Abstract
Drosophila melanogaster is widely used as a model system to study innate immunity and signaling pathways related to innate immunity, including the Toll signaling pathway. Although this pathway is well studied, the precise mechanisms of posttranscriptional regulation of key components of the Toll signaling pathway by microRNAs (miRNAs) remain obscure. In this study, we used an in silico strategy in combination with the Gal80ts-Gal4 driver system to identify microRNA-958 (miR-958) as a candidate Toll pathway regulating miRNA in Drosophila. We report that overexpression of miR-958 significantly reduces the expression of Drosomycin, a key antimicrobial peptide involved in Toll signaling and the innate immune response. We further demonstrate in vitro and in vivo that miR-958 targets the Toll and Dif genes, key components of the Toll signaling pathway, to negatively regulate Drosomycin expression. In addition, a miR-958 sponge rescued the expression of Toll and Dif, resulting in increased expression of Drosomycin. These results, not only revealed a novel function and modulation pattern of miR-958, but also provided a new insight into the underlying molecular mechanisms of Toll signaling in regulation of innate immunity.
Keywords: Toll signaling, miR-958, Toll, Dif, Drosophila melanogaster
drosophila melanogaster is a well-established model system used to decipher the molecular and cellular mechanisms that underlie complex biological processes, such as immune responses (16, 23). Despite the lack of an adaptive immune system, Drosophila is able to mount a rapid and efficient immune reaction in response to microbial infection (5). Fruit flies neutralize potentially harmful microorganisms by producing antimicrobial peptides (AMPs) (15). The expression of AMPs is mainly regulated by two signaling pathways, Toll and immune deficiency (Imd), which show striking similarities to the mammalian Toll-like receptor/interleukin-1 and tumor necrosis factor-α pathways, respectively (29). It is clear that the Toll signaling pathway in Drosophila plays a key role in the innate immune response against Gram-positive bacterial and fungal infections (32). Canonic components of this pathway are the extracellular cytokine Spatzle (which shares structural similarities with the nerve growth factor, NGF), the transmembrane receptor Toll, the Tube and MyD88 adaptors, the Pelle kinase, Cactus (the Drosophila homolog of IκB), and the Dorsal and Dif transactivators (Drosophila homologs of NF-κB) (12). Deletion of any of these components, with the exceptions of Cactus and Dorsal, can cause an immune-deficient phenotype characterized by the lack of expression of several immune effectors, including the antimicrobial peptide Drosomycin (Drs), increasing susceptibility to infection by fungus and Gram-positive bacteria (21). However, overactivation of the immune response can negatively impact individual fitness, and therefore safeguard mechanisms must be put in place to either tolerate or limit the potential for overactive immune responses. Posttranscriptional gene regulation has been proposed as a mechanism to fine tune immune responses and other physiological processes to prevent or limit overactivation (6).
MicroRNAs (miRNAs) are small noncoding RNAs that posttranscriptionally regulate protein synthesis by base pairing to the 3′-untranslated regions (3′-UTR) of target mRNAs (19). Recent studies have demonstrated that miRNAs extensively modulate immune responses against bacteria and parasites across different species. For example, in Drosophila, let-7 directly interacts with the 3′-UTR of an AMP gene Diptericin in S2 cells, and miR-8 negatively regulates the expression of Diptericin and Drosomycin in the absence of pathogen stimulation (7, 14, 20). In Caenorhabditis elegans (C. elegans), miR-233 is required for innate immune response after Pseudomonas aeruginosa infection (8). In human and mouse cells, miR-19b is a critical regulator of the NF-κB signaling pathway, and miR-195 decreases the expression of multiple NF-κB downstream effectors by directly targeting the IKKα and TAB3 genes (9, 13).
Nevertheless, the regulatory mechanisms of miRNAs involved in immune responses remain unclear. To further clarify the regulatory roles of miRNAs involved in immune responses, we aimed to identify miRNAs that regulate the Toll signaling pathway in Drosophila. By combining an in silico screening strategy and the Gal80ts-Gal4 driver system, we identified miR-964, miR-958, and miR-317 as candidate miRNAs that potentially regulate Toll pathway signaling. We report that miR-958 directly targets the Toll and Dif genes to negatively regulate the Toll signaling pathway and the expression of its downstream effector, Drosomycin. Our results demonstrate that miR-958 is a bona fide regulator of the Toll signaling pathway and is critical for the regulation of innate immunity in Drosophila melanogaster.
MATERIALS AND METHODS
Drosophila strains.
Drosophila stocks were maintained under standard culture conditions. All stocks in this study were obtained from the Bloomington Stock Center. The ubiquitous Gal80ts-Gal4 driver system was used to express miRNAs of interest specifically in adult flies. Crosses were performed, and the resulting progeny were initially raised at the permissive temperature (18°C) for the Gal80ts inhibitor to repress Gal4 activity. Following adult elusion, flies were transferred to 29°C to allow activation of Gal4 in the adult stage (17).
In silico prediction of miRNA targets.
Toll pathway genes that are potential targets of Drosophila miRNAs were identified using three different miRNA prediction algorithms, TargetScan, miRanda, and PITA. TargetScan predicts biological targets of miRNAs by searching for the presence of conserved 8mer and 7mer sites that match the seed region of each miRNA (22, 26). miRanda determines interactions through free-energy dynamics across the entire miRNA:mRNA interaction, with increased weight given to the miRNA 5′ seed region (10). PITA requires sequence similarity within the seed region but also determines the accessibility of the mRNA 3′-UTR based on free energy (18).
Infection and survival experiments.
Drosophila adult males, aged 3–4 days, were used for septic injury experiments. Control and miRNA-overexpressing flies were infected by Micrococcus luteus (M. luteus), a widely used bacterial strain that induces activation of the Toll-mediated immune response, to induce the expression of Drosomycin. Septic injury was performed by pricking the thorax of the flies with a pulled glass capillary carrying M. luteus using a Nanoject apparatus (Nanoliter 2010, WPI) (25). Flies were then incubated at 29°C and collected at 24 h postinfection. For the survival experiment, flies were then infected with Enterococcus faecalis (E. faecalis), and survival was monitored for 24 h (31).
RNA extraction and qRT-PCR.
Total RNA was extracted from control and treated adult flies using Trizol (Invitrogen). A small RNA library was generated from the samples using the Illumina Truseq Small RNA Preparation kit at LC Sciences Company. cDNA was synthesized using PrimeScript RT Master Mix kit (Takara). Quantitative RT-PCR was performed using SYBR Premix Ex Taq (Takara) in triplicate on an ABI StepOne plus real-time PCR instrument. SYBR Prime Script miRNA RT-PCR kit (Takara) was used for quantitative RT-PCR of miRNAs. Rp49 and 5s rRNA genes were used as reference controls.
Western blotting.
Western blotting was performed on protein samples pooled from 12 Drosophila. Rabbit anti-α-tubulin primary antibody (Bioworld) was used at a 1:1,000 dilution with an anti-rabbit-horseradish peroxidase-conjugated secondary antibody (Vazyme) at 1:30,000. Rabbit anti-Toll (Santa) was used at 1:200. The antibodies against Drosomycin and Dif were generated by immunizing rabbits with the Escherichia coli-produced recombinant His6-Drosomycin (LSGRYKGPCAVWDNET) and Dif (full-length), respectively. Antibody binding was detected using Super Signal West Femto Maximum Sensitivity substrate (Thermo Fisher Scientific).
Plasmid construction and luciferase assay.
The 3′-UTR reporter plasmids for Toll and Dif were generated by inserting PCR-amplified 3′-UTR fragments downstream of the luciferase gene in the pAc5.1 luciferase vector (a gift from Dr. Jinjun Qian). A miR-958 expression plasmid was constructed from the empty pAc5.1 vector (Promega). Binding-site mutant constructs were generated via Fast Site-Directed Mutagenesis Kit (TIANGEN). Transient transfections were performed in S2 cells using X-treme GENE HP transfection reagent (Roche). For dual-luciferase reporter assays, cells were harvested at 48 h posttransfection. Firefly and Renilla luciferase activity were measured using the Dual-Luciferase Assay Reporter System (Promega). Renilla luciferase was used to normalize the transfection efficiency.
Statistical analyses.
All experimental data in this study were collected from three biological replicates. All statistical analyses are shown as means ± SD. Significant differences between values under different experimental conditions were determined by two-tailed Student’s t-test. Statistical analysis of fly survival experiments was carried out using the log-rank (Mantel-Cox) test. For all tests, a P value <0.05 was considered statistically significant. *P < 0.05; **P < 0.01; ***P < 0.001.
RESULTS
Identification of miRNAs involved in Drosophila Toll signaling.
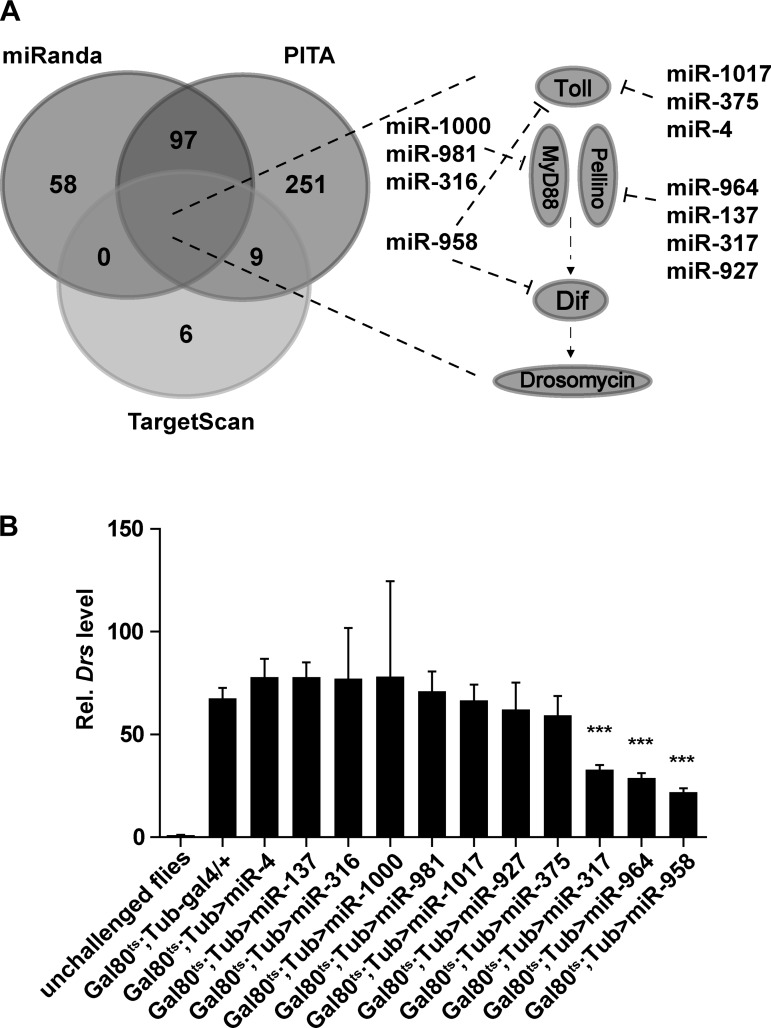
To identify candidate miRNAs involved in regulating the Drosophila Toll signaling pathway, we carried an in silico screening using three different online miRNA target prediction methods (TargetScan, miRanda, and PITA) to reduce the number of false positives. We analyzed potential gene-miRNA pairs for 25 genes in the Toll signaling pathway and 256 Drosophila miRNAs (obtained from miRBase). We found that 11 miRNAs were commonly predicted to target four Toll signaling pathway genes by the prediction methods (Fig. 1A). In particular, it was predicted that four miRNAs (miR-4, miR-375, miR-958, and miR-1017) target Toll, four miRNAs (miR-137, miR-317, miR-927, and miR-964) target Pellino, three miRNAs (miR-316, miR-981, and miR-1000) target MyD88, and one, miR-958, targets Dif (Fig. 1A). It is worth mentioning that miR-958 was predicted to target two key genes in Toll signaling pathway, Toll and Dif.
Fig. 1.
Identification of miRNAs associated with Toll signaling in Drosophila. A: in silico prediction of miRNAs associated with the Toll signaling pathway in Drosophila was performed using TargetScan, miRanda, and PITA software. Eleven putative miRNAs involved in Toll signaling are shown with their target genes (right). Venn diagram indicates miRNAs commonly predicted by all software (created using the online software Draw Venn Diagram). B: miR-317, miR-964, and miR-958 were verified to influence Drosomycin expression using Gal80ts-UAS-miRNA lines. All miRNA-overexpressing flies and control flies were injected with Micrococcus luteus (M. luteus), and Drosomycin expression was analyzed at 24 h postinfection. Data are presented as means ± SD from at least three independent experiments. Statistical significance was assessed by two-tailed Student’s t-test; ***P < 0.001.
To verify whether these 11 predicted miRNAs really are involved in the Toll-mediated immune response, we employed the ubiquitous Gal80ts-Gal4 driver system to overexpress candidate miRNAs in adult flies and to avoid potential embryonic lethality that can be induced by overexpressing miRNAs during development (2). Following a two-step screening strategy, we found that only three of the candidate miRNAs (miR-317, miR-964, and miR-958) were involved in regulating the expression of Drosomycin (Fig. 1B). Interestingly, overexpression of miR-958, which was predicted to target Toll and Dif, led to the largest reduction of the expression level of Drosomycin.
miR-958 inhibits Toll signaling in vivo.
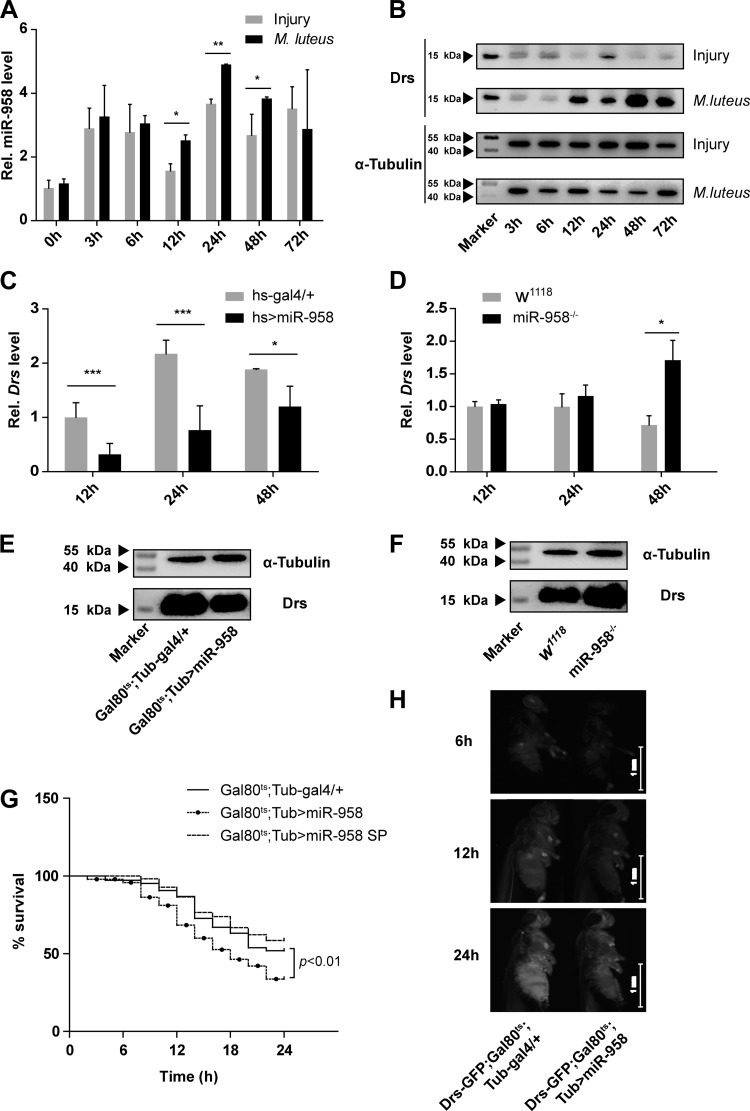
To further confirm that miR-958 is involved in modulating the innate immunity response, temporal expression patterns of miR-958 were analyzed following exposure to M. luteus compared with miR-958 expression patterns following injury. miR-958 expression in flies infected by M. luteus was significantly higher than injured flies at 12, 24, and 48 h after challenge or injury (Fig. 2A). Additionally, a significant reduction of Drosomycin expression was observed at 72 h postinfection (Fig. 2B).
Fig. 2.
miR-958 as a negative regulator of Toll signaling pathway. A and B: time course analysis of miR-958 expression (A) and Drosomycin protein (B) in wild-type (w1118) adults after infection with M. luteus or water. C and D: miR-958 overexpression (C) or knockout (D) flies and controls were infected with M. luteus for 12, 24, and 48 h, and RNA was harvested for quantitative RT-PCR analysis to measure mRNA levels of Drosomycin. E and F: simultaneously, Drosomycin protein of miR-958 overexpression (E) or knockout (F) flies and controls was measured at 24 h and 48 h, respectively. The Toll pathway was activated by challenging flies with M. luteus; 24 h later, the flies were challenged with Enterococcus faecalis. G: the survival of the flies was monitored for 24 h (G). Gal80ts;Tub-gal4/+ flies were used as a control. Gal80ts;Tub-gal4/+ flies (n = 106); Gal80ts;Tub>miR-958 flies (n = 95); Gal80ts;Tub>miR-958 SP flies (n = 111). H: the Drosomycin-green fluorescent protein (GFP) reporter gene is expressed in adult flies during the immune response (H). Less fluorescence is observed in the representative miR-958 overexpression fly (right) than in the control fly (left) following infection with M. luteus for 6, 12, and 24 h. Data (A, C, and D) are presented as means ± SD from at least three independent experiments. Statistical significance was assessed by two-tailed Student’s t-test; *P < 0.05, **P < 0.01, ***P < 0.001.
We employed another Gal4 driver, the heat shock driver, to increase miR-958 expression ubiquitously in adult flies. This method obtained consistent results with the Gal80ts-Gal4 driver system, and we found that overexpression of miR-958 significantly suppressed the expression of Drosomycin at all time points (Fig. 2C). Consistently, miR-958-null mutants (miR-958−/−) exhibited enhanced Toll signaling activity, and Drosomycin expression was upregulated in miR-958−/− flies at 48 h postinfection (Fig. 2D). Western bolt analysis of Drosomycin protein expression was consistent with the gene expression determined by qRT-PCR (Fig. 2, E and F).
To examine whether the effect of miR-958 silencing on the Toll pathway is sufficient to impair the Drosophila survival, we employed a septic injury model involving a challenge with the Gram-positive bacteria E. faecalis. miR-958-overexpressing flies exhibited a statistically significant reduction in survival compared with control flies. There was no significant difference in survival between the miR-958-null flies and controls (Fig. 2G).
In addition, using a Drosomycin–green fluorescent protein (GFP) reporter gene, we observed that overexpression of miR-958 inhibits expression of Drosomycin in live flies (Fig. 2H). Transgenic flies were immunized for 6, 12, and 24 h, and miR-958-overexpressing flies exhibited lower GFP than controls, especially at 24 h after the immunization challenge. Collectively, these results indicate that miR-958 functions as a negative regulator of Toll-mediated innate immunity in Drosophila.
Analysis of miR-958 target sites in Toll and Dif.
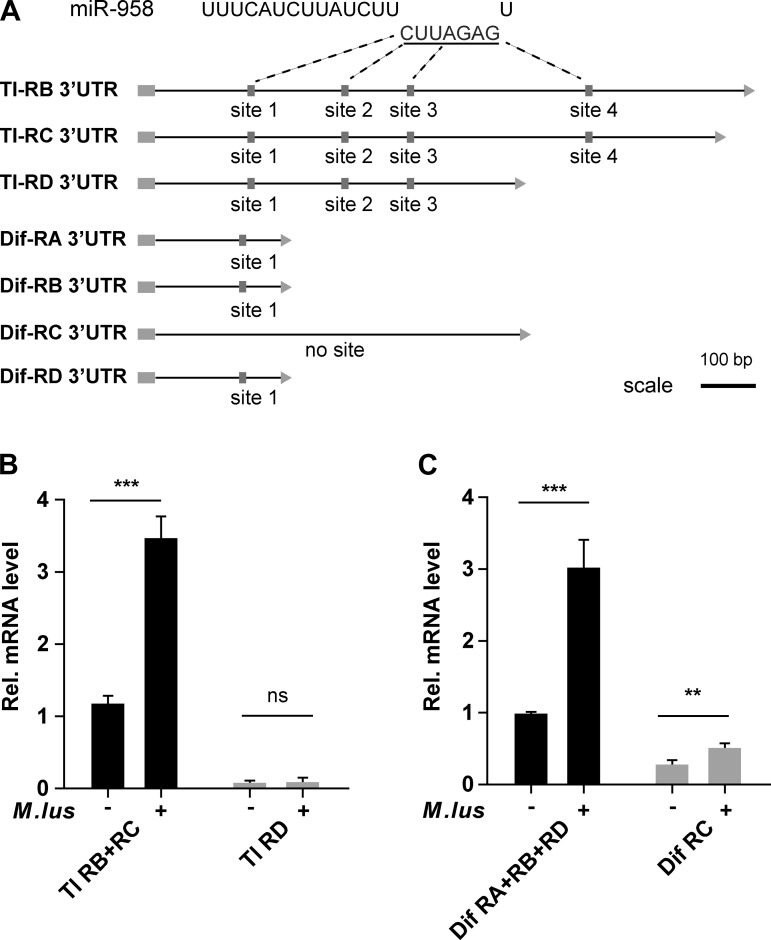
We identified that Toll and Dif are two potential target genes of miR-958. Toll and Dif each consist of numerous transcript variants that may experience different regulation by miR-958, attributable to differences in 3′-UTR target sites in the transcript variants. For example, there are four miR-958 target sites in Toll-RB and Toll-RC but only three in Toll-RD. The three transcript variants of Dif (Dif-RA, Dif-RB, and Dif-RD) have identical 3′-UTR sequences, each containing a single identical miR-958 target site, whereas there is no target site in the Dif-RC 3′-UTR (Fig. 3A). Accordingly, the expression of both Toll-RB and Toll-RC was drastically increased after infection, whereas Toll-RD showed no significant change (Fig. 3B). Additionally, the expression of Dif-RA, Dif-RB, and Dif-RD was dramatically increased after infection with M. luteus, whereas Dif-RC expression increased to a lesser extent (Fig. 3C). These results indicated that different transcript variants of the Toll and Dif genes have different expression levels in responses to Toll signaling and suggest that miR-958 may play a critical role in repressing the expression of these Toll and Dif transcript variants to further affect the expression of Drosomycin.
Fig. 3.
Analysis of transcripts and target sites of Toll and Dif. A: schematic detailing putative miR-958-binding sites in the 3′-untranslated region (UTR) of transcript variants of Toll and Dif (three and four, respectively; data from FlyBase and miRanda). The underlined sequence indicates “seed sequence” of miR-958. Target sites (labeled 1-4) are symbolized by shaded boxes. Scale bar, 100 bp. The transcript variants of Toll (B) and Dif (C) show differential expression when wild-type flies were infected by M. luteus compared with control flies. Data (B and C) are presented as means ± SD from at least three independent experiments. Statistical significance was assessed by two-tailed Student’s t-test; NS, not significant, **P < 0.01, ***P < 0.001.
miR-958 directly targets Toll and Dif.
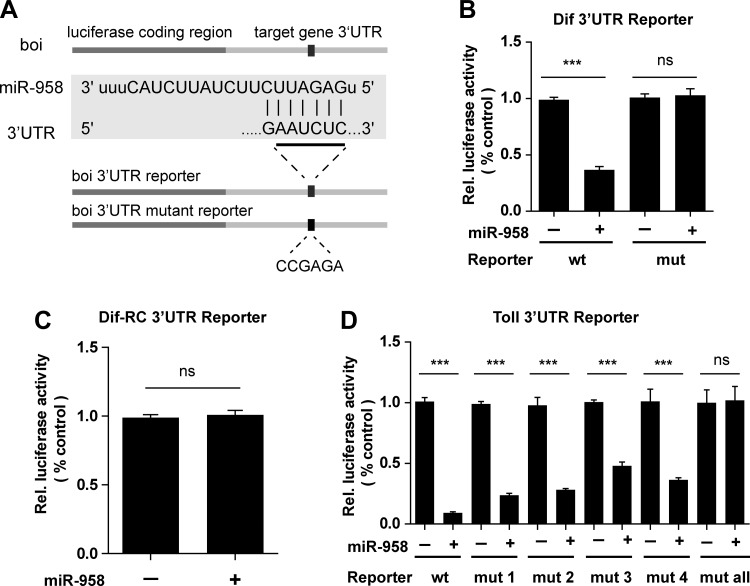
To verify whether miR-958 directly targets Toll or Dif, we cloned the full-length 3′-UTRs of the Drosophila Toll, Dif-RA, and Dif-RC genes into a reporter vector downstream of firefly luciferase cDNA (Fig. 4A). Compared with control S2 cells treated with pAc-empty vector, S2 cells transfected with the Dif-RA 3′-UTR construct exhibited significantly decreased luciferase activity when cotransfected with miR-958 overexpression vector (Fig. 4B). Furthermore, luciferase activity from the Dif-RC 3′-UTR construct did not show any changes after miR-958 overexpression (Fig. 4C), confirming that miR-958 does not regulate Dif-RC expression. Transfection with miR-958 reduced luciferase activity from the Toll 3′-UTR construct to one-tenth of the level of control transfected cells (Fig. 4D).
Fig. 4.
miR-958 targets Toll and Dif-RA, not Dif-RC, in S2 cells. A: construct used to generate the 3′-UTR reporter vectors for Toll, Dif-RA, and Dif-RC. Mutant vectors were constructed by replacing seed sequence components with the mutant CCGAGA sequence. B-D: the interactions between miR-958 and its predicted target sequences in the 3′-UTRs of Dif-RA (B), Dif-RC (C), and Toll (D) were determined in Drosophila S2 cells. 48 h after transfection, cells were lysed for luciferase assays; firefly luciferase was normalized to Renilla luciferase activity. Data (B-D) are presented as means ± SD from at least three independent experiments. Statistical significance was assessed by two-tailed Student’s t-test; NS, not significant, ***P < 0.001.
To determine the effects of miR-958 on each target site, we sequentially introduced single target site mutations into each of the miR-958 seed matches in the Toll and Dif 3′-UTR reporter vectors. We also mutated all four miR-958 target sites in the Toll 3′-UTR. When the regions homologous to the “seed” sequence of miR-958 were mutated in the Dif 3′-UTR reporter constructs, the luciferase activity returned to the levels seen in transfection with the control reporter plasmid (Fig. 4B). Mutation in the four different miR-958 binding sites in the Toll 3′-UTR reporter showed differential levels of rescue of luciferase activity, and complete rescue of luciferase activity was seen when all four sites were mutated simultaneously. Mutation of target site 3 in the Toll 3′-UTR reporter exerted a greater ability to rescue luciferase activity mutation of other single miR-958-binding sites (Fig. 4D), suggesting that this site might be the primary region of miR-958 regulation of Toll expression. Taken together, miR-958 directly targets Toll and Dif by recognizing the corresponding seed-match target sites to engage in posttranscriptional regulation of Toll and Dif.
miR-958 controls Drosomycin production by targeting Toll and Dif in vivo.
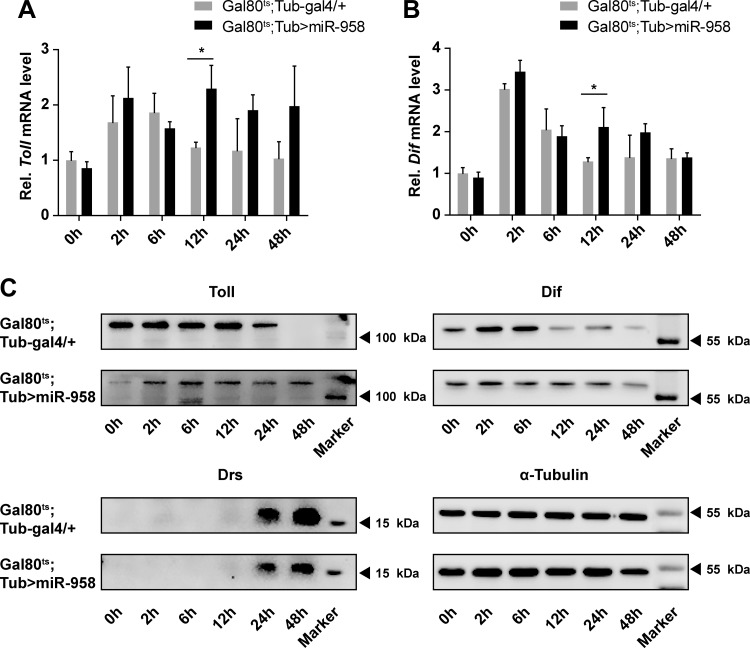
To further determine the effects of miR-958 on regulation of Toll and Dif expression, we analyzed the mRNA and protein levels of Toll and Dif after overexpression of miR-958 in vivo. Flies with miR-958 overexpression exhibited increased mRNA levels of Toll and Dif compared with controls at 12 h after M. luteus infection (Fig. 5, A and B). However, this phenomenon was not observed at other time points after injection. In contrast, Western blot analysis showed that the protein expression levels of Toll and Dif were inhibited significantly in miR-958-overexpressing flies (Fig. 5C). At 0, 2, 6, 12, and 24 h after M. luteus infection, the Toll protein expression was dramatically decreased in miR-958-overexpressing flies, whereas at 48 h Toll protein remained expressed in the miR-985-overexpressing flies but was not expressed in control flies. We also observed similar patterns in the expression of Dif protein. At 0, 2, and 6 h, Dif expression was lower in miR-958-overexpressing flies (Fig. 5C). After 12 h, Dif protein expression in miR-958-overexpressing flies was not significantly different compared with controls. These results suggest that miR-958 overexpression inhibits the basal expression of Toll and Dif, which can inhibit Drosomycin production, which could delay onset of an appropriate immune response in miR-958 overexpression flies, as more time is needed to produce enough Toll and Dif protein.
Fig. 5.
miR-958 inhibits expression of Toll, Dif, and Drosomycin in vivo. A and B: the mRNA expression levels of Toll (A) and Dif (B) were determined in miR-958-overexpressing flies and controls at 0, 2, 6, 12, 24, and 48 h after M. luteus infection. C: protein levels of Toll, Dif, and Drosomycin were determined by Western blotting at the same times after M. luteus infection. Data (A and B) are presented as means ± SD from at least 3 independent experiments. Statistical significance was assessed by two-tailed Student’s t-test; *P < 0.05.
Introduction of miR-958 sponge restores the normal expression of Drosomycin in miR-958-overexpressing flies.
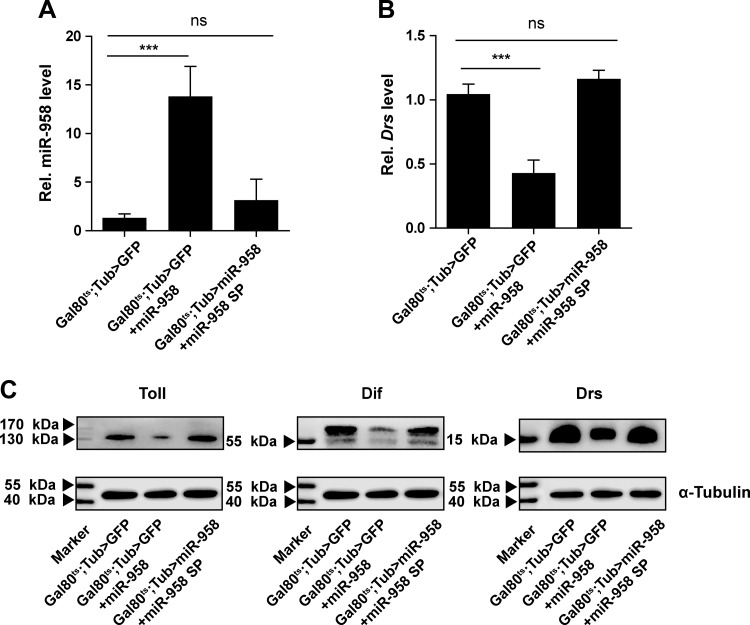
To further determine whether miR-958 inhibits Drosomycin expression and regulates Toll signaling by suppressing Toll and Dif, we simultaneously overexpressed miR-958 and a miR-958 sponge in Drosophila. The UAS-miR-958 sponge specifically reduces miR-958 levels by providing binding targets to engage endogenous and exogenous miR-958 molecules. Overexpression of the miR-958 sponge decreased miR-958 expression to a level similar to basal expression, even in the context of miR-958 overexpression (Fig. 6A). Importantly, this reduction of exogenous miR-958 (Tub>miR-958, miR-958 sponge) rescues Drosomycin mRNA expression, restoring it back to the level of controls (Fig. 6B). Additionally, Western blot analysis demonstrated that the miR-958 sponge rescues the reduced Drosomycin, as well as expression of the miR-958 targets Toll and Dif, in miR-958 overexpression flies (Fig. 6C). These results confirm that miR-958 regulates Toll signaling and Drosomycin expression by targeting Toll and Dif.
Fig. 6.
miR-958 sponge restores Toll signaling in miR-958 overexpression flies. A: the levels of miR-958 were examined in control flies (Gal80ts;Tub>GFP), miR-958-overexpressing flies (Gal80ts;Tub>GFP+miR-958), and miR-958 and miR-958 sponge coexpression flies (Gal80ts;Tub>miR-958+miR-958 sponge) before infection. B: mRNA levels of Drosomycin were determined at 48 h postinfection. C: protein levels of Toll and Dif were determined at 6 h and levels of Drosomycin were determined at 48 h after infection with M. luteus. Data (A and B) are presented as means ± SD from at least three independent experiments. Statistical significance was assessed by two-tailed Student’s t-test; NS, not significant, ***P < 0.001.
DISCUSSION
Although the Toll signaling pathway has been well studied, we have yet to fully elucidate the molecular mechanisms involved in how this pathway fine tunes immune responses (30). miRNAs are posttranscriptional regulatory molecules that play diverse roles in physiological processes, such as homeostasis, development, cancer, and immune responses (27, 28, 33). An accumulating body of evidence indicates that miRNAs regulate immune responses to prevent overactivation of the immune system. Examples of immune-response genes regulated by miRNAs include the endosomal regulation of murine TLR7 and human TLR8 by miR-21 and miR-29 (11), the modulation of TLR-2 protein by miR-105 in human gingival keratinocytes (3), and the reduction of the NFKB1/p105 protein in monocytes overexpressing miR-9 (1). However, there are limited reports of miRNAs involved in regulating immune response in Drosophila.
In this study, we identified miR-958 as a novel negative regulator of Toll signaling of Drosophila. miR-958 is located on a noncoding gene, and little has been reported about it in Drosophila, except that it was reported to control activity-dependent synaptic growth at neuromuscular junctions (24). We demonstrate that miR-958 inhibits the Toll signaling pathway by targeting two genes, Toll and Dif. We demonstrated with reporter studies that the Toll 3′-UTR is significantly inhibited by miR-958 because it harbors four miR-958-binding sites. Of these sites, the one with the strongest regulatory effect is site 3, which is present in all three Toll transcript variants. In addition, site 3 is conserved across 12 Drosophila species, site 2 in nine, and sites 1 and 4 are poorly conserved, suggesting that this specific miR-958-binding site in the Toll 3′-UTR is critical for regulation of Toll and Toll signaling by miR-958.
Overexpression of miR-958 significantly inhibited the expression level of Drosomycin in response to immunization challenge, whereas Drosomycin expression was increased in miR-958−/− mutant flies at 48 h after M. luteus infection. Key characteristics of gene regulation by miRNAs are that 1) different transcripts may be regulated by the same miRNA and 2) different miRNAs may regulate the same mRNA (4), suggesting that other miRNAs, in addition to miR-958, may contribute to the regulation of the Toll signaling pathway and could compensate for any loss of function of miR-958. However, the data presented here indicate that miR-958 is critically involved in returning Drosomycin levels to normal upon completion of an immune response. This return to baseline levels is important for Drosophila to avoid overactivation of the immune response.
In the present study, we identified miR-958 as a novel regulator of the Toll signaling pathway in Drosophila. To further elucidate the complex mechanism of miRNA regulation of immune responses, the roles of additional candidate miRNAs should be examined in the future.
GRANTS
This work was partially supported by the National Natural Science Foundation of China (no. 31572324), the National Natural Science Youth Foundation of China (no. 31501863), the Natural Science Research Project of Jiangsu Higher Education Institutions (no. 16KJB180014), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.L., Y.L., and L.S. performed experiments; S.L., Y.L., and P.J. analyzed data; S.L. interpreted results of experiments; S.L. prepared figures; S.L. drafted manuscript; S.L., Y.L., L.S., P.J., L.C., and F.M. approved final version of manuscript; L.C. and F.M. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Dr. Jinjun Qian (Southeast University, Nanjing, China) for valuable suggestions.
REFERENCES
- 1.Bazzoni F, Rossato M, Fabbri M, Gaudiosi D, Mirolo M, Mori L, Tamassia N, Mantovani A, Cassatella MA, Locati M. Induction and regulatory function of miR-9 in human monocytes and neutrophils exposed to proinflammatory signals. Proc Natl Acad Sci USA 106: 5282–5287, 2009. doi: 10.1073/pnas.0810909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bejarano F, Bortolamiol-Becet D, Dai Q, Sun K, Saj A, Chou Y-T, Raleigh DR, Kim K, Ni J-Q, Duan H, Yang JS, Fulga TA, Van Vactor D, Perrimon N, Lai EC. A genome-wide transgenic resource for conditional expression of Drosophila microRNAs. Development 139: 2821–2831, 2012. doi: 10.1242/dev.079939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benakanakere MR, Li Q, Eskan MA, Singh AV, Zhao J, Galicia JC, Stathopoulou P, Knudsen TB, Kinane DF. Modulation of TLR2 protein expression by miR-105 in human oral keratinocytes. J Biol Chem 284: 23107–23115, 2009. doi: 10.1074/jbc.M109.013862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brennecke J, Stark A, Russell RB, Cohen SM. Principles of microRNA-target recognition. PLoS Biol 3: e85, 2005. doi: 10.1371/journal.pbio.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchon N, Silverman N, Cherry S. Immunity in Drosophila melanogaster–from microbial recognition to whole-organism physiology. Nat Rev Immunol 14: 796–810, 2014. doi: 10.1038/nri3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen CZ, Schaffert S, Fragoso R, Loh C. Regulation of immune responses and tolerance: The microRNA perspective. Immunol Rev 253: 112–128, 2013. doi: 10.1111/imr.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi IK, Hyun S. Conserved microRNA miR-8 in fat body regulates innate immune homeostasis in Drosophila. Dev Comp Immunol 37: 50–54, 2012. doi: 10.1016/j.dci.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Dai LL, Gao JX, Zou CG, Ma YC, Zhang KQ. miR-233 modulates the unfolded protein response in C. elegans during Pseudomonas aeruginosa infection. PLoS Pathog 11: e1004606, 2015. doi: 10.1371/journal.ppat.1004606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding J, Huang S, Wang Y, Tian Q, Zha R, Shi H, Wang Q, Ge C, Chen T, Zhao Y, Liang L, Li J, He X. Genome-wide screening reveals that miR-195 targets the TNF-α/NF-κB pathway by down-regulating IκB kinase alpha and TAB3 in hepatocellular carcinoma. Hepatology 58: 654–666, 2013. doi: 10.1002/hep.26378. [DOI] [PubMed] [Google Scholar]
- 10.Enright AJ, John B, Gaul U, Tuschl T, Sander C, Marks DS. MicroRNA targets in Drosophila. Genome Biol 5: R1, 2003. doi: 10.1186/gb-2003-5-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, Lovat F, Fadda P, Mao C, Nuovo GJ, Zanesi N, Crawford M, Ozer GH, Wernicke D, Alder H, Caligiuri MA, Nana-Sinkam P, Perrotti D, Croce CM. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci USA 109: E2110–E2116, 2012. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrandon D. The complementary facets of epithelial host defenses in the genetic model organism Drosophila melanogaster: From resistance to resilience. Curr Opin Immunol 25: 59–70, 2013. doi: 10.1016/j.coi.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Gantier MP, Stunden HJ, McCoy CE, Behlke MA, Wang D, Kaparakis-Liaskos M, Sarvestani ST, Yang YH, Xu D, Corr SC, Morand EF, Williams BR. A miR-19 regulon that controls NF-κB signaling. Nucleic Acids Res 40: 8048–8058, 2012. doi: 10.1093/nar/gks521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garbuzov A, Tatar M. Hormonal regulation of Drosophila microRNA let-7 and miR-125 that target innate immunity. Fly (Austin) 4: 306–311, 2010. doi: 10.4161/fly.4.4.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann JA, Reichhart J-M. Drosophila innate immunity: An evolutionary perspective. Nat Immunol 3: 121–126, 2002. doi: 10.1038/ni0202-121. [DOI] [PubMed] [Google Scholar]
- 16.Imler JL. Overview of Drosophila immunity: A historical perspective. Dev Comp Immunol 42: 3–15, 2014. doi: 10.1016/j.dci.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 17.Ji S, Sun M, Zheng X, Li L, Sun L, Chen D, Sun Q. Cell-surface localization of Pellino antagonizes Toll-mediated innate immune signalling by controlling MyD88 turnover in Drosophila. Nat Commun 5: 3458, 2014. doi: 10.1038/ncomms4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kertesz M, Iovino N, Unnerstall U, Gaul U, Segal E. The role of site accessibility in microRNA target recognition. Nat Genet 39: 1278–1284, 2007. doi: 10.1038/ng2135. [DOI] [PubMed] [Google Scholar]
- 19.Lau NC, Lim LP, Weinstein EG, Bartel DP. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science 294: 858–862, 2001. doi: 10.1126/science.1065062. [DOI] [PubMed] [Google Scholar]
- 20.Lee GJ, Hyun S. Multiple targets of the microRNA miR-8 contribute to immune homeostasis in Drosophila. Dev Comp Immunol 45: 245–251, 2014. doi: 10.1016/j.dci.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 21.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol 25: 697–743, 2007. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 22.Lewis BP, Burge CB, and Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120: 15–20, 2005. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 23.McKean KA, Yourth CP, Lazzaro BP, Clark AG. The evolutionary costs of immunological maintenance and deployment. BMC Evol Biol 8: 76, 2008. doi: 10.1186/1471-2148-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nesler KR, Sand RI, Symmes BA, Pradhan SJ, Boin NG, Laun AE, Barbee SA. The miRNA pathway controls rapid changes in activity-dependent synaptic structure at the Drosophila melanogaster neuromuscular junction. PLoS One 8: e68385, 2013. doi: 10.1371/journal.pone.0068385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neyen C, Bretscher AJ, Binggeli O, Lemaitre B. Methods to study Drosophila immunity. Methods 68: 116–128, 2014. doi: 10.1016/j.ymeth.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 26.Ruby JG, Stark A, Johnston WK, Kellis M, Bartel DP, Lai EC. Evolution, biogenesis, expression, and target predictions of a substantially expanded set of Drosophila microRNAs. Genome Res 17: 1850–1864, 2007. doi: 10.1101/gr.6597907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shields BB, Pecot CV, Gao H, McMillan E, Potts M, Nagel C, Purinton S, Wang Y, Ivan C, Kim HS, Borkowski RJ, Khan S, Rodriguez-Aguayo C, Lopez-Berestein G, Lea J, Gazdar A, Baggerly KA, Sood AK, White MA. A genome-scale screen reveals context-dependent ovarian cancer sensitivity to miRNA overexpression. Mol Syst Biol 11: 842, 2015. doi: 10.15252/msb.20156308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stefani G, Slack FJ. Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol 9: 219–230, 2008. doi: 10.1038/nrm2347. [DOI] [PubMed] [Google Scholar]
- 29.Tanji T, Ip YT. Regulators of the Toll and Imd pathways in the Drosophila innate immune response. Trends Immunol 26: 193–198, 2005. doi: 10.1016/j.it.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Teixeira L. Whole-genome expression profile analysis of Drosophila melanogaster immune responses. Brief Funct Genomics 11: 375–386, 2012. doi: 10.1093/bfgp/els043. [DOI] [PubMed] [Google Scholar]
- 31.Valanne S, Myllymäki H, Kallio J, Schmid MR, Kleino A, Murumägi A, Airaksinen L, Kotipelto T, Kaustio M, Ulvila J, Esfahani SS, Engström Y, Silvennoinen O, Hultmark D, Parikka M, Rämet M. Genome-wide RNA interference in Drosophila cells identifies G protein-coupled receptor kinase 2 as a conserved regulator of NF-kappaB signaling. J Immunol 184: 6188–6198, 2010. doi: 10.4049/jimmunol.1000261. [DOI] [PubMed] [Google Scholar]
- 32.Valanne S, Wang JH, Rämet M. The Drosophila Toll signaling pathway. J Immunol 186: 649–656, 2011. doi: 10.4049/jimmunol.1002302. [DOI] [PubMed] [Google Scholar]
- 33.Yang D, Sun Y, Hu L, Zheng H, Ji P, Pecot CV, Zhao Y, Reynolds S, Cheng H, Rupaimoole R, Cogdell D, Nykter M, Broaddus R, Rodriguez-Aguayo C, Lopez-Berestein G, Liu J, Shmulevich I, Sood AK, Chen K, Zhang W. Integrated analyses identify a master microRNA regulatory network for the mesenchymal subtype in serous ovarian cancer. Cancer Cell 23: 186–199, 2013. doi: 10.1016/j.ccr.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]