Abstract
Objective:
Allograft rejection is still an important cause of morbidity and mortality after heart transplantation (HTx). Many techniques in cardiac magnetic resonance imaging (CMR) were investigated to diagnose acute cellular rejection (ACR). However, there is not enough information about late gadolinium enhancement (LGE) in the myocardium and ACR.
Methods:
We prospectively analyzed our consecutive 41 heart transplant recipients who were admitted for routine endomyocardial biopsies. CMR was performed maximum 6 h before the scheduled endomyocardial biopsy. Correlation between LGE in the myocardium and ACR was investigated.
Results:
Twenty-seven patients showed no rejection, and nine of them had LGE in the myocardium. Fourteen patients had LGE in the left ventricle (LV), and two patients had LGE also in the right ventricle (RV). There was no correlation between LGE and ACR (p=0.879). There was no difference in the left ventricular ejection fraction (LVEF), right ventricular fractional area change (RVFAC), and cardiac ischemic time between the groups (p=0.825, p=0.370, and p=0.419, respectively). LGE in the myocardium could be due to previous rejection episodes; therefore, all patients were retrospectively searched for previous rejection grades and number of episodes. Thirty-eight of the 41 patients had a history of one ACR episode, but none of them had a statistically significant correlation with LGE (for grade 1R, p=0.964 and grade 3R, p=1) There was also no correlation between number of rejection episodes history and LGE.
Conclusion:
LGE is not suitable to detect ACR in heart transplant patients. LGE and the history of ACR have no correlation.
Keywords: heart transplantation, graft rejection, cardiac magnetic resonance imaging
Introduction
Heart transplantation (HTx) is the gold standard therapy for end-stage heart failure. According to the International Society of Heart and Lung Transplantation (ISHLT) registries, the median survival after HTx has increased up to 10 years, but graft failure still remains to be the most important cause of death (1). Despite the advancement of immunosuppressive therapy, 40%–70% of heart transplants have acute rejection episodes in first 6 months (2). Acute cellular rejection (ACR) occurs because of mismatched histocompatibility between the donor organ and recipient. The hallmark of acute rejection is lymphocytic and monocytic infiltration, followed by endothelial cellular injury to myocardial necrosis (3, 4). Recurrent episodes of acute rejection and severity are strongly associated with the development of chronic rejection within time (5, 6). An important problem is the need to balance allograft rejection and immune deficiency with appropriate immunosuppressive therapy.
Endomyocardial biopsy (EMB) is still considered as a gold standard for the diagnosis of ACR, although it has some limitations: (a) it is an invasive procedure with the risk of cardiac tamponade or death, (b) substantial exposure to radiation, (c) sampling error, (d) interobserver variability, and (e) repeated EMB may result in cardiac scarring or venous thrombosis (7).
ISHLT has published revised ACR grading guidelines and simplified the evaluation of EMB, as well as improving its efficacy. The new ISHLT grading system suggests four grades of ACR: 0R (no rejection), 1R (mild rejection), 2R (moderate rejection), and 3R (severe rejection) (4). While aggressive therapy with intravenous corticosteroids or other immunomodulatory agents are generally recommended for moderate rejection and above, mild rejection is usually managed conservatively, as majority of such episodes resolve on follow-up EMB without the need of increased immunosuppression (8).
New reliable, less invasive tool is needed to replace EMB for diagnosing ACR.
Various imaging modalities, such as echocardiography, positron emission tomography, computed tomography, and cardiac magnetic resonance imaging (CMR) offer great potential for noninvasive detection of ACR (9).
CMR is a promising diagnostic tool to diagnose ACR because it allows excellent tissue contrast with superior image quality. Late gadolinium enhancement (LGE) is obtained with the use of gadolinium (Gd) contrast agents and it further enables high-resolution tissue characterization, as different patterns of myocardial involvement can be differentiated (10, 11).
LGE is commonly used in imaging scar tissue and fibrosis in ischemic heart disease, myocarditis, and hypertrophic cardiomyopathy (12). It is known that, even when there is no sign of coronary disease, LGE can be seen in transplanted hearts. Causes and prognostic values of this pattern are still unclear (13).
In the present study, the correlation between LGE imaging of the myocardium and ACR was investigated in heart transplant patients.
Methods
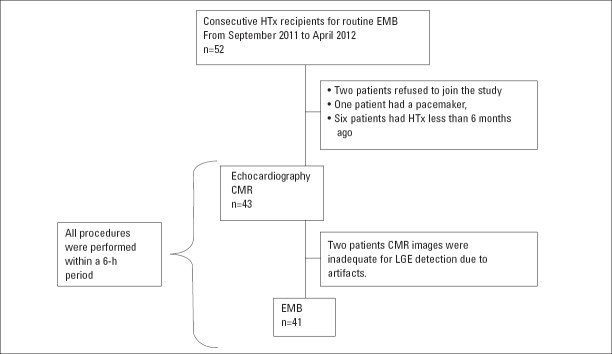
According to our institutional protocol, every heart transplant patient has EMBs periodically. Consecutively, 52 patients attended for usual EMB controls at the HTx clinic between September 2011 and April 2012. Inclusion criteria for the study were to have had HTx more than 6 months ago, age ≥18 years, and to have no contraindication for CMR. We excluded patients with severe claustrophobia, age <18 years, HTx <6 months ago, contraindication to CMR, and contraindication to Gd contrast. Nine patients were excluded (two patients refused to join the study, one patient had a pacemaker, and six patients had HTx less than 6 months ago). Two patients were excluded after study enrollment because CMR images were inadequate for LGE detection due to artifacts. One patient had EMB 1 week late because of a suspicious thrombus in the left atrium. In our institution, every transplant recipient has routine coronary angiogram and stress echocardiography for ischemia and allograft vasculopathy screening. There was no sign of cardiac ischemia in our study population.
Echocardiography, CMR, and EMB were performed within a 6-h period. Biopsies were taken after CMR to avoid the traumatic effect of biopsy on the right ventricle (RV) (Fig. 1).
Figure 1.
Study design, causes, and numbers of the excluded patients
CMR - cardiac magnetic resonance imaging; EMB - endomyocardial biopsy; HTx - heart transplantation; LGE - late gadolinium enhancement
Informed consent was obtained from all patients. The study protocol was approved by the Ege University Review Committee.
Endomyocardial biopsies
All biopsies were performed according to the guidelines, jugular approach being used mostly. A minimum of three samples were taken per session. ACRs were histopathologically graded according to the latest guidelines of International Society of Heart and Lung Transplantation (ISHLT).
Grade 0R, no rejection.
Grade 1 R, mild rejection: Interstitial and/or perivascular infiltrate with up to one focus of myocyte damage.
Grade 2 R, moderate rejection: two or more foci of infiltrate with associated myocyte damage.
Grade 3 R, severe rejection: diffuse infiltrate with multifocal myocyte damage, with or without edema, hemorrhage, or vasculitis (4). An experienced cardiac pathologist analyzed all biopsies, blinded to CMR results and patients’ clinical state.
Cardiac magnetic resonance imaging protocol
Vector-ECG triggered CMR was performed on a 1.5 Telsa whole-body magnetic resonance imaging (MRI) scanner (Siemens Medical Solutions, Erlangen, Germany) employing a cardiac phased-array receiver coil. Generation of imaging planes, assessment of left ventricular function and LGE sequences 10 min after contrast agent administration (0.2 mmoL/kg, Magnevist, Schering, Germany) were performed in a standardized way as previously described (14). Images were then processed on a dedicated workstation using commercially available software (Argus Workstation, Siemens Medical Solutions, and Erlangen, Germany). Images were analyzed and classified into LGE involvement in the RV and left ventricle (LV). Same radiologist, blinded to patients’ clinical state and EMB results, reviewed all CMR images.
Statistical analysis
Continuous data are expressed as mean±SD and discrete variables as absolute numbers and percentages. Continuous variables were assessed for normality using formal skewness and kurtosis testing. Continuous variables were compared using Mann–Whitney U or student's t-test. Dichotomous variables were assessed using chi-square tests or Fisher's exact test where appropriate. Two tailed p values <0.05 were considered statistically significant. All calculations were made using a computerized statistical package.
Results
Overall, 41 heart transplant recipients were enrolled in this study. Thirty three (80.5%) of them were male, mean age was 43.2±12.1 years, and graft age was 35.7±24.6 months (between 6 and 91 months). None of the recipients were in New York Heart Association (NYHA) functional class III or higher [39 (95%) were in NYHA class I and 2 (5%) were in class II]. The most common etiology of heart failure was dilated cardiomyopathy [24 (58.5%)] and 15 (36.6%) of the patients had left ventricular assist device (LVAD) implantation prior to transplantation. A standard biatrial orthotopic HTx procedure was performed in all patients. LGE in the myocardium was detected in 14 (33.3%) patients; all having LV involvement, except for two patients who had both RV and LV involvement (Table 1).
Table 1.
ACR grades and LGE results
| LGE (-) | LGE (+) | |
|---|---|---|
| ACR (-) | 18 | 9 |
| ACR (+) | ||
| Grade 1R | 8 | 4 |
| Grade 2R | 1 | 1 |
| Grade 3R | 0 | 0 |
ACR - acute cellular rejection; LGE - late gadolinium enhancement
There were no statistically significant difference in the graft age (p=0.165), right and left ventricular functions, and donor ischemic time (p=0.419) between LGE positive and negative groups (Table 2).
Table 2.
Comparisons of the baseline characteristics of LGE positive and negative groups
| LGE (-) Mean±SD | LGE (+) Mean±SD | P | |
|---|---|---|---|
| Age, years | 43.1±13.2 (n=27) | 43.6±10.2 (n=14) | 0.978 |
| Male | 21 (77.8%) | 12 (85.7%) | 0.692 |
| NYHA | 0.111 | ||
| Class I | 27 (100%) | 12 (85.7%) | |
| Class II | 0 (0%) | 2 (14.3%) | |
| Graft age, months | 31.8±22.5 (n=27) | 43.2±27.4 (n=14) | 0.165 |
| Donor age, years | 26.1±9.3 (n=25) | 30.1±9.5 (n=14) | 0.168 |
| LVEF, % | 60.1±5.7 (n=27) | 60.5±6.6 (n=14) | 0.825 |
| RVFAC, % | 49.1±7.9 (n=41) | 50.4±7.6 (n=14) | 0.370 |
| SPAP, mm Hg | 28.8±8.4 (n=25) | 28.3±8.1 (n=13) | 0.612 |
| TAPSE, mm | 11.2±2.2 (n=27) | 9.6±2.4 (n=14) | 0.079 |
| Ischemia time, min | 195.7±44.5 (n=26) | 208±60.7 (n=14) | 0.419 |
ACR - acute cellular rejection; LGE - late gadolinium enhancement; LVEF - left ventricular ejection fraction; RVFAC - right ventricular fractional shortening; SPAP - systolic pulmonary arterial pressure; TAPSE - tricuspid annular plane systolic excursion
Twenty-seven patients (65.8%) had no cellular rejection, and of these patients, 18 (44%) had no LGE detected in the myocardium.
Grade ≥1R rejection was detected in 14 (34.2%) patients, and only five of them had late enhancement in the myocardium, resulting in no statistically significant correlation (p=0.879).
History of any ACR episode or recurrent episodes may lead to late enhancement in the myocardium. Thirty-eight patients had a history of grade ≥1R ACR episode and the remaining three patients (two LGE negative and one LGE positive) had no history of ACR. There was no statistically significant difference between the history of ACR episode and LGE in the myocardium (history of no ACR vs. at least one Grade ≥1R rejection, p=1.000). Furthermore, severity of past ACR was compared; three patients had 3R ACR history and of these two had LGE and one did not, resulting in no significant relation between grade 3R rejection history and late enhancement in the myocardium (p=1.000) (Table 3) The mean Grade 1R ACR episode in the LGE negative and positive group were 3.2±2.8 and 3.6±3, respectively (p=0.687). There were also no difference between other rejection grades episode numbers and LGE (Table 4).
Table 3.
Correlation between history of any ACR, Grade 3R ACR and LGE groups
| LGE (-) | LGE (+) | P | |
|---|---|---|---|
| ACR episode history (+)/(-) | 25/2 | 13/1 | 1.000 |
| Grade 3R ACR history (+)/(-) | 1/26 | 2/12 | 1.000 |
ACR - acute cellular rejection; LGE - late gadolinium enhancement Fisher’s Exact test
Table 4.
Relation between numbers of ACR episodes in history and LGE groups
| Numbers of ACR Episodes | |||
|---|---|---|---|
| LGE (-) | LGE (+) | ||
| Mean±SD | Mean±SD | P | |
| Grade 1R episode | 3.2±2.8 | 3.6±3 | 0.687 |
| Grade 2R episode | 1.1±1.3 | 2±1.9 | 0.176 |
| Grade 3R episode | 0.03±0.19 | 0.14±0.36 | 0.223 |
ACR - acute cellular rejection; LGE - late gadolinium enhancement Mann-Whitney U test
Discussion
As our study result demonstrated that ACR, history of ACR, and LGE in transplanted hearts had no correlation. CMR can precisely quantify cardiac size and function and depict tissue changes that are associated with the various forms of myocardial inflammation. Thereby, CMR can often detect myocardial inflammation before contractility is obviously impaired. CMR may be a good candidate to noninvasively diagnose and screen for heart transplant rejection unaware of its degree of severity. Various CMR techniques, including T2-weighted imaging and early- and late-Gd enhanced T1-weighted imaging, are used to assess aspects of inflammation. In the present study, correlation between LGE imaging of the myocardium and ACR was investigated in heart transplant patients.
Gd cannot enter the intact cell owing to its large molecular size. After injection, Gd quickly leaves the vascular compartment and enters the interstitial space, not accumulating in the intracellular or interstitial space results in normal myocardium being free from late contrast enhancement. Gd accumulation may reflect inflammatory and fibrotic tissue increase in the interstitial space, as well as different wash-out kinetics in those areas. LGE has become very popular for imaging myocardial infarction. Technically, it is well standardized and widely available (15). Besides classical infarction scar, non-ischemic lesions, including acute myocarditis, could also be visualized. The signal intensity patterns do not differ between ischemic and non-ischemic lesions, but the distribution does. Non-ischemic lesions are located intramural in the middle layer or within the epicardial portion of the myocardial wall (16). They are often multiple and not confined to a single coronary territory. Multiple small edematous foci may be found in myocarditis corresponding to multiple patches of fibrosis in late enhancement. These LGE lesions can be detected early at the time of active myocarditis and usually do not represent chronic squeal during the late phase of the disease. LGE mostly disappears after healing of active myocarditis (17, 18).
Causes of LGE in heart transplant recipients are still unclear. There are a few studies with small groups about the prognostic value of LGE, correlation with allograft vasculopathy and ACR. Krieghoff et al. (19) studied 73 heart transplant recipients and 69% of them had LGE on the myocardium. Steen et al. (18) found that almost half of the 53 HTx recipients had LGE on the myocardium. In our study group, the rate of LGE was lower than that in other studies. Thirty-four percent (14/41) of our study group had late enhancement on the myocardium. Owing to small study groups and multifactorial variables, exact prevalence of LGE is unknown. Braggion-Santos et al. (13) studied 89 HTx recipients, divided in two groups according to the graft age (earlier vs. later than 30 months) and found that earlier HTx group had statistically significant higher rates of LGE on the myocardium, older donor age (p=0.01), longer ischemia time (p=0.03), and more number of recipients had toxoplasma infection (p<0.001). They speculated that ischemia and reperfusion injury due to longer ischemia time caused more fibrosis in the myocardium and also toxoplasma infection in the immune suppressive HTx recipient could cause silent myocardial injuries (13). Older donor hearts may have more fibrosis in the myocardium and for this reasons earlier group may have more LGE in the myocardium.
Butler et al. (20) compared 38 HTx recipients (19 LGE +/19 LGE-) and found no differences between mean donor ischemia times, but LGE positive group had higher graft ages. In the present study, there were no differences for donor ischemia time between groups (p=0.419). Mean ischemia time for all our study population was longer than that in Butler et al.'s (21) study (250 min vs. 240 min), graft age was higher in the LGE positive group (31.8 vs. 43. 2 mo.), but this differences was not statistically significant (p=0.165). There are conflicting results about ventricular functions and late enhancement. Some studies showed that the left ventricular ejection fraction (LVEF) and right ventricular ejection fraction (RVEF) were lower in the LGE positive groups (20). In our study, no differences were detected between the groups. [LVEF, p=0.825; right ventricular fractional area change p=0.370]. This could be caused by the fact that our study consisted of a healthier cohort (our LGE rate was lower than that in some other study groups) and left and right ventricular functions were measured with echocardiography. Arraiza et al. (22) reported that echocardiography tends to underestimate ventricular volumes and over estimate ejection fractions in HTx patients (21).
All HTx recipients in our institute routinely had coronary angiogram and stress echocardiography for ischemia and allograft vasculopathy screening and none of our patients had ischemia. Four of our recipients had infarct typical LGE, but it has been previously reported that even recipients without stenotic allograft vasculopathy in conventional angiography may have infarct typical LGE on the myocardium (18). Therefore, we did not separate LGE positive recipients in two groups according to the LGE pattern.
There are a few studies which evaluated LGE and ACR in HTx recipients. These studies used different ACR classifications and grades for comparisons of LGE patterns and there is no consensus on LGE and ACR. Taylor et al. (23) compared 68 CMR studies and EMB results and found that LGE was statistically higher in grade 2R positive ACR patients. Butler et al. (21) found no differences in 50 HTx recipients for grade 2R ACR. Also, Krieghoff et al. (19) compared 146 CMR and EMB results of 73 HTx recipients and found no LGE differences for grade 1b ACR. Our comparison groups were different, grade 1R and above (ACR positive) to normal EMB. There was no statistical significance between ACR positive and negative groups. Most of our ACR positive patients were grade 1R. ACR starts at small areas in the myocardium and perhaps these small areas would be too small to detect on LGE imaging (23). Our study and other studies were not able to compare true, specific histological myocardial injuries to LGE imaging. In the future, MRI guided biopsies of the most affected myocardial areas would help us for true correlation. In our study and other LGE studies where LGE and ACR were not correlated, LGE was relatively lower in the ACR positive groups. LGE may have underestimated the true prevalence of fibrosis or necrosis in the myocardium. Altering the inversion time to make apparently normal myocardium appear black maximizes the contrast between the LGE positive and normal myocardium. When fibrosis is diffuse, relatively homogenous with no normal myocardial area, LGE imaging could fail to detect pathology. Both acute processes like necrosis and chronic processes like fibrosis cause increased volume distribution in the myocardium and results in LGE. As a result, LGE imaging has difficulties for differentiating acute and chronic injuries Therefore, we compared past history and different grades of ACR with LGE, but no statistical significant difference was found between groups.
One of the two biventricular LGE positive recipient had positive pathology for ACR. We did not perform statistical analyses between only LV positive and biventricular LGE positive patients because the numbers were insufficient. Although four recipients in the study group had older graft ages, more biopsy procedures showed late enhancement only in the LV myocardium, suggesting that recurrent biopsies may not cause LGE in RV.
Study limitations
Our study is about a special and infrequent patient population; therefore, it has a small sample size. This is the major limitation of our study and limiting the generality of our findings. True histological myocardial comparison of LGE could not be achieved and in future, MRI guided biopsy could help us. We focused on recipient characteristics, ACR, and history of ACR, but myocardial fibrosis may also occur due to other causes.
Conclusion
LGE patterns in the non ischemic transplanted myocardium are still unclear. We also showed that donor ischemic time or donor age is not associated with LGE. Prospective, large-scale studies are needed to find out the relationship between LGE and rejection and other clinical parameters.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept - E.Ş., S.N.; Design - E.Ş., S.N., N.C., M.Z.; Supervision - E.Ş., S.N., H.S.K.; Funding - E.Ş., S.N., M.Z., N.C.; Materials - T.Y., M.Ö., Ç.E.; Data collection &/or processing - E.Ş., S.N., M.Ö., T.Y., Ç.E.; Analyisis and/or interpretation - E.Ş., S.N., N.C., M.Z.; Literature search - E.Ş., S.N., H.S.K.; Writing - E.Ş., H.S.K., M.Z., N.C., Ç.E., M.Ö.; Critical review - M.Ö., T.Y., Ç.E., E.Ş., S.N., H.S.K., M.Z., N.C.

Reden Sie klar, ich bin Arzt. Sie wollen dass ich Sie von Ihrer Schwangerschaft befreie. Solche Versuche sind für beide Teile gefährlich. Es ist mir gesetzlich untersagt. Ich verliere dadurch meine Pension.
- Ich werde sie Inhnen entschädigen.
Talk to me clearly, I am a physician. You expect me to save you from your pregnancy. These kind of actions are risky for both sides. The law forbids me to do this. I might loose my retirement right because of this.
-I will compensate this for you.
The one who made contribution to the Turkish language: Prof. Dr. Selçuk Ünlü
References
- 1.Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report-2012. J Heart Lung Transplant. 2012;31:1052–64. doi: 10.1016/j.healun.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Lindenfeld J, Miller GG, Shakar SF, Zolty R, Lowes BD, Wolfel EE, et al. Drug therapy in the heart transplant recipient:part II:immunosuppressive drugs. Circulation. 2004;110:3858–65. doi: 10.1161/01.CIR.0000150332.42276.69. [DOI] [PubMed] [Google Scholar]
- 3.Nair V, Butany J. Heart transplant biopsies:interpretation and significance. J Clin Pathol. 2010;63:12–20. doi: 10.1136/jcp.2009.072462. [DOI] [PubMed] [Google Scholar]
- 4.Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–20. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Jimenez J, Kapadia SR, Yamani MH, Platt L, Hobbs RE, Rincon G, et al. Cellular rejection and rate of progression of transplant vasculopathy:a 3-year serial intravascular ultrasound study. J Heart Lung Transplant. 2001;20:393–8. doi: 10.1016/s1053-2498(00)00249-7. [DOI] [PubMed] [Google Scholar]
- 6.Yamani MH, Yousufuddin M, Starling RC, Tuzcu M, Ratliff NB, Cook DJ, et al. Does acute cellular rejection correlate with cardiac allograft vasculopathy? J Heart Lung Transplant. 2004;23:272–6. doi: 10.1016/S1053-2498(03)00189-X. [DOI] [PubMed] [Google Scholar]
- 7.Baraldi-Junkins C, Levin HR, Kasper EK, Rayburn BK, Herskowitz A, Baughman KL. Complications of endomyocardial biopsy in heart transplant patients. J Heart Lung Transplant. 1993;12:63–7. [PubMed] [Google Scholar]
- 8.Winters GL, Loh E, Schoen FJ. Natural history of focal moderate cardiac allograft rejection. Is treatment warranted?Circulation. 1995;91:1975–80. doi: 10.1161/01.cir.91.7.1975. [DOI] [PubMed] [Google Scholar]
- 9.Christen T, Shimizu K, Libby P. Advances in imaging of cardiac allograft rejection. Curr Cardiovasc Imaging Rep. 2010;3:99–105. [Google Scholar]
- 10.Jackson E, Bellenger N, Seddon M, Harden S, Peebles C. Ischaemic and non-ischaemic cardiomyopathies-cardiac MRI appearances with delayed enhancement. Clin Radiol. 2007;62:395–403. doi: 10.1016/j.crad.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005;26:1461–74. doi: 10.1093/eurheartj/ehi258. [DOI] [PubMed] [Google Scholar]
- 12.Moon JC. What is late gadolinium enhancement in hypertrophic cardiomyopathy? Rev Esp Cardiol (English Version) 2007;60:1–4. [PubMed] [Google Scholar]
- 13.Braggion-Santos M, Andre F, Lossnitzer D, Hofmann E, Simpfendörfer J, Dösch A, et al. Prevalence of different forms of infarct-atypical late gadolinium enhancement in patients early and late after heart transplantation. Clin Res Cardiol. 2014;103:57–63. doi: 10.1007/s00392-013-0623-9. [DOI] [PubMed] [Google Scholar]
- 14.Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson. 2013;15:91. doi: 10.1186/1532-429X-15-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim RJ, Shah DJ, Judd RM. How we perform delayed enhancement imaging. J Cardiovasc Magn Reson. 2003;5:505–14. doi: 10.1081/jcmr-120022267. [DOI] [PubMed] [Google Scholar]
- 16.Hunold P, Schlosser T, Vogt FM, Eggebrecht H, Schmermund A, Bruder O, et al. Myocardial late enhancement in contrast-enhanced cardiac MRI:distinction between infarction scar and non–infarction-related disease. AJR Am J Roentgenol. 2005;184:1420–6. doi: 10.2214/ajr.184.5.01841420. [DOI] [PubMed] [Google Scholar]
- 17.Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114:1581–90. doi: 10.1161/CIRCULATIONAHA.105.606509. [DOI] [PubMed] [Google Scholar]
- 18.Steen H, Merten C, Refle S, Klingenberg R, Dengler T, Giannitsis E, et al. Prevalence of different gadolinium enhancement patterns in patients after heart transplantation. J Am Coll Cardiol. 2008;52:1160–7. doi: 10.1016/j.jacc.2008.05.059. [DOI] [PubMed] [Google Scholar]
- 19.Krieghoff C, Barten MJ, Hildebrand L, Grothoff M, Lehmkuhl L, Lucke C, et al. Assessment of sub-clinical acute cellular rejection after heart transplantation:comparison of cardiac magnetic resonance imaging and endomyocardial biopsy. Eur Radiol. 2014;24:2360–71. doi: 10.1007/s00330-014-3246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butler CR, Kumar A, Toma M, Thompson R, Chow K, Isaac D, et al. Late gadolinium enhancement in cardiac transplant patients is associated with adverse ventricular functional parameters and clinical outcomes. Can J Cardiol. 2013;29:1076–83. doi: 10.1016/j.cjca.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 21.Butler CR, Kim DH, Chow K, Toma M, Thompson R, Mengel M, et al. Cardiovascular MRI predicts 5-year adverse clinical outcome in heart transplant recipients. Am J Transplant. 2014;14:2055–61. doi: 10.1111/ajt.12811. [DOI] [PubMed] [Google Scholar]
- 22.Arraiza M, Azcarate PM, De Cecco CN, Viteri G, Simon-Yarza I, Hernandez-Estefania R, et al. Assessment of left ventricular parameters in orthotopic heart transplant recipients using dual-source CT and contrast-enhanced echocardiography:comparison with MRI. Eur J Radiol. 2012;81:3282–8. doi: 10.1016/j.ejrad.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Taylor AJ, Vaddadi G, Pfluger H, Butler M, Bergin P, Leet A, et al. Diagnostic performance of multisequential cardiac magnetic resonance imaging in acute cardiac allograft rejection. Eur J Heart Fail. 2010;12:45–51. doi: 10.1093/eurjhf/hfp174. [DOI] [PMC free article] [PubMed] [Google Scholar]