Abstract
Objective:
Mitral annular calcification (MAC) is degeneration of the fibrous annular ring of the mitral valve. Left atrial (LA) function and volume have been evaluated by many methods; however, none have used real-time three-dimensional echocardiography (RT3DE) in patients with MAC. Our study is the first to evaluate LA volume and mechanical function using RT3DE in patients with MAC.
Methods:
Our study was a prospective cross-sectional study. In total, 32 patients with echocardiographic evidence of MAC and 30 volunteers without MAC were enrolled in the study. Kolmogorov–Smirnov test, Student’s t-test, Mann-Whitney U test, chi-square test, Pearson’s correlation test, and multiple linear regression analyses were used in this study.
Results:
LA diameter was significantly higher in patients with MAC (38.5±3.8 vs. 31.1±2.9, p<0.001). Maximum LA volume (49.6±11.2 vs. 35.6±2.5, p<0.001), minimum LA volume (23.8±7.9 vs. 12.6±2.3, p<0.001), and LA volume index (LAVI) (26.9±6.1 vs. 20.5±2.4, p<0.001) were also higher in the MAC group. LAVI was correlated with age (p<0.001), blood urea nitrogen levels (p=0.089), total cholesterol levels (p=0.055), left ventricular systolic myocardial velocity (p=0.048), E/A ratio (p<0.001), and MAC (p<0.001). Multiple linear regression analyses revealed that age (β=0.390, p<0.001) and MAC (β=0.527, p<0.001) were independent predictors of LAVI.
Conclusion:
We found that LA mechanical function was impaired in patients with MAC. Furthermore, age and MAC were independent predictors of increased LAVI according to our RT3DE examination.
Keywords: mitral annular calcification, left atrial volume index, real-time three-dimensional echocardiography
Introduction
Mitral annular calcification (MAC), described for the first time by Bonninger et al. (1), is caused by degeneration of the fibrous annular ring supporting the mitral valve. In addition to atherosclerosis, cardiovascular disease risk factors, including advanced age, hypertension, hyperlipidemia, diabetes, and obesity contribute to the development of MAC (2). MAC is associated with heart failure, coronary artery disease, and endocarditis (3-5). Several pathologies such as left atrial (LA) dilatation, hypertrophic cardiomyopathy, mitral regurgitation, atrial fibrillation, and aortic valve calcification are also associated with MAC (6).
LA function and volume can be evaluated by many methods, including two-dimensional echocardiography (2-DE) and cardiac magnetic resonance imaging (MRI) and angiography (7-9). Real-time three-dimensional echocardiography (RT3DE) is a novel method that has been used to show volume changes in the left atrium during cardiac cycles (10-12). Correlated results can be achieved using RT3DE with MRI, which is the gold standard technique used to determine LA volumes (13, 14). However, more accurate volume measurements have been achieved with RT3DE compared with M-mode and 2-DE (15). Although some studies have evaluated LA function in patients with MAC using echocardiography, none have used RT3DE. Our study is the first to evaluate LA volume and mechanical function using RT3DE in patients with MAC.
Methods
Study design
This study was a prospective cross-sectional study performed at the İnönü University Faculty of Medicine, Turgut Özal Medical Center. The study protocol conformed to the principles of the Declaration of Helsinki and was approved by the Inönü University Faculty of Medicine Ethics Committee. Informed consent was obtained from all participants.
Study population
The study enrolled 32 consecutive patients with MAC who were admitted to our clinic for routine examination and 30 volunteers without MAC. Patients with a mitral valve area of <2.5 cm2 or a mean transvalvular gradient exceeding 2 mm Hg were excluded from this study. Patients whose ejection fraction was <50% or those diagnosed with coronary artery disease, hypertrophic cardiomyopathy, atrial fibrillation, primary or secondary hypertension, moderate or severe rheumatic valvular disease, valvular stenosis, moderate or severe valvular insufficiency as a result of secondary or primary pulmonary hypertension, chronic obstructive pulmonary disease, cor pulmonale, hepatic impairment, or malignancies were also excluded.
Study protocol
Age, gender, and other cardiac risk factors were recorded for all patients. Patients were asked if they had consumed coffee or tea or smoked in the last 30 min, and blood pressure was then measured after they had rested for 5 min. Hypertension was defined as an arterial blood pressure of >140/90 mm Hg on at least 3 separate weeks or the use of anti-hypertensive medications for 3 months. The patients’ heart rate, body mass index (BMI; kg/m2), and body surface area (BSA; m2) were calculated. Fasting blood glucose, creatinine, blood urea nitrogen (BUN), total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and triglyceride levels were measured.
Transthoracic echocardiographic assessment and tissue Doppler measurements were performed on all patients on the left side in the supine position using a Philips IE33 ultrasound with an S5-1 transducer (Philips Medical Systems, USA). Standard parasternal long-axis and apical 4-chamber, 2-chamber, and 5-chamber images were obtained. M-mode, 2-D, Doppler [color flow, PW Doppler (PWD)], and tissue Doppler echocardiography images and measurements were evaluated. MAC was defined as thickness of the intense echo-producing structure, >3 mm in width, located at the junction of the atrioventricular groove and posterior mitral valve leaflet on the parasternal long-axis, apical 4-chamber, or parasternal short-axis views in 2-DE (7).
The ejection fraction was calculated using Simpson’s biplane method (16). All measurements were performed in 3 consecutive cycles, and the mean value was recorded. All Doppler measurements were performed at the end of expiration to avoid exposure to respiratory flow parameters. Em- and Am-wave velocities were measured with the sample volume using PWD from the mitral lateral annulus. From the same tissue, the Doppler image isovolumetric relaxation and contraction times (IVRT and IVCT, respectively) and deceleration time (dT) were calculated.
After electrocardiographic monitoring, real-time volumetric data were recorded from apical 2-chamber and 4-chamber views using a X3-1 matrix array transducer with PureWave technology (iE33 ultrasound, Philips, Andover, MA, USA) at the end of expiration in patients who had held their breath for 4-5 consecutive cardiac beats. Two observers who were unaware of the clinical data evaluated the data using pre-recorded Philips Medical Systems software (QLAB-Philips version 7.1). As previously described (17), the largest end-diastolic volume and smallest LA end-systolic volume were determined. After determining the systolic and diastolic limits, 5 reference points were determined as the atrial anterior, inferior, lateral, septum, and LA apex. After determining the reference point in the program, QLAB automatically determined the LA endocardial border for each frame separately (Fig. 1). The resulting false demarcations were then corrected manually. LA appendage and pulmonary veins were not included in the limits. Maximum LA volume (Vmax; at the end of ventricular systole just before opening the mitral valve), minimum LA volume (Vmin; at the end of ventricular diastole just before closing the mitral valve), atrial volume before contraction (VpreA), LA stroke volume, and left atrium ejection fraction were also calculated using the software.
Figure 1.
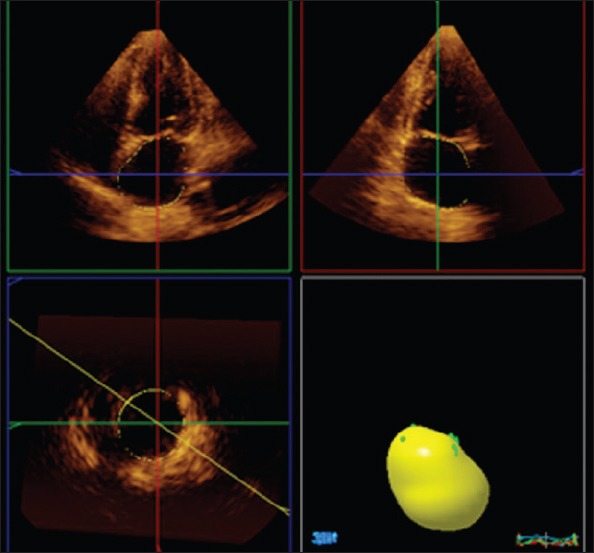
Illustration of the calculation of maximum left atrial volume using the software program
EDV - end-diastolic volume
After the above measurements were obtained, LA functions were calculated using the following formulas: LA total stroke volume (TSV)=Vmax-Vmin; LA total emptying fraction (TEF)=TSV/Vmax×100; LA active stroke volume (ASV)=VpreA-Vmin; LA active emptying fraction (AEF)=ASV/VpreA×100; LA passive emptying fraction (PEF)=(Vmax-VpreA)/Vmax×100; LA expansion index (EI)=TSV/Vmin×100; and maximum LA volume index (LAVI)=Vmax/BSA. The intra- and interobserver coefficients of variation were 3.9% for LAVI.
Statistical analysis
All statistical analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Continuous variables are presented as means±standard deviation, and categorical variables are shown as numbers and percentages. The normal distribution of the data was determined using the Kolmogorov-Smirnov test. To assess quantitative variables, Student’s t-test, Mann-Whitney U test, and chi-square test were used for independent groups and categorical variables as appropriate. Pearson’s correlation coefficient was used to evaluate the association between LAVI and continuous variables. Linear regression analyses using the stepwise method were used for multiple analyses of independent variables that were significantly different in univariate analyses (p<0.1). A p value of <0.05 was considered statistically significant.
Results
In total, 32 patients with MAC (17 males, 15 females; mean age: 58.1±3.9 years) and 30 healthy volunteers (19 males, 11 females; mean age 58.6±4.5 years) were included in this study. The demographic characteristics of the patient and control groups are shown in Table 1. There were no significant differences in age, gender, smoking status, BMI, fasting blood glucose levels, BUN levels, creatinine levels, total cholesterol levels, LDL levels, HDL levels, or triglycerides levels between the groups. However, weight (p=0.034) and BSA (p=0.030) were significantly different between patients and controls.
Table 1.
Demographic characteristics of the study population
| Parameters | MAC group (n=32) | Control group (n=30) | P* |
|---|---|---|---|
| Age, years | 58.1±3.9 | 58.6±4.5 | 0.243 |
| Gender, male (%) | 16 (53.3) | 19 (63.3) | 0.441 |
| Height, cm | 166.2±6.1 | 164.7±6.83 | 0.364 |
| Weight, kg | 73.0±8.2 | 68.0±9.4 | 0.034 |
| Body surface area, m2 | 1.84±0.12 | 1.77±0.14 | 0.030 |
| Body mass index, kg/m2 | 26.4±3.3 | 25.1±3.5 | 0.128 |
| Smoking, n (%) | 11 (36.7) | 4 (13.3) | 0.037 |
| Fasting glucose, mg/dL | 99.3±21.2 | 99.6±17.2 | 0.951 |
| Blood urea nitrogen, mg/dL | 13.7±3.7 | 12.6±3.1 | 0.236 |
| Creatinine, mg/dL | 0.75±0.15 | 0.71±0.1 | 0.237 |
| Total cholesterol, mg/dL | 200.8±31.4 | 182.3±53.1 | 0.113 |
| LDL cholesterol, mg/dL | 118.3±31.2 | 120.6±44.5 | 0.826 |
| HDL cholesterol, mg/dL | 41.7±6.7 | 42.7±12.4 | 0.705 |
| Triglyceride, mg/dL | 184.3±56.3 | 166.8±67.7 | 0.295 |
HDL - high-density lipoprotein; LDL - low-density lipoprotein; MAC - mitral annular calcification. Values are presented as mean±standard error.
Student’s t-test, Mann-Whitney U test, and chi-square test were used
Comparisons of the standard 2-D and Doppler echocardiographic measurements are shown in Table 2. There were no significant differences in left ventricular (LV) end-diastolic diameter, LV end-systolic diameter, interventricular septum thickness, posterior wall thickness, EF, and systolic pulmonary artery pressure between the groups. LA diameter was significantly higher in patients with MAC (p<0.001). Early-diastolic mitral inflow velocity (p<0.001), systolic myocardial velocity (p<0.001), and E/A ratio (p<0.001) were lower in patients with MAC, whereas late-diastolic mitral inflow velocity (p=0.003), IVRT (p=0.039), and E/Em ratio (p=0.017) were higher.
Table 2.
Comparison of echocardiographic data of the study population
| MAC group (n=32) | Control group (n=30) | P* | |
|---|---|---|---|
| LV ejection fraction, % | 65.5±4.1 | 65.3±4.1 | 0.828 |
| LVEDD, mm | 45.3±2.3 | 45.6±3.1 | 0.673 |
| LVESD, mm | 28.8±2.2 | 28.1±3.0 | 0.307 |
| Septal thickness, mm | 10.0±0.8 | 9.7±1.1 | 0.279 |
| PW thickness, mm | 9.8±0.7 | 9.7±1.4 | 0.909 |
| Left atrial diameter, mm | 38.5±3.8 | 31.1±2.9 | <0.001 |
| PAB, mm Hg | 30.4±3.8 | 29.9±5.2 | 0.650 |
| E, cm/s | 60.2±14.8 | 86.3±8.9 | <0.001 |
| A, cm/s | 72.4±16.6 | 62.2±7.5 | 0.003 |
| dT, ms | 262±21.7 | 173.8±9.6 | <0.001 |
| Em, cm/s | 7.7±2.9 | 12.4±1.5 | <0.001 |
| Am, cm/s | 8.9±2.1 | 8.4±1.0 | 0.296 |
| Sm, cm/s | 8.4±1.7 | 10.6±1.5 | <0.001 |
| IVRT, ms | 83.2±12.1 | 79.6±7.6 | 0.039 |
| IVCT, ms | 63.1±12.1 | 65.1±11.2 | 0.525 |
| E/A | 0.87±0.31 | 1.39±0.15 | <0.001 |
| E/Em | 8.4±2.9 | 7.0±1.0 | 0.017 |
A - mitral late-diastolic velocity; Am - LV myocardial late-diastolic velocity; dT – mitral E-wave deceleration time; E - mitral early-diastolic velocity; Em - LV myocardial earlydiastolic velocity; IVCT - isovolumetric contraction time; IVRT - isovolumetric relaxation time; LV - left ventricular; LVEDD - LV end-diastolic dimension; LVESD – LV end-systolic dimension; MAC - mitral annular calcification; PW - posterior wall; Sm - LV systolic myocardial velocity. Values are presented as mean±standard error.
Student’s t-test or Mann-Whitney U test was used
The following parameters were also significantly higher in patients with MAC: LA mechanical function, as assessed by RT3DE, Vmax (p<0.001), Vmin (p<0.001), VpreA (p<0.001), TSV (p<0.005), and LAVI (p<0.001). In contrast, ASV (p=0.040), AEF (p<0.001), TEF (p<0.001), PEF (p=0.012), and EI (p<0.001) were lower in MAC patients (Table 3).
Table 3.
Comparison of left atrial volume parameters
| MAC group (n=32) | Control group (n=30) | P* | |
|---|---|---|---|
| Maximum left atrial volume, mL | 49.6±11.2 | 35.6±2.5 | <0.001 |
| Minimum left atrial volume, mL | 23.8±7.9 | 12.6±2.3 | <0.001 |
| Atrial volume before contraction, mL | 29.8±8.7 | 19.6±1.9 | <0.001 |
| Total stroke volume, mL | 25.5±5.1 | 22.6±1.1 | 0.005 |
| Active stroke volume, mL | 5.9±1.0 | 6.9±2.4 | 0.040 |
| Total emptying fraction, % | 51.7±7.3 | 65.5±2.7 | <0.001 |
| Active emptying fraction, % | 20.8±3.9 | 35.3±11.3 | <0.001 |
| Passive emptying fraction, % | 40.3±6.9 | 44.7±6.1 | 0.012 |
| Expansion index | 115.5±34.1 | 184.8±32.2 | <0.001 |
| Left atrial volume index, mL/m2 | 26.9±6.1 | 20.5±2.4 | <0.001 |
MAC - mitral annular calcification. Values are presented as mean±standard error.
Student’s t-test or Mann-Whitney U test was used
The correlation of study parameters with LAVI is shown in Table 4. LAVI was positively correlated with age (p<0.001), BUN levels (p=0.089), total cholesterol levels (p=0.055), dT (p<0.001), IVRT (p=0.067), and MAC (p<0.001) and negatively correlated with E/A ratio (p<0.001) and Sm velocity (p=0.048). Multiple linear regression analyses revealed that age (β=0.390, p<0.001) and MAC (β=0.527, p<0.001) were independent predictors of LAVI.
Table 4.
Univariate and multivariate analysis to demonstrate independent predictors of left atrial volume index
| Dependent variable: Left atrial volume index | ||||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| Variables | r | P | β | P |
| Age | 0.450 | <0.001 | 0.390 | <0.001 |
| Sex | -0.214 | 0.100 | ||
| Body mass index | -0.138 | 0.295 | ||
| Body surface area | -0.118 | 0.370 | ||
| Smoking status | 0.074 | 0.573 | ||
| Glucose | 0.006 | 0.964 | ||
| Blood urea nitrogen | 0.223 | 0.089 | 0.131 | 0.256 |
| Creatinine | -0.172 | 0.210 | ||
| Total cholesterol | 0.258 | 0.055 | 0.145 | 0.213 |
| High-density lipoprotein | 0.169 | 0.217 | ||
| Triglyceride | -0.053 | 0.697 | ||
| Ejection fraction | -0.090 | 0.492 | ||
| Sm velocity | -0.256 | 0.048 | 0.141 | 0.301 |
| dT | 0.486 | <0.001 | -0.276 | 0.385 |
| IVRT | 0.238 | 0.067 | 0.076 | 0.525 |
| E/A ratio | -0.515 | <0.001 | -0.191 | 0.245 |
| E/Em ratio | 0.016 | 0.905 | ||
Discussion
The main result of this study is that LA mechanical function as assessed by RT3DE was impaired in patients with MAC. LAVI was also increased in this patient group. Age and MAC were independent predictors of LAVI.
MAC is a chronic, degenerative, and non-inflammatory process that occurs in the fibrous ring, and its incidence increases with age (18, 19). In a large-scale study by Movahed et al. (20), LV hypertrophy, mitral regurgitation, tricuspid insufficiency, LA dilatation, and reduced LV diastolic parameters were independently associated with MAC. Consistent with this, Ritschard et al. (21) analyzed 33 dialysis patients and reported that those with MAC had more frequent moderate dilatation of the left atrium. In our study, moderate and severe valvular insufficiencies were excluded, as regurgitation may greatly influence LA dimensions. Therefore, the result are believed to be due to MAC itself, because the mild valve insufficiency that can occur secondary to MAC cannot be distinguished from its pathophysiology. Because the left atrium has an asymmetric structure, the reliability of 2-DE for determining its size and diameter is controversial (22). On the other hand, RT3DE provides dynamic volumetric data and can thus be used to accurately evaluate cardiac function and LA volume (23). In all previous studies of MAC, LA diameters were measured using 2-DE; therefore, this study was the first to evaluate LA function using RT3DE.
The size of the left atrium is an important indicator of mortality and morbidity in cardiomyopathy, LV dysfunction, aortic stenosis, mitral regurgitation, and arrhythmias (24, 25). In addition, changes in LA volume after acute myocardial infarction have been identified as important prognostic markers (26). It is well known that impaired LV diastolic function causes the left atrium to increase its contribution to LV filling; therefore, LA volumes and fractions reflect the severity of LV diastolic dysfunction (26). Tsang et al. (27) demonstrated a significant association between LA and LV diastolic parameters. As diastolic dysfunction increases because of an increased pre-load, the mitral PWD measurements (E- and A-wave velocities) become normal; therefore, a pseudonormal pattern develops. Assessing changes in LA volume may offer considerable advantages in terms of identifying patients with a pseudonormal pattern (27, 28).
Zhong et al. (29) performed a study using RT3DE in healthy subjects to calculate LAVI, which increased with age but was gender independent. Similarly, we found that LAVI was positively correlated with age and that age was also an independent predictor of LAVI. The relationship between active and passive ejection of the left atrium as assessed by RT3DE was a sensitive indicator of the working principle of the left atrium and has been reported to reflect the severity of LV diastolic dysfunction (30). Diastolic dysfunction in individuals with MAC was evidenced by increased Vmax and Vmin. The reason for this increase in Vmax was attributed to the increase in the LV filling pressure. In addition, increased Vmin was reported to be due to atrial contraction defects (31). In our study, Vmax and Vmin were higher in the MAC than in the control group. Moreover, the E/Em ratio was higher, whereas the E/A ratio was lower in this patient group. Therefore, these parameters are believed to be indicators of LA and LV dysfunction in MAC patients. Contrary to expectations, MAC and age, but not Sm, E/A, or the E/Em ratio, were found to be independent predictors of LAVI in multiple regression analyses.
Study limitations
Our study had several limitations. First, this was a single-center, cross-sectional study that enrolled a relatively small number of patients. Second, LA strain, which was not measured, should have been evaluated to consolidate the understanding of LA physiology in the presence of MAC. Therefore, longer-term studies with larger populations are needed. Because the E/A ratio cannot give a clear result in the evaluation of diastolic function in patients with severe MAC, a more comprehensive study including MAC subgroups is necessary.
Conclusion
In conclusion, it was previously shown that 3D-STE allows more accurate measurement of LAVI and LA function than 2D-STE with high reproducibility. Although previous studies have shown increased LA size in MAC patients using 2-DE, LA mechanical function has not been assessed sufficiently. Here we found that LA mechanical function was impaired in patients with MAC. In addition, age and MAC were independent predictors of increased LAVI according to RT3DE examinations.
Acknowledgements:
The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/0ul3PS.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept - A.B., H.T., Y.Ö.O., Ş.H., F.K., A.D., H.P.; Design - A.B., H.T., Y.Ö.O., Ş.H., H.P.; Supervision - A.B., H.T., H.P.; Resource - Y.Ö.O., Ş.H., F.K., A.B., H.P.; Materials - A.B., H.T., Y.Ö.O., Ş.H., H.P.; Data collection &/or processing - A.B., H.T., Y.Ö.O., Ş.H., F.K., A.D., H.P.; Analysis &/or interpretation - A.B., H.T., Y.Ö.O., H.P.; Literature search - A.B., H.T., Y.Ö.O.; Writing - A.B., H.T., Y.Ö.O., Ş.H., R.K., A.D., H.O.; Critical review - A.B., H.T., Y.Ö.O., Ş.H., F.K., A.D., H.P.
References
- 1.Bonninger M. (a) Bluttransfusion bei pernizioser anamie:(b) Zwei Falle von Herzblock. Dtsch Med Wochenschr. 1908;34:2292. [Google Scholar]
- 2.Elmariah S, Budoff MJ, Delaney JA, Hamirani Y, Eng J, Fuster V, et al. Risk factors associated with the incidence and progression of mitral annulus calcification:the multi-ethnic study of atherosclerosis. Am Heart J. 2013;166:904–12. doi: 10.1016/j.ahj.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mellino M, Salcedo EE, Lever HM, Vasudevan G, Kramer JR. Echographic- quantified severity of mitral anulus calcification:prognostic correlation to related hemodynamic, valvular, rhythm and conduction abnormalities. Am Heart J. 1982;103:222–5. doi: 10.1016/0002-8703(82)90495-1. [DOI] [PubMed] [Google Scholar]
- 4.Adler Y, Fisman EZ, Shemesh J, Tanne D, Hovav B, Motro M, et al. Usefulness of helical computed tomography in detection of mitral annular calcification as a marker of coronary artery disease. Int J Cardiol. 2005;101:371–6. doi: 10.1016/j.ijcard.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 5.Burnside JW, Desanctis RW. Bacterial endocarditis on calcification of the mitral anulus fibrosus. Ann Intern Med. 1972;76:615–8. doi: 10.7326/0003-4819-76-4-615. [DOI] [PubMed] [Google Scholar]
- 6.Aronow WS, Koenigsberg M, Kronzon I, Gutstein H. Association of mitral annular calcium with new thromboembolic stroke and cardiac events at 39-month follow-up in elderly patients. Am J Cardiol. 1990;65:1511–2. doi: 10.1016/0002-9149(90)91364-c. [DOI] [PubMed] [Google Scholar]
- 7.Pekdemir H, Cansel M, Yağmur J, Açıkgöz N, Ermiş N, Kurtoğlu E, et al. Assessment of atrial conduction time by tissue Doppler echocardiography and P-wave dispersion in patients with mitral annulus calcification. J Electrocardiol. 2010;43:339–43. doi: 10.1016/j.jelectrocard.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Artang R, Migrino RQ, Harmann L, Bowers M, Woods TD. Left atrial volume measurement with automated border detection by 3-dimensional echocardiography:comparison with magnetic resonance imaging. Cardiovasc Ultrasound. 2009;7:16. doi: 10.1186/1476-7120-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kagawa K, Arakawa M, Miwa H, Noda T, Nishigaki K, Ito Y, et al. Left atrial function during left ventricular diastole evaluated by left atrial angiography and left ventriculography. J Cardiol. 1994;24:317–5. [PubMed] [Google Scholar]
- 10.Marsan NA, Tops LF, Nihoyannopoulos P, Holman ER, Bax JJ. Real-time three-dimensional echocardiography:current and future clinical applications. Heart. 2009;95:1881–90. doi: 10.1136/hrt.2008.151613. [DOI] [PubMed] [Google Scholar]
- 11.Hung J, Lang R, Flachskampf F, Shernan SK, McCulloch ML, Adams DB, et al. 3D echocardiography:a review of the current status and future directions. J Am Soc Echocardiogr. 2007;20:213–33. doi: 10.1016/j.echo.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Murata M, Iwanaga S, Tamura Y, Kondo M, Kouyama K, Murata M, et al. A real-time three-dimensional echocardiographic quantitative analysisof left atrial function in left ventricular diastolic dysfunction. Am J Cardiol. 2008;102:1097–102. doi: 10.1016/j.amjcard.2008.05.067. [DOI] [PubMed] [Google Scholar]
- 13.Keller AM, Gopal AS, King DL. Left and right atrial volume by freehand three-dimensional echocardiography:in vivo validation using magnetic resonance imaging. Eur J Echocardiogr. 2000;1:55–65. doi: 10.1053/euje.2000.0010. [DOI] [PubMed] [Google Scholar]
- 14.Rodevan O, Bjornerheim R, Ljosland M, Maehle J, Smith HJ, Ihlen H. Left atrial volumes assessed by three- and two-dimensional echocardiography compared to MRI estimates. Int J Card Imaging. 1999;15:397–410. doi: 10.1023/a:1006276513186. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins C, Bricknell K, Marwick TH. Use of real-time three-dimensional echocardiography to measure left atrial volume:comparison with other echocardiographic techniques. J Am Soc Echocardiogr. 2005;18:991–7. doi: 10.1016/j.echo.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 16.Grossgasteiger M, Hien MD, Graser B, Rauch H, Gondan M, Motsch J, et al. Assessment of left ventricular size and function during cardiac surgery. An intraoperative evaluation of six two-dimensional echocardiographic methods with real-time three-dimensional echocardiography as a reference. Echocardiography. 2013;30:672–81. doi: 10.1111/echo.12116. [DOI] [PubMed] [Google Scholar]
- 17.Nikitin NP, Witte KK, Thackray SD, Goodge LJ, Clark AL, Cleland JG. Effect of age and sex on left atrial morphology and function. Eur J Echocardiogr. 2003;4:36–42. doi: 10.1053/euje.2002.0611. [DOI] [PubMed] [Google Scholar]
- 18.Ramaraj R, Manrique C, Hashemzadeh M, Movahed MR. Mitral annulus calcification is independently associated with all-cause mortality. Exp Clin Cardiol. 2013;18:e5–7. [PMC free article] [PubMed] [Google Scholar]
- 19.Rossi A, Faggiano P, Amado AE, Cicoira M, Bonapace S, Franceschini L, et al. Mitral and aortic valve sclerosis/calcification and carotid atherosclerosis:results from 1065 patients. Heart Vessels. 2014;29:776–83. doi: 10.1007/s00380-013-0433-z. [DOI] [PubMed] [Google Scholar]
- 20.Movahed MR, Saito Y, Ahmadi-Kashani M, Ebrahimi R. Mitral annulus calcification is associated with valvular and cardiac structural abnormalities. Cardiovasc Ultrasound. 2007;5:14. doi: 10.1186/1476-7120-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ritschard T, Blumberg A, Jenzer HR. Mitral annular calcification in dialysis patients. Schweiz Med Wochenschr. 1987;117:1363–7. [PubMed] [Google Scholar]
- 22.Lester SJ, Ryan EW, Schiller NB, Foster E. Best method in clinical practice and in research studies to determine left atrial size. Am J Cardiol. 1999;84:829–32. doi: 10.1016/s0002-9149(99)00446-4. [DOI] [PubMed] [Google Scholar]
- 23.Gopal AS, Keller AM, Rigling R, King DL, Jr, King DL. Left ventricular volume and endocardial surface area by three-dimensional echocardiography:comparison with two-dimensional echocardiography and nuclear magnetic resonance imaging in normal subjects. J Am Coll Cardiol. 1993;22:258–70. doi: 10.1016/0735-1097(93)90842-o. [DOI] [PubMed] [Google Scholar]
- 24.Rossi A, Cicoira M, Zanolla L, Sandrini R, Golia G, Zardini P, et al. Determinants and prognostic value of left atrial volume in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2002;40:1425. doi: 10.1016/s0735-1097(02)02305-7. [DOI] [PubMed] [Google Scholar]
- 25.Rossi A, Tomaino M, Golia G, Santini F, Pentiricci S, Marino P, et al. Usefulness of left atrial size in predicting postoperative symptomatic improvement in patients with aortic stenosis. Am J Cardiol. 2000;86:567–70. doi: 10.1016/s0002-9149(00)01019-5. [DOI] [PubMed] [Google Scholar]
- 26.Moller JE, Hillis GS, Oh JK, Seward JB, Reeder GS, Wright RS, et al. Left atrial volume:a powerful predictor of survival after acute myocardial infarction. Circulation. 2003;107:2207–2. doi: 10.1161/01.CIR.0000066318.21784.43. [DOI] [PubMed] [Google Scholar]
- 27.Tsang TS, Barnes ME, Gersh BJ, Takemoto Y, Rosales AG, Bailey KR, et al. Prediction of risk for first age-related cardiovascular events in an elderly population:the incremental value of echocardiography. J Am Coll Cardiol. 2003;42:1199–5. doi: 10.1016/s0735-1097(03)00943-4. [DOI] [PubMed] [Google Scholar]
- 28.Reed D, Abbott RD, Smucker ML, Kaul S. Prediction of outcome after mitral valve replacement in patients with symptomatic chronic mitral regurgitation. The importance of left atrial size. Circulation. 1991;84:23–34. doi: 10.1161/01.cir.84.1.23. [DOI] [PubMed] [Google Scholar]
- 29.Zhong L, Tan LK, Finn CJ, Ghista D, Liew R, Ding ZP. Effects of age and gender on left atrial ejection force and volume from real-time three-dimensional echocardiography. Ann Acad Med Singapore. 2012;41:161–9. [PubMed] [Google Scholar]
- 30.Oliveira W, Campos O, Cintra F, Matos L, Vieira ML, Rollim B, et al. Impact of continuous positive airway pressure treatment on left atrial volume and function in patients with obstructive sleep apnoea assessed by real-time three-dimensional echocardiography. Heart. 2009;95:1872–8. doi: 10.1136/hrt.2009.173625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caselli S, Canali E, Foschi ML, Santini D, Di Angelantonio E, Pandian NG, et al. Long-term prognostic significance of three-dimensional echocardiographic parameters of the left ventricle and left atrium. Eur J Echocardiogr. 2010;11:250–6. doi: 10.1093/ejechocard/jep198. [DOI] [PubMed] [Google Scholar]


