Abstract
Objective:
To evaluate the effects of the consumption of energy drinks on cardiovascular parameters in a group of healthy young individuals.
Methods:
In a quasi-experimental study, 44 healthy adult participants aged between 15 and 30 years were evaluated. The blood pressure (BP) as well as electrocardiographic indices, including heart rate (HR), PR interval, QRS duration, corrected QT (QTc) interval, and ST-T changes were recorded before consumption of a caffeine-containing energy drink and at the specific time points over a 4-h test duration.
Results:
We found statistically significant HR decline (p=0.004) and more frequent ST-T changes (p=0.004) after the participants consumed the energy drink. However, readings for systolic BP (p=0.44), diastolic BP (p=0.26), PR interval (p=0.449), QRS duration (p=0.235), and QTc interval (p=0.953) showed no significant change post-consumption.
Conclusion:
In conclusion, we demonstrated that the consumption of energy drinks could contribute to HR decline and ST-T change in healthy young adults.
Keywords: energy drinks, blood pressure, heart rate, ECG
Introduction
Energy drinks usually refer to a specific category of beverages that contain caffeine in varied amounts as well as some other ingredients such as taurine, vitamin B, sugar, and herbal supplements. After being first introduced around 1960 (1), they have been increasingly used all over the world for the promotion of mental concentration and vigilance.
Although few of these claims have been partly demonstrated in a few athlete-targeted surveys (2, 3), many more health concerns are increasingly unfolded about the excessive consumption of energy drinks. Consequences such as epilepsy, mania, stroke, and even sudden death have all been potentially associated with such beverages (4-6). In addition, a number of investigations focused on the hemodynamic effects of the consumption of energy drinks on healthy individuals; some of which found a statistically significant effect on heart rate (HR) and blood pressure (BP) (7, 8), and some others detected either no or minor nonsignificant effects (9, 10). A number of case reports also revealed that catastrophic complications such as aortic dissection are more likely to occur in individuals with underlying cardiovascular problems (11, 12).
Most published studies till date, however, have been limited by their small number of cases. Moreover, potential effects of the consumption of energy drinks on the electrocardiogram (ECG) have not been well described. We thus designed this investigation to evaluate the effects of the consumption of energy drinks on ECG as well as HR and BP in a relatively larger sample size.
Methods
Study design
The present survey was a quasi-experimental study.
Study subjects
Between July 2012 and March 2013, public announcement was made on the internet and in four different university campuses in Tehran, Iran. Healthy volunteers aged between 15 and 30 years were then recruited study subjects. Exclusion criteria were a medical history of cardiovascular disease, taking any medication affecting the cardiovascular system (such as anti-hypertensives and anti-arrhythmics), regular alcohol intake, a history of substance abuse, or taking central nervous system stimulants as well as conditions of pregnancy and lactation.
Study protocol
After enrollment, all study participants (and the guardian of a subject under the legal age) provided informed consent about the survey, and their baseline characteristics were collected. All experiments were performed at the Rasoul-e-Akram General Hospital, Tehran, Iran.
First, the participants were advised not to take alcohol within 48 h prior to the study date. Additionally, as caffeine is known to remain active in the human body for 3 to 6 h (13), participants were asked to not consume any caffeine containing products such as tea, chocolate, or cola drinks for at least 12 h before the study. They also did not consume anything (except water) during the study protocol.
For each study subject, 250 mL of an energy drink containing caffeine (80 mg), taurine, glucuronolactone, vitamins, and sugar was used. BP measurements and ECG recordings were performed just before drinking the beverage and at 30 min, 2 h, and 4 h post-consumption by a single general practitioner. ECG findings were then recorded by a single-blinded cardiologist.
BP (systolic and diastolic) was measured with a mercury sphygmomanometer after participants were seated and had rested for at least 5 min. ECG was performed in a supine position using a Cardioline AR1200 view BT EKG machine (Cavreno (TN), Italy).
The study was performed according to the Declaration of Helsinki and the protocol was approved by our local Ethics Committee.
Study variables
Baseline characteristics included the subjects’ age and sex.
BP was recorded as systolic and diastolic. For ECG, the following parameters were recorded:
Cardiac rhythm: in five categories as normal sinus rhythm (NSR), sinus tachycardia, sinus bradycardia, NSR with premature ventricular contraction (PVC), and other rhythms (atrial fibrillation, flutter, etc.),
HR,
Intervals and durations, including PR interval, QRS duration, and corrected QT (QTc) interval,
ST-T changes as ST depression or elevation, flattening of the T-wave, biphasic T-wave, and T-wave inversion.
Statistical analysis
The study data were analyzed using SPSS 11.5 (SPSS, Inc., Chicago, IL, USA). The Kolmogorov-Smirnov test was used to assess the normal distribution of the variables. For quantitative variables the repeated measures ANOVA and for qualitative variables the Cochran's Q and Kendall's W tests were used where appropriate. Statistical significance was considered as p<0.05.
Results
A total number of 44 subjects were recruited in this study, 20 (46%) of whom were females. No unexpected side effect occurred during the study and 100% of participants completed the study course. Baseline characteristics of the study participants are shown in Table 1.
Table 1.
Baseline characteristics of the study subjects
Values are the mean+standard deviation
Values are the number (percentage)
Analysis of BP changes by repeated measures ANOVA detected no significant difference in systolic BP (p=0.44) (Fig. 1) and diastolic BP (p=0.26) (Fig. 2).
Figure 1.
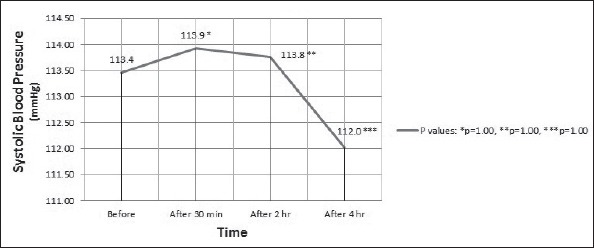
Systolic blood pressure (mean) of the participants during the test. Analysis by repeated measures ANOVA
Figure 2.
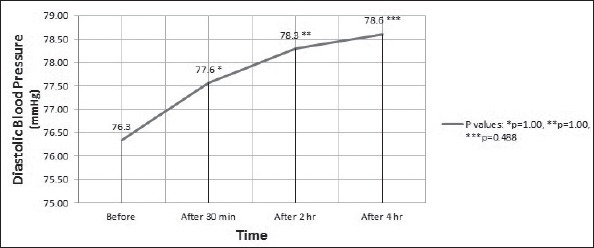
Diastolic blood pressure (mean) of the participants during the test. Analysis by repeated measures ANOVA
Among the all participants, three (7%) had sinus tachycardia, one (2%) had NSR with PVCs, and the other 40 (91%) had NSR as their baseline ECG rhythm. Table 2 summarizes the distribution of different cardiac rhythms in sequential time points during the study. As seen, the most frequent rhythm after NSR was sinus bradycardia at the 2- and 4-h time points post-consumption. The mean blood pressure and heart rate is provided in Table 3 for different rhythm groups. The Kendall's W test was used for analyzing cardiac rhythm and W (3)=0.056 and p=0.07 indicated no significant change in cardiac rhythm before and after the consumption of the energy drink.
Table 2.
Distribution of different cardiac rhythms before and after consumption of the energy drink
| Time point / Cardiac rhythm | Before* | After 30 min* | After 2 h* | After 4 h* |
|---|---|---|---|---|
| Normal sinus rhythm | 40 (91) | 42 (96) | 38 (86) | 36 (82) |
| Sinus tachycardiaα | 3 (7) | 1 (2) | 2 (5) | 3 (7) |
| Sinus bradycardiaβ | 0 (0) | 1 (2) | 3 (7) | 4 (9) |
| Normal sinus rhythm with premature ventricular complexes | 1 (2) | 0 (0) | 1 (2) | 1 (2) |
| Other rhythms | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Total | 44 (100) | 44 (100) | 44 (100) | 44 (100) |
Values are represented as the number (%) of participants.
Sinus tachycardia: HR above 100 bpm, median=107.5
Sinus bradycardia: HR below 60 bpm, median=55.5
Kendall’s W test: W (3)=0.056, p=0.07
Table 3.
Blood pressure and heart rate in different rhythm groups
| Normal sinus rhythm | ||||
|---|---|---|---|---|
| Before | After 30 min | After 2 h | After 4 h | |
| Number of participants | 40 | 42 | 38 | 36 |
| Systolic blood pressure* (mm Hg) | 112.62±10.43 | 113.45±12.22 | 113.11±11.56 | 111.94±9.04 |
| Diastolic blood pressure* (mm Hg) | 76.12±8.58 | 77.61±10.13 | 77.56±9.32 | 78.75±9.05 |
| Heart rate* (beats per minute) | 77.95±11.35 | 75.61±11.30 | 74.28±11.35 | 75.30±11.63 |
| Sinus tachycardia | ||||
| Before | After 30 min | After 2 h | After 4 h | |
| Number of participants | 3 | 1 | 2 | 3 |
| Systolic blood pressure* (mm Hg) | 135.00±8.66 | 135.00 | 135.00±7.07 | 131.67±2.88 |
| Diastolic blood pressure* (mm Hg) | 91.66±2.88 | 90.00 | 95.00±7.07 | 93.33±5.77 |
| Heart rate* (beats per minute) | 110.00±7.81 | 107.00 | 111.00±0 | 105.33±3.78 |
| Sinus bradycardia | ||||
| Before | After 30 min | After 2 h | After 4 h | |
| Number of participants | 0 | 1 | 3 | 4 |
| Systolic blood pressure* (mm Hg) | _ | 110.00 | 106.67±12.58 | 100.00±8.16 |
| Diastolic blood pressure* (mm Hg) | _ | 80.00 | 71.66±10.40 | 69.50±8.22 |
| Heart rate* (beats per minute) | _ | 58.00 | 56.00±2.00 | 55.50±2.51 |
Values are the mean+standard deviation
Readings for HR at 30 min post-consumption, however, showed a statistically significant HR drop (p=0.004) when calculated using within-subjects ANOVA. Pairwise comparisons also demonstrated a significant HR decline with time (p=0.043, p=0.009, and p=0.022 for 30 min, 2 h, and 4 h post-consumption, respectively). Figure 3 summarizes the data related to the sequential HR measurements.
Figure 3.
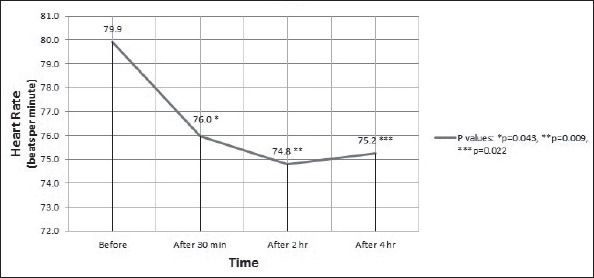
Heart rate (mean) of the participants during the test. Analysis by repeated measures ANOVA
Data for intervals and durations are presented in Table 4. As seen, when compared to baseline values, no significant changes were detected for PR interval (p=0.449), QRS duration (p=0.235), and QTc interval (p=0.953) using the repeated measures ANOVA.
Table 4.
Analysis results of PR interval, QRS duration, and corrected QT (QTc) interval
| Variable | Before* | After 30 min* | After 2 h* | After 4 h* | P |
|---|---|---|---|---|---|
| PR interval | 151.42±22.80 | 145.47±20.97 | 149.04±22.28 | 148.80±22.32 | 0.449 |
| QRS duration | 78.09±12.14 | 79.04±8.78 | 76.90±8.96 | 75.23±11.52 | 0.235 |
| QTc interval | 397.1220±26.18 | 396.9512±23.04 | 399.2683±26.34 | 399.2683±3132 | 0.953 |
Values are represented in milliseconds; ** Repeated measures ANOVA
According to the baseline ECGs, two participants had background T-wave inversions. The first case continued to have T-wave inversion in lead III at 30 min and 2 h post-consumption, but it returned to normal at a 4-h time point. In the second case, however, primary T-wave inversions of leads V1 and V2 disappeared at 30-min and 2-h time points but appeared again at a 4 h post-consumption.
Among the 42 subjects with normal primary ECGs, one (2%), six (14%), and two (5%) had post-consumption ST-T changes at 30-min, 2-h, and 4-h time points, respectively (Table 5). The observed changes were the appearance of U wave and T-wave inversion in leads III, V1, V2, and V3 (Table 6). Results of the Cochran's Q test, Q (3)=13.105 and p=0.004, demonstrated significantly more frequent ST-T changes after the consumption of the energy drink.
Table 5.
Frequencies of ST-T changes among study subjects with normal primary electrocardiogram (ECG)
| Time point for ECG | ST-T changes | ST-T changes |
|---|---|---|
| absent* | present* | |
| Baseline | 42 (100) | 0 (0) |
| 30 min post-consumption | 41 (98) | 1 (2)** |
| 2 h post-consumption | 36 (86) | 6 (14)α |
| 4 h post-consumption | 40 (95) | 2 (5)β |
Values are the number (percentage)
T-wave inversion in V1 and V2
T-wave inversion in III, V1, and V2; U wave in V3
T-wave inversion in V1, V2, and V3 Cochran’s Q test, Q (3)=13.105, p=0.004
Table 6.
Specific post-consumption ST-T changes among the study subjects
| Time point/Participant | Before drink | After 30 min | After 2 h | After 4 h |
|---|---|---|---|---|
| Participant A | None | None | T-wave inversion in III | None |
| Participant B | None | None | T-wave inversion in III U wave in V3 | None |
| Participant C | None | None | T-wave inversion in V1 and V2 | T-wave inversion in V1 and V2 |
| Participant D | None | None | T-wave inversion in V1 and V2 | None |
| Participant E | None | None | T-wave inversion in V1 | None |
| Participant F | None | T-wave inversion in V1 and V2 | T-wave inversion in V1 and V2 | T-wave inversion in V1, V2, and V3 |
(3)=13.105 and p=0.004, demonstrated significantly more frequent ST-T changes after the consumption of the energy drink.
Discussion
The present study was designed to determine the acute effects of the consumption of energy drinks on the cardiovascular system. Based on the results, we found statistically significant declines in HR (p=0.004) and significantly more frequent ST-T changes (p=0.004) at the first hours of consuming the energy drink. However, readings for systolic and diastolic BP (p=0.44 and p=0.26, respectively), PR interval (p=0.449), QRS duration (p=0.235), and QTc interval (p=0.953) showed no significant changes post-consumption.
Energy drinks, as a marketing tool, have been rapidly promoted for their claimed beneficial effects such as metabolic stimulation and improved concentration. Some potential adverse effects related to these beverages, however, have been concerning in recent years; among these effects, cardiovascular effects are of special importance. The impact of energy drinks on the cardiovascular system can be assessed by two main measures: hemodynamic parameters (such as BP and HR) and electrophysiological indices (including cardiac rhythm, PR interval, QRS duration, QTc interval, and ST-T changes).
Regarding the hemodynamic parameters, a literature review indicates that the results of different studies were not always coherent. For example, Alford et al. (14) investigated the impact of the energy drink Red Bull (250 mL) on exercise performance of 36 volunteers and reported no change in resting BP 30 min after consumption. Similarly, Ragsdale et al. (15) in a placebo-controlled study, reported no change in BP throughout a 2-h test period after taking 250 mL of Red Bull. By contrast, Worthley et al. (9) found an increase in BP after the consumption of 250 mL of a sugar-free energy drink compared with water (control). Steinke et al. (8) also reported a significant increase in BP after taking 500 mL of an unspecified energy drink at 2 and 4 h post-consumption. Likewise, Sung et al. (16), who used 3.3 mg/kg of caffeine in 34 healthy men, and Lemery et al. (17), who used 5 mg/kg of caffeine in 80 patients with symptomatic supraventricular tachycardia, both found a significant increase in resting systolic and diastolic BP. However, according to Franks et al. (18), who indicated that energy drink supplementation might increase the mean BP more than a caffeine control, the abovementioned studies are not fully comparable to the others. Some of the discrepancies across the former studies could also be attributed to different types of energy drinks as well as different amounts of the same type of energy drink used for investigation. Additionally, the duration of post-consumption BP monitoring varies among with studies from as early as 15 to 30 min (which mostly indicated no BP change) to even 24 h or more (that mostly demonstrated a rise in BP). Taking these points into consideration, it can be hypothesized that energy drinks increase BP when they are used in sufficiently high amounts and when BP is monitored at sufficiently late time points post-consumption. Thus, it would have been possible to observe a significant elevation in BP in this study if we had used higher amounts of the same energy drink. This hypothesis, however, requires more focused investigations, which can be targeted in future studies.
Similar disparities are also seen for HR between different studies. Although GeiB et al. (19) (who examined 10 endurance athletes during submaximal exercise after the consumption of 500 mL of Red Bull) and Bichler et al. (20) (who used caffeine plus taurine-containing pills for some college students and monitored HR within 45 min) in accordance with our results demonstrated a significant drop in HR, Alford et al. (14) and Ragsdale et al. (15) found a trend toward HR decline, and Grasser et al. (7) (gave 355 mL of Red Bull to 25 healthy subjects with a 2-h HR recording) and Steinke et al. (8) detected a statistically significant increase in HR after the consumption of energy drinks. In addition to the reasons mentioned earlier for BP, some of the discrepancies could be attributed to the different lifestyle and the fitness state of the study subjects as well as the inclusion of energy drink-habituated subjects and the lack of strict control of food and beverage consumption close the experimental period. Interestingly, after focusing only on the caffeine dosage in different studies, it could be speculated that HR is diminished after lower doses (<5 mg/kg) of caffeine, but higher doses would increase HR. Moreover, as also previously supposed by McClaran et al. (21), the final effect of caffeine-containing products on HR varies with different stress levels such that the tendency to reduce HR is reversed when they are consumed during high-stress situations. In this regard, moderate-to-high-intensity exercises might be perceived as stressful conditions (during which HR is increased), and conversely, rest or low-to-moderate-intensity exercises, especially in healthy people, might be perceived as not very stressful ones (during which HR is diminished).
On the other hand, from a pathophysiological point of view, there are some possible explanations for HR decline after the consumption of energy drinks. First, according to the baroreflex mechanism in response to a post-consumption BP elevation, HR is lowered to maintain normal BP (22). Because we found that BP changes were insignificant in the present study, this explanation might not be fully applicable to our study. Second, because of the inconsistent correlation between HR and BP, it has been speculated that the drop in HR is due to direct central stimulation of the vagus nerve (by caffeine) rather than the baroreflex mechanism (23). Third, assuming that the cardiac output does not significantly change after the consumption of caffeine-containing energy drinks (24-26), an augmented post-consumption stroke volume would lead to decline in HR. Then, the most likely mechanisms for increased stroke volume are either the enhancement of myocardial contractility (21) or an increase in the left ventricular preload (27, 28) after the consumption of energy drinks.
Among the electrophysiological indices, most published investigations till date have focused on the effects of caffeine consumption on cardiac rhythm as well as ECG durations and intervals. After studies by Sutherland et al. (29), Newcombe et al. (30), and Chelsky et al. (31), results of a review article by Myers (32) also demonstrated that moderate ingestion of caffeine does not increase the frequency of cardiac arrhythmias. In accordance with those studies (about caffeine) and our findings (about energy drinks), Lemery et al. (17) also recently indicated that caffeine in moderate doses has no significant impact on cardiac conduction and refractoriness. To the best of our knowledge, however, there is no robust investigation about the potential effects of energy drinks on ST-T changes. Given that any dynamic ST-T change could reflect a probable subendocardial damage, our findings of more frequent post-consumption ST-T changes are of high clinical importance. Accordingly, it might be hypothesized that excessive catecholamine release after the consumption of energy drinks is the responsible mechanism for the significant ST-T changes, an issue which is similar to the “catecholamine storm” theory for ECG changes after subarachnoid hemorrhage (33-35). Yet, larger clinical trials are needed to support that claim.
The present study was a quasi-experimental study involving as many as 44 participants, higher than previous investigations. Using a single physician for BP and ECG measurements and a single cardiologist for recording ECG findings, we omitted any interobserver variability. Also, by advising the study subjects to abstain from alcohol and caffeine-containing products in a given period of time prior till the study, we minimized potential confounding effects. As BP and HR readings are influenced by many factors, attempts were made in this study to eliminate the effects of factors, such as activity, eating, and stress, as much as possible.
Study limitations
The lack of an appropriate control group with a placebo drink is one of the most important limitations of the present study. We also followed the participants only for 4 h post-consumption; our results, therefore, are limited to a 4-h time period after the consumption of the energy drink. Not using a method to objectively confirm that the participants successfully abstained from alcohol and caffeine-containing products can be considered as another limitation of this study. Finally, although our sample size is larger than most sample sizes previously reported, it is still extremely small to derive at a strong conclusion. Thus, the inclusion of more subjects is warranted to improve the power of future studies.
Conclusion
We demonstrated that the consumption of energy drinks could contribute to HR decline and ST-T changes in healthy young adults. Nonetheless, systolic and diastolic BP and other ECG parameters do not change significantly after the consumption of the energy drink.
Footnotes
Conflict of interest: None declared. All of the expenses of the present study were covered by agrant from Iran University of Medical Sciences.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept - S.H., F.M.; Design - S.H., F.M.; Supervision - S.H.; Fundings - S.H., F.M.; Materials - F.M., M.J.M., K.K., M.T.; Data collection &/or processing - F.M., M.J.M., K.K., M.T.; Analysis &/or interpretation - F.M., M.J.M., E.R.; Literature search - F.M., M.J.M.; Writing - S.H., F.M., M.J.M., K.K., M.T., E.R.; Critical review - S.H., F.M., M.J.M., K.K., M.T., E.R.
References
- 1.Reissig CJ, Strain EC, Griffiths RR. Caffeinated energy drinks.A growing problem. Drug Alcohol Depend. 2009;99:1–10. doi: 10.1016/j.drugalcdep.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abian-Vicen J, Puente C, Salinero JJ, González-Millán C, Areces F, Muñoz G, et al. A caffeinated energy drink improves jump performance in adolescent basketball players. Amino Acids. 2014;46:1333–41. doi: 10.1007/s00726-014-1702-6. [DOI] [PubMed] [Google Scholar]
- 3.Lara B, Gonzalez-Millán C, Salinero JJ, Abian-Vicen J, Areces F, Barbero-Alvarez JC, et al. Caffeine-containing energy drink improves physical performance in female soccer players. Amino Acids. 2014;46:1385–92. doi: 10.1007/s00726-014-1709-z. [DOI] [PubMed] [Google Scholar]
- 4.Clauson KA, Shields KM, McQueen CE, Persad N. Safety issues associated with commercially available energy drinks. J Am Pharm Assoc (Wash) 2008;48:e55–e63. doi: 10.1331/JAPhA.2008.07055. [DOI] [PubMed] [Google Scholar]
- 5.Garriott JC, Simmons LM, Poklis A, Mackell MA. Five cases of fatal overdose from caffeine-containing “look-alike” drugs. J Anal Toxicol. 1985;9:141–3. doi: 10.1093/jat/9.3.141. [DOI] [PubMed] [Google Scholar]
- 6.Kerrigan S, Lindsey T. Fatal caffeine overdose. two case reports. Forensic Sci Int. 2005;153:67–9. doi: 10.1016/j.forsciint.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Grasser EK, Yepuri G, Dulloo AG, Montani JP. Cardio- and cerebrovascular responses to the energy drink Red Bull in young adults:a randomized cross-over study. Eur J Nutr. 2011;53:1561–71. doi: 10.1007/s00394-014-0661-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinke L, Lanfear DE, Dhanapal V, Klaus JS. Effect of “energy drink” consumption on hemodynamic and electrocardiographic parameters in healthy young adults. Ann Pharmacother. 2009;43:596–602. doi: 10.1345/aph.1L614. [DOI] [PubMed] [Google Scholar]
- 9.Worthley MI, Prabhu A, De Sciscio P, Schultz C, Sanders P, Willoughby SR. Detrimental effects of energy drink consumption on platelet and endothelial function. Am J Med. 2010;123:184–7. doi: 10.1016/j.amjmed.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Nienhueser J, Brown GA, Shaw BS, Shaw I. Effects of energy drinks on metabolism at rest and during submaximal treadmill exercise in college age males. Int J ExercSci. 2011;4:65–76. [Google Scholar]
- 11.Jonjev ZS, Bala G. High-energy drinks may provoke aortic dissection. Coll Antropol. 2013;2:227–9. [PubMed] [Google Scholar]
- 12.Hsue PY, Salinas CL, Bolger AF, Benowitz NL, Waters DD. Acute aortic dissection related to crack cocaine. Circulation. 2002;105:1592–5. doi: 10.1161/01.cir.0000012524.44897.3a. [DOI] [PubMed] [Google Scholar]
- 13.Nawrot P, Jordan S, Eastwood J, Rotstein J, Hugenholtz A, Feeley M. Effects of caffeine on human health. Food Addit Contam. 2003;20:1–30. doi: 10.1080/0265203021000007840. [DOI] [PubMed] [Google Scholar]
- 14.Alford C, Cox H, Wescott R. The effects of red bull energy drink on human performance and mood. Amino Acids. 2001;21:139–50. doi: 10.1007/s007260170021. [DOI] [PubMed] [Google Scholar]
- 15.Ragsdale FR, Gronli TD, Batool N, Haight N, Mehaffey A, McMahon EC, et al. Effect of Red Bull energy drink on cardiovascular and renal function. Amino Acids. 2010;38:1193–200. doi: 10.1007/s00726-009-0330-z. [DOI] [PubMed] [Google Scholar]
- 16.Sung BH, Lovallo WR, Pincomb GA, Wilson MF. Effects of caffeine on blood pressure response during exercise in normotensive healthy young men. Am J Cardiol. 1990;65:909–13. doi: 10.1016/0002-9149(90)91435-9. [DOI] [PubMed] [Google Scholar]
- 17.Lemery R, Pecarskie A, Bernick J, Williams K, Wells GA. A prospective placebo controlled randomized study of caffeine in patients with supraventricular tachycardia undergoing electrophysiologic testing. J Cardiovasc Electrophysiol. 2015;26:1–6. doi: 10.1111/jce.12504. [DOI] [PubMed] [Google Scholar]
- 18.Franks AM, Schmidt JM, McCain KR, Fraer M. Comparison of the effects of energy drink versus caffeine supplementation on indices of 24-hour ambulatory blood pressure. Ann Pharmacother. 2012;46:192–9. doi: 10.1345/aph.1Q555. [DOI] [PubMed] [Google Scholar]
- 19.Geiß KR, Jester I, Falke W, Hamm M, Waag KL. The effect of a taurine-containing drink on performance in 10 endurance-athletes. Amino Acids. 1994;7:45–56. doi: 10.1007/BF00808445. [DOI] [PubMed] [Google Scholar]
- 20.Bichler A, Swenson A, Harris MA. A combination of caffeine and taurine has no effect on short term memory but induces changes in heart rate and mean arterial blood pressure. Amino Acids. 2006;31:471–6. doi: 10.1007/s00726-005-0302-x. [DOI] [PubMed] [Google Scholar]
- 21.McClaran SR, Wetter TJ. Low doses of caffeine reduce heart rate during submaximal cycle ergometry. J Intern Soc Sports Nutrition. 2007;4:11. doi: 10.1186/1550-2783-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosqueda-Garcia R, Tseng CJ, Biaggioni I, Robertson RM, Robertson D. Effects of caffeine on baroreflex activity in humans. Clin Pharmacol Ther. 1990;48:568–74. doi: 10.1038/clpt.1990.193. [DOI] [PubMed] [Google Scholar]
- 23.Whitsett TL, Manion CV, Christensen HD. Cardiovascular effects of coffee and caffeine. Am J Cardiol. 1984;53:918–22. doi: 10.1016/0002-9149(84)90525-3. [DOI] [PubMed] [Google Scholar]
- 24.Pincomb GA, Lovallo WR, Passey RB, Whitsett TL, Silverstein SM, Wilson MF. Effects of caffeine on vascular resistance, cardiac output, and myocardial contractility in young men. Am J Cardiol. 1985;56:119–22. doi: 10.1016/0002-9149(85)90578-8. [DOI] [PubMed] [Google Scholar]
- 25.Engels HJ, Wirth JC, Celik S, Dorsey JL. Influence of caffeine on metabolic and cardiovascular functions during sustained light intensity cycling and at rest. Int J Sport Nutr. 1999;9:361–70. doi: 10.1123/ijsn.9.4.361. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan JJ, Knowlton RG, Brown DD. Caffeine affects heart rate and blood pressure response to prolonged walking. J Cardiopulm Rehabil. 1992;12:418–22. [Google Scholar]
- 27.Gould L, Verkatararaman K, Goswami M, Gomprecht RF. The cardiac effects of coffee. Angiology. 1973;24:455–63. doi: 10.1177/000331977302400801. [DOI] [PubMed] [Google Scholar]
- 28.Robertson D, Frolich JC, Carr RK, Watson JT, Hollifield JW, Shand DG. Effects of caffeine on plasma rennin activity, catecholamines and blood pressure. N Engl J Med. 1978;298:181–6. doi: 10.1056/NEJM197801262980403. [DOI] [PubMed] [Google Scholar]
- 29.Sutherland DJ, McPherson DD, Renton KW, Spencer CA, Montague TJ. The effect of caffeine on cardiac rate, rhythm, and ventricular repolarization. Analysis of 18 normal subjects and 18 patients with primary ventricular dysrhythmia. Chest. 1985;87:319–24. doi: 10.1378/chest.87.3.319. [DOI] [PubMed] [Google Scholar]
- 30.Newcombe PF, Renton KW, Rautaharju PM, Spencer CA, Montague TJ. High-dose caffeine and cardiac rate and rhythm in normal subjects. Chest. 1988;94:90–4. doi: 10.1378/chest.94.1.90. [DOI] [PubMed] [Google Scholar]
- 31.Chelsky LB, Cutler JE, Griffith K, Kron J, McClelland JH, McAnulty JH. Caffeine and ventricular arrhythmias. An electrophysiological approach. JAMA. 1990;264:2236–40. [PubMed] [Google Scholar]
- 32.Myers MG. Caffeine and cardiac arrhythmias. Ann Intern Med. 1991;114:147–50. doi: 10.7326/0003-4819-114-2-147. [DOI] [PubMed] [Google Scholar]
- 33.Smith M. Intensive care management of patients with subarachnoid haemorrhage. Curr Opin Anesthesiol. 2007;20:400–7. doi: 10.1097/ACO.0b013e3282efa686. [DOI] [PubMed] [Google Scholar]
- 34.Tung P, Kopelnik A, Banki N, Ong K, Ko N, Lawton MT, et al. Predictors of neurocardio genic injury after subarachnoid hemorrhage. Stroke. 2004;35:548–53. doi: 10.1161/01.STR.0000114874.96688.54. [DOI] [PubMed] [Google Scholar]
- 35.Macmillan CSA, Grant IS, Andrews PJD. Pulmonary and cardiac sequelae of subarachnoid haemorrhage:time for active management. Intensive Care Med. 2002;28:1012–23. doi: 10.1007/s00134-002-1382-7. [DOI] [PubMed] [Google Scholar]


