Abstract
OBJECTIVE:
Sepsis is still major cause of morbidity and mortality, despite improvements in diagnosis and treatment in modern medicine. Therefore, laboratory examinations that provide correct and rapid results are needed to support the diagnosis. This study was conducted to investigate value of immunological indicators procalcitonin (PCT) and soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) in differential diagnosis of patients with sepsis and systemic inflammatory response syndrome (SIRS), as well as to assess their importance in determining prognosis of patients with sepsis.
METHODS:
Total of 90 patients, 38 with SIRS and 52 with sepsis, who were between the ages 20 to 92, were included in this prospectively planned study. Blood sample was collected from the patients during hospitalization and again in follow-up visit. Enzyme-linked immunosorbent assay (MyBioSource, Inc., San Diego, CA, USA) was used to measure sTREM-1, and PCT was measured using mini VIDAS B.R.A.H.M.S PCT assay (Biomerieux, S.A., Marcy-l’Étoile, France). In addition, patients were clinically assessed using Acute Physiology and Chronic Health Evaluation (APACHE) II scoring system.
RESULTS:
On day of intensive care unit admission, sTREM-1 and PCT levels, as well as APACHE II score were significantly higher in sepsis group than SIRS group (p=0.001, p=0.01, p=0.001, respectively). Values of sTREM-1 and APACHE II score were higher in the patients with positive blood cultures than those with negative culture results (p=0.002, p=0.006, respectively). PCT, C-reactive protein, and sTREM-1 levels were significantly higher in nonsurviving group. In differentiation of SIRS from sepsis, sTREM-1 cut-off value ≥133 pg/mL and PCT cut-off value of 1.57 ng/mL yielded sensitivity of 71.1% and 67.33%, and specificity of 73.3% and 65.79%, respectively.
CONCLUSION:
In patients with suspected sepsis, sTREM-1 and PCT can be used as indicators, in addition to scoring systems such as APACHE II and Sepsis-related Organ Failure Assessment score. However, it would be appropriate to support present findings with studies of larger series.
Keywords: APACHE II, procalcitonin, sepsis, SIRS, sTREM-1
Sepsis is a common cause of morbidity and mortality in critically ill patients [1, 2]. Lack of specific clinical signs and symptoms for sepsis frequently leads to delay in treatment. However, accurate and timely diagnosis could allow for early treatment, reduce misuse of antibiotics and costs, limit morbidity, and improve patient outcomes. Microbiological cultures are still considered optimal for sepsis diagnosis, but this method has low sensitivity and specificity [3, 4]. In this context, it is imperative to identify ideal markers that would prove useful in differentiating sepsis from noninfectious diseases. Therefore, evaluation of sepsis biomarkers in different patient groups is still of great consequence.
C-reactive protein (CRP) has been widely used as an indicator of infection, but it is also elevated by other inflammatory conditions at early stages. Among recent potentially useful sepsis markers, procalcitonin (PCT) has been considered one of the most promising, and cumulative published reports support use of PCT measurement [3–5]. Serum triggering receptor expressed on myeloid cells-1 (TREM-1) is a recently discovered member of the immunoglobulin superfamily, expression of which on neutrophils and monocytes was upregulated by exposure to bacteria and fungi. sTREM-1 is soluble form of TREM-1 and is released from activated phagocytes into body fluids, such as plasma, pleural fluid, bronchoalveolar lavage fluid, urine, and cerebrospinal fluid. Many studies and several meta-analyses have indicated that sTREM-1 could be a valuable diagnostic biomarker for various infectious diseases [5–9].
In this study, sTREM-1, PCT, CRP serum levels and a scoring system, Acute Physiology and Chronic Health Evaluation (APACHE) II, were assessed in terms of their value for sepsis diagnosis and prediction of prognosis for hospitalized patients.
MATERIALS AND METHODS
This prospective observational study was performed in the adult medical-surgical intensive care units (ICUs) of a Training and Research Hospital, which has 725 beds, between May 2013 and January 2014. Approval of the hospital ethics committee and signed informed consent forms were obtained from patients or their families before inclusion in the study.
Patients
Consecutive hospitalized patients who had 2 or more of the following signs during their first 24 hours in the units were diagnosed as SIRS and were included in the study: temperature of >38°C or <36°C, pulse rate of >90 beats/min, respiratory rate of >20 breaths/min or hyperventilation with partial pressure of arterial carbon dioxide of <32 mmHg, or white blood cell (WBC) count of >12 000µL or <4000 µL, or >10% immature cells. Patients exhibiting 2 or more signs of SIRS with proven infection were diagnosed as sepsis. Exclusion criteria were: <18 years of age, acquired immunodeficiency syndrome, neutropenia (polymorphonuclear granulocyte count <500 µL), or died within 24 hours after admission to the hospital, elected not to participate in the study, or declined treatment during the observation period.
All medical records of the patients were retrospectively evaluated and the patients were classified as sepsis or SIRS at the time of submission by 2 clinicians blinded to biomarker results.
Data collection
Upon admission to the units, the following data were recorded for each patient: age, gender, main complaints for admission, symptoms, body temperature, leukocyte count, APACHE II score, etiological factors, and underlying diseases. Survival or death in hospital was evaluated during 28-day follow-up period.
When the patients were admitted, first blood sample was drawn within the first 24 hours and second was taken during treatment when patient had fever or mental state disorder (consistent with sepsis criteria). Blood samples were centrifuged at 3000 rpm for 15 minutes and supernatant was stored at -80ºC.
Assays
BacT/ALERT 3D automated microbial detection system (Biomerieux, S.A., Marcy-l’Étoile, France) was used for blood cultures and pathogens were identified conventionally or using automated (VITEK 2 Compact; Biomerieux, S.A., Marcy-l’Étoile, France) system. WBC was determined using CELL-DYN 3700 hematology analyzer (Abbott Laboratories, Abbott Park, IL, USA). CRP was determined using nephelometric assay (Beckman Coulter, Inc., Brea, CA, USA and Siemens Healthcare Diagnostics Products GmbH, Marburg, Germany). PCT was measured using mini VIDAS B.R.A.H.M.S PCT assay (Biomerieux, S.A., Marcy-l’ Étoile, France), sTREM-1 level was determined in duplicate using an enzyme-linked immunosorbent assay (MyBioSource, Inc., San Diego, CA, USA). All procedures were performed in strict accordance with manufacturers’ instructions and standard microbiology guidelines.
Statistical analysis
Descriptive results of continuous variables with normal distribution are presented as mean± SD or median (interquartile range). Mann-Whitney U test was used for comparison of variables and to evaluate differences between groups. Wilcoxon signed-rank test was used to analyze relationship between second and first measurements, and Yates continuity correction was applied to compare qualitative data. Receiver operating characteristic (ROC) curve was employed to evaluate effects of sTREM-1, PCT, CRP levels, and APACHE II scores on sepsis diagnosis. NCSS 2007 and PASS 2008 (NCSS, LLC, Kaysville, UT, USA) statistical software programs were used for statistical analysis. Two-tailed p<0.05 was considered significant.
RESULTS
A total of 90 consecutive patients admitted to ICUs of the hospital were enrolled in the study. Final diagnosis of 52 patients was established to be sepsis and 38 were diagnosed as SIRS. Forty-seven patients (52.2%) were male and 43 (47.8%) were female. Mean age of the patients was 64.27±15.54 years (range: 20–92 years). Comorbidities were frequent (92.2%). In terms of predisposing factors, only rate of cerebrovascular occlusion was statistically higher in sepsis group than in SIRS group (p=0.03). Blood cultures were positive in 38 (73.3%) patients with sepsis. Most frequently isolated microorganisms were methicillin-resistant coagulase-negative Staphylococcus (36%), Escherichia coli (13%), and Acinetobacter baumannii (13%), respectively. Main source of infection was the lungs (44.2%), followed by the blood (21%). Length of stay in ICU was 14±12 (SD) days. Twenty-two patients (24.4%) died. Mortality rate was much higher in sepsis group than in SIRS group (p=0.01) (Table 1).
Table 1.
Baseline characteristics of the patients
| SIRS n=38 | Sepsis n=52 | p | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age | 65.71±15.23 | 63.21±15.83 | NS | ||
| Gender | |||||
| Male | 18 | 47.4 | 29 | 55.8 | NS |
| Female | 20 | 52.6 | 23 | 44.2 | |
| Positive blood culture | 0 | 0 | 38 | 73.1* | <0.001 |
| APACHE II | 23.84±5.26 | 27.83±5.76 | 0.001 | ||
| Etiological factors | |||||
| Respiratory system infection | – | – | 21 | 40 | – |
| Bacteremia | – | – | 11 | 21 | – |
| Urinary tract infection | – | – | 5 | 9 | – |
| Unknown | – | – | 4 | 8 | – |
| Catheter-related bloodstream infection | – | – | 4 | 8 | – |
| Intraabdominal infection | – | – | 4 | 8 | – |
| Surgical site infection | – | – | 2 | 4 | – |
| Endocarditis | – | – | 1 | 2 | – |
| Predisposal factors | |||||
| CVO | 4 | 10 | 11 | 21 | 0.03 |
| Malignancy | 5 | 15 | 5 | 9 | NS |
| Respiratory disorder | 4 | 10 | 3 | 6 | NS |
| CKD | 4 | 10 | 3 | 6 | NS |
| Trauma | 1 | 3 | 2 | 4 | NS |
| COPD | 2 | 5 | 2 | 4 | NS |
| Neurological disorder | 2 | 5 | 2 | 4 | NS |
| CAD | 1 | 3 | 2 | 4 | NS |
| HT | 2 | 5 | 1 | 2 | NS |
| Length of hospital stay (days) | 25.05±14.19 | 32.7±21.37 | NS | ||
| Mortality | 5 | 13.2 | 17 | 32.7 | 0.01 |
APACHE: Acute Physiology and Chronic Health Evaluation; CAD: Chronic arterial disease; CKD: Chronic kidney disease; COPD: Chronic obstructive pulmonary disease; CVO: Chronic vascular occlusion; HT: Hypertension; SIRS: Systemic inflammatory response syndrome.
sTREM-1, PCT level, and APACHE II score were significantly elevated in sepsis group compared with SIRS group (p=0.001, p=0.01, p=0.001, respectively), on first day of ICU admission. There were no significant differences in median CRP value or WBC count (p=0.498, p=0.180, respectively). Both first and second measurements of sTREM-1 level were significantly higher in sepsis group than in SIRS group (p=0.001, p=0.001, p<0.01, respectively). First and second measurement of PCT level (p=0.01, p=0.01, p<0.05) and APACHE II score (p=0.001, p<0.01) were significantly higher in sepsis group. In terms of differentiation between sepsis and SIRS, there was no significant difference between first and second measurement of sTREM-1, PCT and CRP levels, or WBC count (Table 2).
Table 2.
PCT, sTREM-1, CRP, WBC levels and APACHE II score of patients with sepsis and SIRS
| Sepsis (n=52) | SIRS (n=38) | ap | ||
|---|---|---|---|---|
| PCT | ||||
| 1st measurement | Min-Max (Median) | 0.05–95.03 (3.86) | 0.05–71.95 (1.14) | 0.010* |
| Mean±SD | 11.33±19.31 | 5.86±13.57 | ||
| PCT | ||||
| 2nd measurement | Min-Max (Median) | 0.06–106.70 (2.62) | 0.05–13.34 (0.91) | 0.010* |
| Mean±SD | 11.53±18.96 | 2.48±3.38 | ||
| bp | 0.820 | 0.516 | 0.958 | |
| Difference; Min-Max (Median) | -66.02/105.45 (-0.23) | -61.15/12.21 (-0.05) | ||
| sTREM-1 | ||||
| 1st measurement | Min-Max (Median) | 67.49–2000 (164.66) | 18.33–371.62 (96.78) | 0.001** |
| Mean±SD | 389.82±524.44 | 110.86±57.66 | ||
| sTREM-1 | ||||
| 2nd measurement | Min-Max (Median) | 70.86–2000.00 (171.28) | 37.08–289.36 (99.85) | 0.001** |
| Mean±SD | 397.59±494.75 | 111.45±45.84 | ||
| bp | 0.426 | 0.602 | 0.947 | |
| Difference; Min-Max (Median) | -829.12/797.78 (2.85) | -82.26/49.58 (1.89) | ||
| CRP | ||||
| 1st measurement | Min-Max (Median) | 3.0–42.8 (13.7) | 4.3–32.5 (11.75) | 0.498 |
| Mean±SD | 14.55±7.00 | 13.91±7.18 | ||
| CRP | ||||
| 2nd measurement | Min-Max (Median) | 1.93–42.90 (11.0) | 1.98–30.00 (9.61) | 0.367 |
| Mean±SD | 13.24±8.14 | 11.63±7.21 | ||
| bp | 0.201 | 0.213 | 0.922 | |
| Difference; Min-Max (Median) | -19.80/33.85 (-3.22) | -24.85/16.39 (-1.52) | ||
| WBC | ||||
| 1st measurement | Min-Max (Median) | 6.0–46.7 (14.1) | 2.4–46.2 (13.05) | 0.180 |
| Mean±SD | 15.86±7.97 | 14.10±8.64 | ||
| WBC | ||||
| 2nd measurement | Min-Max (Median) | 2.4–55.9 (12.2) | 3.1–45.1 (10.9) | 0.170 |
| Mean±SD | 14.83±8.95 | 13.14±8.45 | ||
| bp | 0.473 | 0.293 | 0.927 | |
| Difference; Min-Max (Median) | -37.70/38.90 (-1.00) | -24.10/20.39 (-0.86) | ||
| APACHE II | Min-Max (Median) | 10–40 (28.5) | 10–32 (23.5) | 0.001** |
| Mean±SD | 27.83±5.76 | 23.84±5.26 |
APACHE: Acute Physiology and Chronic Health Evaluation; CRP: C-reactive protein; PCT: Procalcitonin; SIRS: Systemic inflammatory response syndrome; sTREM-1: Soluble triggering receptor expressed on myeloid cells-1; WBC: White blood cell;
Mann-Whitney U test;
Wilcoxon signed-rank test;
p<0.05;
p<0.01
sTREM-1 value and APACHE II score were higher in the patients with positive blood cultures than negative ones (177.9 pg/mL vs 113.96 pg/mL, p=0.002; 26 vs 29.5, p= 0.006). PCT, CRP, and WBC levels were found to be higher in the patients with positive blood cultures, but the difference between 2 groups was not statistically meaningful.
PCT and CRP levels were significantly higher (1.07 ng/mL vs 3.11 ng/mL, p=0.01; 9.15 mg/dL vs 17.80 mg/dL, p=0.02) in second measurement, and initial APACHE II score was statistically higher (26 vs 31; p=0.001), in nonsurviving patients. sTREM-1 level increased in second measurement of nonsurviving group (104.37 pg/mL vs 160.74 pg/mL; p <0.01). First and second sTREM-1 levels were higher in nonsurviving group (135.83 pg/mL vs 104.37 pg/mL; 140 pg/mL vs 160.74 pg/mL), but the difference was not statistically significant (p=0.840, p=0.512) between surviving and nonsurviving groups.
ROC was used to determine cut-off values of sTREM-1, PCT, and APACHE II. Area under the ROC curve (AUROC) was 0.78 (95% CI, 0.69–0.86) for sTREM-1, 0.65 (95% CI, 0.53–0.76) for PCT, and 0.71 (95% CI, 0.60-0.81) for APACHE II score. sTREM-1 cut-off value of ≥133 pg/mL yielded sensitivity of 71.15%, specificity of 76.32%, positive predictive value (PPV) of 80.43, and negative predictive value (NPV) of 65.91 for differentiating patients with SIRS from those with infection. When 1.57 ng/mL was set as cut-off value for PCT, sensitivity was 67.31%, specificity established was 65.79%, PPV was 72.92, and NPV was determined to be 59.52. APACHE II score of greater than 25 produced sensitivity of 80.77%, specificity of 52.63%, PPV of 70.00, and NPV of 66.67 (Figure 1).
Figure 1.
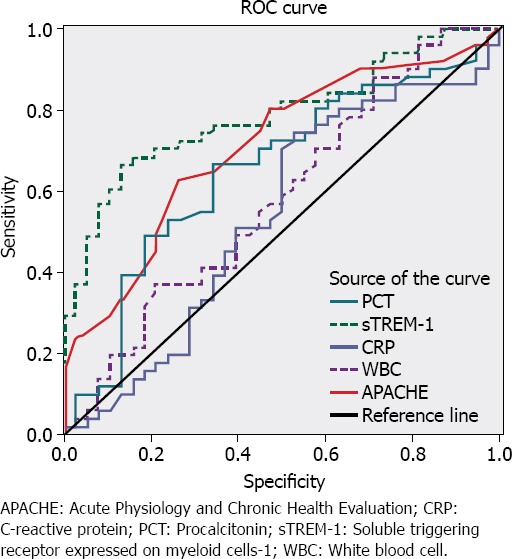
Receiver operating characteristic curve of PCT, sTREM-1, CRP, WBC, and APACHE II values.
DISCUSSION
Sepsis has high mortality rate, with estimated range of 10% to 52%. Early identification of sepsis and appropriate initial treatment has a major effect on clinical course and outcomes of patients [10–14]. Currently, physicians use a variety of tools, such as WBC, CRP, PCT, and severity scores, such as APACHE II, to help discriminate between infectious and noninfectious conditions, as well as to predict clinical outcomes. Many sepsis-related biomarkers have been identified for this purpose; however, it is still controversial which biomarker is best for early sepsis diagnosis in daily practice [15, 16].
Although CRP level has been widely proposed as a useful screening test for sepsis, it is doubtful that it is a good indicator for early diagnosis of sepsis [17]. PCT complies with most of the desirable preanalytical, analytical, and postanalytical features for an ideal laboratory biomarker. PCT level increases in bacterial infection, especially Gram-negative bacterial infections [18]. Compared to other biomarkers of sepsis, PCT has superior diagnostic accuracy and remains unaffected even in the presence of concomitant immunosuppressive therapy. PCT level was found to be the most valuable biomarker for diagnosis of sepsis and bacteremia for patients in the emergency department and ICU [19–21]. Endo et al. [22] reported that if they used serum PCT cutoff point of 2.0 ng/mL to discriminate between sepsis and severe sepsis, overall diagnostic efficiency was 84.3%. On the other hand, several studies have also reported less convincing data, making use of this biomarker controversial [23, 24].
Many studies have indicated that sTREM-1 could be valuable diagnostic biomarker for various infectious diseases [25–31]. Dynamic changes in sTREM-1 level could predict outcome of patients at early stage of sepsis. Rivera-Chavez et al. [26] evaluated 93 patients in a surgical ICU with SIRS symptoms and suspected infection. The patients were classified as having SIRS (n=37) or sepsis (n=56) according to decision of the treating physician and bacteriological evidence. The authors reported that the patients with sepsis had significantly higher sTREM-1 level than those with SIRS. sTREM-1 cut-off value of 230 pg/mL yielded sensitivity of 98% and specificity of 91% in differentiating patients with SIRS from those with infection. Su et al. [27] performed a study involving 144 patients in ICU (60 patients with SIRS and 84 patients with sepsis complicated by new onset of fever). They found that sepsis group had higher serum sTREM-1, PCT, and CRP levels compared with SIRS group (p<0.05) on first day of ICU admission. In study conducted by Li et al. [28], 52 consecutive patients hospitalized in surgical ICU with suspicion of infection included 14 patients with SIRS, 9 patients with sepsis, 14 patients with severe sepsis, and 15 patients with septic shock. They found that in postoperative patients, plasma level of sTREM-1 was higher in patients with sepsis than in patients with SIRS (111.7 pg/mL vs 64.1 pg/mL; p<0.05), with sensitivity, specificity, and predictive values higher than those of PCT and tumor necrosis factor alpha.
In meta-analysis of 13 clinical studies that fulfilled inclusion criteria (Total 980 patients, 557 patients with bacterial and 423 with nonbacterial infection), Jiyong et al. [9] reported that sTREM-1 level for diagnosis of infection in AUC of summary ROC was 0.86, with sensitivity of 0.82, and specificity of 0.86. These findings indicated that sTREM-1 is reliable biomarker for bacterial infection. Wu et al. [16] evaluated 11 studies with total of 1795 patients in a recent meta-analysis. AUC of summary ROC was 0.87, pooled sensitivity and specificity were 79% and 80%, respectively. The authors concluded that plasma sTREM-1 had moderate diagnostic performance in differentiating sepsis from SIRS. Moreover, some researchers have suggested that PCT and CRP are more sensitive biomarkers than sTREM-1 for diagnosis of bacterial infection [32, 33]. In the present study, we found that plasma sTREM-1 and PCT values, as well as APACHE II score, were significantly higher in patients with sepsis than in patients with SIRS. There was no significant difference between median CRP value and WBC count of the 2 groups. Additionally, AUROC was 0.78 for sTREM-1, 0.65 for PCT, and 0.71 for APACHE II score. sTREM-1 cut-off value of ≥133 pg/mL yielded higher sensitivity for differentiating patients with SIRS from those with infection.
Although many studies on predictive value of PCT in diagnosis of bacteremia exist, there are only a few studies about using sTREM-1for the same purpose [19–21, 27, 34]. Ruiz-Gonzales et al. [34] suggested that sTREM-1 is useful biomarker in diagnosis of secondary bacteremia due to community-acquired pneumonia. Su et al. [27] recently reported that there were no significant differences in serum sTREM-1 or PCT levels between blood culture-positive and negative groups with ICU-acquired new onset fever. However, we found that initial sTREM-1level and APACHE II score were significantly higher in patients with positive blood culture than negative ones (p=0.02, p=0.06, respectively).
Gibot et al. [25] evaluated alterations in serum sTREM-1, PCT, and CRP levels in sepsis, severe sepsis, and septic shock in a medical ICU and reported that sTREM-1 level was significantly lower in the survival group. Su et al. [27] found that within bacteremia group, sTREM-1 and PCT levels were significantly higher in nonsurvivors, and they reported that sTREM-1 and PCT levels were useful for predicting prognosis of bacteremia. In this study, we found that APACHE II score of the patients was high, and serum plasma levels of CRP and PCT were elevated in second measurement of nonsurviving group. Interestingly, although sTREM-1 level was higher in nonsurvivors than survivors, the difference between the 2 groups was not statistically meaningful.
Main limitations of this study must also be acknowledged. First, number of enrolled patients was relatively small. Second, severe bacterial sepsis patients with negative blood cultures were not investigated in this study.
In conclusion, present study demonstrated that measurement of sTREM-1 in plasma may be valuable tool for early distinction between sepsis and SIRS in adult patients in daily practice. Our data also suggest that plasma sTREM-1 at admission is the most useful biomarker for prediction of bacterial culture positivity. Initial high APACHE-II score and elevation of CRP and PCT levels are important parameters to predict outcome of ICU patients with sepsis.
Footnotes
Conflict of Interest: None declared.
Financial Disclosure: The authors declared that this study has received no financial support.
Authorship contributions: Concept – S.A., P.A.,; Design – S.A., P.A.; Supervision – S.A., P.A.; Materials – S.A.; Data collection &/or processing – S.A., P.A., A.Ö.; Analysis and/or interpretation – P.A.; Literature search – P.A.; Writing – P.A., A.İ., S.C.; Critical review – S.A.
REFERENCES
- 1.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Eng J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 2.Davis BH. Improved diagnostic approaches to infection/sepsis detection. Expert Rev Mol Diagn. 2005;5:193–207. doi: 10.1586/14737159.5.2.193. [DOI] [PubMed] [Google Scholar]
- 3.Di Somma S, Magrini L, Travaglino F, Lalle I, Fiotti N, Cervellin G, et al. Opinion paper on innovative approach of biomarkers for infectious dieseases and sepsis management in the emergency department. Clin Chem Lab Med. 2013;51:1167–75. doi: 10.1515/cclm-2012-0795. [DOI] [PubMed] [Google Scholar]
- 4.O’Grady NP, Barie PS, Bartlett JG, Bleck T, Carroll K, Kalil AC, et al. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med. 2008;36:1330–49. doi: 10.1097/CCM.0b013e318169eda9. [DOI] [PubMed] [Google Scholar]
- 5.Harbarth S, Holeckova K, Froidevaux C, Pittet D, Ricou B, Grau GE, et al. Diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. Am J Respir Crit Care Med. 2001;164:396–402. doi: 10.1164/ajrccm.164.3.2009052. [DOI] [PubMed] [Google Scholar]
- 6.Muller B, Becker KL, Schachinger H, Rickenbacher PR, Huber PR, Zimmerli W, et al. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit Care Med. 2000;28:977–83. doi: 10.1097/00003246-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Uzzan B, Cohen R, Nicolas P, Cucherat M, Perret GY. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: a systematic review and meta-analysis. Crit Care Med. 2006;34:1996–2003. doi: 10.1097/01.CCM.0000226413.54364.36. [DOI] [PubMed] [Google Scholar]
- 8.Jiyong J, Tiancha H, Wei C, Huahao S. Diagnostic value of the soluble triggering receptor expressed on myeloid cells-1 in bacterial infection: a meta-analysis. Intensive Care Med. 2009;35:587–95. doi: 10.1007/s00134-008-1333-z. [DOI] [PubMed] [Google Scholar]
- 9.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–50. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 10.Padkin A, Goldfrad C, Brady AR, Young D, Black N, Rowan K. Epidemiology of severe sepsis occurring in the first 24 hrs in intensive care units in England, Wales, and Northern Ireland. Crit Care Med. 2003;31:2332–8. doi: 10.1097/01.CCM.0000085141.75513.2B. [DOI] [PubMed] [Google Scholar]
- 11.Pavon A, Binquet C, Kara F, Martinet O, Ganster F, Navellou JC, et al. Profile of the risk of death after septic shock in the present era: an epidemiologic study. Crit Care Med. 2013;41:2600–9. doi: 10.1097/CCM.0b013e31829a6e89. [DOI] [PubMed] [Google Scholar]
- 12.Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–93. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu V, Escobar GJ, Greene JD, Soule J, Whippy A, Angus DC, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312:90–2. doi: 10.1001/jama.2014.5804. [DOI] [PubMed] [Google Scholar]
- 14.Jiyong J, Tiancha H, Wei C, Huahao S. Diagnostic value of the soluble triggering receptor expressed on myeloid cells-1 in bacterial infection: a meta-analysis. Intensive Care Med. 2009;35:587–95. doi: 10.1007/s00134-008-1333-z. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y, Wang F, Fan X, Bao R, Bo L, Li J, et al. Accuracy of plasma sTREM-1 for sepsis diagnosis in systemic inflammatory patients: a systemic review and meta-analysis. Critical care. 2012;29:16–229. doi: 10.1186/cc11884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee CC, Hong MY, Lee NY, Chen PL, Chang CM, Ko WC. Pitfalls in using serum C-reactive protein to predict bacteremia in febrile adults in the ED. Am J Emerg Med. 2012;30:562–9. doi: 10.1016/j.ajem.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Clech’h C, Ferriere F, Karoubi P, Fosse JP, Cupa M, Hoang P, et al. Diagnostic and prognostic value of procalcitonin in patients with septic shock. Crit Care Med. 2004;32:1166–9. doi: 10.1097/01.ccm.0000126263.00551.06. [DOI] [PubMed] [Google Scholar]
- 18.Kim MH, Lim G, Kang SY, Lee WI, Suh JT, Lee HJ. Utility of procalcitonin as an early diagnostic marker of bacteremia in patients with acute fever. Yonsei Med J. 2011;52:276–81. doi: 10.3349/ymj.2011.52.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai CC, Tan CK, Chen SY, Wang CY, Liu WL, Hou CC, et al. Diagnostic performance of procalcitonin for bacteremia in patients with bacterial infection at the emergency department. J Infect. 2010;61:512–5. doi: 10.1016/j.jinf.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Jain S, Sinha S, Sharma KS, Samantaray JC, Aggrawal P, Vikram NK, et al. Procalcitonin as a prognostic marker of sepsis: a prospective observational study. BMC Reseach Notes. 2014;7:458. doi: 10.1186/1756-0500-7-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Endo S, Aikawa N, Fujishima S, Sekine I, Kogawa K, Yamamoto Y, et al. Usefulness of procalcitonin serum level for the discrimination of severe sepsis from sepsis: a multicenter prospective study. J Infect Chemother. 2008;14:244–9. doi: 10.1007/s10156-008-0608-1. [DOI] [PubMed] [Google Scholar]
- 22.Blijlevens NM, Donnelly JP, Meis JF, De Keizer MH, DePauw BE. Procalcitonin does not discriminate infection from inflammation after allogeneic bone marrow transplantation. Clin Diagn Lab Immunol. 2000;7:889–92. doi: 10.1128/cdli.7.6.889-892.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang BM, Eslick GD, Graig JC, McLean AS. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. Lancet Infect Dis. 2007;7:210–7. doi: 10.1016/S1473-3099(07)70052-X. [DOI] [PubMed] [Google Scholar]
- 24.Gibot S, Cravoisy A, Kolopp-Sarda MN, Bene MC, Faure G, Bollaert PE, et al. Time course of sTREM (soluble triggering receptor expressed on myeloid cells)-1, procalcitonin, and C-reactive protein plasma concentrations during sepsis. Crit Care Med. 2005;33:792–6. doi: 10.1097/01.ccm.0000159089.16462.4a. [DOI] [PubMed] [Google Scholar]
- 25.Rivera-Chavez FA, Minei JP. Soluble triggering receptor expressed on myeloid cells-1 is an early marker of infection in the surgical intensive care unit. Surg Infect (Larchmt) 2009;10:435–9. doi: 10.1089/sur.2009.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su L, Han B, Liang L, Zhaoxu J, Deng J, Yan P. Value of soluble TREM-1, procalcitonin and C-reactive protein serum levels as biomarkers for detecting bacteremia among sepsis patients with new fever in intensive care units: a prospective cohort study. BMC Infect Dis. 2012;18:12–157. doi: 10.1186/1471-2334-12-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L, Zhu Z, Chen J, Ouyang B, Chen M, Guan X. Diagnostic value of soluble triggering receptor expressed on myeloid cells-1 in critically-ill, postoperative patients with suspected sepsis. Am J Med Sci. 2013;345:178–84. doi: 10.1097/MAJ.0b013e318253a1a6. [DOI] [PubMed] [Google Scholar]
- 28.Palmiere C, Bardy D, Mangin P, Augsburger M. Value of STREM-1, procalcitonin and CRP as laboratory parameters for postmortem diagnosis of sepsis. J Infect. 2013;67:545–55. doi: 10.1016/j.jinf.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 29.Gibot S, Bene MC, Noel R, Massin F, Guy J, Cravoisy A, et al. Combination biomarkers to diagnose sepsis in the critically ill patient. Am. J Respir Crit Care Med. 2012;186:65–71. doi: 10.1164/rccm.201201-0037OC. [DOI] [PubMed] [Google Scholar]
- 30.Jeong SJ, Song YG, Kim CO, Kim WH, Ku SN, Han HS, et al. Measurement of plasma sTREM-1 in patients with severe sepsis receiving early goal-directed therapy and evaluation of its usefulness. Shock. 2012;37:574–8. doi: 10.1097/SHK.0b013e318250da40. [DOI] [PubMed] [Google Scholar]
- 31.Kofoed K, Andersen O, Kronborg G, Tvede M, Petersen J, Eugen-Olsen J, et al. Use of plasma C-reactive protein, procalcitonin, neutrophils, macrophage migration inhibitory factor, soluble urokinase-type plasminogen activator receptor, and soluble triggering receptor expressed on myeloid cells-1 in combination to diagnose infections: a prospective study. Crit Care. 2007;11:38. doi: 10.1186/cc5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barati M, Bashar FR, Shahrami R, Zadeh MH, Taher MT, Nojomi M. Soluble triggering receptor expressed on myeloid cells 1 and the diagnosis of sepsis. J Crit Care. 2010;25:362–6. doi: 10.1016/j.jcrc.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Ruiz-Gonzalez A, Esquerda A, Falguera M, Abdulghani N, Cabezas P, Bielsa S, et al. Triggering receptor (TREM-1) expressed on myeloid cells predicts bacteremia better than clinical variables in community-acquired pneumonia. Respirology. 2011;16:321–5. doi: 10.1111/j.1440-1843.2010.01905.x. [DOI] [PubMed] [Google Scholar]


