Abstract
The heterogeneity of the developing pancreatic epithelium and low abundance of endocrine progenitors limit the information derived from traditional expression studies. To identify genes that characterize early developmental tissues composed of multiple progenitor lineages, we applied single-cell RNA-Seq to embryonic day (e)13.5 mouse pancreata and performed integrative analysis with single cell data from mature pancreas. We identified subpopulations expressing macrophage or endothelial markers and new pancreatic progenitor markers. We also identified potential α-cell precursors expressing glucagon (Gcg) among the e13.5 pancreatic cells. Despite their high Gcg expression levels, these cells shared greater transcriptomic similarity with other e13.5 cells than with adult α-cells, indicating their immaturity. Comparative analysis identified the sodium-dependent neutral amino acid transporter, Slc38a5, as a characteristic gene expressed in α-cell precursors but not mature cells. By immunofluorescence analysis, we observed SLC38A5 expression in pancreatic progenitors, including in a subset of NEUROG3+ endocrine progenitors and MAFB+ cells and in all GCG+ cells. Expression declined in α-cells during late gestation and was absent in the adult islet. Our results suggest SLC38A5 as an early marker of α-cell lineage commitment.
Keywords: alpha cell marker, pancreas development, single cell RNA-Seq
transcriptome studies have become integral to the investigation of the cellular development of different tissues and organs. Similar to epidemiology, these studies provide valuable information on the state of a population of cells and are most useful when there is a high degree of cellular homogeneity within the tissue studied. However, they fail to detect subtle, biologically important differences among cells that seem otherwise identical and to accurately characterize heterogeneous tissue populations with multiple cell subtypes (27). The advent of high-fidelity nucleic acid amplification techniques, starting from picogram amounts of RNA (11, 19, 30), and of high-throughput sequencing has transformed transcriptome investigation by allowing analysis at the single cell level. The single cell technique is uniquely suited to the exploration of a complex developing tissue such as the pancreas with its different progenitor and precursor lineages.
During embryonic development of the endocrine pancreas, pancreatic progenitors give rise to unipotent Neurogenin3 (Neurog3)-expressing endocrine progenitors during temporal windows of differentiation, with endocrine progenitors giving rise to α-cells appearing during early development and those giving rise to the other endocrine lineages appearing later (10, 20). At midgestation, around embryonic day (e)13.5, the mouse pancreatic epithelium is at the beginning of the “secondary transition,” a massive wave of differentiation and lineage allocation (24). To characterize the transcriptome of pancreatic progenitors and endocrine cells, we employed RNA sequencing (RNA-Seq) of single mouse pancreatic cells at e13.5, isolated by the Fluidigm C1 platform. When we compared the overall transcriptional profile of e13.5 pancreatic cells with that of adult endocrine cells, we were able to identify subpopulations of cells in both groups. Subsequently, we performed an unsupervised ordering of e13.5 single cells along a trajectory of differentiation that identified 151 differentially expressed genes, most notably glucagon (Gcg). Expression of the sodium-dependent neutral amino acid transporter Slc38a5 correlated highly with Gcg along the trajectory and subsequent analysis suggested that SLC38A5 is a novel α-cell differentiation marker. Thus, this transcriptomic approach has utility to identify new markers and to characterize rare cellular subpopulations when applied to heterogeneous tissues such as the developing pancreas.
MATERIALS AND METHODS
Single cell RNA-Seq.
The dorsal pancreas was dissected from e13.5 CD1 embryos, stored briefly in PBS on ice, and then digested in Trypsin 0.05% for 5 min at 37°C. After addition of RPMI/FBS medium, cells were counted and diluted to a concentration of 200 cells/μl. The Fluidigm C1 requires at least 1,000 cells to be loaded (5 μl), of which ~10% fill the 96 wells of the 10–17 μm diameter chip. Capture efficiency was briefly evaluated, and then the chip was loaded onto the C1 for cDNA preparation using Clontech SMARTer kit (Takara-Clontech). We excluded 15 capture events because they represented cell debris or clusters of two or more cells. Hence, 81 individual single cell RNA libraries were generated from 150 pg cDNA, using the low throughput Nextera XT SNA library prep kit (Illumina). Cells were loaded and analyzed in random order. Individual bar-coded libraries were sequenced by a Illumina HiSeq 2500. In total, 493,090,926 reads (average 6 million reads/cell) were obtained from two sequencing runs and aligned to an average of 5,500 genes/cell. The mRNA expression level, expressed as reads per kilobase per million mapped reads (RPKM), was calculated for each gene. Caution should be taken in comparing expression levels among different transcripts because of the limitations of normalization. All 81 cells expressed the β-actin gene (ActB) with RPKM >2,000 and were considered viable. Overall, the depth of sequencing and library quality were similar to other published studies (37). Data are available for download from GEO database (GSE78510).
Bioinformatic analysis.
Sequencing data were mapped using RUM (15). Four e13.5 pancreatic cells with fewer than 1 million mapped reads were removed. RPKM values were calculated over Refseq gene annotation after merging two technical replicates of the single cell RNA-Seq data. Seurat R toolkit was applied to perform t-distributed stochastic neighbor embedding (t-SNE) clustering using the single cell RNA-Seq data from e13.5 and adults islets (38) (GSE77980). Adult endocrine cell doublets were also excluded as presented in (38). t-SNE was implemented over the 340 genes whose dispersion and the average gene expression are >2.
We used nonnegative least squares (NNLS) (22) to understand the transcriptomic similarity of single cells with known annotated cells using coefficients that represent the contribution of each reference transcriptome from the ENCODE database (http://www.encodeproject.org). NNLS is a multiple linear regression with only positive coefficients. To model transcriptome (y) of a cell with a reference transcriptome x, NNLS finds arg minx‖Ax − y‖2 subject to x ≥ 0. A cell is called ambiguous if the largest coefficient is <0.3.
We applied Monocle for cell ordering and visualization using 142 genes known to be expressed in the pancreas (Supplemental Table S4). (The online version of this article contains supplemental material.) The Monocle algorithm has been described (32). We used Gcg expression level to identify the beginning, intermediate and ending states. We obtained differentially expressed genes using “differentialGeneTest” against “pseudotime” after removing cells belong to the intermediate states (q-value cutoff = 0.1). The Wilcoxon rank sum test was applied to identify genes differentially expressed between the beginning (state 1) and ending states (the last 20 cells in state 3) using a P value cut-off of 0.05. Hierarchical clustering analysis was performed for differentially expressed genes using expression values from all ordered cells. Ward’s criterion for genes with 1- (correlation coefficient) was used as a distance measure. A clustering heat map was generated using the z-score that is scaled across all cells for each gene.
RNA-Seq data for mature α-cells were obtained from GEO (GSE54973) (5). Monocle was applied to the RNA-Seq data from single cells as well as the mature α- and β-cells using the 142 gene set (Supplemental Table S4).
Protein expression.
We obtained CD1 timed pregnant females from Jackson Laboratories. Whole embryos (e9.5–e13.5) or individual pancreata (e15.5–e17.5 and adult) were fixed for 4 h in 4% paraformaldehyde at 4°C, washed in PBS, and placed in 30% sucrose overnight. Tissues were then embedded in OCT, frozen on dry ice, and stored at −80°C until sectioning. OCT-embedded tissues were cut at 7–8 μm on a cryostat, and sections were stored at −80°C. Sections were blocked in 1% bovine serum albumin in PBS containing 5% normal donkey serum. Primary antibodies were: goat anti-PDX1 (1:10,000, gift from C. Wright), guinea pig anti-PDX1 (1:500, gift from C. Wright), chicken anti-NEUROG3 (1:500, Βeta Cell Biology Consortium – from Michael Ray, Vanderbilt University), rabbit anti-MAFB (1:250, Bethyl), goat anti-SLC38A5 (1:500, Santa Cruz Biotechnology, used for the immunofluorescence staining in Figs. 4; 5, A–F; and 6), rabbit anti-SLC38A5 (1:500, Millipore, used for the immunofluorescence staining in Fig. 5, G–I), rabbit anti-glucagon (Santa Cruz Biotechnology), mouse anti-glucagon (1:500, Abcam), guinea pig anti-glucagon (1:500, Millipore), guinea pig anti-insulin (1:500, Abcam), goat and rabbit anti-ghrelin (1:500, Santa Cruz Biotechnology), anti-somatostatin (Santa Cruz Biotechnology, 1:500), rabbit anti-PP (Linco, 1:200). Secondary labeling was performed with species-specific secondary antisera raised in donkey, conjugated to Cy2, Cy3, or Cy5 fluorophores (Jackson ImmunoResearch) and diluted 1:500. Digital images were acquired using the same exposure settings on a Nikon 600 microscope at ×20 or ×40 magnification, using MetaMorph software.
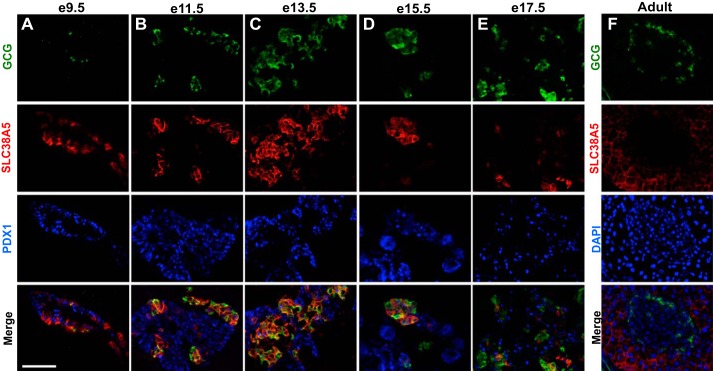
Fig. 4.
SLC38A5 is expressed in GCG-positive cells from the onset of endocrine differentiation. Immunofluorescence staining for GCG (green), SLC38A5 (red), and PDX1 (blue) in mouse pancreatic epithelium at e9.5 (A), e11.5 (B), e13.5 (C), e15.5 (D), and e17.5 (E). Images shown are representative of ≥3 epithelia imaged at each age. F: representative adult islet stained for GCG and SLC38A5. DAPI nuclear counterstain shown in blue. Merge of all 3 channels is shown at the bottom of each panel. Images taken at ×20 magnification. Scale bar represents 50 μm.
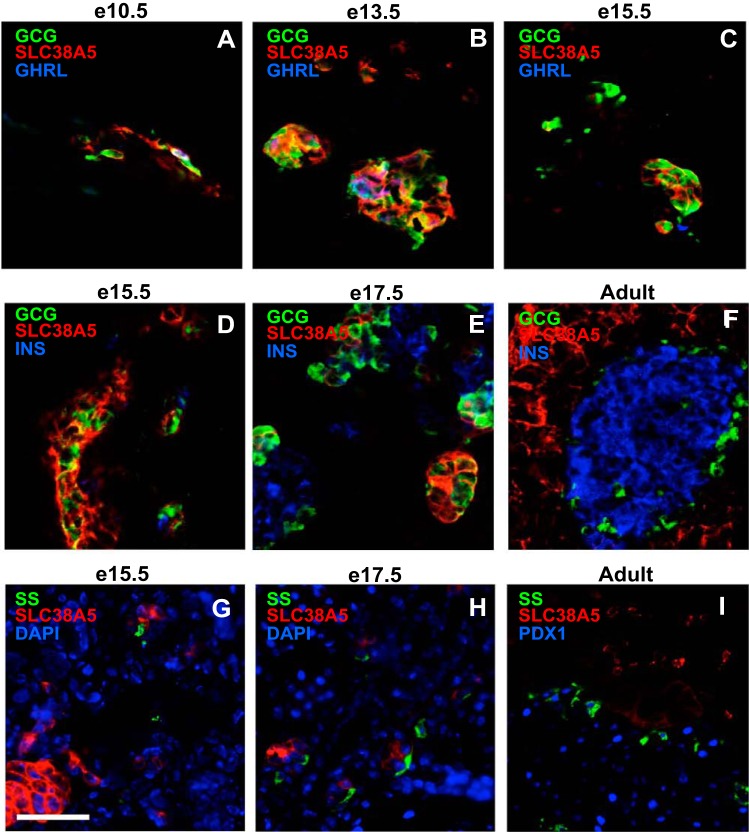
Fig. 5.
SLC38A5 is not expressed in ε-, β-, or δ-cells in the pancreatic bud and adult islet. A–C: SLC38A5 is expressed in GCG-only and GCG-GHRL cells, and not in GHRL-only cells. Immunofluorescence staining for GHRL (blue), SLC38A5 (red), and GCG (green) at e10.5 (A), e13.5 (B), and e15.5 (C). D–E: SLC38A5 is not expressed in β-cells. Immunofluorescence staining for GCG, SLC38A5 (red), and INS (blue) at e15.5 (D), e17.5 (E), and adult pancreas (F). G–I: SLC38A5 is not expressed in δ-cells. Immunofluorescence staining for somatostatin (SST) (green), SLC38A5 (red), and DAPI or PDX1 (blue) at e15.5 (G), e17.5 (H), and adult pancreas (I). Scale bar represents 20 μm.
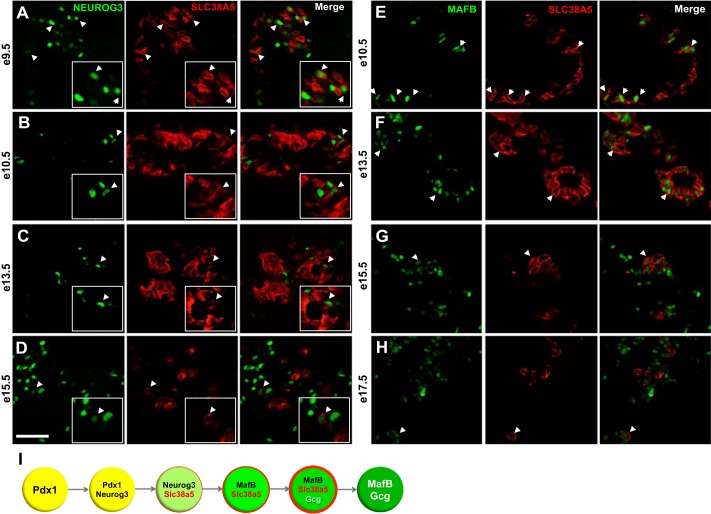
Fig. 6.
Colocalization of SLC38A5 with NEUROG3, MAFB, and GCG during early–midgestation. NEUROG3 (green) and SLC38A5 (red) colocalize in a subset of pancreatic cells at e9.5 (A), e10.5 (B), e13.5 (C), and e15.5 (D) (white arrowheads). MAFB (green) and SLC38A5 (red) colocalize at e10.5 (E), e13.5 (F), e15.5 (G), and e17.5 (H). Images taken at ×40 magnification. Scale bar represents 100 μm. I: model depicting SLCA38A5 expression during pancreas development.
RESULTS
We used a microfluidic device (Fluidigm C1, Fluidigm) to capture 96 individual pancreatic cells at e13.5 of mouse embryonic development. Fifteen capture events represented cell debris or clusters and were therefore excluded from further analysis. RNA from each cell was isolated to construct a library, which was followed by deep sequencing. In total, 493,090,926 reads were obtained from two sequencing runs. Four cells were removed due to a low number of mapped reads (<1 million mapped reads). The remaining 77 cells were subsequently analyzed. The mRNA expression level, expressed as RPKM was calculated for each gene.
Transcriptomic heterogeneity of pancreatic cells at e13.5.
To evaluate the robustness of the single cell approach, we compared average gene expression across our single cell e13.5 RNA-Seq data set with previously published expression by whole pancreas e13.5 RNA-Seq (16). Overall, gene expression levels in the single cell and whole pancreas were highly correlated [correlation coefficient (r) 0.82] (Fig. 1A). We found 3,348 genes were underrepresented and 103 genes were overrepresented in the scRNA-Seq data (Fig. 1A, Supplemental Table S1) (adjusted P value <0.0001 and fold difference >3). Although genes such as Ins1, Pdx1, and Neurog3 were underrepresented among the single cells, genes such as Gcg, MafB, Sox9, Isl1, Hnf1a, Hnf1b, Rfx6, NeuroD1 were highly correlated between the two data sets. This finding is not unexpected, as the C1 methodology has been shown to detect only ~40% of genes detected by bulk RNA-Seq using the same cDNA kit (37). This may due to Fluidigm C1 cell size selection, to cell loss during enzymatic dispersion, and/or to low expression of some genes at this developmental age, below the limit of detection by the single cell transcriptomic approach. Although the correlation coefficient between single cell multiplexed quantitative PCR and C1-based amplification using SMARTer chemistry was high (37), it is possible that transcripts with low copy number are unevenly amplified and hence underrepresented in this type of analysis.
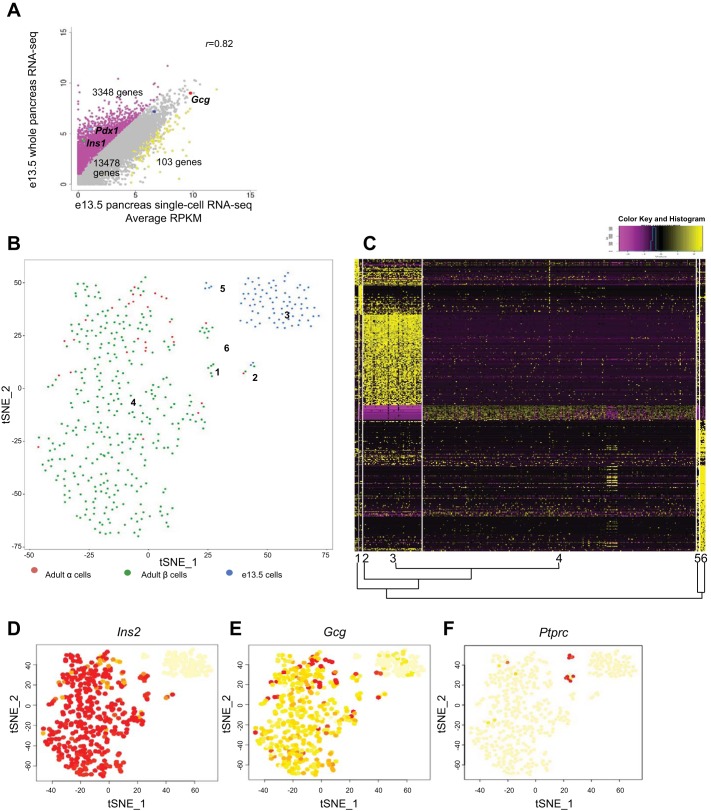
Fig. 1.
Clustering of single pancreatic embryonic day (e)13.5 cells, adult α- and β-cells using t-distributed stochastic neighbor embedding (t-SNE). A: correlation plot of average transcript levels between e13.5 single cell RNA sequencing (RNA-Seq) and published whole pancreas RNA-Seq (14) Each dot represents an unique transcript. B: single e13.5 cells and adult α- and β-cells analyzed by t-SNE and represented in 2-dimensional space. Six clusters were identified, labeled 1–6. Each dot represents the position of a single cell. C: heat map depicting the transcript levels of genes that were uniquely up or downregulated in each cluster (upregulated, yellow; downregulated, purple). The cluster number and relationship to other clusters is shown along the bottom border. D–F: expression of Ins2, Gcg, and Ptprc in the 6 t-SNE clusters. The vast majority of cells expressing Ins2 and Gcg are found in cluster 4. Two cells expressing Gcg are seen in cluster 3, while Ptprc was specific to clusters 5 and 6. The color of each cell is proportional to expression level of represented transcript (yellow, no expression; red, highest expression).
To characterize the e13.5 single cells and to identify subgroups, we incorporated published single cell data from adult α- and β-cells (38) and performed t-SNE analysis using the Seurat toolkit (34). t-SNE can capture nonlinear relationships in data by representing the high-dimensional scRNA-Seq data in two-dimensional space where cells with similar transcriptomic profiles are located nearby and dissimilar objects are located distantly. To limit potential confounding of results, we prepared the data sets chosen for comparison by the same methodology.
The t-SNE approach identified two large and four small groups among the mixed population of adult and embryonic cells (Fig. 1, B and C). The two large groups were composed solely of e13.5 single pancreatic cells (cluster 3) and the adult α- and β-cells (cluster 4) (Fig. 1, B, D, E). The transcriptional distances among the populations shown by the t-SNE analysis suggests that the mature endocrine and fetal pancreatic cells are more different, while adult α- and β-cells are more similar. Two e13.5 cells exhibited high Gcg expression (Fig. 1, C and E), comparable with adult single α-cells. However, these cells were still found in cluster 3, suggesting greater transcriptomic similarity to the rest of the e13.5 pancreatic cells than with adult α-cells.
Genes involved in developmental pathways, such as epithelial-mesenchymal transition (Fgfr1, Fzd2, Wnt5a, Snai2), HIPPO signaling (Yap1, Tead2), and axonal guidance (Sema5a, Sema6a, Slit2, Mmp2, Gli3) were uniquely expressed in cluster 3 cells (Fig. 1C, Supplemental Table S2). The transcripts most highly associated with cluster 3 were Cdkn1c (P57Kip2), Gpc3 (glypican3), and Ptn (pleiotrophin) (Supplemental Table S2). While Cdkn1c upregulation has been shown to promote cell cycle exit of pancreatic progenitors (13), the functions of PTN and GPC3 in pancreas development have not been previously appreciated.
Clusters 1, 2, 5, and 6 represented minor populations of adult and embryonic cells that have not previously been characterized transcriptomically. The gene most strongly associated with cluster 1 was Postn (periostin) (Supplemental Table S2), considered a marker of activated pancreatic stellate cells (6). Cluster 1 was enriched for genes implicated in “cellular growth and proliferation” and “cancer,” although no clear cellular signature was found. Cluster 2, containing both adult and embryonic cells, was characterized by an endothelial transcriptomic signature (Pecam1, Flt1, Cdh5, Robo4, Fabp4, and others) (Supplemental Table S2). Only a few genes were upregulated in cluster 4 and downregulated in cluster 3 (Fig. 1C). Among them were components of secretory granule exocytosis (Scg3, Syt4, ChgB) and known β-cell surface markers (Disp2, Ptprn, Ppp1r1a). Genes involved in immune regulation were unique to clusters 5 (e13.5 cells) and 6 (adult α- and β-cells) (Supplemental Table S2). A closer look at the set of genes enriched in cluster 5 (four e13.5 pancreatic cells), revealed signatures of macrophages [Ptprc (CD45) and Emr1 (F4/80)] (Fig. 1F, Supplemental Table S2). Embryonic macrophage precursors have been previously described as early as e12.5 in murine pancreas and are thought to play a role in islet development (4, 14). The seven adult Ins+ cells in cluster 6 also expressed Ptprc (Fig. 1F).
To further characterize cell populations and to identify potential doublets without relying on multihormonal gene expression, we investigated the transcriptomic similarity of the single cells with published transcriptomic data, using a method based on nonnegative least squares (NNLS) (22). For this we used the reference transcriptomes derived from adult mouse cells [mouse ENCODE and the α-, β-, δ-. and PP cells (38)]. All cells in cluster 3 were classified as “ambiguous,” indicating that these cells are not similar to known, mainly adult reference transcriptomes (Supplemental Table S3). Interestingly, of all adult cell types used for comparison, the e13.5 pancreatic cells in cluster 3 were most similar to adult brain cells, although the correlation coefficient was low. Comparison with ENCODE RNA-Seq transcriptomic data also confirmed that the cells in cluster 5 were more similar to macrophages than to other adult cell types (Supplemental Table S3). Cluster 6 was enriched for complement system components, monocyte, and macrophage factors. These cells were computationally closer to adult β-cells and/or macrophages (Supplemental Table S3).
Dynamic differentiation analysis models aspects of α-cell development.
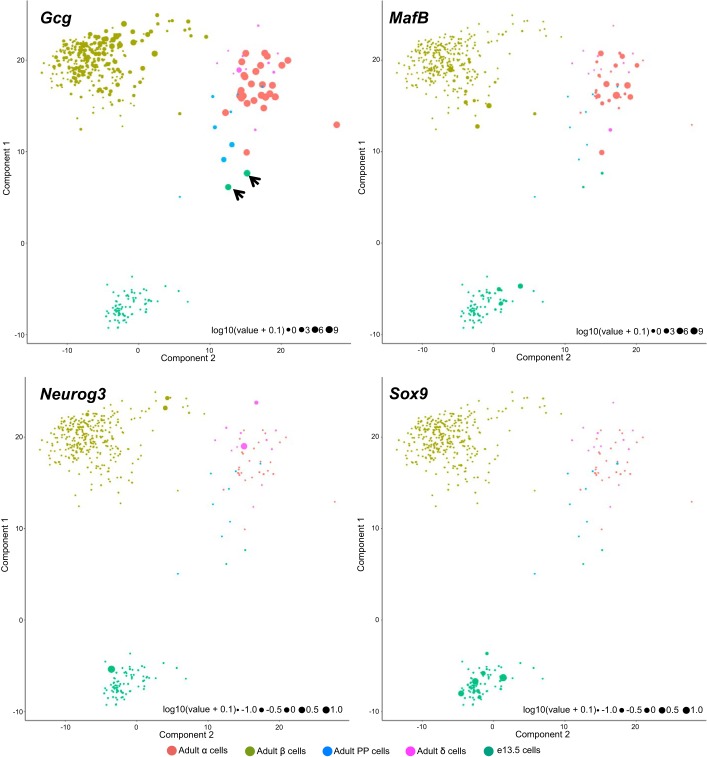
To identify potential differentiation dynamics in the e13.5 pancreatic cell population, we performed independent component analysis (ICA) using a set of 142 genes with known biological roles in the pancreas (Supplemental Table S4). The ICA showed separate populations for α-, β-, and e13.5 cells (Fig. 2). Interestingly, the two e13.5 cells expressing the highest Gcg levels were located near the α/PP/δ cell cluster (Fig. 2, black arrows). These cells were clustered with the other e13.5 cells based on t-SNE (Fig. 1, B and E). Hence, while their total transcriptome was most similar to that of embryonic pancreatic cells, the pancreatic transcriptional program classified them in the α-lineage, suggesting that they are α-cell precursors. We observed that several adult β- and δ-cells expressed Neurog3, MafB, and Sox9 at similar levels to e13.5 cells (Fig. 2); however, the adult Neurog3+, MafB+, or Sox9+ cells were transcriptomically quite distant from e13.5 progenitors (Fig. 2). Whether these represent adult endocrine precursor cells requires further investigation.
Fig. 2.
e13.5 pancreatic cells and adult islet cells ordered in a 2-dimensional independent component space. Expression level of Gcg, MafB, Neurog3, and Sox9 in individual cells represented by the size of the circle in a log2 scale. Black arrows indicate 2 e13.5 pancreatic cells that express high levels of Gcg.
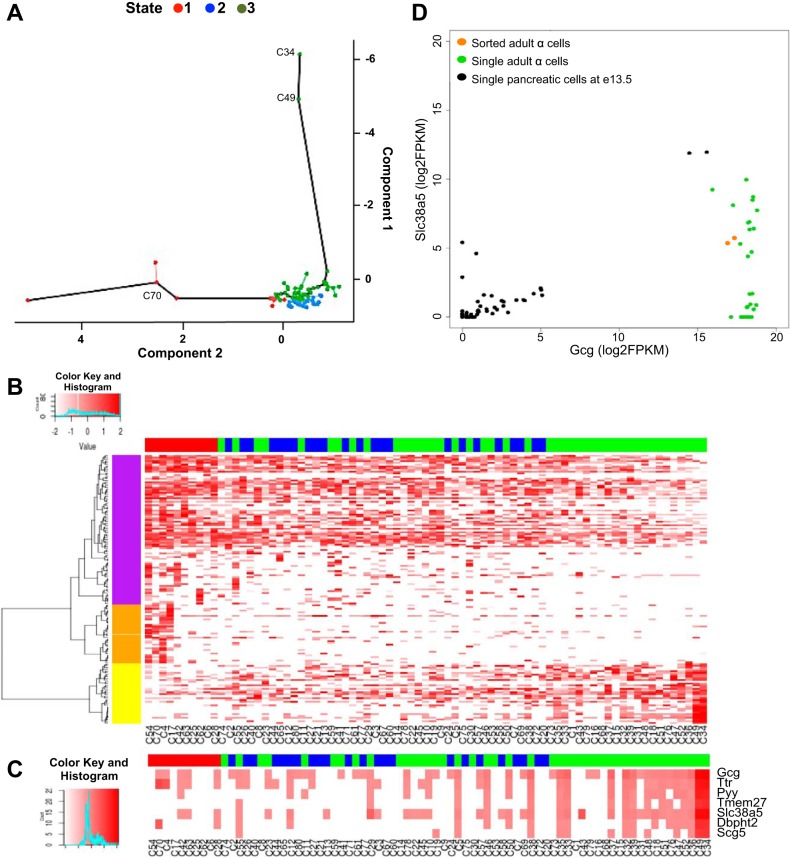
Pseudotime has been used to model the temporal dynamics of single cell transcriptomes for myoblast differentiation (32) and ear cell development (7). Hypothesizing that the two e13.5 cells with strong Gcg expression levels are α-cell precursors, we investigated the potential developmental dynamics across the e13.5 cells using Monocle (32). The 77 e13.5 cells were arranged according to the transcriptomic distances among them. This led to a theoretical trajectory of transcriptomic differentiation and grouped these cells into three states (Fig. 3A). States 1 and 3 contained cells arranged at the two extremes of the trajectory. Of the 142 genes initially used for this analysis, 20 genes were differentially expressed between these two states (Supplemental Table S5). Gcg was one of the most differentially expressed genes along this trajectory (P value: 1.3e-6).
Fig. 3.
Differential expression analysis of single pancreatic cells at e13.5. A: cells ordered in a 2-dimensional independent component space by Monocle. In Monocle, cells were interconnected using the minimum spanning tree algorithm that organizes cells into hierarchies of related phenotypes. The solid black line connects the longest continuous subset of the vertices from the tree of hierarchies. Three states were defined in this ordered trajectory (state 1, red; state 2, blue; state 3, green). The positions of cells 34 and 49 expressing the highest levels of Gcg and of cell C70 expressing Neurog3 are indicated. B: heat map representation of the transcript levels of 151 differentially expressed genes along the ordered trajectory. Clustering dendrogram is depicted along the left margin. C: heat map representation of 6 transcripts that are highly correlated with Gcg along the ordered trajectory. The state designation of each cell is represented above each heat map (B, C) (state 1, red; state 2, blue; state 3, green). D: scatter plot representation of the correlation between Gcg and Slc38a5 (Pearson correlation coefficient 0.86 – red line) in e13.5 single cells (black dots). The level of Gcg and Slc38a5 in sorted adult α-cells (orange dots) (5) or single adult α-cells (light green dots) (38) is shown for comparison.
Considering the ordered trajectory as pseudodevelopmental time for α-cells, we defined state 3 as the “late” state, since it contained cells expressing Gcg, and state 1 as the “early” state. Expanding the analysis to the entire transcriptome, we found 151 genes that were statistically differentially expressed between states 1 and 3 (Fig. 3B, Supplemental Table S6). Of these, 117 genes were downregulated along the ordered trajectory (purple and orange clusters in Fig. 3B). These included Hnf1b, Notch1, and FoxM1, factors associated with pancreatic progenitor differentiation or maintenance of the endocrine progenitor pool (2, 9, 40). Another 34 genes were upregulated between state 1 and 3, including Gcg. This group included genes with known involvement in α-cell commitment or function (yellow cluster in Fig. 3B) (1, 3, 33, 35).
To identify potential genes characterizing α-cell precursors, we analyzed the single cell transcriptomic data for genes whose expression correlated with Gcg expression (Fig. 3C). Among these, transmembrane protein 27 (Tmem27), transthyretin (Ttr), polypeptide YY (Pyy), neuroendocrine protein 7B2/secretogranin 5 (Scg5) (Fig. 3C) have previously described roles in α-cell maturation or function (1, 21, 28, 33, 35). Two factors, solute carrier 38 domain 5 (Slc38a5) and DNA-binding protein with histidine and threonine domain (Dbpht2), have yet unknown roles in pancreas development. Notably, when compared with the two highest Gcg cells in our data set, fluorescence-activated cell sorting (FACS)-sorted or single adult α-cells (5, 38) showed similar Gcg transcript levels, but markedly lower levels of Slc38a5 (Fig. 3D), suggesting that Slc38a5 may be a marker of early α-cell commitment.
SLC38A5 marks α-cell progenitor and differentiated cells during development.
We further investigated SLC38A5 (also known as SNAT5/SN2), a member of the system N family of transporters able to cotransport sodium and amino acids (glycine, glutamine, alanine, serine, and histidine) in exchange for H+. In the adult pancreas of mice and humans, SLC38A5 is expressed in the basolateral membrane of acinar cells (8, 26); however, a large transcriptomic atlas of mouse development (www.eurexpress.org) shows that Slc38a5 is highly expressed in the developing pancreas at e15, and analysis of SAGE libraries of murine pancreas between e10 and e18 clusters Slc38a5 with classical endocrine and proendocrine markers, including as Arx, Gcg, Isl1, Neurog3, Pax4, and Pax6 (18). Expression peaks at e13 and is distributed in the endocrine progenitor-rich trunk region by in situ hybridization (18, 36). In further support of its role in the endocrine lineage, Slc38a5 was downregulated almost ninefold at e10.5 in the dorsal pancreatic bud of Pdx1−/− (29) mice and fourfold downregulated in e13.5 Neurog3−/− pancreas compared with controls (25), suggesting its position downstream of PDX1 and NEUROG3, respectively.
To describe the pattern of Slc38a5 expression during pancreas development and to validate its specificity for maturing α-cells, we performed immunofluorescence staining of the pancreatic bud during development. SLC38A5 was uniquely and highly expressed in the developing pancreatic bud until late gestation. Abundant SLC38A5+GCG− cells were found in the dorsal pancreatic bud at e9.5, whereas all GCG+ cells expressed SLC38A5 (Fig. 4A). Between e11.5 and e15.5, the expression of GCG and SLC38A5 largely overlapped, with the majority of cells expressing both factors (Fig. 4, B–D). At e17.5, SLC38A5 expression decreased, with only about half of GCG+ cells still expressing this transporter (Fig. 4E). In adult pancreas, Slc38a5 expression was no longer observed in adult islets (Fig. 4F), although low-level SLC38A5 could be seen across the acinar domain as previously described (26). Furthermore, SLC38A5 was expressed in GCG+GHRL+ cells and not in GHRL+ only cells between e10.5 and e13.5 (Fig. 5, A and B). At e15.5, the few GHRL-only cells did not express SLC38A5 (Fig. 5C). Throughout embryonic development and in adult islets, SLC38A5 never colocalized with insulin (Fig. 5, D–F) or somatostatin (Fig. 5, G–I), underscoring its specific localization to the developing α-cell lineage. Thus, beginning in early development, SLC38A5 progressively colocalized with GCG, peaking at e13.5 when all GCG+ are SLC38A5+.
Since abundant SLC38A5+GCG− cells were seen very early in development, we hypothesized that SLC38A5 is expressed in pancreatic endocrine progenitors that are committed to an α-cell fate, even before the onset of Gcg expression. Consistent with the early competency window for α-cell lineage differentiation (20), SLC38A5 was expressed in most NEUROG3 endocrine progenitors between e9.5 and e10.5 (Fig. 6, A and B). At e13.5 and e15.5, only rare SLC38A5+NEUROG3+ progenitors were identified (Fig. 6, C and D). Early in development, SLC38A5 may label the subpopulation of NEUROG3+ cells that are already committed to an α-cell fate. In agreement with this concept, all or most MAFB+ cells expressed SLC38A5 at e9.5–e13.5 (Fig. 6, E and F), while after e15.5 only rare MAFB-positive cells still expressed SLC38A5 (Fig. 6, G and H). Together, our results support a model in which SLC38A5 marks early α-cell precursors and suggest roles for SLC38A5 during α-cell maturation (Fig. 6I).
DISCUSSION
The ability to analyze the transcriptome of single pancreatic progenitor cells poses great advantages; it allows for the identification and analysis of cells in a highly heterogeneous tissue with multiple lineages that undergo asynchronous differentiation. We analyzed single cells to understand the differences between embryonic and adult endocrine cells and to identify markers correlated with maturation. Clustering of cells based on total transcriptomic profiles identified genes differentially expressed in embryonic vs. adult cells and also distinguished small subpopulations of presumptive resident macrophages and endothelial cells. We further arranged e13.5 pancreatic cells based on a computationally calculated pseudodevelopmental trajectory. Along this ordered trajectory, pancreatic progenitor genes were downregulated and α-cell maturation genes were upregulated, suggesting that the trajectory mimics aspects of α-cell differentiation. FACS according to GCG or a genetic reporter would have grouped these cells into a single population, and the differences and new markers we observe would not have been revealed.
Most importantly, the analysis of the single e13.5 pancreatic cell transcriptome revealed several factors with an expression pattern highly correlated with that of Gcg. Among them Slc38a5 and Dbpht2 have not been previously described in relation to α-cell differentiation. Our expression analysis suggests that SLC38A5 expression identifies the subpopulation of NEUROG3+ cells that are committed toward the α-cell fate. We show that later in development SLC38A5+ cells lose NEUROG3 and gain initially MAFB and GCG expression. As α-cells mature further toward the end of gestation, they extinguish SLC38A5 protein expression. However, the analysis of the adult α-cell transcriptome (38) suggests that some of these cells continue to express Slc38a5 mRNA. It is possible that in the adult islet, SLC38A5 undergoes posttranslational modifications and/or is not positioned in the plasma membrane, possibly masking critical epitopes required for visualization by immunofluorescence.
The Slc38a5 pattern of expression suggests a specific, time-limited role in the specification and/or maturation of α-cells. In the central nervous system, SLC38A5 mediates the electroneutral and bidirectional pH-dependent transport of glycine and glutamine at perisynaptic astroglial membranes. Together with SLC38A3, it is thought to play a crucial role in the glutamate/GABA-glutamine cycle between neurons and adjoining glial cells (17). We speculate that during embryonic endocrine maturation SLC38A5 could similarly function in an autocrine or paracrine manner to mediate shuttling of glycine or glutamine among α-cells and/or between α-cells and newly emerging β-cells. Notably, glycine and glutamine both regulate glucagon and insulin secretion (12, 23, 31, 39).
These findings highlight the utility and limitations of the single cell transcriptomic approach. It can provide a wealth of information for building high-throughput expression blueprints of maturation, for identifying novel factors with roles in pancreas development and for identifying maturational cell surface markers that could be used for positive or negative enrichment of stem cell derived progenitor cells to increase the yield of stem cell differentiation protocols. The extension of single cell transcriptomics to other pancreatic developmental ages, in mouse or human, will shed light on the transcriptomic continuum of β- and other endocrine cells, with the potential to reveal new maturation factors that could be used to optimize cell replacement strategies for diabetes.
GRANTS
These studies were supported by a Diabetes Career Development Award 5K12DK-094723-03 and Pediatric Endocrine Society Clinical Scholar Award (D. E. Stanescu), NIH R01DK-106027 (K.-J. Won), NIH U01DK-089540 and R01DK-105689 (D. A. Stoffers). Single cell isolation, cDNA and library preparation and sequencing was performed by the FGC of the University of Pennsylvania Diabetes Research Center (DK-019525).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.E.S. performed experiments; D.E.S., R.Y., and K.J.W. analyzed data; D.E.S., K.J.W., and D.A.S. interpreted results of experiments; D.E.S., R.Y., and K.J.W. prepared figures; D.E.S. and D.A.S. drafted manuscript; D.E.S., R.Y., K.J.W., and D.A.S. edited and revised manuscript; D.E.S., K.J.W., and D.A.S. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Olga Smirnova and Jonathan Schug [UPenn Functional Genomics Core (FGC)], Samira Tliba for technical support, Michael Ray (Vanderbilt University) for providing immunofluorescence antibodies, and Klaus Kaestner for critical review of the manuscript.
REFERENCES
- 1.Akpinar P, Kuwajima S, Krützfeldt J, Stoffel M. Tmem27: a cleaved and shed plasma membrane protein that stimulates pancreatic beta cell proliferation. Cell Metab 2: 385–397, 2005. doi: 10.1016/j.cmet.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature 400: 877–881, 1999. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- 3.Artner I, Le Lay J, Hang Y, Elghazi L, Schisler JC, Henderson E, Sosa-Pineda B, Stein R. MafB: an activator of the glucagon gene expressed in developing islet alpha- and beta-cells. Diabetes 55: 297–304, 2006. doi: 10.2337/diabetes.55.02.06.db05-0946. [DOI] [PubMed] [Google Scholar]
- 4.Banaei-Bouchareb L, Gouon-Evans V, Samara-Boustani D, Castellotti MC, Czernichow P, Pollard JW, Polak M. Insulin cell mass is altered in Csf1op/Csf1op macrophage-deficient mice. J Leukoc Biol 76: 359–367, 2004. doi: 10.1189/jlb.1103591. [DOI] [PubMed] [Google Scholar]
- 5.Benner C, van der Meulen T, Cacéres E, Tigyi K, Donaldson CJ, Huising MO. The transcriptional landscape of mouse beta cells compared to human beta cells reveals notable species differences in long non-coding RNA and protein-coding gene expression. BMC Genomics 15: 620, 2014. doi: 10.1186/1471-2164-15-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchholz M, Kestler HA, Holzmann K, Ellenrieder V, Schneiderhan W, Siech M, Adler G, Bachem MG, Gress TM. Transcriptome analysis of human hepatic and pancreatic stellate cells: organ-specific variations of a common transcriptional phenotype. J Mol Med (Berl) 83: 795–805, 2005. doi: 10.1007/s00109-005-0680-2. [DOI] [PubMed] [Google Scholar]
- 7.Burns JC, Kelly MC, Hoa M, Morell RJ, Kelley MW. Single-cell RNA-Seq resolves cellular complexity in sensory organs from the neonatal inner ear. Nat Commun 6: 8557, 2015. doi: 10.1038/ncomms9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danielsson A, Pontén F, Fagerberg L, Hallström BM, Schwenk JM, Uhlén M, Korsgren O, Lindskog C. The human pancreas proteome defined by transcriptomics and antibody-based profiling. PLoS One 9: e115421, 2014. doi: 10.1371/journal.pone.0115421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Vas MG, Kopp JL, Heliot C, Sander M, Cereghini S, Haumaitre C. Hnf1b controls pancreas morphogenesis and the generation of Ngn3+ endocrine progenitors. Development 142: 871–882, 2015. doi: 10.1242/dev.110759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desgraz R, Herrera PL. Pancreatic neurogenin 3-expressing cells are unipotent islet precursors. Development 136: 3567–3574, 2009. doi: 10.1242/dev.039214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eberwine J, Yeh H, Miyashiro K, Cao Y, Nair S, Finnell R, Zettel M, Coleman P. Analysis of gene expression in single live neurons. Proc Natl Acad Sci USA 89: 3010–3014, 1992. doi: 10.1073/pnas.89.7.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao ZY, Li G, Najafi H, Wolf BA, Matschinsky FM. Glucose regulation of glutaminolysis and its role in insulin secretion. Diabetes 48: 1535–1542, 1999. doi: 10.2337/diabetes.48.8.1535. [DOI] [PubMed] [Google Scholar]
- 13.Georgia S, Soliz R, Li M, Zhang P, Bhushan A. p57 and Hes1 coordinate cell cycle exit with self-renewal of pancreatic progenitors. Dev Biol 298: 22–31, 2006. doi: 10.1016/j.ydbio.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 14.Geutskens SB, Otonkoski T, Pulkkinen MA, Drexhage HA, Leenen PJ. Macrophages in the murine pancreas and their involvement in fetal endocrine development in vitro. J Leukoc Biol 78: 845–852, 2005. doi: 10.1189/jlb.1004624. [DOI] [PubMed] [Google Scholar]
- 15.Grant GR, Farkas MH, Pizarro AD, Lahens NF, Schug J, Brunk BP, Stoeckert CJ, Hogenesch JB, Pierce EA. Comparative analysis of RNA-Seq alignment algorithms and the RNA-Seq unified mapper (RUM). Bioinformatics 27: 2518–2528, 2011. doi: 10.1093/bioinformatics/btr427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hale MA, Swift GH, Hoang CQ, Deering TG, Masui T, Lee YK, Xue J, MacDonald RJ. The nuclear hormone receptor family member NR5A2 controls aspects of multipotent progenitor cell formation and acinar differentiation during pancreatic organogenesis. Development 141: 3123–3133, 2014. doi: 10.1242/dev.109405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamdani el H, Gudbrandsen M, Bjørkmo M, Chaudhry FA. The system N transporter SN2 doubles as a transmitter precursor furnisher and a potential regulator of NMDA receptors. Glia 60: 1671–1683, 2012. doi: 10.1002/glia.22386. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman BG, Zavaglia B, Witzsche J, Ruiz de Algara T, Beach M, Hoodless PA, Jones SJ, Marra MA, Helgason CD. Identification of transcripts with enriched expression in the developing and adult pancreas. Genome Biol 9: R99, 2008. doi: 10.1186/gb-2008-9-6-r99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iscove NN, Barbara M, Gu M, Gibson M, Modi C, Winegarden N. Representation is faithfully preserved in global cDNA amplified exponentially from sub-picogram quantities of mRNA. Nat Biotechnol 20: 940–943, 2002. doi: 10.1038/nbt729. [DOI] [PubMed] [Google Scholar]
- 20.Johansson KA, Dursun U, Jordan N, Gu G, Beermann F, Gradwohl G, Grapin-Botton A. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev Cell 12: 457–465, 2007. doi: 10.1016/j.devcel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Kato M, Kato K, Blaner WS, Chertow BS, Goodman DS. Plasma and cellular retinoid-binding proteins and transthyretin (prealbumin) are all localized in the islets of Langerhans in the rat. Proc Natl Acad Sci USA 82: 2488–2492, 1985. doi: 10.1073/pnas.82.8.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawson CL, Hanson RJ. Solving Least Squares Problems. Philadelphia: SIAM, 1995, p. xii. doi: 10.1137/1.9781611971217 [DOI] [Google Scholar]
- 23.Li C, Liu C, Nissim I, Chen J, Chen P, Doliba N, Zhang T, Nissim I, Daikhin Y, Stokes D, Yudkoff M, Bennett MJ, Stanley CA, Matschinsky FM, Naji A. Regulation of glucagon secretion in normal and diabetic human islets by γ-hydroxybutyrate and glycine. J Biol Chem 288: 3938–3951, 2013. doi: 10.1074/jbc.M112.385682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan FC, Wright C. Pancreas organogenesis: from bud to plexus to gland. Dev Dyn 240: 530–565, 2011. doi: 10.1002/dvdy.22584. [DOI] [PubMed] [Google Scholar]
- 25.Petri A, Ahnfelt-Rønne J, Frederiksen KS, Edwards DG, Madsen D, Serup P, Fleckner J, Heller RS. The effect of neurogenin3 deficiency on pancreatic gene expression in embryonic mice. J Mol Endocrinol 37: 301–316, 2006. doi: 10.1677/jme.1.02096. [DOI] [PubMed] [Google Scholar]
- 26.Rooman I, Lutz C, Pinho AV, Huggel K, Reding T, Lahoutte T, Verrey F, Graf R, Camargo SM. Amino acid transporters expression in acinar cells is changed during acute pancreatitis. Pancreatology 13: 475–485, 2013. doi: 10.1016/j.pan.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Saliba AE, Westermann AJ, Gorski SA, Vogel J. Single-cell RNA-seq: advances and future challenges. Nucleic Acids Res 42: 8845–8860, 2014. doi: 10.1093/nar/gku555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su Y, Jono H, Misumi Y, Senokuchi T, Guo J, Ueda M, Shinriki S, Tasaki M, Shono M, Obayashi K, Yamagata K, Araki E, Ando Y. Novel function of transthyretin in pancreatic alpha cells. FEBS Lett 586: 4215–4222, 2012. doi: 10.1016/j.febslet.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 29.Svensson P, Williams C, Lundeberg J, Rydén P, Bergqvist I, Edlund H. Gene array identification of Ipf1/Pdx1-/- regulated genes in pancreatic progenitor cells. BMC Dev Biol 7: 129, 2007. doi: 10.1186/1471-213X-7-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang F, Barbacioru C, Nordman E, Li B, Xu N, Bashkirov VI, Lao K, Surani MA. RNA-Seq analysis to capture the transcriptome landscape of a single cell. Nat Protoc 5: 516–535, 2010. doi: 10.1038/nprot.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tolhurst G, Zheng Y, Parker HE, Habib AM, Reimann F, Gribble FM. Glutamine triggers and potentiates glucagon-like peptide-1 secretion by raising cytosolic Ca2+ and cAMP. Endocrinology 152: 405–413, 2011. doi: 10.1210/en.2010-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trapnell C, Cacchiarelli D, Grimsby J, Pokharel P, Li S, Morse M, Lennon NJ, Livak KJ, Mikkelsen TS, Rinn JL. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat Biotechnol 32: 381–386, 2014. doi: 10.1038/nbt.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Upchurch BH, Aponte GW, Leiter AB. Expression of peptide YY in all four islet cell types in the developing mouse pancreas suggests a common peptide YY-producing progenitor. Development 120: 245–252, 1994. [DOI] [PubMed] [Google Scholar]
- 34.van der Maaten L, Hinton G. Visualizing data using t-SNE. J Mach Learn Res 9: 2579–2605, 2008. [Google Scholar]
- 35.Westphal CH, Muller L, Zhou A, Zhu X, Bonner-Weir S, Schambelan M, Steiner DF, Lindberg I, Leder P. The neuroendocrine protein 7B2 is required for peptide hormone processing in vivo and provides a novel mechanism for pituitary Cushing’s disease. Cell 96: 689–700, 1999. doi: 10.1016/S0092-8674(00)80579-6. [DOI] [PubMed] [Google Scholar]
- 36.White P, May CL, Lamounier RN, Brestelli JE, Kaestner KH. Defining pancreatic endocrine precursors and their descendants. Diabetes 57: 654–668, 2008. doi: 10.2337/db07-1362. [DOI] [PubMed] [Google Scholar]
- 37.Wu AR, Neff NF, Kalisky T, Dalerba P, Treutlein B, Rothenberg ME, Mburu FM, Mantalas GL, Sim S, Clarke MF, Quake SR. Quantitative assessment of single-cell RNA-sequencing methods. Nat Methods 11: 41–46, 2014. doi: 10.1038/nmeth.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xin Y, Kim J, Ni M, Wei Y, Okamoto H, Lee J, Adler C, Cavino K, Murphy AJ, Yancopoulos GD, Lin HC, Gromada J. Use of the Fluidigm C1 platform for RNA sequencing of single mouse pancreatic islet cells. Proc Natl Acad Sci USA 113: 3293–3298, 2016. doi: 10.1073/pnas.1602306113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zawalich WS, Yamazaki H, Zawalich KC, Cline G. Comparative effects of amino acids and glucose on insulin secretion from isolated rat or mouse islets. J Endocrinol 183: 309–319, 2004. doi: 10.1677/joe.1.05832. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H, Ackermann AM, Gusarova GA, Lowe D, Feng X, Kopsombut UG, Costa RH, Gannon M. The FoxM1 transcription factor is required to maintain pancreatic beta-cell mass. Mol Endocrinol 20: 1853–1866, 2006. doi: 10.1210/me.2006-0056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.