Abstract
Objective:
Fragmented QRS (fQRS) complexes that have numerous RSR´ patterns represent alteration of ventricular depolarization. We evaluated the relationship between fQRS and poor coronary collateral circulation and the diagnostic ability of fQRS for myocardial scar detection in patients with chronic total occlusion (CTO) without a history of myocardial infarction.
Methods:
The study population consisted of patients undergoing coronary angiography with a suspicion of CAD. Seventy-nine patients with one totally occluded major coronary artery were enrolled. Exclusion criteria were history of MI; recent acute coronary syndrome; pathologic Q wave on 12-lead ECG; cardiomyopathy or severe valvular disease; coronary artery bypass surgery or percutaneous coronary angioplasty. Collateral circulation was scored on the basis of Rentrop's classification. All patients were assessed by myocardial perfusion SPECT. Fragmented QRS was characterized as existence of an R´ or R wave or S wave notch in two adjacent leads related to the location of a major coronary artery region. Single and multiple logistic regression analyses were completed in the forward method.
Results:
Forty-nine patients had poor and 30 had well-developed collateral circulation. Fragmented QRS complexes were significantly higher in the poor collateral group (81% vs. 20%, p<0.001). Sensitivity, specificity, and the positive and negative predictive values of fQRS for myocardial scar identification were 89.4%, 87.5%, and 91.3% and 84.8%, respectively. The summed stress score and the summed rest score on SPECT were significantly higher in the poor collateral group than in the well-developed group (p<0.001) as well as in the fQRS group than the non-fQRS group (p<0.001). Logistic regression analysis revealed that the presence of fQRS was significantly and independently associated with poor collateral circulation and myocardial scar in patients with CTO.
Conclusion:
Fragmented QRS is independently related to poor coronary collateral circulation in patients with CTO without prior myocardial infarction. Notably, it can be a good predictor of myocardial scar rather than merely ischemia, with high diagnostic accuracy.
Keywords: fragmented QRS, coronary artery disease, coronary collateral circulation, SPECT
Introduction
Some studies showed that a slight abnormality within the QRS, known as fragmented QRS (fQRS), can indicate conduction disturbance and localized myocardial scar especially in non-Q-wave myocardial infarction (MI) (1, 2). Coronary collaterals are small anastomotic vessels providing retrograde flow in distal segments in patients who develop total obstruction of coronary arteries (3) and may protect the myocardium during ischemia (4, 5). Such obstructions sometimes occur gradually without acute MI; these obstructions are known as chronic total occlusions (CTOs) (6).
The relation of fQRS in patients with coronary artery disease (CAD) with myocardial scar detected on single photon emission computed tomography (SPECT) (7) or collaterals in patients with CTO detected on coronary angiography (2) have been shown. However, such a relation has not been assessed with both SPECT and angiography in any study; moreover, one study failed to show the value of fQRS in scar detection (8). Also, some studies have reported that it may be associated with all-cause mortality and recurrent cardiovascular events (9) as well as ventricular arrhythmia in patients with nonischemic cardiomyopathy (10).
We postulated that fQRS on 12-lead electrocardiogram (ECG) may be associated with inadequate collateral circulation detected on coronary angiography and myocardial scar identified on SPECT.
Methods
The present study was cross-sectional and was conducted prospectively (study power=0.80) in the cardiology clinic of Heshmat Heart Center affiliated to the Guilan University of Medical Sciences. The study population consisted of patients undergoing coronary angiography with a suspicion of CAD at our center. Patients with one totally occluded major coronary artery were enrolled. All patients had chest pain or angina-equivalent symptoms with positive exercise test or myocardial perfusion scan. All enrolled patients underwent myocardial perfusion SPECT examination. Clinical characteristics, including patient history and physical examination, were then recorded by cardiologists and put into a database at our cardiac catheterization laboratory. Exclusion criteria were history of MI; history of acute coronary syndrome during 1 month prior to presentation; pathologic Q wave on 12-lead ECG (defined as a duration of more than 0.04 s and more than 25% of the R wave voltage); history of known cardiomyopathy or severe valvular disease; history of coronary artery bypass surgery or percutaneous coronary angioplasty; and complete or incomplete right bundle branch block (RBBB), typical left bundle branch block (LBBB), or nonspecific intraventricular conduction delay with QRS width more than 120 ms on 12-lead ECG.
The Ethics Committee approved the study protocol and patients gave written informed consent prior to enrollment in the study.
Routine laboratory tests
Blood samples were drawn for routine blood tests after 8 h of fasting the day before coronary angiography. Fasting blood glucose and serum total cholesterol were measured following standard methods.
fQRS definition and ECG
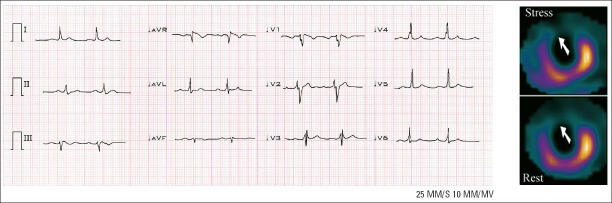
Resting 12-lead ECG (0.5-150 Hz, 25 mm/s, 10 mm/mV) was obtained for each patient. All ECGs were interpreted by two experienced cardiologists blinded to study. There was 99.9% concordance for ECG interpretation of fQRS. Fragmented QRS was defined as the existence of R’ or a notch in the nadir of the R or S wave, or the existence of more than one R’ (fragmented) in two adjacent leads related to the location of a major coronary region (Fig. 1 and 2). The existence of an fQRS in two adjacent anterior leads (V1 toV5) was related to the left anterior descending territory. The existence of an fQRS in two adjacent lateral leads (I, aVL, and V6) was related to the left circumflex territory. Also, the existence of an fQRS in two adjacent inferior leads (II, III, and aVF) was related to the right coronary artery territory. On the basis of this definition, only one fQRS was considered as absent fQRS.
Figure 1.
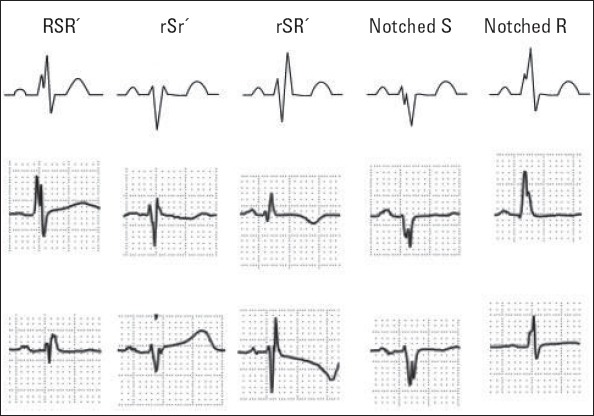
Classification of fragmented QRS (various RSR’ patterns). Fragmented QRS was defined as an additional spike of QRS complexes without bundle branch block. Reproduced with permission (1)
Figure 2.
Twelve-lead ECG showing an fQRS (various RSR’ patterns) in leads V1-V4 correlated with a fixed perfusion defect of anteroseptal segment on an MPI. There is no Q wave
Myocardial SPECT imaging and analysis
Patients were instructed to fast for at least 4 h before the study. Beta-blockers and calcium channel blockers were discontinued for 48 h, and the patients were advised not to take caffeine or aminophylline 24 h before the test. A sestamibi kit of Atomic Energy Organization of Iran was applied according to the manufacturer's instructions. All patients underwent a rest/stress (low-dose/high-dose) technetium 99 sestamibi stress test on a single-day basis. The ECG-gated frames were obtained on post-stress images in the supine position at 8 frames in each R-R interval 60 min after a peak stress Tc-99m injection using a rotating, dual head SPECT (E.cam, Siemens Medical Systems, Erlangen, Germany) equipped with a low-energy, high resolution parallel-hole collimator. Tomograms were reconstructed by filtered software in different axes. Visual interpretation of different slices on monitors and final film reconstruction of SPECT images was performed by two experienced readers who had no information about the patients’ medical history.
The assessment were performed independently by 2 expert nuclear medicine specialist who were unaware of patient identity or the results of angiography. The inter-observer concordance and intra-observer concordance was 97.9% and 99%, respectively. Discrepancies were resolved by mutual agreement. For assessment, sum stress score (SSS), sum rest score (SRS), and sum difference score (SDS) were measured on the basis of the pattern of 17-segment with the following scaling: 0, normal; 1, mildly abnormal; 2, moderately abnormal; 3, severely abnormal; and 4, absent. The territory of the left anterior descending artery (seven segments) denoted by leads V1 to V5 (anterior segments); the right coronary artery (five segments) denoted by leads II, III, and aVF (inferior segments); the left circumflex artery (five segments) denoted by leads I, aVL, and V6 (lateral segments); and were scored regarding the conventional nomenclature (11, 12). On myocardial perfusion imaging (MPI), myocardial scar was characterized by a sum difference score of ≤2 and territorial SSS and SRS of ≥3 related to the location of a major coronary artery. On the basis of the overall evaluation, including the above criteria, we identified the study as negative or positive for scar (Fig. 2). By adding the scores of respective segments in the provided images, the summed rest and stress scores were calculated (11, 13). The sum of the differences between different segments was characterized as SDS, depicting the quantity of ischemia.
Coronary angiography and coronary collateral grading
Selective coronary angiography was performed using Judkins method. Analysis of coronary angiographies and assessment to score collaterals was performed by two expert cardiologists blinded to the study. There was a 98% inter-observer concordance in interpretation of coronary angiography and collaterals. Intra-observer concordance was 98.8%. Discrepancies were resolved by mutual agreement. Patients with CTO of one major coronary artery were included into the study. Collateral flow from the patent arteries to the totally occluded artery was graded using Rentrop classification (14); grade 0: no filling of collaterals is visible, grade 1: side branches of the obstructed artery are filled but the major artery is not visible, grade 2: the major coronary artery is partially filled, and grade 3: collaterals are filled but artery is completely. Patients who had grade 0 and 1 were classified as the poorly developed collateral group and those who had grade 2 or 3 were classified as the well-developed collateral group.
Statistical analysis
To analyze data we used SPSS for Windows software (SPSS version 18.0; SPSS Inc., Chicago, IL, USA). We tested continuous variables for normal distribution by the Kolmogorov-Smirnov test considered as mean ± standard deviation (SD); categorical variables were specified as a frequency or percentage. Continuous variables were analyzed using the independent samples t-test and categorical variables in the groups were analyzed using the chi-squared test. Single and multiple logistic regression analyses were completed in the forward method to assess whether the presence of fQRS as an independent variable can predict collateral development and myocardial scar as dependent variables. The sensitivity, specificity, and positive and negative predictive values were calculated. The phi coefficient (a chi-square-based measure of association) was used to assess the relationship between nominal data (relation between collateral development and MPI scar).
Results
Of the 3340 consecutive patients who underwent selective coronary angiography at our center, 208 patients had CTO; 117 patients were excluded for the following reasons: history of MI or Q wave in baseline ECG (117 patients), typical LBBB (five patients), RBBB (four patients), severe aortic stenosis (one patient), and severe heart failure (two patients). Finally, 79 patients were included the study. Thirty patients had well-developed and 49 had poor collaterals. Patients with poor collateral development were significantly elder (68±8 vs. 59±11 years, p<0.001) and lower left ventricular ejection fraction than those with adequate collateral circulation (40±8% vs. 49±8%, p<0.001). fQRS was present in 46 (58%) patients. The presence of fQRS presence was more common in the poor collateral group than in the well-developed collateral group (82% vs. 20%, p<0.001). A demographic comparison of the two collateral groups is outlined in Table 1. Forty-five patients (57%) had poorly developed collateral and positive MPI scar, 28 patients (35%) had well-developed collateral and negative MPI scar, two patients (3%) had well-developed collateral and positive MPI scar, and four patients (5%) had poorly developed collateral and negative MPI scar. There was a significant relation between collateral development and MPI scar (r=0.84, p<0.001).
Table 1.
Comparison of demographic characteristics between patients
| Demographic variables | Poorly developed collateral (n=49) | Well-developed collateral (n=30) | P |
|---|---|---|---|
| Age, years | 68±8 | 59±11 | <0.001 |
| Gender, male | 71% | 74% | NS |
| Hypertension | 41% | 38% | NS |
| Diabetes mellitus | 31% | 40% | NS |
| Hyperlipidemia | 33% | 28% | NS |
| Smoking | 47% | 26% | NS |
| Familial history of CAD | 18% | 15% | NS |
| Coronary angiography | |||
| LAD | 51% | 49% | NS |
| LCX | 18% | 17% | NS |
| RCA | 31% | 34% | NS |
| ECG | |||
| Presence of fQRS | 82% | 20% | <0.001 |
| Ejection fraction, % | 40±8 | 49±8 | <0.001 |
| Medication | |||
| Insulin | 18% | 15% | NS |
CAD - coronary artery disease; fQRS - fragmented QRS; HDL - high-density lipoprotein; LCX - left circumflex; LAD - left anterior descending; LDL - low-density lipoprotein; NS - not significant; RCA - right coronary artery. Continuous variables were analyzed using the independent samples t-test and categorical variables in the groups were analyzed using the chi-squared test.
Binary logistic regression analysis with the existence of myocardial scar as a dependent and the presence of fQRS as independent variables showed that fQRS was significantly associated with myocardial scar (OR=58.8, 95% CI=14.5-238.2; p<0.001). Positive and negative predictive values, sensitivity, and specificity of fQRS for myocardial scar identification were 91.3% and 84.8%, 89.4%, and 87.5%, respectively; whereas these indices were lower for the detection of collateral circulation adequacy (Table 2). Multiple binary regression analysis with the existence of myocardial scar as a dependent and the presence of fQRS, ejection fraction, and age as independent variables also revealed a significant and independent association between fQRS and myocardial scar in patients with CTO (OR=26.1, 95% CI=4.7-142.9; p<0.001). SSS and SRS on scintigraphy were significantly higher in the poor collateral group than in the well-developed group (p<0.001), whereas SDS was nonsignificant (p=0.074). Also, SSS and SRS predictive of myocardial scar was significantly higher in the fQRS group than in the group without fQRS (p<0.001), although SDS showed no significant difference again (p=0.082) (Figs. 3 and 4).
Table 2.
Sensitivity, specificity, and positive and negative predictive values of fQRS for myocardial scar detection and degree of collateral development
| Test | Poorly developed collateral | Well- developed collateral | MPI scar (+) | MPI scar (-) |
|---|---|---|---|---|
| fQRS (+), n | TP=40 | FP=6 | TP=42 | FP=4 |
| fQRS (-), n | FN=9 | TN=24 | FN=5 | TN=28 |
| Sensitivity, % | 81.6 | 89.4 | ||
| Specificity, % | 80.0 | 87.5 | ||
| PPV, % | 86.9 | 91.3 | ||
| NPV, % | 72.7 | 84.8 |
fQRS - fragmented QRS; FP - false-positive; FN - false-negative; PPV - positive predictive value; MPI - myocardial perfusion imaging; NPV - negative predictive value; TP - true-positive; TN - true-negative. The sensitivity, specificity, and positive and negative predictive values of fQRS were calculated for myocardial scar detection and degree of collateral development.
Figure 3.
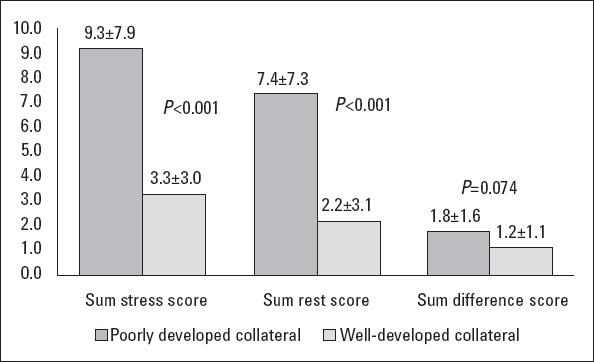
Bar graph showing the SPECT variables in the two collateral groups
Figure 4.
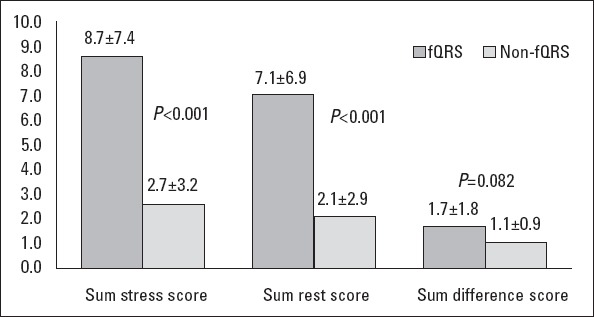
Bar graph showing the SPECT variables in the presence and absence of fQRS
Binary logistic regression analysis with collateral development as a dependent and the presence of fQRS as independent variables showed a significant association between the existence of fQRS with poorly developed collateral (OR=17.8, 95% CI=5.6-56.2, p<0.001). Fragmented QRS percentages corresponding to each CTO vessel are shown in Table 3. The positive and negative predictive values, sensitivity, and specificity of fQRS in each myocardial segment were calculated, and they showed lower sensitivity for the LCX territory but high specificity for the anterior territory (Fig. 5). Multiple binary regression analysis with collateral development as a dependent and the presence of fQRS, ejection fraction, and age as independent variables also revealed a significant and independent association between fQRS with poorly developed collateral in patients with CTO (OR=13.7, 95% CI=3.9-48.3; p<0.001).
Table 3.
Percentage of fragmented QRSs on ECG with related occluded coronary vessel
| fQRS percentage on ECG territories | ||||
|---|---|---|---|---|
| Vessel | CTO % | Lead I, AVL and V6, % | Lead V1-V5, % | Lead II, III, and AVF, % |
| LAD | 54 | 55 | 88 | 22 |
| LCX | 13 | 38 | 8 | 8 |
| RCA | 33 | 7 | 4 | 70 |
CTO - chronic total occlusion; fQRS - fragmented QRS; RCA - right coronary artery; LCX - left circumflex; LAD - left anterior descending
Figure 5.
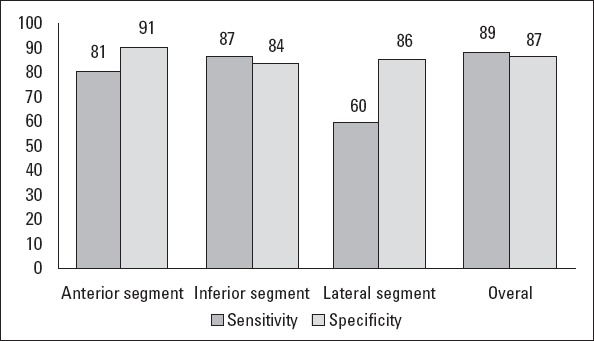
Sensitivity and specificity of fQRS are shown for scar detected on SPECT in each myocardial segment as well as the whole heart
Discussion
Our study showed that the presence of fQRS on ECG was independently related to poor collateral circulation in patients with CTO and no MI history. Collateral flow plays a key role in blood supply to the segment distal to obstruction when the occluded artery fails to provide adequate blood flow (15). It has been shown that the degree of collateral filling could predict the presence of residual viable myocardium in patients with an old MI (16, 17). The number of coronary collaterals increased significantly in the cases where coronary obstruction evolved slowly (18). It has been indicated that good coronary collaterals may protect the myocardium from infarction during episodes of ischemia (3) and notably, in patients with one occluded coronary artery without prior MI, no resting perfusion defects appears. Thus, resting localized abnormalities in wall motion are not seen, whereas stress-induced perfusion defects may appear (19). Although the mechanism of fQRS has not yet been fully understood, it may be due to irregular and erratic conduction around the scarred or fibrosed myocardium, generating several wave potentials inside QRS (7, 20-22) reflecting heterogeneous myocardial electrical activation (23-25). The goal in viability assessment is to optimize the selection of patients who benefit maximally from revascularization. Currently, there are generally four methods of myocardial viability evaluation in routine practice. Dobutamine stress echocardiography is more specific, SPECT imaging appears to be slightly more sensitive, and PET modality appears to have a higher accuracy (26). PET modality required equipment and radiotracer production on the location, and the protocols are specifically challenging in terms of technical view in some groups of patients, such as those with diabetics (27). Currently, late gadolinium enhancement using cardiac magnetic resonance (CMR) has been presented as the gold standard for the detection of myocardial scar (28). However, a meta-analysis of observational studies on myocardial viability showed no difference among the modalities commonly used to evaluate viability (PET vs. dobutamine echocardiography vs. CMR or SPECT) with respect to mortality reduction (29). It seems that the theoretical value of CMR and PET is merely their better spatial resolution. To the best of our knowledge, this is the first study to evaluate the relation of fQRS with poor collaterals and myocardial scar in patients with CTO using a combination of coronary angiography and myocardial SPECT imaging. Das et al. (9) demonstrated that compared with the Q wave, fQRS shows a remarkably higher negative predictive value and sensitivity for myocardial scar detection. They demonstrated that the presence of fQRS in different ECG leads related to their coronary artery territories can predict myocardial scar (7). However, one study reported a poor value for fQRS for scar detection (8) and the reason is not clearly defined. Nevertheless, there is some evidence that patchy fibrosis in the myocardium may be a result of chronic ischemia without the development of infarction (30) although it has been proposed that severe ischemia itself can cause nonhomogenous conduction, resulting in fQRS formation. It is remarkable that our data showed no significant difference in the SDS value between the two groups, indicating that the amount of ischemia is not considerably different. This finding indicates that ischemia without scar is less likely to lead to fQRS development. Mahenthiran et al. (31) demonstrated that the presence of fragmented QRS could be an indicator of myocardial perfusion with acceptable specificity, sensitivity, and accuracy for the prediction of regional myocardial scar on a stress MPI scan. However, their study showed that all stress MPI perfusion scores including SDS, SSS, and SRS were statistically higher in all coronary artery territories in the fQRS group. Also, similar results were found in another study in which the aforementioned quantitative scores were significantly higher in the fQRS patients, implying a relationship between fQRS and ischemia or/and infarction (32). Recently Kadı et al. (2) showed that fragmented QRS was associated with poorly grown collateral coronary circulation in patients with CTO without prior MI. However, they did not enroll any patient with grade 0 coronary flow and only made a comparison between patients with grade 3 and grade 1. The present study demonstrated the presence of fQRS on ECG in 81% of patients with poor collateral circulation compared with only 20% of patients with well-developed collateral who presented with fQRS. Notably, all patients with grade 0 collateral had fQRS, whereas only 9% of patients with grade 3 had fQRS. According to the logistic regression analysis, fQRS patients were more likely to have poor collateral circulation. We found that the presence of fQRS was significantly higher in the poor collateral group than the well-developed collateral group (p<0.001); also, the logistic regression analysis demonstrated an independent relation between fQRS and collateral development, which is consistent with other studies (33-35). Overall, these data indicate that poor collaterals could not possibly prevent severe ischemia and MI, and consequently, myocardial micro-infarction and scars, which may be the main cause of fQRS development, was more likely to occur in patients with CTO.
We excluded patients with Q wave in order to demonstrate the value of fQRS to better detect myocardial scar. Our results showed that the positive predictive value, specificity, and sensitivity of fQRS for diagnosing myocardial scar assessed on SPECT was substantially high, which is against another study (8). Of note, the specificity was lower in the inferior territory (84% vs. 91% vs. 86%; Fig. 5), which may be due to the more probable nonspecific conduction abnormality in this segment. Previous silent infarct sometimes can be predicted just by the presence of fQRS; this is more likely seen in women, patients with diabetes mellitus, patients with dementia, and people with mental disabilities. Therefore, greater localized fixed defects on SPECT are associated with fQRS, which could possibly improve the detection of previous silent infarcts. Furthermore, we found a reverse relation between ejection fraction and fQRS (OR=0.91, 95% CI=0.859-0.96; p=0.002), which is concordant with other studies (36, 37). However, some of the studies mentioned, have been conducted in patients with a history of MI, whereas our results have been obtained from patients without a history of MI. From a clinical viewpoint, the presence of fQRS on a simple 12-lead ECG can be used as a valuable predictor clue for collateral circulation condition and myocardial scar in such patients so that it guides the clinician to the next diagnostic approach.
Study limitations
First, the sample size of our study was small. A second limitation is that we used myocardial SPECT for the detection of myocardial scar although MRI is the standard modality for this, as already cited. Further studies with larger numbers of subjects using MRI are warranted to better clarify this issue. Another limitation is the cross-sectional observational nature of our study because of which we could not conclude a cause-effect relationship. Finally, the patients who presented with bundle branch block or a nonspecific conduction delay with QRS longer than 120 ms may have potential myocardial scar but were not enrolled because of the absence of suitable selection criteria.
Conclusion
fQRS was independently related to insufficient coronary collateral circulation in patients with CTO and myocardial scar. fQRS is a good predictor of poor collaterals, with high sensitivity and specificity, and can be used to identify regional scar instead of using expensive and time consuming imaging modalities such as MRI and SPECT.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept - H.B., J.K., M.A.R., A.B.; Design - H.M.; Supervision - J.K., H.B., E.A., M.A.R.; Materials - H.B., J.K., E.A., A.S.; Data collection&/or processing - B.S., E.A., M.A.R.; Analysis &/or interpretation - H.M., B.S.; Literature search - B.S., A.S.; Writing - A.S.; Critical review - J.K., H.M., A.B.
References
- 1.Take Y, Morita H. Fragmented QRS: What is the meaning? Indian Pacing and Electrophysiology J. 2012;12:213–25. doi: 10.1016/s0972-6292(16)30544-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kadı H, Ceyhan K, Fatih K, Önalan O. Relation between fragmented QRS and collateral circulation in patients with chronic total occlusion without prior myocardial infarction. Anatol J Cardiol. 2011;11:300–4. doi: 10.5152/akd.2011.079. [DOI] [PubMed] [Google Scholar]
- 3.Koer Selman I, Van der Graaf Y, Jaegere PP, Grobbee DE. Coronary collaterals: An Important and underexposed aspect of coronary artery disease. Circulation. 2003;107:2507–11. doi: 10.1161/01.CIR.0000065118.99409.5F. [DOI] [PubMed] [Google Scholar]
- 4.Werner GS, Ferrari M, Betge S, Gastmann O, Richartz BM, Figulla HR. Collateral function in chronic total coronary occlusions is related to regional myocardial function and duration of occlusion. Circulation. 2001;104:2784–90. doi: 10.1161/hc4801.100352. [DOI] [PubMed] [Google Scholar]
- 5.Kornowski R. Collateral formation and clinical variables in obstructive coronary artery disease: The influence of hypercholesterolemia and diabetes mellitus. Coron Artery Dis. 2003;14:61–4. doi: 10.1097/00019501-200302000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Shah B. Management of coronary chronic total occlusion. Circulation. 2011;123:1780–4. doi: 10.1161/CIRCULATIONAHA.110.972802. [DOI] [PubMed] [Google Scholar]
- 7.Das MK, Khan B, Jacob S, Kumar A, Mahenthiran J. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation. 2006;113:2495–501. doi: 10.1161/CIRCULATIONAHA.105.595892. [DOI] [PubMed] [Google Scholar]
- 8.Wang DD, Buerkel DM, Corbett JR, Gurm HS. Fragmented QRS complex has poor sensitivity in detecting myocardial scar. Ann Noninvasive Electrocardiol. 2010;15:308–14. doi: 10.1111/j.1542-474X.2010.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das MK, Saha C, Masry H, Peng J, Dandamudi G, Mahenthiran J, et al. Fragmented QRS on a 12-lead ECG: a predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm. 2007;4:1385–92. doi: 10.1016/j.hrthm.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 10.Michael M, Das M. Fragmented QRS (fQRS) on 12-lead ECG is a predictor of arrhythmic events and mortality in patients with dilated cardiomyopathy. Heart Rhythm. 2006;3:S103. doi: 10.1016/j.hrthm.2006.02.313. [DOI] [Google Scholar]
- 11.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 12.Aepfelbacher FC, Johnson RB, Schwartz JG, Chen L, Parker RA, Parker JA, et al. Validation of a model of left ventricular segmentation for interpretation of SPECT myocardial perfusion images. Eur J Nucl Med. 2001;28:1624–9. doi: 10.1007/s002590100618. [DOI] [PubMed] [Google Scholar]
- 13.Germano G, Kiat H, Kavanagh PB, Moriel M, Mazzanti M, Su HT, et al. Automatic quantification of ejection fraction from gated myocardial perfusion SPECT. J Nucl Med. 1995;36:2138–47. [PubMed] [Google Scholar]
- 14.Rentrop KP, Cohen M, Blancke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol. 1985;5:587–92. doi: 10.1016/S0735-1097(85)80380-6. [DOI] [PubMed] [Google Scholar]
- 15.Sasayama S, Fujita M. Recent insights into coronary collateral circulation. Circulation. 1992;85:1197–204. doi: 10.1161/01.CIR.85.3.1197. [DOI] [PubMed] [Google Scholar]
- 16.Fukai M, Ii M, Nakakoji T, Kawakatsu M, Nariyama J, Yokota N, et al. Angiographically demonstrated coronary collaterals predict residual viable myocardium in patients with chronic myocardial infarction: a regional metabolic study. J Cardiol. 2000;35:103–11. [PubMed] [Google Scholar]
- 17.Sabia PJ, Powers ER, Ragosta M, Sarembock IJ, Burwell LR, Kaul S. An association between collateral blood flow and myocardial viability in patients with recent myocardial infarction. N Engl J Med. 1992;327:1825–31. doi: 10.1056/NEJM199212243272601. [DOI] [PubMed] [Google Scholar]
- 18.Baroldi G, Mantero O, Scomazzoni G. The collaterals of the coronary arteries in normal and pathologic hearts. Circ Res. 1956;4:223–9. doi: 10.1161/01.RES.4.2.223. [DOI] [PubMed] [Google Scholar]
- 19.Aboul-Enein F, Kar S, Hayes SW, Sciammarella M, Abidov A, Makkar R, et al. Influence of angiographic collateral circulation on myocardial perfusion in patients with chronic total occlusion of a single coronary artery and no prior myocardial infarction. J Nucl Med. 2004;45:950–5. [PubMed] [Google Scholar]
- 20.Das MK, Suradi H, Maskoun W, Michael MA, Shen C, Peng J, et al. Fragmented wide QRS on a 12-lead ECG: a sign of myocardial scar and poor prognosis. Circ Arrhythm Electrophysiol. 2008;1:258–68. doi: 10.1161/CIRCEP.107.763284. [DOI] [PubMed] [Google Scholar]
- 21.Morita H, Kusano KF, Miura D, Nagase S, Nakamura K, Morita ST, et al. Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation. 2008;118:1697–704. doi: 10.1161/CIRCULATIONAHA.108.770917. [DOI] [PubMed] [Google Scholar]
- 22.Das MK, Maskoun W, Shen C, Michael MA, Suradi H, Desai M, et al. Fragmented QRS on twelve-lead electrocardiogram predicts arrhythmic events in patients with ischemic and non-ischemic cardiomyopathy. Heart Rhythm. 2010;7:74–80. doi: 10.1016/j.hrthm.2009.09.065. [DOI] [PubMed] [Google Scholar]
- 23.Flowers NC, Horan LG, Thomas JR, Tolleson WJ. The anatomic basis for high-frequency components in the electrocardiogram. Circulation. 1969;39:531–9. doi: 10.1161/01.CIR.39.4.531. [DOI] [PubMed] [Google Scholar]
- 24.Basaran Y, Tigen K, Karaahmet T, Işıklar I, Çevik C, Gürel E, et al. Fragmented QRS complexes are associated with cardiac fibrosis and significant intraventricular systolic dyssynchrony in nonischemic dilated cardiomyopathy patients with a narrow QRS interval. Echocardiography. 2011;28:62–8. doi: 10.1111/j.1540-8175.2010.01242.x. [DOI] [PubMed] [Google Scholar]
- 25.Wiener I, Mindich B, Pitchon R. Fragmented endocardial electrical activity in patients with ventricular tachycardia: a new guide to surgical therapy. Am Heart J. 1984;107:86–90. doi: 10.1016/0002-8703(84)90138-8. [DOI] [PubMed] [Google Scholar]
- 26.Siebelink HM, Blanksma PK, Crijns HJ, Bax JJ, van Boven AJ, Kingma T, et al. No difference in cardiac event-free survival between positron emission tomography and single-photon emission computed tomography-guided patient management. J Am Coll Cardiol. 2001;37:81–8. doi: 10.1016/S0735-1097(00)01087-1. [DOI] [PubMed] [Google Scholar]
- 27.Kobylecka M, Maczewska J, Fronczewska-Wieniawska K, Mazurek T, Plazinska MT, Królicki L. Myocardial viability assessment in 18FDG PET/CT study (18FDG PET myocardial viability assessment) Nuclear Medicine Review. 2012;15:52–60. doi: 10.5603/NMR.2012.0010. [DOI] [PubMed] [Google Scholar]
- 28.Kwong R, Farzaneh-Far A. Measuring myocardial scar by CMR. J Am Coll Cardiol Img. 2011;4:157–60. doi: 10.1016/j.jcmg.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Allman K, Shaw L, Hachamovitch R, Udelson JE. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: A meta-analysis. J Am Coll Cardiol. 2002;39:1151–8. doi: 10.1016/S0735-1097(02)01726-6. [DOI] [PubMed] [Google Scholar]
- 30.Weinberg SL, Reynolds RW, Rosenman RH, Katz LN. Electrocardiographic changes associated with patchy myocardial fibrosis in the absence of confluent myocardial infarction; an anatomic correlative study. Am Heart J. 1950;40:745–59. doi: 10.1016/0002-8703(50)90203-1. [DOI] [PubMed] [Google Scholar]
- 31.Mahenthiran J, Khan BR, Sawada SG, Das MK. Fragmented QRS complexes not typical of a bundle branch block: a marker of greater myocardial perfusion tomography abnormalities in coronary artery disease. J Nucl Cardiol. 2007;14:347–53. doi: 10.1016/j.nuclcard.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Özdemir S, Tan YZ, Çölkesen Y, Temiz A, Türker F, Akgöz S. Comparison of fragmented QRS and myocardial perfusion-gated SPECT findings. Nucl Med Commun. 2013;34:1107–15. doi: 10.1097/MNM.0b013e3283653884. [DOI] [PubMed] [Google Scholar]
- 33.Torigoe K, Tamura A, Kawano Y, Shinozaki K, Kotoku M, Kadota J. The number of leads with fragmented QRS is independently associated with cardiac death or hospitalization for heart failure in patients with prior myocardial infarction. J Cardiol. 2012;59:36–41. doi: 10.1016/j.jjcc.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Erdogan T, Kocaman SA, Çetin M, Çanga A, Durakoglugil ME, Çiçek Y, et al. Relationship of fragmented QRS complexes with inadequate coronary collaterals in patients with chronic total occlusion. J Cardiovasc Med. 2012;13:499–504. doi: 10.2459/JCM.0b013e328353683c. [DOI] [PubMed] [Google Scholar]
- 35.Çetin M, Kocaman SA, Kiris T, Erdogan T, Canga A, Durakoglugil ME, et al. Absence and resolution of fragmented QRS predict reversible myocardial ischemia with higher probability of ST segment resolution in patients with ST segment elevation myocardial infarction. Korean Circ J. 2012;42:674–83. doi: 10.4070/kcj.2012.42.10.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Canga A, Kocaman SA, Durakoglugil ME, Çetin M, Erdogan T, Kiris T, et al. Relationship between fragmented QRS complexes and left ventricular systolic and diastolic functions. Herz. 2013;38:665–70. doi: 10.1007/s00059-012-3739-1. [DOI] [PubMed] [Google Scholar]
- 37.Cheema A, Khalid A, Wimmer A, Bartone C, Chow T, Spertus JA, et al. Fragmented QRS and mortality risk in patients with left ventricular dysfunction. Circ Arrhythm and Electrophysiol. 2010;3:339–44. doi: 10.1161/CIRCEP.110.940478. [DOI] [PubMed] [Google Scholar]