Abstract
Objective:
The neutrophil/lymphocyte ratio (NLR) has been evaluated as a new predictor of cardiovascular risk. Inflammation has been shown to be associated with various arrhythmias including supraventricular tachycardias (SVTs). In this study, we aimed to investigate the relation between NLR and SVT in patients with a documented atrial tachyarrhythmia.
Methods:
The study used a retrospective cross-sectional design. Patients who had SVT but were otherwise healthy were included. The exclusion criteria included drug use (except antiarrhythmic agents), morbid obesity, acute or chronic infection, inflammatory diseases, systemic diseases, and cancer. Total and differential leukocyte counts and routine biochemical tests were performed before the ablation procedure.
Results:
The study included 150 patients with SVT and 98 healthy controls. The biochemical and hematological parameters were comparable between the groups, except neutrophil and lymphocyte counts. The neutrophil count was significantly higher (4.7±1.5×103/µL versus 4.1±1.0×103/µL; p<0.001) and lymphocyte count was significantly lower (2.2±0.6×103/µL versus 2.5±0.6×103/µL; p=0.001) in the SVT group than in the control group. As a result, the SVT group had significantly higher NLR values than the control group (2.2±0.9 versus 1.7±0.5; p<0.001). In addition, NLR values were higher in patients in whom tachycardia was induced during an electrophysiological study (EPS) (2.3±0.9 versus 2.0±0.8; p=0.02). The association between NLR and SVT remained significant after multivariate analysis (odds ratio: 1.5, 95% confidence interval: 1.001-2.263, p=0.049).
Conclusion:
Our study indicated that NLR values were significantly higher in patients with documented SVT than in control subjects. Inducibility of SVT during EPS was associated with higher NLR values.
Keywords: supraventricular tachycardia, inflammation, neutrophil/lymphocyte ratio
Introduction
The term supraventricular tachycardia (SVT) includes many tachycardias in which the atrial or atrioventricular (AV) nodal tissue is essential for sustaining arrhythmia. SVTs usually manifest themselves as recurrent palpitations, can seriously impair the quality of life, and often prompt visits to primary care doctors and acute medical units (1). An increase in the count of white blood cells (WBCs) and its subtypes is an important cardiovascular risk factor (2, 3). The neutrophil/lymphocyte ratio (NLR) has been evaluated as a new predictor of cardiovascular risk (2). Previous studies have shown that inflammation acts as a facilitator in the induction of SVT and a recent study has shown that increased inflammatory markers may have a role in predicting atrial tachycardia (AT) in selected patients (4). Data about the relationship between NLR and SVT is lacking. Therefore, in this study, we aimed to investigate the relation between NLR and SVT in patients with a documented atrial tachyarrhythmia.
Methods
The study used a retrospective cross-sectional design. All patients who underwent catheter ablation of SVT between June 2012 and July 2014 at our center were included in the study. Age- and sex-matched healthy volunteers (n=98) were recruited for the control group. The investigation complied with the principles outlined in the Declaration of Helsinki. The study was approved by the local ethics committee, and all participants provided written informed consent before participating. Patient information regarding indication, procedural details, and possible complications of the ablation procedure was provided during an outpatient visit at our institution or at the referring institution prior to the planned procedure. SVT included AV nodal reentry tachycardia (AVNRT), AV reentry tachycardia (AVRT), and AT. An initial diagnostic electrophysiological study (EPS) with standard diagnostic catheters was performed in patients with a prediagnosis of SVT. In patients with detected arrhythmia focus, radiofrequency (RF) ablation therapy was performed. All patients in whom the ablation procedure was performed were given intravenous 5000 IU heparin. We described procedural success as being unable to provoke a tachycardia after ablation, even after an atropine challenge. Patients who had SVT but were otherwise healthy were included. The exclusion criteria included drug use (except antiarrhythmic agents), morbid obesity (body mass index of ≥35 kg/m2), diabetes mellitus, metabolic syndrome, dyslipidemia, renal dysfunction (serum creatinine levels of >1.5 mg/dL, blood urea nitrogen levels of >30 mg/dL), heart failure, valvular diseases, asthma, chronic obstructive pulmonary disease, peripheral and cerebral vascular disease, hematological disorders, acute or chronic infection, cancer, inflammatory diseases, and hepatic dysfunction.
Biochemical measurements
Venous blood samples were drawn from the antecubital vein at initial presentation. Total and differential leukocyte counts and routine biochemical tests were performed before the ablation procedure.
Statistical analysis
Continuous variables are presented as mean±standard deviation. Categorical variables are expressed as number and percentage. The variables were compared using the chi-square (χ2) or Fisher’s exact test as appropriate. Procedural outcomes and complications were assessed according to one patient group treatment. Continuous variables were compared between the groups using the one-sample Student’s t-test or Mann–Whitney U test for non-normally distributed variables. Multivariate logistic regression analysis was performed to determine the independent predictors of SVT. Statistical analyses were performed using SPSS 15.0 (SPSS Inc., Chicago, Illinois, USA). A p value of <0.05 was considered significant. The study was conducted in accordance with the regulations of the institutional Ethics Committee.
Results
The study population consisted of 248 subjects (64% female; mean age: 40±14 years). The baseline demographic, biochemical, and hematological characteristics of the study population are shown in Table 1. There were no statistically significant differences between the groups with respect to age, sex, body mass index, smoking status, or hemoglobin levels. More than 93% of the patients with SVT had symptoms persisting for more than a year and more than 85% of those had at least 2 episodes per month. Overall, 81% of the patients with SVT were on at least 1 antiarrhythmic agent (beta blockers and calcium channel blockers commonly) and 5 patients were on propafenone. Angiography was performed in 16 SVT patients (mean age: 55±13 years) who had chest pain or were at a higher risk (age or family history) to exclude ischemia. These patients had normal coronary arteries (NCAs). With respect to SVTs, 90 (60%) patients had AVNRT, 18 (12%) patients had concealed AVRT, 37 (24.7%) patients had Wolff-Parkinson-White (WPW) syndrome, and 5 (3.3%) patients had AT. RF ablation treatment was given to 150 patients.
Table 1.
Baseline demographic, biochemical, and hematological
| SVT (n=150) | Control (n=98) | P | |
|---|---|---|---|
| Age, years | 39±15 | 41±11 | 0.26 |
| Femaleγn (%) | 101 (67) | 58 (59) | 0.28 |
| Smokingγn (%) | 8 (6) | 11 (11) | 0.18 |
| Body mass index, kg/m2 | 25.3±2.7 | 24.9±2.7 | 0.45 |
| Hemoglobin, g/L | 13.7±3.7 | 13.7±1.3 | 0.76 |
| Fasting glucose, mg/dL | 97±11 | 99±10 | 0.15 |
| *Creatinine, mg/dL | 0.71±0.12 | 0.73±0.11 | 0.14 |
| *Red-cell distribution width, % | 16.04±2.75 | 15.79±1.43 | 0.73 |
| Platelet count, /mm3 | 264±79 | 257±57 | 0.51 |
| Mean platelet volume, fL | 8.6±1.8 | 8.1±1.3 | 0.08 |
| White blood cell count, ×103/µL | 7.2±1.7 | 7.2±1.4 | 0.78 |
| Neutrophil count, ×103/µL | 4.7±1.5 | 4.1±1.0 | <0.001 |
| Lymphocyte count, ×103/µL | 2.2±0.6 | 2.5±0.6 | 0.001 |
| Neutrophil/lymphocyte ratio | 2.2±0.9 | 1.7±0.5 | <0.001 |
Mann-Whitney U test was performed.
Chi-square (χ2) was performed. One-sample Student’s t-test was performed for the others parameters. SVT – supraventricular tachycardia
The biochemical and hematological parameters were comparable between the groups, except neutrophil and lymphocyte counts. The neutrophil count was significantly higher (4.7±1.5×103/µL versus 4.1±1.0×103/µL; p<0.001) and the lymphocyte count was significantly lower (2.2±0.6×103/µL versus 2.5±0.6×103/µL; p=0.001) in the SVT group than in the control group. As a result, the SVT group had significantly higher NLR values compared with the control group (2.2±0.9 versus 1.7±0.5; p<0.001) (Fig. 1). The association between NLR and SVT remained significant after multivariate analysis (odds ratio: 1.5, 95% confidence interval: 1.001-2.263, p=0.049) (Table 2).
Figure 1.
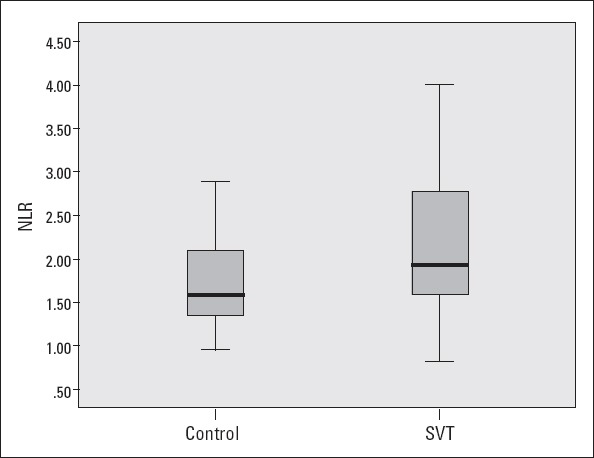
Comparison of the neutrophil/lymphocyte ratio in patients with supraventricular tachycardia and control subjects
NLR - neutrophil/lymphocyte ratio; SVT - supraventricular tachycardia One-sample Student’s t-test was performed.
Table 2.
Independent predictors of supraventricular tachycardia
| Odds ratio | 95% CI | P | |
|---|---|---|---|
| Neutrophil/lymphocyte ratio | 1.505 | 1.001-2.263 | 0.04 |
| Female sex | 1.649 | 0.921-2.955 | 0.09 |
| Age | 0.985 | 0.965-1.006 | 0.16 |
| Mean platelet volume | 1.177 | 0.972-1.426 | 0.09 |
Multivariate logistic regression analysis was performed.
SVT patients were further categorized into 2 groups depending on whether or not tachycardia was induced during EPS (Table 3). Patients in whom tachycardia was induced during EPS showed higher NLR values than those in whom tachycardia was not induced (2.3±0.9 versus 2.0±0.8; p=0.02). However, C-reactive protein (CRP) levels were comparable between the groups.
Table 3.
Comparison between subgroups of patients with supraventricular tachycardia in terms of inducibility
| Induced (n=112) | Non-induced (n=38) | P | |
|---|---|---|---|
| Age, years | 40±15 | 35±15 | 0.06 |
| Femaleγn (%) | 73 (65) | 28 (74) | 0.22 |
| Smoking€, n (%) | 7 (7) | 1 (3) | 0.68 |
| Body mass index, kg/m2 | 25.5±2.7 | 24.9±2.8 | 0.33 |
| Hemoglobin, g/L | 13.6±3.4 | 13.8±4.5 | 0.74 |
| Fasting glucose, mg/dL | 98 ±11 | 97±10 | 0.73 |
| Creatinine*, mg/dL | 0.71±0.11 | 0.71±0.13 | 0.61 |
| C-reactive protein§, mg/L | 0.46±0.31 | 0.47±0.33 | 0.98 |
| Red-cell distribution width*% | 15.8±1.8 | 16.7±4.3 | 0.47 |
| Platelet count, /mm3 | 260±75 | 274±89 | 0.39 |
| Mean platelet volume, fL | 8.6±1.7 | 8.5±1.9 | 0.71 |
| White blood cell count,×103/µL | 7.3±1.7 | 6.9±1.9 | 0.24 |
| Neutrophil count,×103/µL | 4.8±1.4 | 4.4±1.7 | 0.09 |
| Lymphocyte count,×103/µL | 2.2±0.6 | 2.3±0.5 | 0.40 |
| Neutrophil/lymphocyte ratio | 2.3±0.9 | 2.0±0.8 | 0.02 |
Fisher’s exact test was performed. One-sample Student’s t-test was performed for the others parameters.
Chi-square (χ2) was performed.
Mann-Whitney U test was performed.
C-reactive protein levels were available for 44 patients
The receiver-operating characteristic (ROC) curve explored the relationship between NLR and inducibility of SVT during EPS. Using a cut-off point of 1.74, NLR predicted the inducibility of SVT with a sensitivity of 68% and specificity of 61% (area under the ROC curve: 0.651, 95% CI: 0.542-0.760, p=0.005; Fig. 2).
Figure 2.
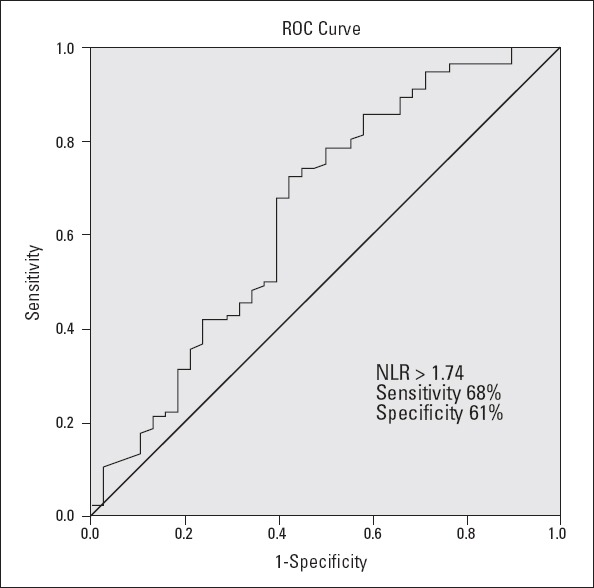
Receiver-operating characteristic curve of the neutrophil/lymphocyte ratio for inducible supraventricular tachycardia
Discussion
In the present study, higher values of NLR, a reliable marker of inflammation, were found to be statistically associated with the presence of SVT. In addition, we detected higher NLR values in patients in whom tachycardia was induced during EPS. To the best of our knowledge, this is one of the first studies in the literature to evaluate the relationship between NLR and SVT.
The term SVT is generally used to refer to AVNRT, AVRT, and AT. People of all ages, sex, and ethnicity can develop SVT. The prevalence of the different SVT mechanisms varies. Based on a study of 1754 patients undergoing catheter ablation of 1856 SVTs [excluding atrial fibrillation (AF), atrial flutter, and inappropriate sinus tachycardia] between 1991 and 2003, Porter et al. (5) found AVNRT as the predominant SVT mechanism (56%), followed by AVRT (27%), and AT (17%).
SVTs are the most common disturbance seen in the cardiology department. In general, improvement in the quality of life is a major therapeutic goal in case of SVTs, and treatment strategies are selected according to symptoms and patient preference. Patients troubled by recurrent symptomatic episodes are offered treatment, with options including drug therapy or catheter ablation. Patients can learn Valsalva maneuvers and some find this helpful in controlling their symptoms. There are no large-scale randomized studies comparing these treatments. However, data from prospective non-randomized studies suggest that catheter ablation results in a greater reduction in symptoms and higher quality of life scores compared with medical treatment (6-8).
Leukocyte subtype counts and NLR are also indicators of systemic inflammation (9, 10). Recent studies have demonstrated the predictive and prognostic significance of NLR in a wide range of cardiovascular diseases (11-15).
Inflammation has been shown to have an important role in SVTs in previous studies (16, 17). They reported that increased inflammatory markers, such as CRP, may have a predictive value for the presence of AT in some patients. According to their results, patients with lone atrial arrhythmias had higher CRP values than control subjects. In addition, they proposed that increased CRP levels may be associated with AF burden (18). In another study, histological evaluation of the atria of patients with lone AF revealed infiltration of inflammatory cells (19). Furthermore, some studies have reported diminished inflammation after the treatment of atrial arrhythmias (16, 20). Although a causal relationship could not be identified in these studies, we can deduce that atrial tachyarrhythmias have are associated with the inflammatory status of the body. On the other hand, premature ventricular and atrial contractions usually cause the initiation of SVTs. There has been a close relationship between presence of premature ventricular contraction, NLR, and other myocardial inflammatory conditions (14, 21, 22). As premature contractions are the most common triggering factor for SVTs, the inflammatory status causing premature contractions may have a role in the initiation of SVTs. Consistent with these data, we speculated that an increased inflammatory status of the body, as measured by NLR in this study, may have a role in the inducibility of SVT during EPS.
In addition, in a previous study, the levels of circulating proinflammatory cytokines (interleukin-6 and tumor necrosis factor-α) were found to be higher in the serum of young patients with ventricular arrhythmias and without structural heart disease than in healthy participants (23). AVNRT and AVRT constitute the vast majority of SVTs. It has been reported that dual AV nodal physiology can be demonstrated in up to 10% of patients without a history of AVNRT (24-26). Although some patients have dual nodal physiology or an accessory pathway, they do not develop tachycardia. Inflammation may have a role in the initiation of SVT in patients having dual AV nodal physiology or an accessory pathway.
In some patients, AVNRT coexists with AF (27). Recent reports have suggested that it may serve as a trigger for AF and its elimination may help to treat AF (reduce AF burden) (27, 28). In particular, this condition may be related to the inflammatory status.
Study limitations
The major limitation of our study was its retrospective design. In addition, the study was single centered and included a relatively small number of patients. Our study provided no data regarding causality between NLR and SVT. Lack of CRP measurements in all patients and postprocedural assessment of inflammatory markers is another limitation. Furthermore, we did not perform coronary angiography in all patients. Despite these limitations, to our knowledge, this is one of the first studies to evaluate the relationship between NLR and SVT.
Conclusion
We found significantly higher NLR values in patients with documented SVT compared with control subjects. In addition, we detected higher NLR values in patients in whom tachycardia was induced during EPS. Further large-scale, prospective, and multicenter studies are needed to elucidate and confirm the association between NLR and SVT.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship Contributions: Concept - M.A., A.Y., M.Y., N.P., A.A., Y.İ.; Design - M.A., A.Y., M.Y., N.P., A.A., Y.İ.; Supervision - M.A., A.Y., M.Y., N.P., A.A., Y.İ.; Resource - M.A., A.Y.; Materials - Y.İ., M.A.; Data collection &/or processing - M.A., A.A.; Analysis &/or interpretation - M.Y., N.P.; Literature search - M.A., N.P.; Writing - M.A., A.Y.; Critical review - M.Y., Y.İ.
References
- 1.Walfridsson U, Strömberg A, Janzon M, Walfridsson H. Wolff-Parkinson-White syndrome and atrioventricular nodal re-entry tachycardia in a Swedish population:consequences on health-related quality of life. Pacing Clin Electrophysiol. 2009;32:1299–306. doi: 10.1111/j.1540-8159.2009.02476.x. [DOI] [PubMed] [Google Scholar]
- 2.Tamhane UU, Aneja S, Montgomery D, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102:653–7. doi: 10.1016/j.amjcard.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Uthamalingam S, Patvardhan EA, Subramanian S, Ahmed W, Martin W, Daley M, et al. Utility of the neutrophil to lymphocyte ratio in predicting long-term outcomes in acute decompensated heart failure. Am J Cardiol. 2011;107:433–8. doi: 10.1016/j.amjcard.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 4.Zellweger MJ, Schaer BA, Cron TA, Pfisterer ME, Osswald S. Elevated troponin levels in absence of coronary artery disease after supraventricular tachycardia. Swiss Med Wkly. 2003;133:439–41. doi: 10.4414/smw.2003.10288. [DOI] [PubMed] [Google Scholar]
- 5.Porter MJ, Morton JB, Denman R, Lin AC, Tierney S, Santucci PA, et al. Influence of age and gender on the mechanism of supraventricular tachycardia. Heart Rhythm. 2004;1:393–6. doi: 10.1016/j.hrthm.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Bathina MN, Mickelsen S, Brooks C, Jaramillo J, Hepton T, Kusumoto FM. Radiofrequency catheter ablation versus medical therapy for initial treatment of supraventricular tachycardia and its impact on quality of life and healthcare costs. Am J Cardiol. 1998;82:589–93. doi: 10.1016/s0002-9149(98)00416-0. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg AS, Bathina MN, Mickelsen S, Nawman R, West G, Kusumoto FM. Long-term outcomes on quality-of-life and health care costs in patients with supraventricular tachycardia (radiofrequency catheter ablation versus medical therapy) Am J Cardiol. 2002;89:1120–3. doi: 10.1016/s0002-9149(02)02285-3. [DOI] [PubMed] [Google Scholar]
- 8.Tanboğa IH, Kurt M, Işık T, Kaya A, Aksakal E, Ekinci M, et al. Catheter ablation treatment of atrioventricular nodal re-entrant tachycardia. Dicle Med J. 2012;39:166–73. doi: 10.5603/cj.2012.0049. [DOI] [PubMed] [Google Scholar]
- 9.Momiyama Y, Kawaguchi A, Kajiwara I, Ohmori R, Okada K, Saito I, et al. Prognostic value of plasma high-sensitivity C-reactive protein levels in Japanese patients with stable coronary artery disease:the Japan NCVC-Collaborative Inflammation Cohort (JNIC) Study. Atherosclerosis. 2009;207:272–6. doi: 10.1016/j.atherosclerosis.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Hartaigh B, Bosch JA, Thomas GN, Lord JM, Pilz S, Loerbroks A, et al. Which leukocyte subsets predict cardiovascular mortality? From the Ludwigshafen RIsk and Cardiovascular health (LURIC) study. Atherosclerosis. 2012;224:161–9. doi: 10.1016/j.atherosclerosis.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Şen N, Afsar B, Özcan F, Büyükkaya E, İşleyen A, Akçay AB, et al. The neutrophil to lymphocyte ratio was associated with impaired myocardial perfusion and long-term adverse outcome in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Atherosclerosis. 2013;228:203–10. doi: 10.1016/j.atherosclerosis.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 12.Kaya H, Ertaş F, İslamoğlu Y, Kaya Z, Atılgan ZA, Çil H, et al. Association between neutrophil to lymphocyte ratio and severity of coronary artery disease. Clin Appl Thromb Hemost. 2014;20:50–4. doi: 10.1177/1076029612452116. [DOI] [PubMed] [Google Scholar]
- 13.Yıldız A, Kaya H, Ertaş F, Oylumlu M, Bilik MZ, Yüksel M, et al. Association between neutrophil to lymphocyte ratio and pulmonary arterial hypertension. Turk Kardiyol Dern Ars. 2013;41:604–9. doi: 10.5543/tkda.2013.93385. [DOI] [PubMed] [Google Scholar]
- 14.Yıldız A, Oylumlu M, Yüksel M, Aydın M, Polat N, Acet H, et al. The Association between the neutrophil to-lymphocyte ratio and the presence of ventricular premature contractions in young adults. Clin Appl Thromb Hemost. 2013 Nov 6; doi: 10.1177/1076029613509478. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Polat N, Yüksel M, Bilik MZ, Aydın M, Acet H, Akıl MA, et al. Association of neutrophil-lymphocyte ratio with the presence and severity of rheumatic mitral valve stenosis. Clin Appl Thromb Hemost. 2014;20:793–8. doi: 10.1177/1076029613514131. [DOI] [PubMed] [Google Scholar]
- 16.Marcus GM, Smith LM, Glidden DV, Wilson E, McCabe JM, Whiteman D, et al. Markers of inflammation before and after curative ablation of atrial flutter. Heart Rhythm. 2008;5:215–21. doi: 10.1016/j.hrthm.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Psychari SN, Apostolou TS, Sinos L, Hamodraka E, Liakos G, Kremastinos DT. Relation of elevated C-reactive protein and interleukin-6 levels to left atrial size and duration of episodes in patients with atrial fibrillation. Am J Cardiol. 2005;95:764–7. doi: 10.1016/j.amjcard.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 18.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–4. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 19.Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, et al. C-reactive protein elevation in patients with atrial arrhythmias:inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–91. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 20.Kallergis EM, Manios EG, Kanoupakis EM, Mavrakis HE, Kolyvaki SG, Lyrarakis GM, et al. The role of the post-cardioversion time course of hs-CRP levels in clarifying the relationship between inflammation and persistence of atrial fibrillation. Heart. 2008;94:200–4. doi: 10.1136/hrt.2006.108688. [DOI] [PubMed] [Google Scholar]
- 21.Stulova MA, Konstantinova EV. Ventricular extrasystole as manifestation of viral myocarditis and myopericarditis in young patients [in Russian] Ter Arkh. 2007;79:28–34. [PubMed] [Google Scholar]
- 22.Cui S, Chen XL, Jiang MX. Study on pathological rhythm of traditional Chinese medicine about circadian distribution of premature ventricular contractions in 240 patients with viral myocarditis [in Chinese] Zhong Xi Yi Jie He Xue Bao. 2005;3:355–8. doi: 10.3736/jcim20050505. [DOI] [PubMed] [Google Scholar]
- 23.Kowalewski M, Urban M, Mroczko B, Szmitkowski M. Proinflammatory cytokines (IL-6, TNF-alpha) and cardiac troponin I (cTnI) in serum of young people with ventricular arrhythmias[in Polish] Pol Arch Med Wewn. 2002;108:647–51. [PubMed] [Google Scholar]
- 24.Thapar MK, Gillette PC. Dual atrioventricular nodal pathways:a common electrophysiologic response in children. Circulation. 1979;60:1369–74. doi: 10.1161/01.cir.60.6.1369. [DOI] [PubMed] [Google Scholar]
- 25.Brooks R, Goldberger J, Kadish A. Extended protocol for demonstration of dual AV nodal physiology. Pacing Clin Electrophysiol. 1993;16:277–84. doi: 10.1111/j.1540-8159.1993.tb01577.x. [DOI] [PubMed] [Google Scholar]
- 26.Bissett JK, de Soynza N, Kane JJ, Murphy ML. Atrioventricular conduction patterns in patients with paroxysmal supraventricular tachycardia. Am Heart J. 1976;91:287–91. doi: 10.1016/s0002-8703(76)80210-4. [DOI] [PubMed] [Google Scholar]
- 27.Hurwitz JL, German LD, Packer DL, Wharton JM, McCarthy EA, Wilkinson WE, et al. Occurrence of atrial fibrillation in patients with paroxysmal supraventricular tachycardia due to atrioventricular nodal reentry. Pacing Clin Electrophysiol. 1990;13:705–10. doi: 10.1111/j.1540-8159.1990.tb02094.x. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Josephson ME. Atrioventricular nodal tachycardia occurring during atrial fibrillation. J Cardiovasc Electrophysiol. 2000;11:812–5. doi: 10.1111/j.1540-8167.2000.tb00053.x. [DOI] [PubMed] [Google Scholar]


