and jump muscles (41). Drosophila IFMs are asynchronous muscles that power flight and generate high FSA (27, 44). In contrast, jump muscles are synchronous muscles that power jumping and only show minimal FSA generation (11, 48), which is the case for most vertebrate skeletal muscles. We recently improved the jump muscle skinned fiber preparation, first developed by Peckham et al. (26), by using only a bundle of six to eight fibers instead of the entire muscle. This reduction in preparation size improved its performance during mechanical testing, including the SA response (11). The myosin isoform endogenous to the jump muscle and the isoforms found in all other Drosophila muscles are generated by alternative splicing from a single myosin heavy chain (MHC) gene (4). This genetic simplicity, along with Drosophila transgenic techniques and the existence of muscle-specific myosin-null lines, enables us to create viable fly lines with myosin isoforms from various muscle types expressed in the mechanically testable IFM and jump muscles (38). We found that expressing an embryonic (EMB) myosin in Drosophila jump muscle transformed it into a moderately SA muscle type (48). Expressing EMB increased FSA and enabled positive cyclical power production, which is not characteristic of the wild-type jump muscle. Because EMB only conferred greater FSA at Pi levels above ~4 mM Pi, this suggested an attachment rate-based mechanism. We proposed that differences in Pi affinity between myosin isoforms, when negatively strained, were responsible for the altered SA response.
Because IFM generates higher FSA and cyclical power than our transgenic EMB-expressing jump muscle, we hypothesized that the MHC isoform from the IFM (IFI) would increase the SA response more than what occurred from expressing EMB. To test this hypothesis, in the present study, we expressed IFI in the jump muscle. Surprisingly, we found that IFI is insufficient to transform the jump muscle into even a moderately stretch-activated muscle type. The transgenic IFI fibers slightly increased FSA only at a high concentration of Pi (16 mM). This increase was not enough to produce any positive power under oscillatory conditions, much less the very-high-power generation found in IFM muscles. These results suggest that the high FSA in the IFM is not solely attributable to an MHC-based kinetic mechanism.
METHODS
Transgenic Drosophila Lines
The control fly line (pwMhc2) expresses the wild-type myosin transgene in a Drosophila line (Mhc10) null for myosin in the IFM and jump muscles. Because of the alternative splicing mechanism, this results in the jump muscle expressing its native isoform. Detailed methods of pwMhc2 creation have been described previously (38). The IFI transgenic Drosophila line was created by modifying the pwMhc2 transgene. With the use of standard cloning techniques, the entire exon 11 coding region and flanking introns were replaced by only the alternative exon 11a sequence, the alternative version of the converter region found in the indirect flight muscle. This is the only region that differs between the IFM and jump muscle MHC isoforms. The IFI transgenic construct was microinjected into Drosophila embryos and incorporated into genomic DNA using P element-mediated transformation as previously described (38). The resultant lines were crossed with Mhc10, resulting in Drosophila lines that expressed only the IFI myosin isoform in the jump muscle. All transgenic fly lines were maintained on a 12-h:12-h light/dark regimen at 25°C.
Jump Muscle Preparation
Jump muscle fiber dissection and mounting were performed as described previously (11, 36). Briefly, the jump muscle was removed from 2- to 3-day-old female flies. Fibers were separated and chemically demembranated in dissection solution [pCa 8.0, 5.00 mM MgATP, 1.00 mM free Mg2+, 0.25 mM phosphate, 5.00 mM EGTA, 20.00 mM N, N-Bis(2-hydroxyethyl)-2-aminoethanesulfonic acid (BES, pH 7.0), 175.00 mM ionic strength, adjusted with Na methane sulfonate, 1.00 mM DTT, 50% glycerol, and 0.5% Triton X-100] for 1 h at 4°C. Jump muscle fibers were attached to the mechanics apparatus using aluminum T-clips (36). Fibers were stretched in relaxing solution (pCa 8.0, 10.00 mM MgATP, 0.00 mM phosphate, 45.00 mM creatine phosphate, 450.00 U/ml creatine phosphokinase, 1.00 mM free Mg2+, 5.00 mM EGTA, 20.00 mM pH 7.0 BES, 260.00 mM ionic strength, adjusted with Na methane sulfonate, and 1.00 mM DTT) to the optimal sarcomere length (3.6 µm), at which length the fiber dimensions were measured. The relaxing solution was exchanged for preactivation solution (EGTA decreased to 0.5 mM) to help maintain sarcomere homogeneity during activation. Fibers were activated by exchanging preactivation solution with activating solution (pCa 5.0, 20.00 mM MgATP, 0.00, 8.00, or 16.00 mM phosphate, 25.00 mM creatine phosphate, 450.00 U/ml creatine phosphokinase, 1.00 mM free Mg2+, 5.00 mM EGTA, 20.00 mM pH 7.0 BES, 260.00 mM ionic strength, adjusted with Na methane sulfonate, and 1.00 mM DTT). The 260 mM ionic strength was needed to accommodate 16 mM Pi and 20 mM ATP. Pi competes with MgATP for rigor myosin (37), thus high [ATP] is needed to minimize competition from Pi when working at higher Pi concentrations. Isometric tension measurements, step analysis, sinusoidal analysis, and work loop assays were started at 3.6-µm sarcomere length and performed at 15°C.
Muscle Mechanics Protocols
Isometric tension measurements.
Total Ca2+-activated isometric tension (A0) of skinned jump muscle fibers expressing the native (control) and IFI myosin isoforms was measured in activation solution at various Pi concentrations (0, 8, or 16 mM) and in relaxation solution. The fiber was held at a constant sarcomere length of 3.6 µm, and A0 was recorded. All measurements of A0 occurred immediately before the subsequent step increase in length for measuring SA force. A0 is composed of two components, passive tension (P0) and calcium-activated tension (F0). P0 was measured at pCa 8.0, and F0 was obtained by subtracting P0 from the total isometric tension (A0) measured at pCa 5.0 (F0 = A0 – P0). To control for possible effects of fiber degradation during the experiment, half the experiments were started at 16 mM Pi and the other half at 0 mM, which produced no differences in the results. Fibers producing an initial isometric tension <15 mN/mm2 at a starting [Pi] of 0 mM or 10 mN/mm2 at a starting [Pi] of 16 mM were discarded. One jump muscle preparation per fly was mechanically analyzed.
SA tension measurements.
Step analysis was performed in activation solution, pCa 5.0, at 0, 8, and 16 mM Pi, and in relaxing solution, pCa 8.0, to measure the SA response of jump muscle fibers. A length step of 1% muscle length (ML) over 0.5 ms was applied, and the corresponding tension response was recorded. The length step was held for 300 ms, and fibers were slowly returned to their original length over 500 ms. Isometric tension before and after each length step was measured at every [Pi]. If the isometric tension decreased >10% of the previous measurement, the fiber was discarded. The total SA magnitude (ASA) was measured by subtracting the total Ca2+-activated isometric tension (A0) immediately before the step from the peak value of phase 3 (Fig. 1). The net active SA response (FSA) was calculated by subtracting the passive SA (PSA), obtained at pCa 8.0, from the total SA response (ASA) obtained at pCa 5.0 (FSA = ASA – PSA). The rate of SA tension generation (r3, rate constant of phase 3) and the rates of phase 2 (r2) and 4 (r4) were obtained by fitting phases 2–4 of the tension transient to the sum of three exponential curves: a3[1 - exp(-r3t)] + a2exp(-r2t) + a4exp(-r4t) + offset (Fig. 1) using Sigma Plot, v11.0.
Work loop assay.
The work loop assay was performed to determine the mechanical power output of muscle fibers under relatively high ML amplitudes (36). Fibers were oscillated in a sinusoidal pattern at a series of strain amplitudes (0.25–1.00% ML) and frequencies (1–60 Hz). Ten identical consecutive cycles of sinusoidal waveforms were applied to fibers, and values from the eighth cycle were used to determine the work and power output because power became consistent by the sixth or seventh cycle. Negative work (work absorbed by the fiber during lengthening, calculated as W = ∫FdL, where F is tension and dL is fiber length change) was subtracted from positive work (work produced by the fiber during shortening) to obtain net work production. Power was calculated as the product of work and frequency (36). Both tension and length change traces were recorded at a sampling rate of 8 kHz. We performed work loop assays at 0, 8, and 16 mM Pi at pCa 5.0.
Sinusoidal analysis.
Sinusoidal analysis was performed to determine the complex stiffness characteristics of the muscle fiber and as a second method of determining whether net positive oscillatory work could be produced by the fibers. A low strain amplitude at 0.25% peak-to-peak sinusoidal length change and a frequency series ranging from 0.50-500.00 Hz were applied to fibers. Viscous and elastic moduli were calculated as previously described (36).
RESULTS
IFI Transgenic Line Creation
We generated the IFI line by P element-mediated Drosophila transformation. After it was crossed into Mhc10, we verified that the correct amount of myosin was being produced by using SDS PAGE gels as described previously (23, 25, 38), which included normalizing myosin levels of each line to their respective actin levels. The amount of myosin produced by the IFI line was 97 ± 5% of wild-type (100 ± 3%), which is not a statistically significant difference (Student’s t-test, P > 0.05). Because the mechanical assays did not show many mechanical differences from the control line, to ensure there were no problems with the IFI Drosophila line, we ran a second, independently generated IFI line through many of the same experiments as the first line. This second line produced results that were not statistically different from the first IFI line for all of the mechanical assays performed, except for a small difference in the amount of power generated at 0 mM Pi (Table 1).
Table 1.
Summary of data from the second independently generated IFI line
| Pi, mM | F0, mN/mm2 | FSA, mN/mm2 | FSA/F0 | Power, W/mm3 | n | |
|---|---|---|---|---|---|---|
| IFI line 2 | 0 | 31.6 ± 3.2 | 2.3 ± 0.4 | 0.07 ± 0.01 | −212.9 ± 27.1* | 8 |
| 8 | 17.0 ± 2.2 | 2.6 ± 0.3 | 0.15 ± 0.03 | −67.8 ± 11.8 | 8 | |
| 16 | 13.8 ± 2.9 | 2.5 ± 0.3 | 0.18 ± 0.03 | −30.1 ± 6.5 | 8 |
Values are means ± SE. n, no. of fibers.
Statistically different compared with the first indirect flight muscle myosin heavy chain isoform (IFI) line at the given Pi concentration; P < 0.05 (Student’s t-test). All other values are not statistically different from the first line.
Isometric Tension
At 0 mM Pi, expressing IFI decreased A0 (total isometric tension) and F0 (Ca2+-activated isometric tension) of jump muscle fiber bundles by 31% compared with the control line, but increasing the Pi concentration to 8 or 16 mM eliminated these differences (Table 2). A0 and F0 of control and IFI fibers both decreased with increasing [Pi], but a greater effect was observed for control fibers because F0 decreased by 60% from 0 mM Pi to 16 mM Pi compared with 46% for IFI fibers. The passive isomeric tension (P0) values of control and IFI fibers were both low, contributing only ~10% of A0 (Table 2). P0 was unaffected by expressing IFI or varying [Pi] (Table 2).
Table 2.
Isometric tension of Drosophila jump muscles
| Pi, mM | A0, mN/mm2 | P0, mN/mm2 | F0, mN/mm2 | n | |
|---|---|---|---|---|---|
| Control | 0 | 45.4 ± 4.7 | 2.8 ± 0.6 | 42.6 ± 4.8 | 9 |
| 8 | 24.9 ± 2.1* | 3.2 ± 0.8 | 21.8 ± 2.2* | 10 | |
| 16 | 19.9 ± 2.3* | 3.1 ± 0.6 | 16.9 ± 2.4* | 8 | |
| IFI | 0 | 32.3 ± 3.0# | 3.0 ± 0.3 | 29.3 ± 3.0# | 11 |
| 8 | 21.1 ± 1.8* | 3.1 ± 0.4 | 18.1 ± 1.8* | 11 | |
| 16 | 18.8 ± 1.6* | 3.1 ± 0.37 | 15.7 ± 1.7* | 11 |
Values are means ± SE. n, no. of fibers; A0, total isometric tension measured at pCa 5.0, 15°C; P0, passive tension measured at pCa 8.0; F0, net active isometric tension after subtracting passive tension (F0 = A0 - P0).
Statistically significant compared with 0 mM Pi, P < 0.05 (Student’s t-test).
Statistically significant compared with the control value at the given Pi concentration, P < 0.05 (Student’s t-test).
Stretch Activation
The control and transgenic IFI Drosophila jump muscles displayed qualitatively similar SA responses (Fig. 1A). The transgenic fibers displayed all four phases of the tension transient response (Fig. 1B). ASA and FSA values of IFI fibers were the same as control values at 0 and 8 mM Pi (Fig. 2A and Table 3). However, at 16 mM Pi, the IFI ASA and FSA values were 1.5-fold and 1.7-fold higher than the control values, respectively. The reason for the difference at 16 mM Pi was that the control FSA values decreased with increasing [Pi], whereas IFI values did not decrease (Fig. 2A). We normalized FSA to isometric tension to ensure that the apparent difference in FSA was not simply attributable to a general difference in tension-generating ability, a possibility suggested by the observed changes in isometric tension generation (Table 3). Normalized SA magnitude (FSA/F0) for IFI at 16 mM Pi was also significantly higher, 1.7-fold, suggesting that expressing IFI specifically alters SA force generation (Table 3). Passive SA (PSA) was not different between fiber types at any [Pi].
Fig. 2.
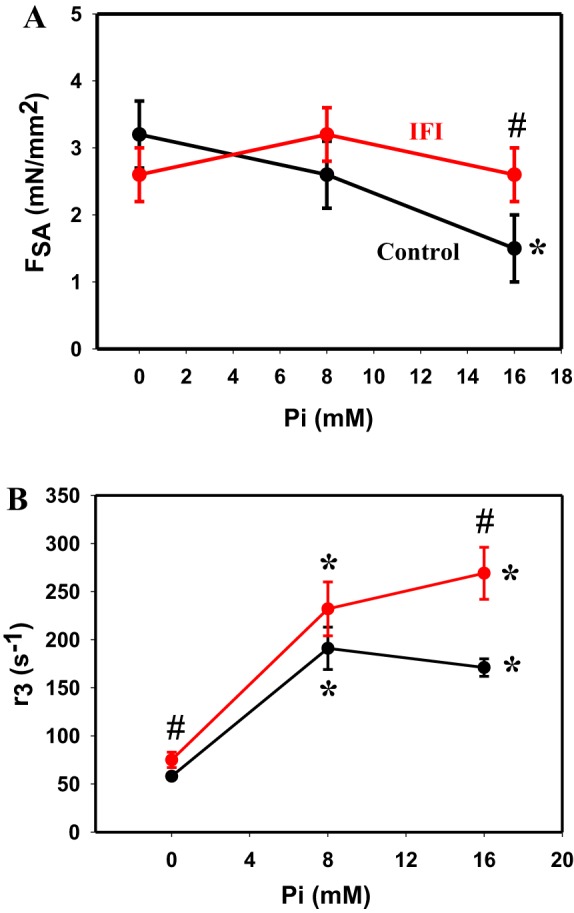
SA tension and kinetics of IFI and control jump muscle fibers. A: stretch-activated tension (FSA) of IFI (red) and control fibers (black) plotted vs. Pi concentration. Error bars show SE. B: rate of SA tension generation, r3 (rate of phase 3), of control (black) and IFI (red) fibers at three Pi concentations. Individual tension traces of SA responses following a 1% ML step over 0.5 ms were fitted to the sum of 3 exponential curves: a3[1 − exp(−r3t)] + a2exp(−r2t) + a4exp(−r4t) + offset. #P < 0.05 compared with control. *P < 0.05 compared with 0 mM Pi.
Table 3.
Stretch activation magnitude of Drosophila jump muscle fibers
| Pi, mM | ASA, mN/mm2 | PSA, mN/mm2 | FSA, mN/mm2 | FSA/F0 | n | |
|---|---|---|---|---|---|---|
| Control | 0 | 3.9 ± 0.4 | 0.7 ± 0.2 | 3.2 ± 0.5 | 0.08 ± 0.02 | 7 |
| 8 | 3.8 ± 0.4 | 1.2 ± 0.3 | 2.6 ± 0.5 | 0.12 ± 0.02 | 10 | |
| 16 | 2.6 ± 0.5* | 1.1 ± 0.3 | 1.5 ± 0.5* | 0.09 ± 0.03 | 10 | |
| IFI | 0 | 3.8 ± 0.3 | 1.3 ± 0.2 | 2.6 ± 0.4 | 0.09 ± 0.02 | 9 |
| 8 | 4.6 ± 0.3 | 1.5 ± 0.3 | 3.2 ± 0.4 | 0.18 ± 0.04 | 9 | |
| 16 | 4.0 ± 0.3# | 1.5 ± 0.3 | 2.6 ± 0.4# | 0.16 ± 0.04# | 9 |
Values are means ± SE. n, no. of fibers; ASA, total stretch-activated tension measured at pCa 5.0; PSA, passive component of stretch-activated tension measured at pCa 8.0; FSA, net stretch-activated tension (FSA = ASA – PSA).
Statistically significant compared with 0 mM Pi, P < 0.05 (Student’s t-test).
Statistically significant compared with the control value at the given Pi concentration, P < 0.05 (Student’s t-test).
Replacing the native jump muscle MHC with IFI slightly increased the jump muscle rate of SA tension generation (r3) (Table 4 and Fig. 2B). Expressing IFI increased r3 1.3-fold at 0 mM Pi and 1.6-fold at 16 mM Pi relative to the control fibers. There was a strong general effect of [Pi] on r3 for both control and IFI fibers, as r3 increased 2.9-fold from 0 to 16 mM Pi for control fibers and 3.6-fold for IFI fibers (Table 4 and Fig. 2B). Most or all of the increase occurred between 0 and 8 mM Pi. The other two tension-transient rates also showed significant changes with Pi. r2 decreased, and r4 increased with increasing Pi concentration.
Table 4.
Stretch activation kinetics of Drosophila jump muscles
| Pi, mM | r2/s | r3/s | r4/s | n | |
|---|---|---|---|---|---|
| Control | 0 | 1,103 ± 88 | 58 ± 2 | 0.3 ± 0.7 | 6 |
| 8 | 725 ± 84* | 191 ± 22* | 7.7 ± 0.2* | 9 | |
| 16 | 761 ± 105* | 171 ± 9* | 8 ± 1.4* | 8 | |
| IFI | 0 | 984 ± 59 | 75 ± 8# | 2.7 ± 1.0# | 9 |
| 8 | 608 ± 68* | 232 ± 28* | 7.2 ± 0.7* | 9 | |
| 16 | 529 ± 52* | 269 ± 27#* | 8.5 ± 0.6*# | 8 |
Values are means ± SE. n, no. of fibers; r2, rate of phase 2; r3, rate of phase 3; r4, rate of phase 4 (see Fig. 1).
Statistically significant compared with 0 mM Pi, P < 0.05 (Student’s t-test).
Statistically significant compared with the control value at the given Pi concentration, P < 0.05 (Student’s t-test).
Power and Viscous Modulus
Work loop analysis.
Because of SA, the indirect flight muscles of Drosophila can generate positive work and power without calcium cycling with each contraction. Thus, as an additional test to see whether the IFI isoform enabled jump muscle fibers to have SA characteristics, we examined whether the IFI-expressing jump muscle could produce positive work or power at pCa 5.0. First, using the work loop assay, we tested a range of muscle length changes (0.25- 1.00% ML) and oscillation frequencies (1–60 Hz) for positive power generation. These conditions included ones that had previously enabled positive power when the EMB isoform was expressed (48). None of these conditions produced positive power (Fig. 3A). Control and IFI fibers produced clockwise work loops at all tested [Pi]s, 0, 8, and 16 mM Pi, indicating that they absorbed work under these conditions.
Fig. 3.
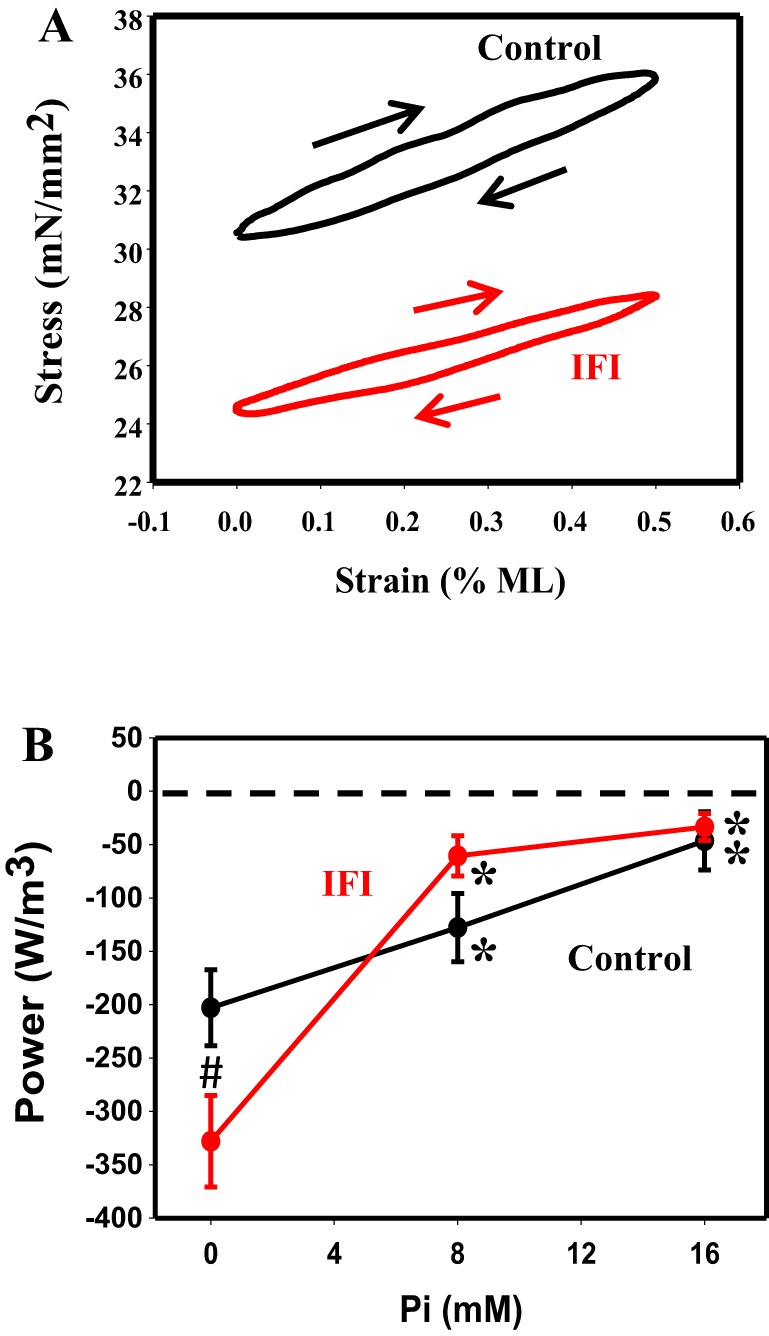
Work and power generation. A: representative work loop traces at 8 mM Pi. Arrows indicate the work loops were generated clockwise, indicating negative work (work absorption) and negative power. B: influence of [Pi] on work loop power generation. #P < 0.05 compared with control. *P < 0.05 compared with 0 mM Pi.
To characterize the negative power generation by the fibers, we focused on a more limited set of conditions at 0.5% ML. The limited focus was required because these negative work and power loops damage the muscle and degrade the subsequent performance of the muscle. We found that both control and IFI fibers showed minor differences in negative power generation at 0, 8, and 16 mM Pi under these conditions (Fig. 3B and Table 5). The only significant differences attributable to expressing IFI were 1.6-fold increases in both absorbed (negative) work and power at 0 mM Pi. The amount of work and power absorbed was highly [Pi] dependent, especially for the IFI fibers. Work and power increased (less negative) 3.7-fold and 10-fold, respectively, for IFI, whereas the increases were only 3.3-fold and 4.4-fold for control fibers. The larger changes in power generation compared with work are due to a Pi dependency of the optimal frequency in IFI fibers, decreasing by 62%, whereas optimal frequency did not change with [Pi] for control fibers (Table 5).
Table 5.
Work and power generation of Drosophila jump muscles measured using the work loop technique
| Pi, mM | Work, J/m3 | Power, W/m3 | Frequency, Hz | n | |
|---|---|---|---|---|---|
| Control | 0 | −5.7 ± 0.7 | −202.9 ± 32.4 | 35.6 ± 3.7 | 9 |
| 8 | −4.0 ± 1.0 | −127.8 ± 31.2* | 31.9 ± 5.2 | 8 | |
| 16 | −1.7 ± 0.3* | −46.5 ± 6.9* | 27.3 ± 6.7 | 7 | |
| IFI | 0 | −7.5 ± 0.7# | −328.6 ± 42.9# | 43.9 ± 4.5 | 9 |
| 8 | −3.2 ± 0.2* | −60.5 ± 18.9* | 17.8 ± 4.8* | 9 | |
| 16 | −2.0 ± 0.2* | −33.5 ± 12.6* | 16.7 ± 4.9* | 9 |
Values are means ± SE. n, no. of fibers; work, power and frequency at which the maximal work and power was generated for 0.5% strain.
Statistically significant compared with 0 mM Pi, P < 0.05 (Student’s t-test). Measurements were made at pCa 5.0 and 15°C.
Statistically significant compared with the control value at the given Pi concentration, P < 0.05 (Student’s t-test).
Sinusoidal analysis.
In addition to the work loop assay, we performed sinusoidal analysis as another method for determining whether the IFI could enable positive power generation. Sinusoidal analysis applies a smaller ML change than work loops, employs a broader frequency range (0.5–500.0 Hz), and causes little to no degradation in IFM fiber performance (36). Control and IFI fiber results were similar in that, at all tested [Pi]s and frequencies, none of the viscous modulus values dipped below zero (Fig. 4). A negative viscosity value at any frequency would have indicated positive work production and power generation. In the absence of Pi, the viscous modulus of jump muscles was slightly increased, at 40, 45, and 50 Hz, when IFI was expressed (Fig. 4A), whereas no differences were observed at 8 and 16 mM Pi (Fig. 4, B and C). Increasing [Pi] depressed the viscous modulus amplitude of both fiber types.
Fig. 4.
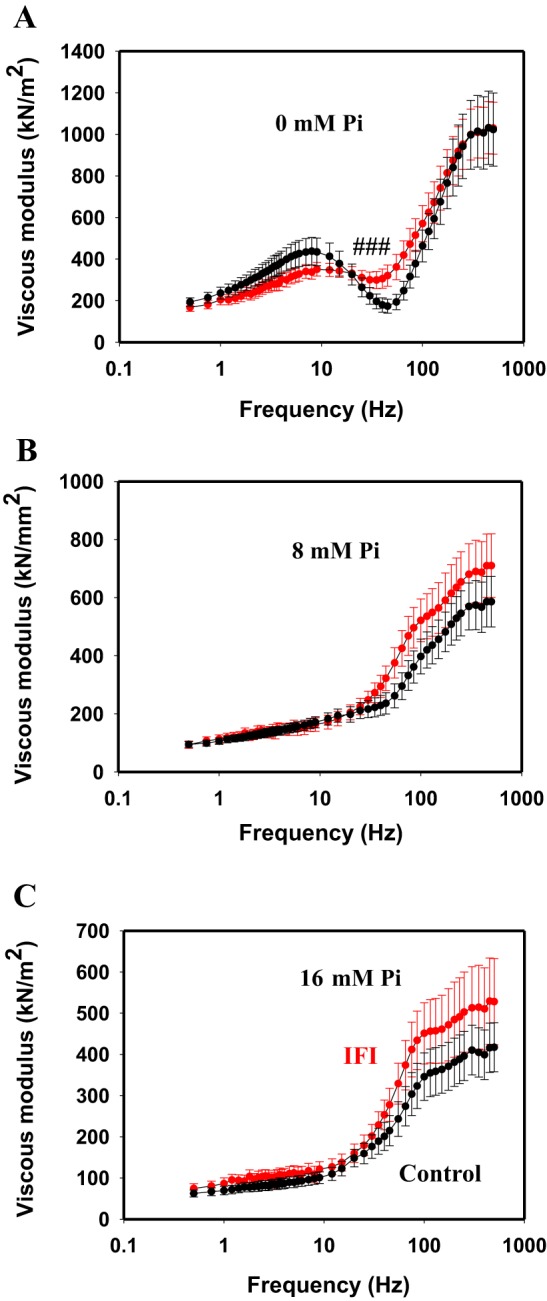
Viscous modulus values for control and IFI fibers. A positive viscous modulus for all frequencies tested, 0.5–500.0 Hz at 0.125% ML, showed that no positive work or power was produced. A–C: viscous modulus of control and IFI fibers over a frequency range of 0.5–500.0 Hz at 0 mM (A), 8 mM (B), and 16 mM (C) Pi. Note the different y-axis scaling. Control is in black and IFI is in red. #P < 0.05 compared with control.
DISCUSSION
IFM Compared with Jump Muscle
IFM, the source of IFI, produces the highest FSA of any muscle type. SA increases Drosophila IFM force twofold relative to calcium-activated force (44). This delayed force increase, along with SD, enables positive power generation during oscillatory contractions at relatively constant calcium concentrations. Only occasional nerve impulses are required to maintain the appropriate calcium concentration. The nerve impulses are not synchronous with each muscle contraction, hence the designation of the muscle as an asynchronous muscle type (22). The role of the motor nerve is to initiate flight and to adjust calcium levels to modify muscle and aerodynamic power output (17). Increasing stimulation frequency increases calcium concentration, which increases FSA, causing greater muscle power output, higher wing beat frequency, and more aerodynamic power output (44).
In contrast to the high IFM FSA, the jump muscle produces minimal FSA. SA increases force only ~12% relative to calcium-activated tension in the jump muscle (48). Without significant SA, and likely minimal SD, when we force an isolated wild-type jump muscle to undergo cyclical contractions at a fixed calcium concentration, no positive power is produced (11). The reason for having low FSA is that the jump muscle is used in a manner that does not benefit from FSA. The jump muscle powers jumps and the take-offs that initiate flight (49). These are one-shot, fast shortening actions with minimal muscle lengthening before contraction. Without stretch before shortening, the SA mechanism cannot be initiated.
Functional Changes Due to Expressing IFI
When we expressed IFI in the jump muscle, we found that calcium-activated isometric tension generation was ~30% lower at 0 mM Pi. A decrease in tension attributable to myosin isoform exchange could either be due to a lower myosin duty ratio (less myosin attached to actin at any given time) or a decrease in myosin cross-bridge stiffness. Given that the tension difference was eliminated as [Pi] increased, this suggests that the duty ratio is more likely the cause. Changing Pi concentration should not change myosin stiffness, but it could alter how many myosins are attached to actin, presumably by a net decrease in rate of attachment (myosins driven backward in the cross-bridge cycle) (8, 24). However, the amount of calcium-activated isometric tension lost at 0 mm Pi was not anywhere close to the amount that it differs between the IFM and jump muscle types. Jump muscle F0 is 30-fold higher than IFM F0, whereas the IFI expressing jump muscle still produced over 10-fold more F0 than IFM produces (11, 44). This indicates that the MHC isoform is not the sole determinant of isometric tension difference between the muscle types. Another mechanism, perhaps the IFM-specific myosin essential light chain (ELC) or a thin filament mechanism (1), is likely decreasing the number of myosins that can bind to actin during isometric contractions of the IFM.
Expressing the IFI did not change passive tension generation; rather it remained low, which is characteristic of the jump muscle compared with the high passive tension values seen in the IFM (42). Our previous work suggests that some of the high IFM passive tension is due to cross-bridge formation at low calcium concentrations (in addition to shorter isoforms of connecting proteins) (6). However, our present results do not suggest that there is any increase in cross-bridge binding under low calcium conditions in the jump muscle when IFI is expressed.
When jump muscle fibers expressing IFI were stretched, while generating active tension, the shape of the resultant tension transient was not obviously altered compared with control fibers (Fig. 1). Focusing on the amplitude of phase 3 (FSA), we observed no change at low [Pi] compared with control fibers. The only change was at 16 mM Pi. The change at high [Pi] was due to IFI FSA not being as negatively affected by increasing [Pi] compared with control fiber values. However, this small change, a 16% increase above isometric tension compared with a 9% increase above isometric tension for jump (FSA/F0, Table 3), is nowhere near the twofold increase above isometric tension seen in IFM fibers (44). Thus IFI alone is likely not responsible for the high SA magnitude seen in IFM.
Even with the increase in FSA at 16 mM Pi, positive power generation by IFI fibers was not possible, nor did it occur at any other [Pi]. Positive power generation at high calcium is a key feature of highly SA muscle types (21). This enables SA muscles to dispense with calcium cycling with each contraction, which enables greater contraction speed and efficiency. At 0 mM Pi, power was actually decreased by expressing the IFI in the jump muscle, even though FSA remained unaltered. One possible reason could be that SD is less prominent at 0 mM Pi. SD is also important for positive power in asynchronous muscle types because it reduces force during muscle lengthening and hence reduces resistive work (22). Perhaps IFI SD at 0 mM Pi is less effective at reducing force during the lengthening portion of the cycle than at higher Pi concentrations. Investigators have not focused on SD as much as SA, and it is not clear if the mechanism responsible for SA is the same as for SD.
For both IFI and control fibers, power became less negative with increasing [Pi]. Interestingly, the improved power generation was greater for IFI expressing jump muscles. The reasons may include the smaller IFI decrease in FSA with [Pi] and perhaps its larger decrease in isometric tension with [Pi]. Is there any possible physiological role for this small increase in power? Perhaps the [Pi] response is an adaptation for fatigue conditions. Increasing FSA might help maintain the ability of IFMs to generate power during long flights.
The rate of SA force generation, r3, increased when IFI was expressed, as one would expect from the myosin isoform from the fastest known muscle type. However, the rate is not nearly as high as IFM r3. At 8 mm Pi, IFM r3 is ~6.5-fold greater than r3 of the jump muscle expressing IFI. Similarly, work loop fmax, another measure of muscle and myosin kinetics, of IFMs is ~135 Hz, whereas IFI in jump was 35 Hz, a 3.8-fold difference. Thus the background muscle type and associated protein isoforms must have a large impact in setting muscle kinetics. The most obvious candidate is the IFM-specific light chain. Recent work by the Moore laboratory suggests myosin light chains are important in setting cross-bridge rate constants when under load (19). Thus perhaps when IFI myosin is operating under load, as is the case for r3 and fmax, it needs its unique ELC to achieve faster muscle kinetics.
Mechanism by Which Myosins Increase FSA
Previously, we have shown that expressing an embryonic myosin converts the jump muscle into a moderately SA muscle type. EMB expression caused a 2.6-fold increase in FSA at 8 mM Pi or, expressed relative to isometric, a 20% increase in active tension (48). Even more convincingly, this enabled the jump muscle to produce positive work and power at a constant high calcium concentration, which is not possible with native jump muscle. Because increasing [Pi] increased EMB FSA and power, but control fiber FSA deceased with increasing [Pi], we proposed that the mechanism for the increased SA response was due to a difference in myosin isoform Pi affinity when under negative strain (muscle is stretched). An increased affinity would allow more myosin heads to be driven backward (during phase 2) into weakly bound (prepower stroke) state(s) by muscle stretch. This would increase the number of heads available to rapidly bind to actin and contribute to SA force generation (phase 3).
Could a similar mechanism account for the small changes we observed for the IFI fibers at 16 mM Pi? We again found that control fiber FSA decreased with increasing [Pi], but IFI FSA had a flat response to [Pi]. Additionally, IFI power generation and isometric tension responses to [Pi] were slightly different than control fibers. These all suggest a difference in Pi affinity between IFI and jump myosins, at least when myosin is under load. Combined with our previous data showing that EMB FSA increases with increasing [Pi], it appears that EMB has the highest Pi affinity, the jump muscle the lowest, and IFI has intermediate Pi affinity.
The finding that EMB increases FSA more than IFI might not seem logical given that the IFI is very highly stretch activated, whereas EMB-expressing muscles may only have modest SA. Perhaps a myosin mechanism that generates a small SA response is enough for the moderate amount of FSA that is likely helpful in the synchronous larval body wall muscles. The EMB-expressing larval body wall muscles, using cyclical contractions, power the movement of the larvae through its environment. A different mechanism capable of generating much higher FSA is likely needed for the asynchronous IFM. Recently, evidence for a thin filament-based mechanism has emerged (1, 28), including our recent work on troponin C isoforms (10). In brief, stretch may be further activating a partially calcium-activated thin filament. A unique troponin C isoform in IFM, which only binds one calcium, appears to play a role in setting how much calcium activation and SA occurs in IFM (1). How the stretch would be sensed by the thin filament is presently unknown, but myosin has been proposed to play a role as part of a stretch-sensing link between the thick and thin filaments (20, 28).
Myosin Structural Differences
Understanding the structural mechanisms for how EMB and IFI influence FSA is made easier in Drosophila because the mechanism of Drosophila of generating muscle MHC isoforms, alternative splicing from one gene, points us to structural regions responsible for functional differences (3). Only the converter differs between IFI and jump MHC (47). Exon 11, which encodes 39 residues of the Drosophila converter, differs at 19 positions between the IFI and jump isoform. Thus the converter must be solely responsible for the differences observed in this study. Although EMB has four additional structural regions that differ from the IFI and jump muscle MHCs, it uses a different converter version than either IFI or jump MHC (45). Thus the converter is likely responsible for some, if not all, of the SA characteristic differences between all three isoforms. Specifically, if our proposed mechanism for the differences in FSA is correct, then the converter is either altering Pi affinity when under load or the ability of myosin to sense stretch.
Although expressing IFI MHC did not endow the jump muscle with high FSA, perhaps working with its specific ELC might produce higher FSA. Studies to date suggest that all Drosophila MHC isoforms are associated with the same splice variant of the myosin ELC gene except for the IFM (12, 13). The IFI ELC differs from the other splice form in its last 14 amino acids. The converter directly interacts with the ELC and perhaps directly with the ELC COOH terminus (16). ELCs are known to influence velocity of shortening and force-generating ability (39, 42), and recent work suggests that the light chains and lever arm play a role in sensing load (19). Thus perhaps the IFI isoforms need its specific ELC to produce a sizable increase in FSA.
Thus, unless the ELC has a drastic impact, we conclude that a myosin isoform mechanism alone is likely not responsible for high IFM FSA. Myosin is likely part of the mechanism, perhaps as part of the troponin bridge proposed to be the stretch sensor or by increasing FSA a small amount, but IFI is not sufficient to impart the very high FSA observed in IFM.
GRANTS
This work was supported by National Institutes of Health National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR-064274 (to D. Swank) and American Heart Association Predoctoral Fellowship 11PRE5630000 (to C. Zhao).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.Z. performed experiments; C.Z. and D.M.S. analyzed data; C.Z. and D.M.S. interpreted results of experiments; C.Z. prepared figures; C.Z. drafted manuscript; C.Z. and D.M.S. approved final version of manuscript; D.M.S. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Amy Loya for helping with transient force response kinetics analysis and Seemanti Ramanath and Qian Wang for helping make the IFI construct and lines.
REFERENCES
- 1.Agianian B, Krzic U, Qiu F, Linke WA, Leonard K, Bullard B. A troponin switch that regulates muscle contraction by stretch instead of calcium. EMBO J 23: 772–779, 2004. doi: 10.1038/sj.emboj.7600097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andruchova O, Stephenson GM, Andruchov O, Stephenson DG, Galler S. Myosin heavy chain isoform composition and stretch activation kinetics in single fibres of Xenopus laevis iliofibularis muscle. J Physiol 574: 307–317, 2006. doi: 10.1113/jphysiol.2006.109926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernstein SI, Milligan RA. Fine tuning a molecular motor: the location of alternative domains in the Drosophila myosin head. J Mol Biol 271: 1–6, 1997. doi: 10.1006/jmbi.1997.1160. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein SI, Mogami K, Donady JJ, Emerson CP Jr. Drosophila muscle myosin heavy chain encoded by a single gene in a cluster of muscle mutations. Nature 302: 393–397, 1983. doi: 10.1038/302393a0. [DOI] [PubMed] [Google Scholar]
- 5.Bullard B, Pastore A. Regulating the contraction of insect flight muscle. J Muscle Res Cell Motil 32: 303–313, 2011. doi: 10.1007/s10974-011-9278-1. [DOI] [PubMed] [Google Scholar]
- 6.Burkart C, Qiu F, Brendel S, Benes V, Hååg P, Labeit S, Leonard K, Bullard B. Modular proteins from the Drosophila sallimus (sls) gene and their expression in muscles with different extensibility. J Mol Biol 367: 953–969, 2007. doi: 10.1016/j.jmb.2007.01.059. [DOI] [PubMed] [Google Scholar]
- 7.Campbell KB, Chandra M. Functions of stretch activation in heart muscle. J Gen Physiol 127: 89–94, 2006. doi: 10.1085/jgp.200509483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dantzig JA, Goldman YE, Millar NC, Lacktis J, Homsher E. Reversal of the cross-bridge force-generating transition by photogeneration of phosphate in rabbit psoas muscle fibres. J Physiol 451: 247–278, 1992. doi: 10.1113/jphysiol.1992.sp019163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickinson MH, Hyatt CJ, Lehmann FO, Moore JR, Reedy MC, Simcox A, Tohtong R, Vigoreaux JO, Yamashita H, Maughan DW. Phosphorylation-dependent power output of transgenic flies: An integrated study. Biophys J 73: 3122–3134, 1997. doi: 10.1016/S0006-3495(97)78338-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eldred CC, Katzemich A, Patel M, Bullard B, Swank DM. The roles of troponin C isoforms in the mechanical function of Drosophila indirect flight muscle. J Muscle Res Cell Motil 35: 211–223, 2014. doi: 10.1007/s10974-014-9387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eldred CC, Simeonov DR, Koppes RA, Yang C, Corr DT, Swank DM. The mechanical properties of Drosophila jump muscle expressing wild-type and embryonic Myosin isoforms. Biophys J 98: 1218–1226, 2010. doi: 10.1016/j.bpj.2009.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falkenthal S, Graham M, Wilkinson J. The indirect flight muscle of Drosophila accumulates a unique myosin alkali light chain isoform. Dev Biol 121: 263–272, 1987. doi: 10.1016/0012-1606(87)90158-8. [DOI] [PubMed] [Google Scholar]
- 13.Falkenthal S, Parker VP, Davidson N. Developmental variations in the splicing pattern of transcripts from the Drosophila gene encoding myosin alkali light chain result in different carboxyl-terminal amino acid sequences. Proc Natl Acad Sci USA 82: 449–453, 1985. doi: 10.1073/pnas.82.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukuda N, Wu Y, Farman G, Irving TC, Granzier H. Titin-based modulation of active tension and interfilament lattice spacing in skinned rat cardiac muscle. Pflugers Arch 449: 449–457, 2005. doi: 10.1007/s00424-004-1354-6. [DOI] [PubMed] [Google Scholar]
- 15.Galler S, Hilber K, Pette D. Stretch activation and myosin heavy chain isoforms of rat, rabbit and human skeletal muscle fibres. J Muscle Res Cell Motil 18: 441–448, 1997. doi: 10.1023/A:1018646814843. [DOI] [PubMed] [Google Scholar]
- 16.Geeves MA, Holmes KC. The molecular mechanism of muscle contraction. Adv Protein Chem 71: 161–193, 2005. doi: 10.1016/S0065-3233(04)71005-0. [DOI] [PubMed] [Google Scholar]
- 17.Gordon S, Dickinson MH. Role of calcium in the regulation of mechanical power in insect flight. Proc Natl Acad Sci USA 103: 4311–4315, 2006. doi: 10.1073/pnas.0510109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granzier HLM, Wang K. Interplay between passive tension and strong and weak binding cross-bridges in insect indirect flight muscle. A functional dissection by gelsolin-mediated thin filament removal. J Gen Physiol 101: 235–270, 1993. doi: 10.1085/jgp.101.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenberg MJ, Kazmierczak K, Szczesna-Cordary D, Moore JR. Cardiomyopathy-linked myosin regulatory light chain mutations disrupt myosin strain-dependent biochemistry. Proc Natl Acad Sci USA 107: 17403–17408, 2010. doi: 10.1073/pnas.1009619107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwamoto H, Inoue K, Yagi N. Fast x-ray recordings reveal dynamic action of contractile and regulatory proteins in stretch-activated insect flight muscle. Biophys J 99: 184–192, 2010. doi: 10.1016/j.bpj.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Josephson RK. Comparative physiology of insect flight muscle. In: Nature's Versatile Engine: Insect Flight Muscle Inside and Out, edited by Vigoreaux JO. New York, NY: Springer, 2005, p. 34–43. [Google Scholar]
- 22.Josephson RK, Malamud JG, Stokes DR. Asynchronous muscle: A primer. J Exp Biol 203: 2713–2722, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Kronert WA, Dambacher CM, Knowles AF, Swank DM, Bernstein SI. Alternative relay domains of Drosophila melanogaster myosin differentially affect ATPase activity, in vitro motility, myofibril structure and muscle function. J Mol Biol 379: 443–456, 2008. doi: 10.1016/j.jmb.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mansfield C, West TG, Curtin NA, Ferenczi MA. Stretch of contracting cardiac muscle abruptly decreases the rate of phosphate release at high and low calcium. J Biol Chem 287: 25696–25705, 2012. doi: 10.1074/jbc.M112.373498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller BM, Zhang S, Suggs JA, Swank DM, Littlefield KP, Knowles AF, Bernstein SI. An alternative domain near the nucleotide-binding site of Drosophila muscle myosin affects ATPase kinetics. J Mol Biol 353: 14–25, 2005. doi: 10.1016/j.jmb.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Moore JR. Stretch activation: toward a molecular mechanism. In: Nature's Versatile Engine: Insect Flight Muscle Inside and Out, edited by Vigoreaux JO. New York: Springer, 2005, p. 44–60. [Google Scholar]
- 27.Peckham M, Molloy JE, Sparrow JC, White DC. Physiological properties of the dorsal longitudinal flight muscle and the tergal depressor of the trochanter muscle of Drosophila melanogaster. J Muscle Res Cell Motil 11: 203–215, 1990. doi: 10.1007/BF01843574. [DOI] [PubMed] [Google Scholar]
- 28.Perz-Edwards RJ, Irving TC, Baumann BA, Gore D, Hutchinson DC, Kržič U, Porter RL, Ward AB, Reedy MK. X-ray diffraction evidence for myosin-troponin connections and tropomyosin movement during stretch activation of insect flight muscle. Proc Natl Acad Sci USA 108: 120–125, 2011. doi: 10.1073/pnas.1014599107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pringle JWS. The Croonian Lecture, 1977. Stretch activation of muscle: Function and mechanism. Proc R Soc Lond B Biol Sci 201: 107–130, 1978. doi: 10.1098/rspb.1978.0035. [DOI] [PubMed] [Google Scholar]
- 30.Rüegg JC, Steiger GJ, Schädler M. Mechanical activation of the contractile system in skeletal muscle. Pflugers Arch 319: 139–145, 1970. doi: 10.1007/BF00592492. [DOI] [PubMed] [Google Scholar]
- 31.Schädler M, Steiger GJ, Rüegg JC. Mechanical activation and isometric oscillation in insect fibrillar muscle. Pflugers Arch 330: 217–229, 1971. doi: 10.1007/BF00588613. [DOI] [PubMed] [Google Scholar]
- 32.Steiger GJ. Stretch activation and myogenic oscillation of isolated contractile structures of heart muscle. Pflugers Arch 330: 347–361, 1971. doi: 10.1007/BF00588586. [DOI] [PubMed] [Google Scholar]
- 33.Steiger GJ. Stretch activation and tension transients in cardiac, skeletal and insect flight muscle. In: Insect Flight Muscle, edited by Tregear RT. Amsterdam, The Netherlands: North Holland, 1977, p. 221–268. [Google Scholar]
- 34.Stelzer JE, Brickson SL, Locher MR, Moss RL. Role of myosin heavy chain composition in the stretch activation response of rat myocardium. J Physiol 579: 161–173, 2007. doi: 10.1113/jphysiol.2006.119719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stelzer JE, Larsson L, Fitzsimons DP, Moss RL. Activation dependence of stretch activation in mouse skinned myocardium: Implications for ventricular function. J Gen Physiol 127: 95–107, 2006. doi: 10.1085/jgp.200509432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swank DM. Mechanical analysis of Drosophila indirect flight and jump muscles. Methods 56: 69–77, 2012. doi: 10.1016/j.ymeth.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swank DM, Vishnudas VK, Maughan DW. An exceptionally fast actomyosin reaction powers insect flight muscle. Proc Natl Acad Sci USA 103: 17543–17547, 2006. doi: 10.1073/pnas.0604972103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swank DM, Wells L, Kronert WA, Morrill GE, Bernstein SI. Determining structure/function relationships for sarcomeric myosin heavy chain by genetic and transgenic manipulation of Drosophila. Microsc Res Tech 50: 430–442, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 39.Sweeney HL, Kushmerick MJ, Mabuchi K, Sréter FA, Gergely J. Myosin alkali light chain and heavy chain variations correlate with altered shortening velocity of isolated skeletal muscle fibers. J Biol Chem 263: 9034–9039, 1988. [PubMed] [Google Scholar]
- 40.Thorson J, White DCS. Distributed representations for actin-myosin interaction in the oscillatory contraction of muscle. Biophys J 9: 360–390, 1969. doi: 10.1016/S0006-3495(69)86392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tohtong R, Yamashita H, Graham M, Haeberle J, Simcox A, Maughan D. Impairment of muscle function caused by mutations of phosphorylation sites in myosin regulatory light chain. Nature 374: 650–653, 1995. doi: 10.1038/374650a0. [DOI] [PubMed] [Google Scholar]
- 42.VanBuren P, Waller GS, Harris DE, Trybus KM, Warshaw DM, Lowey S. The essential light chain is required for full force production by skeletal muscle myosin. Proc Natl Acad Sci USA 91: 12403–12407, 1994. doi: 10.1073/pnas.91.26.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vemuri R, Lankford EB, Poetter K, Hassanzadeh S, Takeda K, Yu ZX, Ferrans VJ, Epstein ND. The stretch-activation response may be critical to the proper functioning of the mammalian heart. Proc Natl Acad Sci USA 96: 1048–1053, 1999. doi: 10.1073/pnas.96.3.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Q, Zhao C, Swank DM. Calcium and stretch activation modulate power generation in Drosophila flight muscle. Biophys J 101: 2207–2213, 2011. doi: 10.1016/j.bpj.2011.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wells L, Edwards KA, Bernstein SI. Myosin heavy chain isoforms regulate muscle function but not myofibril assembly. EMBO J 15: 4454–4459, 1996. [PMC free article] [PubMed] [Google Scholar]
- 46.Wray JS. Filament geometry and the activation of insect flight muscles. Nature 280: 325–326, 1979. doi: 10.1038/280325a0. [DOI] [Google Scholar]
- 47.Zhang S, Bernstein SI. Spatially and temporally regulated expression of myosin heavy chain alternative exons during Drosophila embryogenesis. Mech Dev 101: 35–45, 2001. doi: 10.1016/S0925-4773(00)00549-9. [DOI] [PubMed] [Google Scholar]
- 48.Zhao C, Swank DM. An embryonic myosin isoform enables stretch activation and cyclical power in Drosophila jump muscle. Biophys J 104: 2662–2670, 2013. doi: 10.1016/j.bpj.2013.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zumstein N, Forman O, Nongthomba U, Sparrow JC, Elliott CJ. Distance and force production during jumping in wild-type and mutant Drosophila melanogaster. J Exp Biol 207: 3515–3522, 2004. doi: 10.1242/jeb.01181. [DOI] [PubMed] [Google Scholar]


