Abstract
Angiotensin-converting enzyme inhibitors (ACEi) and mineralocorticoid receptor (MR) antagonists are FDA-approved drugs that inhibit the renin-angiotensin-aldosterone system (RAAS) and are used to treat heart failure. Combined treatment with the ACEi lisinopril and the nonspecific MR antagonist spironolactone surprisingly improves skeletal muscle, in addition to heart function and pathology in a Duchenne muscular dystrophy (DMD) mouse model. We recently demonstrated that MR is present in all limb and respiratory muscles and functions as a steroid hormone receptor in differentiated normal human skeletal muscle fibers. The goals of the current study were to begin to define cellular and molecular mechanisms mediating the skeletal muscle efficacy of RAAS inhibitor treatment. We also compared molecular changes resulting from RAAS inhibition with those resulting from the current DMD standard-of-care glucocorticoid treatment. Direct assessment of muscle membrane integrity demonstrated improvement in dystrophic mice treated with lisinopril and spironolactone compared with untreated mice. Short-term treatments of dystrophic mice with specific and nonspecific MR antagonists combined with lisinopril led to overlapping gene-expression profiles with beneficial regulation of metabolic processes and decreased inflammatory gene expression. Glucocorticoids increased apoptotic, proteolytic, and chemokine gene expression that was not changed by RAAS inhibitors in dystrophic mice. Microarray data identified potential genes that may underlie RAAS inhibitor treatment efficacy and the side effects of glucocorticoids. Direct effects of RAAS inhibitors on membrane integrity also contribute to improved pathology of dystrophic muscles. Together, these data will inform clinical development of MR antagonists for treating skeletal muscles in DMD.
Keywords: Duchenne muscular dystrophy, mineralocorticoid receptor, spironolactone, eplerenone, microarray, sarcolemma
duchenne muscular dystrophy (DMD) is a fatal genetic muscle disease that results in progressive degeneration of skeletal and cardiac muscles and affects 1:5,000 boys (20). DMD is caused by complete absence of the dystrophin protein, which stabilizes muscle membranes by linking the subsarcolemmal cytoskeleton to a transmembrane glycoprotein complex. Currently, the standard-of-care treatment for DMD is prednisone, a glucocorticoid that delays loss of ambulation by an average of 2 yr but has numerous serious side effects (6). Glucocorticoids are widely used to treat both acute and chronic inflammation because of their anti-inflammatory and immunosuppressive effects; however, the underlying mechanism by which these glucocorticoid receptor (GR) agonists benefit skeletal muscles is not fully understood. Several laboratories have demonstrated that prednisone even worsens damage to both skeletal and cardiac muscles in DMD mouse models (13, 14, 31).
The angiotensin-converting enzyme inhibitor (ACEi) lisinopril and the mineralocorticoid receptor (MR) antagonist spironolactone are FDA-approved drugs with a long history of safety and efficacy in treating heart diseases and have minimal side effects (9, 21). Lisinopril and spironolactone indirectly and directly, respectively, target the steroid hormone MR through the renin-angiotensin-aldosterone system (RAAS). Lisinopril is now recommended for use in DMD patients starting at the age of 10 yr (19), and the addition of an MR antagonist has recently been demonstrated to further slow the progression of cardiomyopathy in DMD patients (27).
Our group has previously shown that combined treatment with lisinopril and spironolactone improved skeletal muscle function and pathology, in addition to the heart, in the utrn+/−;mdx (“het”) mouse model of DMD (26). Mice treated with a combination of these drugs showed 80% of normal muscle force generation in both respiratory and limb muscles compared with only 40% of normal force observed in untreated het mice (26). Treatment also led to a significant reduction in ongoing skeletal muscle damage, but a direct effect on membrane integrity has not been tested.
We recently showed that MRs, not previously investigated in skeletal muscles, were present in limb and respiratory muscles from wild-type and dystrophic mice (8). The endogenous MR agonist aldosterone was able to induce a large number of gene-expression changes in normal differentiated human myotubes, supporting MR functions as a steroid hormone receptor in skeletal muscles (8). Together, these data suggest that ACEi and MR antagonists can have a direct therapeutic effect on skeletal muscles.
Inflammation exacerbates muscle damage and contributes to myonecrosis in dystrophic muscles (7). Previous studies have shown that treatment with anti-inflammatory glucocorticoids can shift macrophages from a proinflammatory M1 phenotype to an anti-inflammatory M2 phenotype and improve muscle pathology (7). MR has also been shown to regulate macrophage polarization in other tissues but has not been investigated in skeletal muscles (3). MR activation shifts macrophages to a proinflammatory M1 phenotype, whereas treatment with the nonspecific MR antagonist spironolactone or selective MR antagonist eplerenone promotes an anti-inflammatory M2 phenotype (3). Together, these data support that MR antagonism can result in anti-inflammatory effects similar to glucocorticoids, which may contribute to the improved muscle pathology observed in lisinopril plus spironolactone (LS)-treated dystrophic mice. The goal of this study was to begin to define the cellular and molecular effects of RAAS inhibitors on skeletal muscles. RAAS inhibitors, used in combination clinically in cardiology, were also compared with molecular changes resulting from standard-of-care glucocorticoid treatment.
MATERIALS AND METHODS
Animals.
All protocols were approved by the Institutional Animal Care and Use Committee of The Ohio State University, are in compliance with the laws of the United States, and conform to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. Dystrophin-deficient, utrophin, haplo-insufficient het male mice (38) on a C57BL/10 background were bred in house and used for treated groups or untreated controls. Flexor digitorum brevis (FDB) muscles were used for membrane integrity assays, since they are small, relatively thin muscles that can be dissected cleanly at tendons, allowing for intact surgical removal from the animal, and are typically used for laser-injury experiments in dystrophic mice. Quadriceps muscles were used for the genomic studies, since they are larger muscles that provide sufficient tissue to conduct gene-expression measurements and can be compared with previously published data sets available from this muscle type from various studies on dystrophic mice.
Confocal microscopy membrane integrity assay.
Eight 4-wk-old het male mice, housed two per cage, were given water bottles containing 132 mg/l lisinopril (CAS no. 83915-83-7; SBH Medical, Worthington, OH) + 250 mg/l spironolactone (S3378; Sigma-Aldrich, St. Louis, MO; dissolved in 0.1% ethanol) in reverse-osmosis water (n = 4) or reverse-osmosis water only (n = 4), as described in our previous preclinical studies (17, 26), for 2–4 wk. Medicated water bottles were replaced three times/week, and the volume of water consumed was recorded. Mice were weighed once/week. Mice consumed approximately the predicted dosages of 20 mg·kg−1·day−1 lisinopril and 37.5 mg· kg−1·day−1 spironolactone. Membrane integrity assays were performed on isolated FDB muscle bundles to evaluate the effect of LS on treated versus untreated het mice. A group of four C57BL/6J (C57) male mice were used as wild-types for comparison with untreated and treated het mice. FDB muscles were surgically isolated and placed in minimal Ca2+ Tyrode solution (140 mM NaCl, 5 mM KCl, 2 mM MgCl2, and 10 mM HEPES, pH 7.2). Muscle bundles were mechanically separated at the tendon and then adhered on MatTek glass-bottomed Petri dishes using liquid bandage on the exposed tendons (New-Skin). Membrane disruption was induced in the presence of Tyrode supplement with 2.5 µM FM 4-64 dye (Thermo Fisher Scientific, Waltham, MA) and 2 mM Ca2+, with a FluoView FV1000 multi-photon confocal laser-scanning microscope (Olympus, Melville, NY). A circular region of interest was selected along the edge of the sarcolemma and irradiated at 24% of maximum infrared laser power for 3 s. Pre- and postdamage images were captured every 3 s for a total of 60 s. Extent of membrane damage was analyzed using the ImageJ Fiji software (National Institutes of Health, Bethesda, MD) by measuring the fluorescence intensity encompassing the site of damage with results represented as change in fluorescence signal relative to its starting signal (ΔF/F0), as described previously (34). Fibers for untreated het mice (n = 20), for LS-treated het mice (n = 24), and for C57 mice (n = 16 fibers, bred in house; The Jackson Laboratory, Bar Harbor, ME) were measured from the four mice used for each group. GraphPad Prism (GraphPad Software, La Jolla, CA) was used to fit the ΔF/F0 curve for each animal, calculate the area under the curve, and perform statistical analysis. The area under the curve represents a quantification of total dye accumulation during the entire period of laser damage. This calculation provides an index of the overall extent of sarcolemmal membrane disruption in each muscle fiber tested. Technical replicates for each biological replicate were averaged. All laser-injury experiments and accompanying analyses were performed by an operator blinded to the treatment groups.
Microarray analysis.
Het mice (n = 3/group) were treated from 4 to 6 wk of age with Teklad Rodent Chow (no. 7912), containing 133 mg/kg lisinopril (CAS no. 83915-83-7; SBH Medical) and either 666.66 mg/kg LS (S3378; Sigma-Aldrich) or 2,000 mg/kg eplerenone plus lisinopril (EL; Compound Transfer Program; Pfizer, New York, NY, prepared by Research Diets, New Brunswick, NJ) or water bottles containing 10 mg/l prednisolone (P6004; Sigma-Aldrich; dissolved in 0.04% ethanol) in reverse-osmosis water, replaced three times/week, or left untreated. Chow was used to compare treatment between MR antagonists, since eplerenone is not soluble in water. Mice were weighed weekly, and volume of water or chow consumed was recorded to calculate an average drug dosage of lisinopril (20 mg·kg−1·day−1), spironolactone (100 mg·kg−1·day−1), eplerenone (200 mg·kg−1·day−1), and prednisolone (1.5 mg·kg−1·day−1). All mice were dissected during the middle of the light cycle between 9 AM and 3 PM. One quadriceps muscle from each mouse was removed for RNA processing, and the other quadriceps muscle was embedded in optimal-cutting temperature medium and frozen on liquid nitrogen-cooled isopentane for histological analyses.
RNA from mouse quadriceps muscles was isolated using TRIzol reagent (Thermo Fisher Scientific), according to the manufacturer’s instructions. Samples were DNAse treated using RQ1 DNAse (Promega, Madison, WI) and further purified using the RNeasy mini kit (Qiagen, Germantown, MD) cleanup protocol, and integrity was verified using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). A 100-ng aliquot of total RNA was linearly amplified, and 5.5 µg cDNA was labeled and fragmented using the GeneChip WT PLUS Reagent Kit (Affymetrix, Santa Clara, CA), following the manufacturer's instructions. Labeled cDNA targets were hybridized to Affymetrix GeneChip Mouse Transcriptome Array 1.0 for 16 h at 45°C, rotating at 60 rpm. The arrays were washed and stained using the GeneChip Fluidics Station 450 and scanned using the GeneChip Scanner 3000. Arrays were normalized using the gene-level SST RMA algorithm in Expression Console software and comparisons made in Transcriptome Analysis Console software (Affymetrix). The microarray data have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (accession no. GSE84876). Gene groups were assigned using the Functional Annotation clustering tool from the Database for Annotation, Visualization and Integrated Discovery (DAVID).
Immunofluorescence.
Quadriceps muscle cryosections (8 µm) were stained with an antibody against mouse IgG (Alexa 488 goat anti-mouse IgG, 1:200; Thermo Fisher Scientific) to visualize ongoing muscle damage. Samples were mounted using Vectashield mounting medium (Vector Laboratories, Burlingame, CA) with 1 µl/ml 4′,6-diamidino-2-phenylindole and viewed using an Eclipse E800 microscope (Nikon Instruments, Melville, NY). Images were taken under a ×20 objective using a SPOT RT Slider digital camera and SPOT software.
RESULTS
Direct assessment of membrane stability effects of LS treatment on dystrophic skeletal muscles.
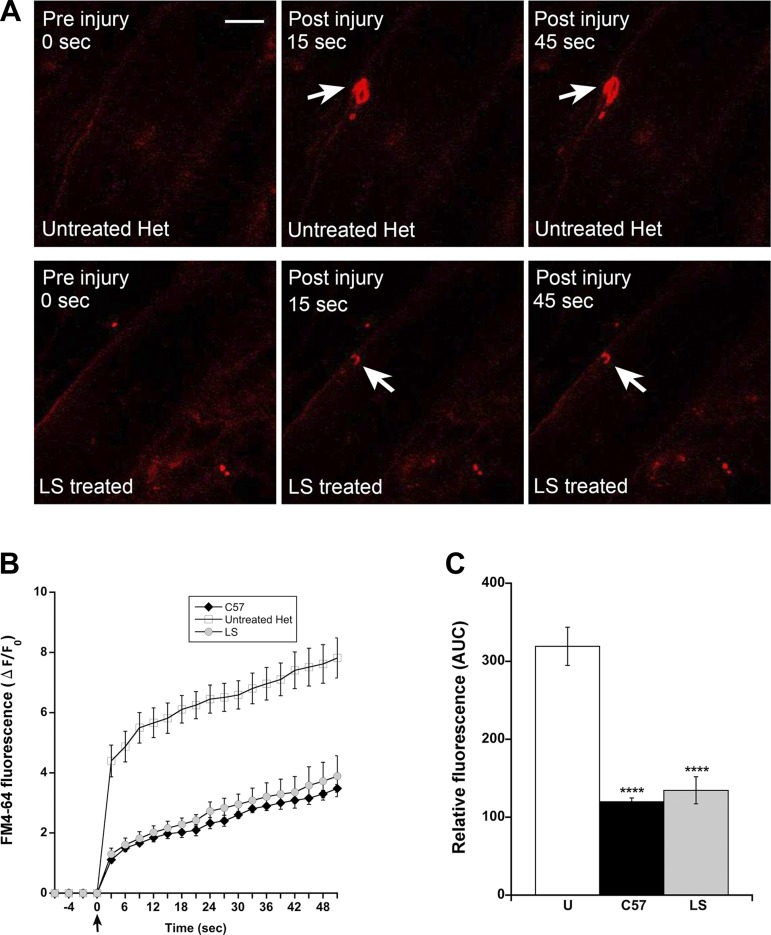
The results from previous preclinical studies support that treatment with lisinopril plus an MR antagonist reduces ongoing muscle damage, but a direct effect on membrane stability has not been investigated. To confirm the loss of membrane integrity in the het model, FDB muscle bundles from het mice were subjected to laser-induced sarcolemmal membrane damage. An infrared multiphoton laser produced localized damage to the sarcolemmal membrane of intact muscle fibers in the presence of a lipophilic FM4-64 dye, which is nonfluorescent in aqueous solution but fluoresces intensely once it enters the cell. When the membrane re-seals, dye entry ceases, and the fluorescent signal stabilizes. The tracking of the fluorescence intensity allows resolution of the extent of membrane disruption. Laser-injury assays demonstrated the predicted compromised membrane stability in untreated het mice compared with wild-type controls (Fig. 1). FDB muscles isolated from het mice treated for 4 wk with LS show a significant improvement in membrane integrity compared with untreated het mice (P < 0.0001; Fig. 1).
Fig. 1.
Lisinopril plus spironolactone (LS) treatment improves membrane integrity in het mice. A: representative confocal microscopy images of isolated FDB muscle bundles from het mice left untreated (top) or treated with LS (bottom) at indicated time points following injury by an infrared multiphoton laser (arrows) in the presence of FM4-64 dye. Original scale bar, 10 µm. B: FM4-64 dye influx over time in muscle fibers is quantified as the change/increase in red fluorescent signal over baseline (ΔF/F0) over a time course following induction of injury (time = 0 s; arrow). Fibers for injury experiments were isolated from 4 mice from each group: untreated het, LS-treated het, and C57 wild-type mice. Data are presented as means ± SE. C: quantification of the area under the curve (AUC) among untreated het (U), LS-treated hets, or C57 wild-type mice. One-way ANOVA, followed by Dunnett’s post hoc test, was used to identify significance between untreated hets and the 2 other groups. ****P < 0.0001 compared with untreated hets.
Treatment with LS or EL results in overlapping gene-expression changes.
Whereas increased membrane integrity could underlie some of the improved structure and function observed in dystrophic skeletal muscle following LS treatment, the molecular basis for the efficacy of RAAS inhibition is not clear. We have previously shown gene-expression differences between LS-treated and untreated het mice at the conclusion of a 16-wk study after phenotypes had significantly diverged (8, 26). These gene-expression differences represent the secondary benefits of treatment, in addition to the potential underlying therapeutic gene-expression changes. We have also shown that the endogenous MR agonist aldosterone can change gene expression in normal human myotubes (8). Here, we sought to begin to define the molecular changes that may underlie the efficacy of targeting MR in skeletal muscle. We compared treatment with LS, which can also bind glucocorticoid and androgen receptors, with lisinopril, plus the specific MR antagonist EL. These two treatments were recently shown to have equivalent efficacy in het mice (16).
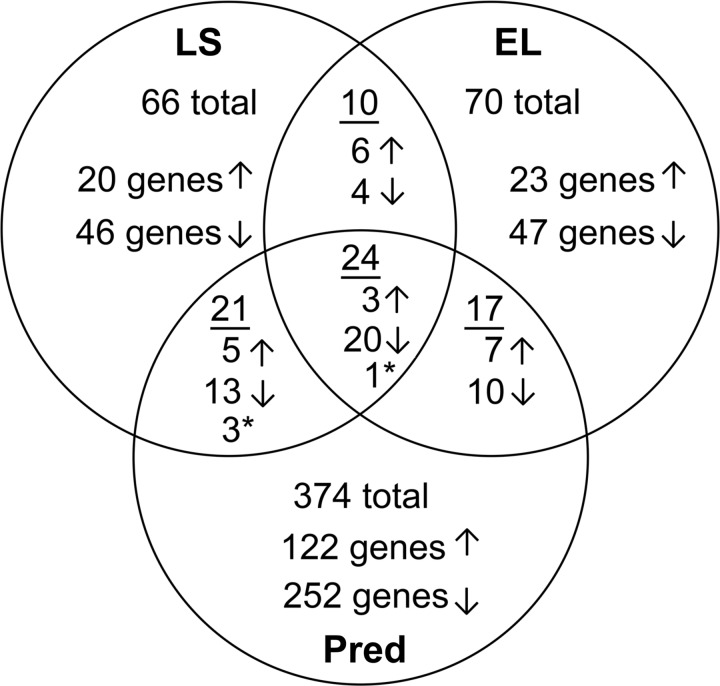
We performed gene-expression microarray on 6-wk-old male het mice that had been treated for 2 wk with LS or EL or left untreated. These studies also included a group of het mice treated with prednisolone, the active metabolite of prednisone, to begin to differentiate between gene-expression changes that may underlie efficacy versus side effects of standard-of-care glucocorticoids. There were 66 gene-expression changes in quadriceps muscles between LS-treated and untreated het mice (Fig. 2). Treatment increased expression of 20 genes (2- to 7-fold) and decreased expression of 46 genes (2- to 7-fold). Similarly, EL treatment led to 70 gene-expression changes compared with untreated—with 23 genes increased (2- to 5-fold) and 47 decreased—with 45 of these reduced <7-fold (Fig. 2). S100a8 was decreased 160-fold by EL treatment, which was the largest fold change observed out of any of our previous microarrays. We reanalyzed the raw gene-expression data to determine whether this change was also present with LS treatment. We observed that there was an outlier in the untreated group, and when removed from the analysis, the S100a8 gene was decreased 93-fold in the LS group. In addition, S100a9, which functions as a dimer with S100a8, was now decreased in both EL and LS compared with untreated het mice (64- and 50-fold, respectively).
Fig. 2.
Microarray analysis of 2 wk-treated dystrophic het mice with a nonspecific or specific MR antagonist plus lisinopril results in a comparable number of gene-expression changes. Treatment with lisinopril plus spironolactone (LS) results in decreased expression of 46 genes and increased expression of 20 genes (66 genes total) compared with untreated het. Eplerenone plus lisinopril (EL) treatment results in a comparable number of gene-expression changes, 23 increased and 47 decreased (70 genes total). Thirty-three (50%) of these gene changes are conserved in EL-treated mice compared with untreated het. Treatment with standard-of-care prednisolone (Pred) results in 374 gene-expression changes, 122 increased and 252 decreased compared with untreated het. Only 62 genes of these genes are conserved with LS- or EL-treated mice versus untreated het; 312 genes are specific to prednisolone treatment. Ten gene-expression changes are present in both the LS and EL compared with untreated het but not in the prednisolone group. *Genes that are changed in the opposite directions with MR antagonist and prednisolone treatment. Dbp is represented in both the group of 10 gene changes conserved between LS and EL and as the gene-expression change (*) conserved with prednisolone but in the opposite direction.
Thirty-three gene-expression changes were conserved between LS and EL compared with untreated (Table 1 and Fig. 3A). Only 10 gene-expression changes conserved between LS and EL compared with untreated were not present or changed in the same direction in the comparison of prednisolone and untreated. Scd1, Scd2, Dbp, Lrtm1, Prkag3, and Nr1d1 were all increased by LS or EL treatment (5.3-, 4.4-, 3.1-, 3.0-, 3.0-, and 2.3-fold, respectively, in EL). Mt1, Pim1, Mt2, and Clu were reduced by LS or EL treatment (2.3-, 2.4-, 2.8-, and 2.9-fold, respectively, in EL). These genes were functionally classified as either ion binding or transcription factors and may underlie MR-specific efficacy.
Table 1.
Fold changes of gene-expression differences conserved in microarray comparing quadriceps muscles from lisinopril plus spironolactone (LS) and eplerenone plus lisinopril (EL) mice versus untreated het controls (Un)
| Gene Symbol | Full Gene Name | Fold Change (LS/Un) | Fold Change (EL/Un) |
|---|---|---|---|
| Immune response and homeostasis | |||
| Cd14 | CD14 antigen | −2.8 | −2.8 |
| Cd80 | CD80 antigen | −2.9 | −3.1 |
| Csf2rb | Colony-stimulating factor 2 receptor, beta, low-affinity (granulocyte-macrophage) | −3.8 | −3.5 |
| Fcgr3 | Fc receptor, IgG, low-affinity III | −2.2 | −2.2 |
| Il6 | Interleukin 6 | −3.9 | −2.9 |
| Mt1 | Metallothionein 1 | −2.1 | −2.3 |
| Nfkbia | Nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor, alpha | −2.8 | −2.1 |
| Tfpi2 | Tissue factor pathway inhibitor 2 | −2.3 | −2.4 |
| Thbs1 | Thrombospondin 1 | −6.2 | −6.8 |
| Scd2 | Stearoyl-coenzyme A desaturase 2 | 4.1 | 4.4 |
| Wfdc17 | Whey acidic protein (WAP) 4-disulfide core domain 17 | −2.1 | −2.6 |
| Regulation of transcription | |||
| Dbp | D Site albumin promoter-binding protein | 4.2 | 3.1 |
| Litaf | LPS-induced TNF-α factor | −2.4 | −2.3 |
| Mxd1 | Myc-associated factor X (MAX) dimerization protein 1 | −2.3 | −2.6 |
| Nr1d1 | Nuclear receptor subfamily 1, group D, member 1 | 2.4 | 2.3 |
| Nr4a3 | Nuclear receptor subfamily 4, group A, member 3 | −5.6 | −4.0 |
| Ion binding and transport | |||
| Adamts1 | A disintegrin-like and metallopeptidase (reprolysin-type) with thrombospondin type 1 motif, 1 | −2.5 | −2.2 |
| Mt2 | Metallothionein 2 | −2.4 | −2.8 |
| Pim1 | Proviral integration site 1 | −2.0 | −2.4 |
| Scd1 | Stearoyl-coenzyme A desaturase 1 | 7.1 | 5.3 |
| Zfp36 | Zinc finger protein 36 | −4.7 | −3.5 |
| Apoptosis and proteolysis | |||
| Clu | Clusterin | −2.1 | −2.9 |
| Alternative splicing | |||
| Asb15 | Ankyrin repeat and SOCS box-containing 15 | 2.8 | 3.4 |
| Plaur | Plasminogen activator, urokinase receptor | −2.4 | −2.7 |
| Prkag3 | Protein kinase, AMP-activated, gamma 3 noncatalytic subunit | 3.1 | 3.0 |
| Transmembrane | |||
| Atp1b4 | ATPase, Na+/K+ transporting, beta 4 polypeptide | 2.0 | 2.6 |
| Ifitm1 | Interferon-induced transmembrane protein 1 | −2.2 | −2.1 |
| Lrtm1 | Leucine-rich repeats and transmembrane domain 1 | 2.5 | 3.0 |
| Ms4a4a | Membrane-spanning 4-domains, subfamily A, member 4A | −2.7 | −2.8 |
| Tmem252 | Transmembrane protein 252 | −2.9 | −3.0 |
| Unknown or specific functions | |||
| Dusp5 | Dual-specificity phosphatase 5 | −2.5 | −2.3 |
| Hdc | Histidine decarboxylase | −6.6 | −6.0 |
| Sec14l5 | SEC14-like 5 (Saccharomyces cerevisiae) | 2.7 | 2.1 |
Genes were functionally clustered using the Functional Annotation clustering tool from the Database for Annotation, Visualization and Integrated Discovery (DAVID). Positive fold changes indicate that gene expression was increased with treatment and vice versa. The 10 gene-expression changes conserved between LS and EL, but not changed by prednisolone, are shown in bold.
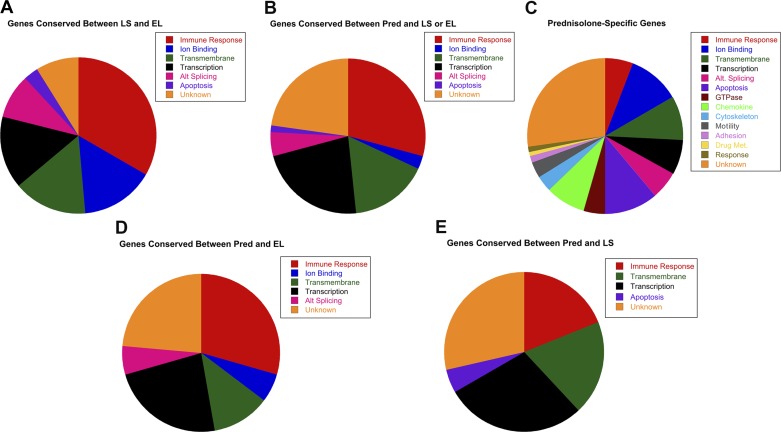
Fig. 3.
Genes involved in immune response and transcriptional regulation are conserved between MR antagonists and standard-of-care glucocorticoid agonists. A: thirty-three gene-expression changes were conserved between lisinopril plus spironolactone (LS) and eplerenone plus lisinopril (EL). Out of these 33, only 10 were also not conserved with prednisolone treatment. B: there were 62 genes conserved between prednisolone (Pred) and LS or EL treated versus untreated het mice. C: an additional 312 genes were detected only in the Pred versus untreated het group. These genes encompass similar functional groups to those in A and B, including immune response, transcriptional regulators, ion binding, transmembrane, and alternative (Alt.) splicing. However, several functional groups specific to prednisolone treatment were also represented, including 35 genes involved in apoptosis or proteolysis (11.1%), 26 cytokines with chemokine activity (8.3%), 10 cytoskeleton (3.2%), 11 cell migration/motility (3.5%), 4 cell adhesion (1.3%), 3 drug metabolism (Met.; 1%), 3 behavioral response (1%), and 14 genes with GTPase activity (4.5%). D: seventeen gene-expression changes were conserved between Pred and EL, and (E) 21 were conserved between Pred and LS, subsets of the genes represented in B.
Short-term prednisolone treatment increases apoptotic genes and damages skeletal muscles.
Sixty-two genes were conserved between prednisolone and either LS or EL compared with untreated (Figs. 2 and 3B and Table 2). Of these 62 genes, 18 were increased with prednisolone treatment, and 44 were decreased. The highest fold change for increased genes was 3.9-fold, and only two genes were increased >3-fold. The largest fold change for decreased genes was 42-fold, and 23 of the 44 genes were reduced >3-fold. Overall, all three drug treatments reduced expression of more genes than they increased. Of the 62 genes conserved between prednisolone and either LS or EL compared with untreated het mice, the majority of genes could be classified into the functional groups of cytokine production/immune response (29%) or regulation of transcription (22.6%; Fig. 3B). One of the genes conserved among all three groups (Dbp), and three of the genes conserved between LS and prednisolone were changed in the opposite direction with mineralocorticoid antagonists compared with prednisolone (Fig. 2 and Table 2).
Table 2.
Fold changes of gene-expression differences in microarray comparing quadriceps muscles from prednisolone (P)-treated and untreated het mice conserved with either lisinopril plus spironolactone (LS) or eplerenone plus lisinopril (EL) versus untreated het controls (Un)
| Fold Change |
||||
|---|---|---|---|---|
| Gene Symbol | Full Gene Name | P/Un | LS/Un | EL/Un |
| Regulation of cytokine production/immune response | ||||
| Adora2b | Adenosine A2b receptor | −2.4 | X | −2.1 |
| Ccl9 | Chemokine (C-C motif) ligand 9 | −4.3 | X | −5.2 |
| Cd14 | CD14 antigen | −2.4 | −2.8 | −2.8 |
| Cd80 | CD80 antigen | −3.4 | −2.9 | −3.1 |
| Csf2rb | Colony stimulating factor 2 receptor, β, low-affinity (granulocyte-macrophage) | −3.5 | −3.8 | −3.5 |
| Csf2rb2 | Colony stimulating factor 2 receptor, β2, low-affinity (granulocyte-macrophage) | −3.0 | X | −2.6 |
| Fcgr3 | Fc receptor, IgG, low-affinity III | −2.6 | −2.2 | −2.2 |
| Il1b | Interleukin 1β | −42.1 | X | −40.3 |
| Il6 | Interleukin-6 | −3.6 | −3.9 | −2.9 |
| Klrb1b | Killer cell lectin-like receptor subfamily B member 1B | −2.3 | −2.3 | X |
| Nfkbia | Nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor, alpha | −4.7 | −2.8 | −2.1 |
| Oas1a | 2′-5′ oligoadenylate synthetase 1A | −3.2 | −2 | X |
| Oas1g | 2′-5′ oligoadenylate synthetase 1G | −2.9 | −2 | X |
| Srgn | Serglycin | −2.6 | X | −2.1 |
| Thbs1 | Thrombospondin 1 | −10.5 | −6.2 | −6.8 |
| Tlr8 | Toll-like receptor 8 | −2.3 | −2.1 | X |
| Wfdc17 | WAP four-disulfide core domain 17 | −3.6 | −2.1 | −2.6 |
| Zfp36 | Zinc finger protein 36 | −5.2 | −4.7 | −3.5 |
| Regulation of transcription | ||||
| Arntl | Aryl hydrocarbon receptor nuclear translocator-like | 2.8 | −2.3 | X |
| Cebpd | CCAAT/enhancer binding protein (C/EBP), delta | −7.4 | −4.5 | X |
| Cited2 | Cbp/p300-interacting transactivator, with Glu/Asp-rich carboxy-terminal domain, 2 | 2.6 | X | 2.5* |
| Dbp | D site albumin promoter binding protein | −2.2 | 4.2 | 3.1 |
| Dmrt2 | Doublesex and mab-3 related transcription factor 2 | 2.1 | −2 | X |
| Fgfrl1 | Fibroblast growth factor receptor-like 1 | 2.3 | X | 2.1 |
| Id1 | Inhibitor of DNA binding 1 | −2.9 | X | −2.6 |
| Id2 | Inhibitor of DNA binding 2 | −3.4 | −2.4 | X |
| Litaf | LPS-induced TN factor | −3.0 | −2.4 | −2.3 |
| Mxd1 | MAX dimerization protein 1 | −3.2 | −2.3 | −2.6 |
| Npas2 | Neuronal PAS domain protein 2 | 2.5 | −2.2 | X |
| Nr4a3 | Nuclear receptor subfamily 4, group A, member 3 | −10.7 | −5.6 | −4 |
| Pyhin1 | Pyrin and HIN domain family, member 1 | −4.5 | −2 | X |
| Rcor3 | REST corepressor 3 | 2.2 | X | 2 |
| Ion-binding and transport | ||||
| Adamts1 | A disintegrin-like and metallopeptidase (reprolysin type) with thrombospondin type 1 motif, 1 | −3.1 | −2.5 | −2.2 |
| F5 | Coagulation factor V | −3.2 | X | −2.2 |
| Apoptosis | ||||
| Casp8 | Caspase 8 | −2.1 | −3.4* | X |
| Alternative splicing | ||||
| Asb14 | Ankyrin repeat and SOCS box-containing 14 | 2.0 | 2.8* | 3.37* |
| Fgfrl1 | Fibroblast growth factor receptor-like 1 | 2.3 | X | 2.1 |
| Plaur | Plasminogen activator, urokinase receptor | −2.5 | −2.4 | −2.7 |
| Transmembrane | ||||
| Atp1b4 | ATPase, Na+/K+ transporting, beta 4 polypeptide | 3.6 | 2 | 2.6 |
| Fcgr4 | Fc receptor, IgG, low affinity IV | −4.2 | −2.3 | X |
| Ifitm1 | Interferon induced transmembrane protein 1 | −2.3 | −2.2 | −2.1 |
| Ifrd1 | Interferon-related developmental regulator 1 | −2.6 | X | −2.3 |
| Ms4a4a | Membrane-spanning 4-domains, subfamily A, member 4A | −3.3 | −2.7 | −2.8 |
| Pnpla3 | Patatin-like phospholipase domain containing 3 | 2.2 | 2.1 | X |
| Slc15a3 | Solute carrier family 15, member 3 | −2.0 | −2.2 | X |
| Sntb1 | Syntrophin, basic 1 | 2.3 | 2.5 | X |
| Tmem252 | Transmembrane protein 252 | −4.6 | −2.9 | −3 |
| Ugcg | UDP-glucose ceramide glucosyltransferase | −2.9 | X | −2.2 |
| Unknown or specific functions | ||||
| Dhrs7c | Dehydrogenase/reductase (SDR family) member 7C | 3.9 | X | 2.2 |
| Dusp5 | Dual-specificity phosphatase 5 | −2.6 | −2.5 | −2.3 |
| Gm4980 | Predicted gene 4980 | 2.5 | X | 2.1 |
| Gm6307 | Predicted gene 6307 | 2.6 | X | 2.5 |
| Hdc | Histidine decarboxylase | −7.7 | −6.6 | −6 |
| Irs2 | Insulin receptor substrate 2 | −2.5 | −2.4 | X |
| Lrrc30 | Leucine rich repeat containing 30 | 2.7 | 2.1 | X |
| Lrrc39 | Leucine rich repeat containing 39 | 2.3 | 2.2 | X |
| Prr33 | Proline rich 33 | 2.4 | X | 2 |
| Ptpn1 | Protein tyrosine phosphatase, nonreceptor type 1 | −2.1 | −2.4 | X |
| Sec14l5 | SEC14-like 5 (S. cerevisiae) | 2.3 | 2.7 | 2.1 |
| Synpo2l | Synaptopodin 2-like | 2.0 | 2.1 | X |
| Tfpi2 | Tissue factor pathway inhibitor 2 | −3.0 | −2.3 | −2.4 |
| Tnfaip6 | Tumor necrosis factor-α-induced protein 6 | −5.5 | −7 | X |
Genes were functionally clustered using the Functional Annotation clustering tool from the Database for Annotation, Visualization and Integrated Discovery (DAVID). Positive fold changes indicate gene expression was increased with treatment and vice versa. Bold X's represent a lack of a 2-fold gene-expression change in the LS or EL treatment groups. Values underlined and italicized highlight gene-expression changes going in the opposite direction with P versus LS or EL treatment.
Functionally conserved gene family members in LS or EL comparisons (Cited4, Casp4, and Asb15).
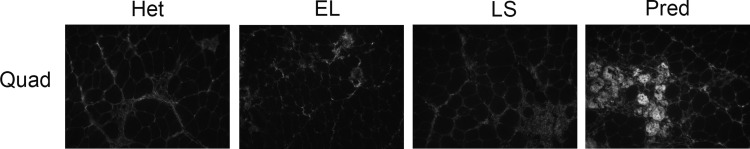
Gene-expression changes (312) were present only in the prednisolone versus untreated het comparison and may represent changes associated with either glucocorticoid-specific muscle benefits or side effects (Figs. 2 and 3C and Table 3). Staining for IgG in quadriceps muscles of het mice treated for 2 wk and used for this microarray experiment (Fig. 4) recapitulated the increased skeletal muscle damage previously observed after longer treatment with prednisolone (14, 31). Genes (103) were increased by prednisolone, and 209 were reduced. Increases ranged from 2- to 6.9-fold with 17 gene-expression changes >3-fold. Decreased genes were reduced between 2- and 57-fold, with 64 genes reduced >3-fold. Classification of these genes identified several functional groups that were not present in the LS or EL versus untreated het comparison. The functional groups specific to prednisolone treatment were apoptosis or proteolysis (11.1%), cytokine/chemokine activity (8.3%), GTPase activity (4.5%), cytoskeleton (3.2%), cell migration and motility (3.5%), and cell adhesion (1.3%; Fig. 3C).
Table 3.
Fold changes of gene-expression differences specific to microarray comparing quadriceps muscles from prednisolone (Pred)-treated het mice versus untreated het controls (Un)
| Gene Symbol | Full Gene Name | Fold Change (Pred/Un) |
|---|---|---|
| Immune response/regulation of immune response | ||
| Cd274 | CD274 antigen | −2.0 |
| Cd300ld | CD300 molecule-like family, member d | −2.2 |
| Clec4n | C-Type lectin domain family 4, member n | −4.1 |
| Cmip | c-Maf-inducing protein | −2.1 |
| Ddx58 | Asp-Glu-Ala-Asp (DEAD) box polypeptide 58 | −2.1 |
| Fcgr2b | Fc receptor, IgG, low-affinity IIb | −2.6 |
| H2-Eb1 | Histocompatibility 2, class II antigen E β | −2.7 |
| Ifih1 | Interferon-induced with helicase C domain 1 | −2.4 |
| Ifit1 | Interferon-induced protein with tetratricopeptide repeats 1 | −2.7 |
| Ifit2 | Interferon-induced protein with tetratricopeptide repeats 2 | −2.2 |
| Ifit3 | Interferon-induced protein with tetratricopeptide repeats 3 | −2.7 |
| Oas2 | 2′-5′ Oligoadenylate synthetase 2 | −5.5 |
| Oasl2 | 2′-5′ Oligoadenylate synthetase-like 2 | −3.2 |
| Rnf125 | Ring finger protein 125 | −3.1 |
| Rsad2 | Radical S-adenosyl methionine domain-containing 2 | −3.4 |
| Samhd1 | SAM domain and HD domain 1 | −2.0 |
| Tpd52 | Tumor protein D52 | −2.2 |
| Trim25 | Tripartite motif-containing 25 | −2.4 |
| Cytokines with chemokine activity | ||
| Adamts9 | A disintegrin-like and metallopeptidase (reprolysin-type) with thrombospondin type 1 motif, 9 | −5.0 |
| Ccl8 | Chemokine (C–C motif) ligand 8 | −4.1 |
| Ccl11 | Chemokine (C–C motif) ligand 11 | −3.2 |
| Cfb | Complement factor B | −2.1 |
| Cxcl1 | Chemokine (C–X–C motif) ligand 1 | −2.7 |
| Cxcl9 | Chemokine (C–X–C motif) ligand 9 | −8.0 |
| Cxcl13 | Chemokine (C–X–C motif) ligand 13 | 6.9 |
| Cxcr4 | Chemokine (C–X–C motif) receptor 4 | −2.9 |
| Egf | Epidermal growth factor | 2.1 |
| Entpd1 | Ectonucleoside triphosphate diphosphohydrolase 1 | −2.1 |
| Hck | Hemopoietic cell kinase | −2.0 |
| Hif1a | Hypoxia-inducible factor 1, alpha subunit | −3.7 |
| Igfbp3 | Insulin-like growth factor-binding protein 3 | 2.7 |
| Igh-VJ558 | Immunoglobulin heavy chain (J558 family) | 2.1 |
| Il1r1 | Interleukin 1 receptor, type I | −3.6 |
| Il13ra1 | Interleukin 13 receptor, alpha 1 | −2.5 |
| Il33 | Interleukin 33 | −8.7 |
| Irf7 | Interferon regulatory factor 7 | −2.6 |
| Lep | Leptin | 2.3 |
| Lrrc32 | Leucine-rich repeat containing 32 | −2.2 |
| Ly86 | Lymphocyte antigen 86 | −2.8 |
| Lyn | Yamaguchi sarcoma viral (v-yes-1) oncogene homolog | −3.1 |
| Rap1b | RAS-related protein 1b | −2.1 |
| Stat1 | Signal transducer and activator of transcription 1 | −2.5 |
| Stat2 | Signal transducer and activator of transcription 2 | −2.9 |
| Thbd | Thrombomodulin | −2.3 |
| Regulation of transcription | ||
| Ankrd2 | Ankyrin repeat domain 2 (stretch-responsive muscle) | 3.5 |
| Bach1 | BTB and CNC homology 1 | −2.0 |
| Baz1a | Bromodomain adjacent to zinc finger domain 1A | −2.6 |
| Bhlhe40 | Basic helix-loop-helix family, member e40 | −2.1 |
| C7 | Complement component 7 | 3.0 |
| Ccnl1 | Cyclin L1 | −2.0 |
| Crem | cAMP-responsive element modulator | −2.1 |
| Ell2 | Elongation factor RNA polymerase II 2 | −2.0 |
| Erg | Avian erythroblastosis virus E-26 (v-ets) oncogene related | −2.3 |
| Fos | FBJ osteosarcoma oncogene | −5.1 |
| Klf2 | Kruppel-like factor 2 (lung) | −3.0 |
| Mafb | v-maf Musculoaponeurotic fibrosarcoma oncogene family, protein B (avian) | −2.8 |
| Mamstr | MEF2-activating motif and SAP domain-containing transcriptional regulator | 2.0 |
| Mnda | Myeloid cell nuclear differentiation antigen | −4.5 |
| Nrip1 | Nuclear receptor-interacting protein 1 | −2.8 |
| Parp14 | Poly (ADP-ribose) polymerase family, member 14 | −2.4 |
| Per1 | Period circadian clock 1 | −6.4 |
| Perm1 | PPARGC1 and ESRR-induced regulator, muscle 1 | 2.5 |
| Rcor3 | REST corepressor 3 | 2.2 |
| Rcor3 | REST corepressor 3 | 2.4 |
| Rorc | RAR-related orphan receptor gamma | 2.4 |
| Rxrg | Retinoid X receptor gamma | 3.2 |
| Tigd4 | Tigger transposable element-derived 4 | 2.3 |
| Ion binding and transport | ||
| Ano5 | Anoctamin 5 | 2.4 |
| Apobec2 | Apolipoprotein B mRNA editing enzyme, catalytic polypeptide 2 | 2.3 |
| Cacna1s | Calcium channel, voltage-dependent, L type, alpha 1S subunit | 2.0 |
| Car14 | Carbonic anhydrase 14 | 6.6 |
| Cd93 | CD93 antigen | −2.5 |
| Cdh5 | Cadherin 5 | −3.7 |
| Clcn1 | Chloride channel 1 | 2.6 |
| Csrp3 | Cysteine and glycine-rich protein 3 | 2.4 |
| Cyp27a1 | Cytochrome P450, family 27, subfamily a, polypeptide 1 | 2.1 |
| Dtna | Dystrobrevin alpha | 2.0 |
| Fmo2 | Flavin containing monooxygenase 2 | 2.1 |
| Gabrr2 | Gamma-aminobutyric acid (GABA) C receptor, subunit rho 2 | 2.1 |
| Kcng4 | Potassium voltage-gated channel, subfamily G, member 4 | 2.7 |
| Kcnj2 | Potassium inwardly rectifying channel, subfamily J, member 2 | 3.5 |
| Kcnj12 | Potassium inwardly rectifying channel, subfamily J, member 12 | 2.7 |
| Mpi | Mannose phosphate isomerase | 2.3 |
| Mrc1 | Mannose receptor, C type 1 | −3.2 |
| Parp12 | Poly (ADP-ribose) polymerase family, member 12 | −2.2 |
| Pgm1 | Phosphoglucomutase 1 | −2.1 |
| Plod2 | Procollagen lysine, 2-oxoglutarate 5-dioxygenase 2 | −2.4 |
| Ppp2r3a | Protein phosphatase 2, regulatory subunit B, alpha | 2.1 |
| Prkd2 | Protein kinase D2 | −2.4 |
| Rnf213 | Ring finger protein 213 | −3.4 |
| S100a11 | S100 calcium-binding protein A11 (calgizzarin) | −2.5 |
| Slc10a6 | Solute carrier family 10 (sodium/bile acid cotransporter family), member 6 | −3.3 |
| Slc16a3 | Solute carrier family 16 (monocarboxylic acid transporters), member 3 | 2.4 |
| Slc25a25 | Solute carrier family 25 (mitochondrial carrier, phosphate carrier), member 25 | −3.5 |
| Slc30a2 | Solute carrier family 30 (zinc transporter), member 2 | 2.6 |
| Slc38a3 | Solute carrier family 38, member 3 | 2.0 |
| Slc38a4 | Solute carrier family 38, member 4 | 2.4 |
| Sln | Sarcolipin | 3.8 |
| Trim54 | Tripartite motif-containing 54 | 2.1 |
| Zfp366 | Zinc finger protein 366 | −2.0 |
| Zswim6 | Zinc finger SWIM-type containing 6 | −2.3 |
| GTPase activity | ||
| Arhgap20 | Rho GTPase-activating protein 20 | 4.1 |
| Gbp2 | Guanylate-binding protein 2 | −2.2 |
| Gbp3 | Guanylate-binding protein 3 | −2.3 |
| Gbp7 | Guanylate-binding protein 7 | −2.3 |
| Gbp8 | Guanylate-binding protein 8 | −2.3 |
| Gbp9 | Guanylate-binding protein 9 | −2.7 |
| Gbp10 | Guanylate-binding protein 10 | −2.9 |
| Gbp11 | Guanylate-binding protein 11 | −2.1 |
| Gm12185 | Predicted gene 12185 | −2.5 |
| Gvin1 | GTPase, very large interferon-inducible 1 | −2.3 |
| Rac2 | RAS-related C3 botulinum substrate 2 | −2.1 |
| Rapgef5 | Rap guanine nucleotide-exchange factor (GEF) 5 | −2.9 |
| Rgs5 | Regulator of G-protein signaling 5 | −2.7 |
| Tgtp2 | T Cell-specific GTPase 2 | −3.2 |
| Apoptosis and proteolysis/regulation of apoptosis | ||
| Agt | Angiotensinogen (serpin peptidase inhibitor, clade A, member 8) | 2.1 |
| Aldh1a1 | Aldehyde dehydrogenase family 1, subfamily A1 | 5.9 |
| Asb2 | Ankyrin repeat and SOCS box-containing 2 | 2.3 |
| Asb11 | Ankyrin repeat and SOCS box-containing 11 | 2.2 |
| Asb12 | Ankyrin repeat and SOCS box-containing 12 | 2.1 |
| Atp1a2 | ATPase, Na+/K+ transporting, alpha 2 polypeptide | 2.1 |
| Bcl3 | B Cell leukemia/lymphoma 3 | 3.2 |
| Ctla2a | Cytotoxic T lymphocyte-associated protein 2 alpha | −2.4 |
| Ctla2b | Cytotoxic T lymphocyte-associated protein 2β | −3.1 |
| Dapk2 | Death-associated protein kinase 2 | 2.2 |
| Ddit4 | DNA damage-inducible transcript 4 | −5.2 |
| Dpep1 | Dipeptidase 1 (renal) | 2.5 |
| Egfbp2 | Epidermal growth factor binding protein type B | 4.0 |
| Egln3 | Egl-9 family hypoxia-inducible factor 3 | 2.1 |
| Eif2ak2 | Eukaryotic translation initiation factor 2-alpha kinase 2 | −2.6 |
| Errfi1 | ERBB receptor feedback inhibitor 1 | −11.5 |
| Fbxo32 | F-Box protein 32 | 2.7 |
| Fcer1g | Fc receptor, IgE, high affinity I, gamma polypeptide | −2.0 |
| Fcgr1 | Fc receptor, IgG, high affinity I | −6.1 |
| Gadd45a | Growth arrest and DNA-damage-inducible 45 alpha | −2.0 |
| Gadd45g | Growth arrest and DNA-damage-inducible 45 gamma | −10.0 |
| Herc3 | Hect domain and RLD 3 | 2.0 |
| Hmox1 | Heme oxygenase (decycling) 1 | −4.4 |
| Ifi204 | Interferon-activated gene 204 | −3.8 |
| Mmp3 | Matrix metallopeptidase 3 | −3.6 |
| Myc | Myelocytomatosis oncogene | −2.5 |
| Nr4a1 | Nuclear receptor subfamily 4, group A, member 1 | −5.3 |
| Pdia3 | Protein disulfide isomerase-associated 3 | −2.3 |
| Pnp | Purine-nucleoside phosphorylase | −2.7 |
| Sh3rf2 | SH3 domain-containing ring finger 2 | 3.5 |
| Smtnl1 | Smoothelin-like 1 | 2.7 |
| Stk17b | Serine/threonine kinase 17b (apoptosis inducing) | −2.5 |
| Tsc22d3 | TSC22 domain family, member 3 | −3.4 |
| Usp18 | Ubiquitin-specific peptidase 18 | −2.8 |
| Xaf1 | X-Linked inhibitor of apoptosis (XIAP)-associated factor 1 | −2.9 |
| Drug metabolism | ||
| Gsta3 | Glutathione S-transferase, alpha 3 | 2.6 |
| Gstk1 | Glutathione S-transferase kappa 1 | 2.1 |
| Mgst3 | Microsomal glutathione S-transferase 3 | 2.1 |
| Cytoskeleton | ||
| Arpc3 | Actin-related protein 2/3 complex, subunit 3 | −2.1 |
| Cnn2 | Calponin 2 | −2.5 |
| Fhl3 | Four and one-half LIM domains 3 | 2.6 |
| Msn | Moesin | −2.2 |
| Myo5c | Myosin VC | 2.2 |
| Myoz1 | Myozenin 1 | 2.2 |
| Ptpn4 | Protein tyrosine phosphatase, nonreceptor type 4 | 2.2 |
| Tagln2 | Transgelin 2 | −2.1 |
| Tpm4 | Tropomyosin 4 | −2.2 |
| Ttll7 | Tubulin tyrosine ligase-like family, member 7 | 2.6 |
| Cell adhesion | ||
| Col15a1 | Collagen, type XV, alpha 1 | −2.5 |
| Cytip | Cytohesin 1-interacting protein | −2.6 |
| Lgals3bp | Lectin, galactoside-binding, soluble, 3 binding protein | −2.1 |
| Vcan | Versican | −2.6 |
| Behavioral response to stimulus | ||
| Amot | Angiomotin | 2.1 |
| Amot | Angiomotin | 2.3 |
| Sncg | Synuclein, gamma | 2.3 |
| Cell migration and motility | ||
| Bin2 | Bridging integrator 2 | −2.1 |
| Ctgf | Connective tissue growth factor | −2.3 |
| Dnaja1 | DnaJ (Hsp40) homolog, subfamily A, member 1 | −3.0 |
| Itga1 | Integrin alpha 1 | −2.7 |
| Itga6 | Integrin alpha 6 | −2.5 |
| Myh9 | Myosin, heavy polypeptide 9, nonmuscle | −2.3 |
| Nus1 | Nuclear undecaprenyl pyrophosphate synthase 1 homolog (S. cerevisiae) | −2.3 |
| Ret | Ret proto-oncogene | −2.7 |
| Rhob | Ras homolog gene family, member B | −2.0 |
| S1pr1 | Sphingosine-1-phosphate receptor 1 | −3.2 |
| Sema6c | Sema domain, transmembrane domain (TM), and cytoplasmic domain (semaphorin), 6C | 2.2 |
| Alternative splicing | ||
| BC094916 | cDNA sequence BC094916 | −3.3 |
| Coq10b | Coenzyme Q10 homolog B (S. cerevisiae) | −2.9 |
| Far1 | Fatty acyl CoA reductase 1 | −2.1 |
| Htra3 | HtrA serine peptidase 3 | 2.2 |
| Ifi203 | Interferon-activated gene 203 | −2.8 |
| Lgals9 | Lectin, galactose-binding, soluble 9 | −3.0 |
| Lpcat2 | Lysophosphatidylcholine acyltransferase 2 | −2.0 |
| Midn | Midnolin | −2.3 |
| Mgat4a | Mannoside acetylglucosaminyltransferase 4, isoenzyme A | −2.1 |
| Nnat | Neuronatin | 3.2 |
| Picalm | Phosphatidylinositol-binding clathrin assembly protein | −2.3 |
| Pla2g4e | Phospholipase A2, group IVE | 2.2 |
| Ptbp3 | Polypyrimidine tract-binding protein 3 | −2.2 |
| Rtp4 | Receptor transporter protein 4 | −2.5 |
| Sp100 | Nuclear antigen Sp100 | −2.5 |
| Trib1 | Tribbles homolog 1 (Drosophila) | −2.2 |
| Trp53inp2 | Transformation-related protein 53-inducible nuclear protein 2 | 2.2 |
| Zbp1 | Z-DNA-binding protein 1 | −2.9 |
| Transmembrane | ||
| Abcb4 | ATP-binding cassette, subfamily B (MDR/TAP), member 4 | 2.3 |
| Alox5ap | Arachidonate 5-lipoxygenase activating protein | −3.8 |
| Aqp4 | Aquaporin 4 | 2.5 |
| B3galt2 | UDP-Gal: βGlcNAc β 1,3-galactosyltransferase, polypeptide 2 | 2.6 |
| B4galt5 | UDP-Gal: βGlcNAc β 1,4-galactosyltransferase, polypeptide 5 | −4.6 |
| Bst2 | Bone marrow stromal cell antigen 2 | −5.3 |
| Cd53 | CD53 antigen | −2.7 |
| Chsy1 | Chondroitin sulfate synthase 1 | −2.2 |
| Cpt1b | Carnitine palmitoyltransferase 1b, muscle | 2.1 |
| Ctxn3 | Cortexin 3 | 3.6 |
| Emp1 | Epithelial membrane protein 1 | −2.7 |
| Gpr65 | G-Protein-coupled receptor 65 | −2.4 |
| Gprc5a | G-Protein-coupled receptor, family C, group 5, member A | −2.1 |
| Has1 | Hyaluronan synthase1 | −2.2 |
| Ier3 | Immediate early response 3 | −2.7 |
| Ifitm3 | Interferon-induced transmembrane protein 3 | −2.3 |
| Mc5r | Melanocortin 5 receptor | 3.0 |
| Ms4a4b | Membrane-spanning 4-domains, subfamily A, member 4B | −2.3 |
| Ms4a4c | Membrane-spanning 4-domains, subfamily A, member 4C | −7.6 |
| Ms4a6b | Membrane-spanning 4-domains, subfamily A, member 6B | −2.6 |
| Ms4a6c | Membrane-spanning 4-domains, subfamily A, member 6C | −2.5 |
| Ms4a6d | Membrane-spanning 4-domains, subfamily A, member 6D | −3.5 |
| Slc2a4 | Solute carrier family 2 (facilitated glucose transporter), member 4 | 3.0 |
| Slc7a8 | Solute carrier family 7 (cationic amino acid transporter, y+ system), member 8 | −2.7 |
| Sptlc2 | Serine palmitoyltransferase, long-chain base subunit 2 | −2.1 |
| St3gal5 | ST3 β-galactoside alpha-2,3-sialyltransferase 5 | 2.2 |
| Tecr | Trans-2,3-enoyl-CoA reductase | 2.1 |
| Tyrobp | TYRO protein tyrosine kinase binding protein | −2.1 |
| Unknown or specific functions | ||
| 1810011O10Rik | RIKEN cDNA 1810011O10 gene | −2.2 |
| 2310002L09Rik | RIKEN cDNA 2310002L09 gene | 2.3 |
| 8430408G22Rik | RIKEN cDNA 8430408G22 gene | −7.3 |
| 9430020K01Rik | RIKEN cDNA 9430020K01 gene | −2.1 |
| A730049H05Rik | RIKEN cDNA A730049H05 gene | −2.1 |
| AA467197 | Expressed sequence AA467197 | −2.1 |
| AI607873 | Expressed sequence AI607873 | −3.5 |
| Aldh1a7 | Aldehyde dehydrogenase family 1, subfamily A7 | 2.9 |
| Apold1 | Apolipoprotein L domain-containing 1 | −9.8 |
| Arrdc2 | Arrestin domain-containing 2 | −2.5 |
| Cox7b2 | Cytochrome c oxidase subunit VIIb2 | −2.2 |
| Dcaf4 | DDB1 and CUL4-associated factor 4 | 2.1 |
| Dennd4a | DENN/MADD-containing 4A | −2.3 |
| Dusp1 | Dual-specificity phosphatase 1 | −3.7 |
| Dusp10 | Dual-specificity phosphatase 10 | 2.9 |
| Eif1a | Eukaryotic translation initiation factor 1A | −2.2 |
| Erdr1 | Erythroid differentiation regulator 1 | −10.0 |
| Exd2 | Exonuclease 3–5 domain-containing 2 | 2.7 |
| Fam46a | Family with sequence similarity 46, member A | −2.0 |
| Fam101b | Family with sequence similarity 101, member B | −2.3 |
| Fcrls | Fc receptor-like S, scavenger receptor | −3.0 |
| Gbas | Glioblastoma-amplified sequence | 2.1 |
| Gm22 | Predicted gene, 22 | −2.2 |
| Gm4899 | Predicted gene, 4899 | −2.4 |
| Gm4955 | Predicted gene, 4955 | −3.4 |
| Gm6377 | Predicted gene, 6377 | −2.5 |
| Gm6904 | Predicted gene, 6904 | −2.1 |
| Gm10110 | Predicted gene, 10110 | 2.2 |
| Gm10999 | Predicted gene, 10999 | 2.0 |
| Gm11037 | Predicted gene, 11037 | −2.4 |
| Gm11710 | Predicted gene, 11710 | −2.0 |
| Gm11711 | Predicted gene, 11711 | −2.0 |
| Gm21742 | Predicted gene, 21742 | −9.7 |
| Gm21748 | Predicted gene, 21748 | −56.6 |
| Gm21750 | Predicted gene, 21750 | 2.1 |
| Gm21857 | Predicted gene, 21857 | −4.2 |
| Gm21860 | Predicted gene, 21860 | −56.6 |
| Gm21887 | Predicted gene, 21887 | −9.3 |
| Gys1 | Glycogen synthase 1, muscle | 2.1 |
| Herc6 | Hect domain and RLD 6 | −2.6 |
| I830012O16Rik | RIKEN cDNA I830012O16 gene | −3.5 |
| Ifi44 | Interferon-induced protein 44 | −6.0 |
| Ifi202b | Interferon-activated gene 202B | −3.4 |
| Inmt | Indolethylamine N-methyltransferase | 2.9 |
| Kbtbd13 | Kelch repeat and BTB (POZ) domain containing 13 | 2.1 |
| Klhdc3 | Kelch domain-containing 3 | 2.2 |
| Klhl38 | Kelch-like 38 | 2.6 |
| Lrrc14b | Leucine-rich repeat containing 14B | 2.2 |
| Lsmem2 | Leucine-rich single-pass membrane protein 2 | 2.0 |
| Mettl7a1 | Methyltransferase-like 7A1 | 2.4 |
| Mettl21e | Methyltransferase-like 21E | 6.6 |
| Mndal | Myeloid nuclear differentiation antigen like | −2.2 |
| Mup3 | Major urinary protein 3 | 2.4 |
| Myom3 | Myomesin family, member 3 | 2.3 |
| Odf3l2 | Outer dense fiber of sperm tails 3-like 2 | 5.3 |
| Pde4b | Phosphodiesterase 4B, cAMP specific | −2.1 |
| Phf11b | PHD finger protein 11B | −3.1 |
| Phf11d | PHD finger protein 11D | −2.3 |
| Plac8 | Placenta-specific 8 | −2.5 |
| Pnp2 | Purine-nucleoside phosphorylase 2 | −2.7 |
| Ppp1r3c | Protein phosphatase 1, regulatory (inhibitor) subunit 3C | 3.0 |
| Prob1 | Proline-rich basic protein 1 | 2.1 |
| Ptma | Prothymosin alpha | −2.4 |
| Pydc3 | Pyrin domain-containing 3 | −3.0 |
| Pydc4 | Pyrin domain-containing 4 | −3.3 |
| Retnlg | Resistin-like gamma | −5.7 |
| Rilpl1 | Rab-interacting lysosomal protein-like 1 | 2.2 |
| Rragd | Ras-related GTP-binding D | 2.1 |
| Sbk2 | SH3-binding domain kinase family, member 2 | 2.6 |
| Serpine1 | Serine (or cysteine) peptidase inhibitor, clade E, member 1 | −9.6 |
| Sh3pxd2b | SH3 and PX domains 2B | −2.0 |
| Slfn1 | Schlafen 1 | −3.2 |
| Slfn2 | Schlafen 2 | −3.7 |
| Slfn4 | Schlafen 4 | −9.7 |
| Slfn5 | Schlafen 5 | −2.3 |
| Slfn8 | Schlafen 8 | −2.7 |
| Tpm3-rs7 | Tropomyosin 3, related sequence 7 | −2.6 |
| Tra2a | Transformer 2 alpha homolog (Drosophila) | −2.5 |
| Trim30a | Tripartite motif-containing 30A | −3.9 |
| Trim30b | Tripartite motif-containing 30B | −3.6 |
| Trim30c | Tripartite motif-containing 30C | −3.4 |
| Trim30d | Tripartite motif-containing 30D | −3.8 |
| Wee1 | WEE 1 homolog 1 (Schizosaccharomyces pombe) | −2.1 |
| Xlr3b | X-Linked lymphocyte-regulated 3B | 2.4 |
| Xlr3c | X-Linked lymphocyte-regulated 3C | 2.2 |
This list excludes the gene-expression changes also conserved with either LS or EL treatment, listed in Table 2. Genes were functionally clustered using the Functional Annotation clustering tool from the Database for Annotation, Visualization and Integrated Discovery (DAVID). Positive fold changes indicate that gene expression was increased with treatment and vice versa.
Fig. 4.
Short-term prednisolone treatment worsens ongoing muscle damage in dystrophic het mice. Immunofluorescence staining for serum IgG on representative quadriceps (Quad) sections from het mice treated for 2 wk with LS, EL, or prednisolone (Pred) and used for microarray analysis. Mice treated with 1.5 mg·kg−1·day−1 prednisolone (n = 3) had more ongoing damage than LS-treated (n = 3), EL-treated (n = 3), and untreated het mice (n = 3).
DISCUSSION
To determine whether the improvement in ongoing damage previously observed in dystrophic mice treated with LS was a direct or indirect effect of the drugs on striated muscle fiber sarcolemmal membranes, we carried out a laser-injury membrane-integrity assay. We found that het mice have reduced membrane integrity, as predicted by the loss of dystrophin and reduction of utrophin, similar to that seen in dystrophin-deficient mice (34). LS treatment for <4 wk is able to restore membrane integrity of muscles isolated from treated het mice to levels not different from that of wild-type mice. These data indicate that this drug treatment can decrease sarcolemmal membrane fragility through direct effects on skeletal muscle fibers or through modification of the muscle ECM. Since muscle bundles were used in these experiments, the native cellular connections to the matrix remain intact, and matrix characteristics could also contribute to improved sarcolemmal membrane integrity.
This increase in membrane integrity could result from multiple mechanisms. Furthermore, there are additional pathways that could contribute to improved muscle structure and function following treatment with LS. We used an unbiased gene-expression analysis approach to establish the effects of LS on the molecular level. Treatment of dystrophic het mice with EL or LS resulted in a comparable number of gene-expression changes (70 and 66, respectively), and ~50% of these changes were conserved between the two treatments. There were only 10 gene-expression changes conserved between LS and EL compared with untreated, which were not changed in the same direction by prednisolone compared with untreated.
Not included in this group of 10 genes is S100a8, the highest fold decrease in the EL group (160-fold), which was also not changed by prednisolone but required removal of an outlier to reveal a 93-fold change in LS. S100a8 works as a dimer with S100a9 to form calprotectin, a regulator of immune reactions and inflammatory processes and a marker of neutrophils. Previous studies have shown that infusion of IL-6 cytokine increases S100A8 expression in human skeletal muscle (22). IL-6 expression is also decreased approximately threefold in both LS and EL compared with untreated. Over 50% of gene-expression changes resulting from LS or EL treatment were also conserved in prednisolone-treated mice, and the majority of these genes are functionally classified as either cytokine production/immune response or transcriptional regulators. These data support that MR antagonists likely exhibit anti-inflammatory effects similar to prednisolone and that MR and GR may interact with each other both pre- and post-transcriptionally, particularly in inflammatory cells (35, 37).
Two of the 10 gene-expression changes specific to the LS and EL groups have known associations with altered membrane integrity in dystrophic muscles. Metallothioneins (Mt) 1 and 2, which are decreased by LS and EL treatment, are known to be increased in mdx limb muscles and reduced in mdx mice expressing a therapeutic utrophin transgene (2, 25). However, a recent study showed that an Mt1 mimetic in a laser-injury assay is protective in a mouse model of limb-girdle muscular dystrophy (1). Since Mt1 is also regulated by IL-6, different levels of metallothioneins may be protective at different stages of disease.
The remainder of the gene changes conserved between LS and EL, but not prednisolone, have known roles in muscle metabolism. Scd1, which encodes an enzyme essential for skeletal muscle lipid metabolism, showed the highest fold increase with treatment. The increase of Scd1 expression in skeletal muscle can enhance muscle triglyceride synthesis, glucose uptake, and exercise capacity (28). Diminished Scd1 activity can lead to increased levels of saturated fatty acids that result in inflammation, lipotoxicity, and insulin resistance (28).
Nr1d1 encodes the rev-ERBα nuclear receptor expressed in oxidative muscle and is known to increase exercise capacity (36). Pim1 encodes a serine/threonine kinase that regulates metabolism and is associated with high intramuscular fat in beef, and knockout of Clu, encoding clusterin in mice, is protective against a high-fat diet (15, 30). Prkag3 encodes the regulatory subunit of AMP kinase, which is known to play a major role in muscle metabolism and also to regulate the muscle circadian clock (33). Dbp, decreased by prednisolone and in muscle atrophy but increased by both LS and EL treatment, and Per3, increased in LS only, are circadian clock-controlled genes in muscle (24, 33). Clock genes have recently been shown to play an important purpose in normal muscle function and metabolism (12). These metabolic gene-expression changes may underlie the improved recovery from fatigue observed in diaphragm and extensor digitorum longus muscles from LS- and EL-treated mice, although the contribution of each gene product will require further investigation.
Two gene-expression changes were conserved between the het mice treated with LS or EL for 2 wk and mice treated for 16 wk with LS from our previous study compared with untreated hets (8). Cited4, which was decreased by 2-wk EL and 16-wk LS treatment, encodes a cAMP response element-binding protein-binding protein/p300 transcriptional coactivator-binding protein that has not been directly investigated in skeletal muscle. However, Cited4 was increased in exercised-induced cardiac hypertrophy and controls myocyte elongation and proliferation (4, 29). Adamst1, decreased by 2- and 16-wk LS treatment, EL treatment, and prednisolone treatment, is an anti-angiogenic regulator transiently increased during myotube maturation. Adamst1 is known to be drastically reduced in skeletal muscle by exercise and predicted to be involved in ECM remodeling (18).
There were 312 gene-expression changes present only in the prednisolone versus untreated het comparison. Functional groups specific to prednisolone treatment include genes involved in apoptosis or proteolysis, cytokines with chemokine activity, GTPase activity, cell migration and motility, cell adhesion, and cytoskeleton. Almost all apoptotic and proteolytic genes are increased with prednisolone treatment, whereas genes that negatively regulate apoptosis are all decreased. GR-induced increases in proapoptotic gene expression are seen in other tissues, including skin, bone, and neurons (5, 10, 23). The reduction of genes involved in cell motility and migration by prednisolone could contribute to impaired regeneration and explain the increased muscle damage associated with prednisolone treatment in animal models of DMD (11). Treatment with prednisolone also decreased expression of GTPase genes, including several members of the Gbp family that are induced by IFN-γ and play a key role in protective immunity (32).
This study has demonstrated that short-term treatment with LS is able to restore membrane integrity of isolated dystrophic mouse muscles and decrease inflammatory genes. The combination of these effects could contribute to the cell and molecular mechanisms that lead to the improved muscle structure and function in dystrophic muscle following treatment with LS. Microarray analysis identified several potential gene targets that may specifically underlie the efficacy of RAAS inhibitors on dystrophic muscles, while also identifying gene-expression changes that may contribute to the side effects of prednisolone on skeletal muscles. This information may be used to develop biomarkers to monitor RAAS inhibitor or glucocorticoid agonist treatment, develop a temporally coordinated treatment with both drug classes, or identify novel therapeutics with these more-specific treatment targets.
GRANTS
Funding for this study was provided by the National Institute of Neurological Disorders and Stroke Grant R01 NS082868) and National Heart, Lung, and Blood Institute Grant R01 HL116533 (to J. A. Rafael-Fortney), National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant R01 AR063084 (to N. Weisleder), and Department of Defense Grant MD120063 (to J. A. Rafael-Fortney). Additional support was provided through a postdoctoral fellowship from The Ohio State University/Nationwide Children’s Hospital Center for Muscle Health and Neuromuscular Disorders (to S. Bhattacharya). Eplerenone (no longer under patent) was provided by the Pfizer Compound Transfer Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.A.C., S.B., and J.L. performed experiments; J.A.C., S.B., and J.A.R-F. analyzed data; J.A.C., N.W., and J.A.R-F. interpreted results of experiments; J.A.C. and S.B. prepared figures; J.A.R-F. drafted manuscript; J.A.C., J.L., N.W., and J.A.R-F. edited and revised manuscript; J.A.C., S.B., J.L., N.W., and J.A.R-F. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Feni Kadakia and Jonathan Zins for assistance with mouse treatments and The Ohio State University Genomics Shared Resource, and in particular, S. Warner for microarray processing.
REFERENCES
- 1.Accornero F, Kanisicak O, Tjondrokoesoemo A, Attia AC, McNally EM, Molkentin JD. Myofiber-specific inhibition of TGFβ signaling protects skeletal muscle from injury and dystrophic disease in mice. Hum Mol Genet 23: 6903–6915, 2014. doi: 10.1093/hmg/ddu413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baban D, Davies KE. Microarray analysis of mdx mice expressing high levels of utrophin: therapeutic implications for dystrophin deficiency. Neuromuscul Disord 18: 239–247, 2008. doi: 10.1016/j.nmd.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Bene NC, Alcaide P, Wortis HH, Jaffe IZ. Mineralocorticoid receptors in immune cells: emerging role in cardiovascular disease. Steroids 91: 38–45, 2014. doi: 10.1016/j.steroids.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boström P, Mann N, Wu J, Quintero PA, Plovie ER, Panáková D, Gupta RK, Xiao C, MacRae CA, Rosenzweig A, Spiegelman BM. C/EBPβ controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell 143: 1072–1083, 2010. doi: 10.1016/j.cell.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budunova IV, Kowalczyk D, Pérez P, Yao YJ, Jorcano JL, Slaga TJ. Glucocorticoid receptor functions as a potent suppressor of mouse skin carcinogenesis. Oncogene 22: 3279–3287, 2003. doi: 10.1038/sj.onc.1206383. [DOI] [PubMed] [Google Scholar]
- 6.Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, Kaul A, Kinnett K, McDonald C, Pandya S, Poysky J, Shapiro F, Tomezsko J, Constantin C; DMD Care Considerations Working Group . Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol 9: 77–93, 2010. doi: 10.1016/S1474-4422(09)70271-6. [DOI] [PubMed] [Google Scholar]
- 7.Carvalho SC, Apolinário LM, Matheus SM, Santo Neto H, Marques MJ. EPA protects against muscle damage in the mdx mouse model of Duchenne muscular dystrophy by promoting a shift from the M1 to M2 macrophage phenotype. J Neuroimmunol 264: 41–47, 2013. doi: 10.1016/j.jneuroim.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Chadwick JA, Hauck JS, Lowe J, Shaw JJ, Guttridge DC, Gomez-Sanchez CE, Gomez-Sanchez EP, Rafael-Fortney JA. Mineralocorticoid receptors are present in skeletal muscle and represent a potential therapeutic target. FASEB J 29: 4544–4554, 2015. doi: 10.1096/fj.15-276782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chitnis AS, Aparasu RR, Chen H, Johnson ML. Comparative effectiveness of different angiotensin-converting enzyme inhibitors on the risk of hospitalization in patients with heart failure. J Comp Eff Res 1: 195–206, 2012. doi: 10.2217/cer.12.5. [DOI] [PubMed] [Google Scholar]
- 10.Crochemore C, Lu J, Wu Y, Liposits Z, Sousa N, Holsboer F, Almeida OF. Direct targeting of hippocampal neurons for apoptosis by glucocorticoids is reversible by mineralocorticoid receptor activation. Mol Psychiatry 10: 790–798, 2005. doi: 10.1038/sj.mp.4001679. [DOI] [PubMed] [Google Scholar]
- 11.Dadgar S, Wang Z, Johnston H, Kesari A, Nagaraju K, Chen YW, Hill DA, Partridge TA, Giri M, Freishtat RJ, Nazarian J, Xuan J, Wang Y, Hoffman EP. Asynchronous remodeling is a driver of failed regeneration in Duchenne muscular dystrophy. J Cell Biol 207: 139–158, 2014. doi: 10.1083/jcb.201402079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harfmann BD, Schroder EA, Esser KA. Circadian rhythms, the molecular clock, and skeletal muscle. J Biol Rhythms 30: 84–94, 2015. doi: 10.1177/0748730414561638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heier CR, Damsker JM, Yu Q, Dillingham BC, Huynh T, Van der Meulen JH, Sali A, Miller BK, Phadke A, Scheffer L, Quinn J, Tatem K, Jordan S, Dadgar S, Rodriguez OC, Albanese C, Calhoun M, Gordish-Dressman H, Jaiswal JK, Connor EM, McCall JM, Hoffman EP, Reeves EK, Nagaraju K. VBP15, a novel anti-inflammatory and membrane-stabilizer, improves muscular dystrophy without side effects. EMBO Mol Med 5: 1569–1585, 2013. doi: 10.1002/emmm.201302621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janssen PM, Murray JD, Schill KE, Rastogi N, Schultz EJ, Tran T, Raman SV, Rafael-Fortney JA. Prednisolone attenuates improvement of cardiac and skeletal contractile function and histopathology by lisinopril and spironolactone in the mdx mouse model of Duchenne muscular dystrophy. PLoS One 9: e88360, 2014. doi: 10.1371/journal.pone.0088360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon MJ, Ju TJ, Heo JY, Kim YW, Kim JY, Won KC, Kim JR, Bae YK, Park IS, Min BH, Lee IK, Park SY. Deficiency of clusterin exacerbates high-fat diet-induced insulin resistance in male mice. Endocrinology 155: 2089–2101, 2014. doi: 10.1210/en.2013-1870. [DOI] [PubMed] [Google Scholar]
- 16.Lowe J, Floyd KT, Rastogi N, Schultz EJ, Chadwick JA, Swager SA, Zins JG, Kadakia FK, Smart S, Gomez-Sanchez EP, Gomez-Sanchez CE, Raman SV, Janssen PM, Rafael-Fortney JA. Similar efficacy from specific and non-specific mineralocorticoid receptor antagonist treatment of muscular dystrophy mice. J Neuromuscul Dis 3: 395–404, 2016. doi: 10.3233/JND-160173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowe J, Wodarcyk AJ, Floyd KT, Rastogi N, Schultz EJ, Swager SA, Chadwick JA, Tran T, Raman SV, Janssen PM, Rafael-Fortney JA. The angiotensin converting enzyme inhibitor lisinopril improves muscle histopathology but not contractile function in a mouse model of Duchenne muscular dystrophy. J Neuromuscul Dis 2: 257–268, 2015. doi: 10.3233/JND-150099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malek MH, Hüttemann M, Lee I, Coburn JW. Similar skeletal muscle angiogenic and mitochondrial signalling following 8 weeks of endurance exercise in mice: discontinuous versus continuous training. Exp Physiol 98: 807–818, 2013. doi: 10.1113/expphysiol.2012.070169. [DOI] [PubMed] [Google Scholar]
- 19.McNally EM, Kaltman JR, Benson DW, Canter CE, Cripe LH, Duan D, Finder JD, Groh WJ, Hoffman EP, Judge DP, Kertesz N, Kinnett K, Kirsch R, Metzger JM, Pearson GD, Rafael-Fortney JA, Raman SV, Spurney CF, Targum SL, Wagner KR, Markham LW, Working Group of the National Heart, Lung, and Blood Institute; Parent Project Muscular Dystrophy . Contemporary cardiac issues in Duchenne muscular dystrophy. Working Group of the National Heart, Lung, and Blood Institute in collaboration with Parent Project Muscular Dystrophy. Circulation 131: 1590–1598, 2015. doi: 10.1161/CIRCULATIONAHA.114.015151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendell JR, Shilling C, Leslie ND, Flanigan KM, al-Dahhak R, Gastier-Foster J, Kneile K, Dunn DM, Duval B, Aoyagi A, Hamil C, Mahmoud M, Roush K, Bird L, Rankin C, Lilly H, Street N, Chandrasekar R, Weiss RB. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol 71: 304–313, 2012. doi: 10.1002/ana.23528. [DOI] [PubMed] [Google Scholar]
- 21.Messaoudi S, Azibani F, Delcayre C, Jaisser F. Aldosterone, mineralocorticoid receptor, and heart failure. Mol Cell Endocrinol 350: 266–272, 2012. doi: 10.1016/j.mce.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 22.Mortensen OH, Andersen K, Fischer C, Nielsen AR, Nielsen S, Akerström T, Aastrøm MB, Borup R, Pedersen BK. Calprotectin is released from human skeletal muscle tissue during exercise. J Physiol 586: 3551–3562, 2008. doi: 10.1113/jphysiol.2008.153551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moutsatsou P, Kassi E, Papavassiliou AG. Glucocorticoid receptor signaling in bone cells. Trends Mol Med 18: 348–359, 2012. doi: 10.1016/j.molmed.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Nakao R, Yamamoto S, Horikawa K, Yasumoto Y, Nikawa T, Mukai C, Oishi K. Atypical expression of circadian clock genes in denervated mouse skeletal muscle. Chronobiol Int 32: 486–496, 2015. doi: 10.3109/07420528.2014.1003350. [DOI] [PubMed] [Google Scholar]
- 25.Porter JD, Merriam AP, Leahy P, Gong B, Khanna S. Dissection of temporal gene expression signatures of affected and spared muscle groups in dystrophin-deficient (mdx) mice. Hum Mol Genet 12: 1813–1821, 2003. doi: 10.1093/hmg/ddg197. [DOI] [PubMed] [Google Scholar]
- 26.Rafael-Fortney JA, Chimanji NS, Schill KE, Martin CD, Murray JD, Ganguly R, Stangland JE, Tran T, Xu Y, Canan BD, Mays TA, Delfín DA, Janssen PM, Raman SV. Early treatment with lisinopril and spironolactone preserves cardiac and skeletal muscle in Duchenne muscular dystrophy mice. Circulation 124: 582–588, 2011. doi: 10.1161/CIRCULATIONAHA.111.031716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raman SV, Hor KN, Mazur W, Halnon NJ, Kissel JT, He X, Tran T, Smart S, McCarthy B, Taylor MD, Jefferies JL, Rafael-Fortney JA, Lowe J, Roble SL, Cripe LH. Eplerenone for early cardiomyopathy in Duchenne muscular dystrophy: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 15: 153–161, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogowski MP, Flowers MT, Stamatikos AD, Ntambi JM, Paton CM. SCD1 activity in muscle increases triglyceride PUFA content, exercise capacity, and PPARδ expression in mice. J Lipid Res 54: 2636–2646, 2013. doi: 10.1194/jlr.M035865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryall KA, Bezzerides VJ, Rosenzweig A, Saucerman JJ. Phenotypic screen quantifying differential regulation of cardiac myocyte hypertrophy identifies CITED4 regulation of myocyte elongation. J Mol Cell Cardiol 72: 74–84, 2014. doi: 10.1016/j.yjmcc.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sadkowski T, Ciecierska A, Majewska A, Oprządek J, Dasiewicz K, Ollik M, Wicik Z, Motyl T. Transcriptional background of beef marbling - novel genes implicated in intramuscular fat deposition. Meat Sci 97: 32–41, 2014. doi: 10.1016/j.meatsci.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 31.Sali A, Guerron AD, Gordish-Dressman H, Spurney CF, Iantorno M, Hoffman EP, Nagaraju K. Glucocorticoid-treated mice are an inappropriate positive control for long-term preclinical studies in the mdx mouse. PLoS One 7: e34204, 2012. doi: 10.1371/journal.pone.0034204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vestal DJ, Jeyaratnam JA. The guanylate-binding proteins: emerging insights into the biochemical properties and functions of this family of large interferon-induced guanosine triphosphatase. J Interferon Cytokine Res 31: 89–97, 2011. doi: 10.1089/jir.2010.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vieira E, Nilsson EC, Nerstedt A, Ormestad M, Long YC, Garcia-Roves PM, Zierath JR, Mahlapuu M. Relationship between AMPK and the transcriptional balance of clock-related genes in skeletal muscle. Am J Physiol Endocrinol Metab 295: E1032–E1037, 2008. doi: 10.1152/ajpendo.90510.2008. [DOI] [PubMed] [Google Scholar]
- 34.Weisleder N, Takizawa N, Lin P, Wang X, Cao C, Zhang Y, Tan T, Ferrante C, Zhu H, Chen PJ, Yan R, Sterling M, Zhao X, Hwang M, Takeshima M, Cai C, Cheng H, Takeshima H, Xiao RP, Ma J. Recombinant MG53 protein modulates therapeutic cell membrane repair in treatment of muscular dystrophy. Sci Transl Med 4: 139ra85, 2012. doi: 10.1126/scitranslmed.3003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson P, Morgan J, Funder JW, Fuller PJ, Young MJ. Mediators of mineralocorticoid receptor-induced profibrotic inflammatory responses in the heart. Clin Sci (Lond) 116: 731–739, 2009. doi: 10.1042/CS20080247. [DOI] [PubMed] [Google Scholar]
- 36.Woldt E, Sebti Y, Solt LA, Duhem C, Lancel S, Eeckhoute J, Hesselink MK, Paquet C, Delhaye S, Shin Y, Kamenecka TM, Schaart G, Lefebvre P, Nevière R, Burris TP, Schrauwen P, Staels B, Duez H. Rev-erb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat Med 19: 1039–1046, 2013. doi: 10.1038/nm.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young MJ, Morgan J, Brolin K, Fuller PJ, Funder JW. Activation of mineralocorticoid receptors by exogenous glucocorticoids and the development of cardiovascular inflammatory responses in adrenalectomized rats. Endocrinology 151: 2622–2628, 2010. doi: 10.1210/en.2009-1476. [DOI] [PubMed] [Google Scholar]
- 38.Zhou L, Rafael-Fortney JA, Huang P, Zhao XS, Cheng G, Zhou X, Kaminski HJ, Liu L, Ransohoff RM. Haploinsufficiency of utrophin gene worsens skeletal muscle inflammation and fibrosis in mdx mice. J Neurol Sci 264: 106–111, 2008. doi: 10.1016/j.jns.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]