Abstract
Objective:
We examined the change in apelin concentration and its relationship with left ventricular diastolic function in patients treated for hypertension.
Methods:
Ninety treatment-naive patients with newly diagnosed hypertension and 33 age- and sex-matched control subjects were prospectively enrolled. Patients with hypertension were randomized to treatment either with telmisartan 80 mg or amlodipine 10 mg. Apelin concentration was measured and echocardiography was performed at baseline and after 1 month of treatment.
Results:
The data of 77 patients and 33 controls were analyzed. Mean age, gender, baseline blood pressure, apelin levels, and echocardiographic measurements were similar between the treatment groups (p>0.05 for all). Apelin concentration was significantly lower in patients with hypertension than in controls. There was a significant increase in apelin level after 1 month of treatment in both groups (0.32±0.17 vs. 0.38±0.17 ng/dL in telmisartan group, p=0.009, and 0.27±0.13 vs. 0.34±0.18 ng/dL in amlodipine group, p=0.013). Diastolic function improved significantly in both groups (p<0.05) but was not significantly associated with change in apelin concentration.
Conclusion:
Apelin concentration increased significantly after 1 month of effective treatment with telmisartan or amlodipine to a similar extent. Change in apelin concentration was not associated with improvement in diastolic function.
Keywords: apelin, hypertension, diastolic function
Introduction
Apelin is an endogenous peptide first isolated from bovine stomach extracts, and is reported to have a variety of functions in the cardiovascular system. Apelin has been identified as endogenous ligand for the G-protein coupled receptor apelin receptor (APJ), which has structural similarities with the angiotensin II type 1 receptor (1, 2). It has been shown that the apelin/APJ system plays a role in the pathophysiology of atherosclerosis, coronary artery disease, heart failure, hypertension, and pulmonary hypertension (3). It has been reported that plasma apelin concentration is decreased in patients with hypertension (4, 5). The apelin/APJ pathway influences blood pressure by an endothelium-dependent, nitric oxide (NO)-mediated mechanism that causes arterial vasodilatation (6).
The relationship between apelin concentration and left ventricular (LV) diastolic function in patients with essential hypertension is not completely understood, although recently it has been reported that circulating apelin concentration is associated with LV systolic and diastolic impairment (5).
We hypothesized that plasma apelin concentration would be lower in patients with untreated arterial hypertension than patients with normal blood pressure and that apelin concentration would rise in response to effective treatment of hypertension independent of the drug choice. We also sought to examine the relationship between apelin concentration and LV diastolic function before and after treatment.
In order to achieve these goals, we 1) compared the plasma apelin concentration of patients with hypertension and healthy controls; 2) examined the change in plasma apelin concentration in patients with newly diagnosed hypertension after 1 month of drug therapy, and compared the effect of two anti-hypertensive drug regimens on apelin concentration (telmisartan or amlodipine); and 3) examined the relationship between plasma apelin concentration and improvements in LV diastolic function after 1 month of treatment.
Methods
Patients
We prospectively enrolled 90 patients (50 men and 40 women), aged over 18 years referred to our outpatient cardiology clinic and diagnosed with stage 1 or 2 essential hypertension based on the European Society of Cardiology guidelines (7). Thirty-three age- and sex-matched participants with normal blood pressure who were admitted to our outpatient cardiology clinic with atypical chest pain or palpitation served as the control group. The local Ethics Committee approved the study protocol, and written informed consent was obtained from each participant. At the first visit, each patient underwent a medical evaluation, including clinical history, physical examination, routine blood analysis, lipid profile, electrocardiography, and echocardiography. Blood pressure was measured with an appropriately sized arm cuff and a manual mercury sphygmomanometer after 5 minutes of rest. The same physician performed all measurements, and the mean of two sitting measurements at 2-minute intervals was used. Hypertension was defined as a systolic blood pressure (SBP) ≥140 mm Hg and/or a diastolic blood pressure (DBP) ≥90 mm Hg on repeated measurements. Apelin concentration has been shown to be associated with several conditions, including coronary artery stenosis, diabetes, obesity, aortic stenosis, chronic kidney disease, and heart failure (8–13). Therefore, patients who had a history of coronary artery disease, secondary hypertension, diabetes mellitus, moderate or severe valvular heart disease, body mass index above 30 kg/m2, LV ejection fraction <45%, or serum creatinine concentration >1.6 mg/dL were excluded. None of the patients were taking any antihypertensive medication at baseline. Patients diagnosed with essential hypertension were randomly assigned to treatment with either 80 mg telmisartan or 10 mg amlodipine orally once daily. Randomization was performed using a random number table. In addition to routine blood analysis, another blood sample for apelin measurement was obtained from each patient and stored in our laboratory for later analysis.
Thirteen patients were excluded from the final analysis: five were lost to follow-up (three in the amlodipine group and two in the telmisartan group); five stopped treatment due to side effects (three in the amlodipine group and two in the telmisartan group); and three required additional drug therapy to bring blood pressure under control (one in the amlodipine group and two in the telmisartan group).
After 1 month of treatment, patients with hypertension were recalled for echocardiography and blood sampling for measurement of follow-up plasma apelin concentration. Ambulatory blood pressure monitoring was performed to evaluate the effectiveness of antihypertensive therapy using an oscillometric device (Tracker NIBP2, DelMar Reynolds Medical, Morrisville, USA) that recorded blood pressure every 30 minutes during the day and every 45 minutes at night.
Blood biochemistry and measurement of plasma apelin concentration
All blood samples were taken in the morning after 12 hours of fasting. Complete blood count, blood glucose levels, lipid profile, liver enzyme, and creatinine concentrations were analyzed on the same day. For apelin analysis, blood samples were immediately centrifuged, and plasma was separated and stored at –80°C for analysis as a batch after randomization. Plasma apelin concentration was quantified by enzyme-linked immunosorbent assay (Human apelin-12 EIA Kit, Phoenix Pharmaceuticals, USA), with a minimum detectable concentration of 0.07 ng/mL, intra-assay variation of ±10% and inter-assay variation of ±15%.
Echocardiography
Echocardiographic imaging was performed using Philips Sonos 7500 Ultrasound System equipment with a 2–4-MHz multifrequency transducer (Andover, MA, USA). The same experienced echocardiographer performed all the studies with the patients in left lateral decubitus position. Cardiac chamber dimensions and wall thicknesses were measured from parasternal long-axis and apical four-chamber views according to the American Society of Echocardiography guidelines (14). Left atrial (LA) volumes were measured from the apical four-chamber view using the biplane area-length method, and indexed for body surface area. Left ventricular end-systolic and end-diastolic volumes, and ejection fraction, were estimated using a modified Simpson’s biplane method. Left ventricular mass (LVM) was calculated according to the Devereux formula, and corrected for body surface area to determine the LVM index (LVMI).
Transmitral pulsed Doppler was recorded from the apical four-chamber view, with the sample volume positioned at the tips of the mitral valve leaflets. Peak early filling (E) and atrial filling (A) velocities, the deceleration time of the peak E wave (MDT), the duration of the mitral A wave (A time), and isovolumic relaxation time (IVRT) were measured from the transmitral flow pattern, and the E/A ratio was calculated.
Pulsed-wave tissue Doppler imaging was performed on the lateral and septal sites of the mitral annulus from the apical four-chamber view. Mean values of three consecutive beats were used for analysis. Peak systolic myocardial velocity (s), and peak early and late diastolic velocities (e’ and a’), were measured. Mean values of the tissue Doppler measurements from the lateral and septal sites were calculated. The ratio of mitral inflow early diastolic velocity to mitral annular peak early diastolic velocity (E/e’) was calculated.
The myocardial performance index (MPI, also known as the Tei-Doppler index), an index of combined systolic and diastolic function, was defined as the sum of the isovolumic contraction time and the isovolumic relaxation time divided by the ejection time (15). The MPI for the LV was calculated from the pulsed-wave tissue Doppler recordings on the lateral (lateral MPI) and septal (septal MPI) sites of the mitral annulus from the apical four-chamber view.
Statistical analysis
The frequency (percent) of categorical variables, and mean±standard deviation for continuous variables are presented as descriptive statistics. The nature of the distribution of continuous variables was evaluated using the Shapiro-Wilk test. The chi-squared test and Kruskal-Wallis variance analysis were used to compare more than two independent groups for categorical variables and continuous variables, respectively. Bonferroni corrected Mann-Whitney U test was used after Kruskal-Wallis variance analysis in the case of significant differences between groups. The paired t-tests were performed to compare two dependent groups. In the presence of covariates, covariance analysis was undertaken to compare independent groups for continuous variables. The Spearman correlation coefficient was used to assess the degree of association between two variables. All statistical analyses were performed using SPSS 17.0 software program (SPSS Inc., Chicago, IL, USA). A p value <0.05 was considered statistically significant.
Results
Data obtained from 77 patients (of median age 48 years, 44 of whom were men) with hypertension and 33 controls (21 men, median age: 49 years) were analyzed. Thirty-eight patients were randomized to treatment with amlodipine, and 39 to treatment with telmisartan. The demographic and clinical characteristics of the study population at baseline were similar between control and treatment groups except blood pressure measurements which were lower in the control group (Table 1). Baseline mean apelin concentration and hemoglobin level was significantly higher in the control group than the treatment groups (Table 1).
Table 1.
Demographic, clinical and laboratory characteristics of telmisartan and amlodipine groups at baseline
| Telmisartan group (n=39) | Amlodipine group (n=38) | Control group (n=33) | P | |
|---|---|---|---|---|
| Age, years | 48±11 | 48±9 | 49±5 | 0.735 |
| Female, n,% | 17 (43) | 16 (42) | 12 (36) | 0.810 |
| Height, cm | 170±9 | 170±9 | 165±5 | 0.078 |
| Weight, kg | 75±12 | 78±11 | 72±5 | 0.089 |
| BMI, kg/m2 | 25.9±2.4 | 26.8±2.1 | 26.4±1.5 | 0.175 |
| Glucose, mg/dL | 95±7 | 93±6 | 92±4 | 0.182 |
| HDL, mg/dL | 46±11 | 49±18 | 40±8 | 0.163 |
| LDL, mg/dL | 145±41 | 130±42 | 134±25 | 0.227 |
| Triglyceride, mg/dL | 126±32 | 131±34 | 118±27 | 0.342 |
| Creatinine, mg/dL | 0.7±0.1 | 0.7±0.1 | 0.7±0.1 | 0.333 |
| Hemoglobin, mg/dL | 14.4±1.3 | 15.0±1.4 | 15.8±1.3 | 0.0011 |
| Apelin, ng/mL | 0.27±0.13 | 0.32±0.17 | 0.56±0.17 | <0.0011 |
| SBP, mm Hg | 154±7 | 154±10 | 118±7 | <0.0011 |
| DBP, mm Hg | 92±6 | 95±9 | 78±4 | <0.0011 |
Values are expressed as mean±standard deviation. BMI - body mass index; BSA - body surface area; DBP - diastolic blood pressure; HDL - high-density lipoprotein; LDL - low-density lipoprotein; SBP - systolic blood pressure.
- Significant difference between control-amlodipine and control-telmisartan groups
Baseline echocardiographic parameters are shown in Table 2. The LVMI, interventricular septum thickness, and posterior wall thickness were similar between the telmisartan and amlodipine groups at baseline, and were significantly higher than the control group (Table 2). Left ventricular end-diastolic diameter was higher in the control group than the treatment groups, whereas EF was similar among 3 groups. The mean lateral and septal annular peak early diastolic velocities (lat-sep e’) at baseline were 8.9±1.8 cm/s and 8.3±2.3 cm/s in the telmisartan and amlodipine groups, respectively (p=0.239). Indices of LV diastolic function before treatment were similar between the telmisartan and amlodipine groups (Table 2).
Table 2.
Echocardiographic parameters of study groups at baseline
| Telmisartan group (n=39) | Amlodipine group (n=38) | Control group (n=33) | P | ||
|---|---|---|---|---|---|
| Left atrial size, cm | 3.4±0.2 | 3.5±0.2 | 3.4±0.2 | 0.251 | |
| LV end-diastolic dimension, cm | 4.3±0.3 | 4.3±0.5 | 4.6±0.4 | 0.0031 | |
| IST, cm | 1.1±0.1 | 1.1±0.1 | 0.8±0.1 | <0.0011 | |
| PWT, cm | 1.1±0.1 | 1.0±0.1 | 0.8±0.1 | <0.0011 | |
| LVEF, % | 60±3 | 60±2 | 64±4 | 0.252 | |
| E/A ratio | 1.0±0.3 | 0.9±0.2 | 0.9±0.3 | 0.289 | |
| IVRT, ms | 98±14 | 101±12 | 84±6 | <0.0011 | |
| Lat-sep e’, cm/s | 8.9±1.8 | 8.3±2.3 | 12.6±1.5 | <0.0011 | |
| Lateral IVRT, ms | 96±11 | 99±9 | 86±5 | <0.0011 | |
| Septal IVRT, ms | 95±9 | 100±12 | 85±7 | <0.0011 | |
| Lateral s’, cm/s | 11.5±0.6 | 11.1±0.8 | 12.7±0.5 | 0.189 | |
| Septal s’, cm/s | 10.9±0.6 | 10.8±0.7 | 11.5±0.6 | 0.354 | |
| E/e’ ratio | 7.8±1.5 | 8.6±2.2 | 5.2±0.9 | <0.0011 | |
| LAVI, mL/ m2 | 21.0±3.6 | 21.1±3.0 | 20.0±2.1 | 0.283 | |
| Lateral MPI | 0.64±0.16 | 0.64±0.11 | 0.41±0.05 | <0.0011 | |
| Septal MPI | 0.61±0.10 | 0.63±0.08 | 0.41±0.05 | <0.0011 | |
| LVMI, gr/ m2 | 114±23 | 112±24 | 97±13 | <0.0011 |
Values are expressed as mean±standard deviation. A - peak atrial filling velocity; E - peak early filling velocity; IST - interventricular septum thickness; IVRT - isovolumic relaxation time; Lat/sep e’ - mean of lateral and septal peak early diastolic velocities; LAVI - left atrial volume index; LV - left ventricle; LVEF - LV ejection fraction; LVMI - LV mass index; MPI - myocardial performance index; s’ - peak systolic annular velocity; PWT - Posterior wall thickness.
- Significant difference between control-amlodipine and control-telmisartan groups
The mean plasma apelin concentration before treatment in the telmisartan, amlodipine, and control groups was 0.27±0.13 ng/mL, 0.32±0.17 ng/mL, and 0.56±0.17 ng/mL, respectively (p<0.001). Apelin concentrations were similar between treatment groups (p=0.150). Apelin concentration was significantly increased in whole patients taking antihypertensive therapy (from 0.29±0.15 ng/mL at baseline to 0.36±0.17 ng/mL at 1 month, p<0.001). There was also a significant increase in plasma apelin concentration after 1 month of treatment in both the amlodipine (from 0.32±0.17 ng/mL at baseline to 0.38±0.17 ng/mL at 1 month, p=0.013) and telmisartan groups (from 0.27±0.13 ng/mL at baseline to 0.34±0.18 ng/mL at 1 month, p=0.009). The incremental change was similar in each of the treatment groups (p=0.922, Fig. 1 and Table 3).
Figure 1.
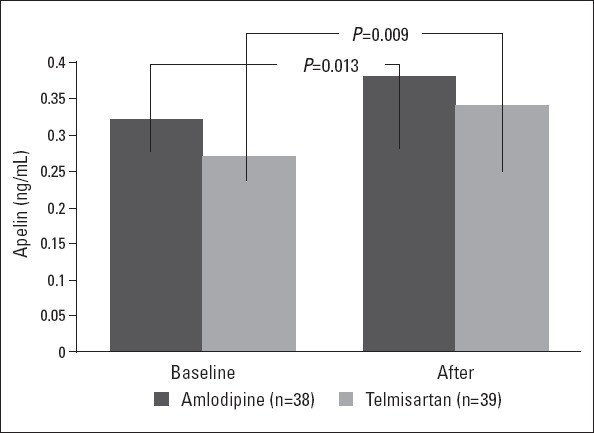
Effects of a month of treatment with amlodipine or telmisartan on plasma apelin levels. Data are shown as means
Table 3.
Comparison of apelin concentration, systolic and diastolic blood pressures before and after treatment
| Group | Baseline treatment | After | P* | P** | |
|---|---|---|---|---|---|
| Apelin, ng/mL | Telmisartan | 0.27±0.13 | 0.34±0.18 | 0.009 | 0.922 |
| Amlodipine | 0.32±0.17 | 0.38±0.17 | 0.013 | ||
| SBP, mm Hg | Telmisartan | 154±7 | 122±8 | <0.001 | 0.658 |
| Amlodipine | 154±10 | 123±10 | <0.001 | ||
| DBP, mm Hg | Telmisartan | 92±6 | 74±9 | <0.001 | 0.271 |
| Amlodipine | 95±9 | 76±8 | <0.001 |
Values are expressed as mean±standard deviation. DBP - diastolic blood pressure; SBP - systolic blood pressure.
P value of paired samples test;
P value of comparison of groups in terms of after treatment values corrected for baseline values
After 1 month of treatment, significant reductions in SBP and DBP were observed in patients with hypertension (p<0.001 for both); the extent of the reduction was similar for both in each of the treatment groups (p=0.658 for SBP and p=0.271 for DBP, Table 3).
Changes in diastolic function after treatment are presented in Table 4: there were significant improvements in IVRT, lat-sep e’, lateral IVRT, septal IVRT, E/e’, left atrial volume index, lateral MPI and septal MPI in both treatment groups, and the extent of the improvements were similar in each of the groups. The proportional change in plasma apelin concentration after antihypertensive therapy was not significantly associated with the proportional change in blood pressure or diastolic indices, except lateral MPI (Table 5).
Table 4.
Echocardiographic indices for the assessment of diastolic function before and after treatment
| Telmisartan group | Amlodipine group | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | After treatment | P* | Baseline | After treatment | P* | P** | |
| E wave, cm/s | 68±12 | 67±11 | 0.326 | 68±14 | 66±13 | 0.344 | 0.795 |
| A wave, cm/s | 72±16 | 69±13 | 0.070 | 78±13 | 69±13 | <0.001 | 0.057 |
| MDT, ms | 226±25 | 221±16 | 0.122 | 227±20 | 222±25 | 0.217 | 0.922 |
| IVRT, ms | 98±14 | 89±10 | <0.001 | 101±12 | 91±10 | <0.001 | 0.773 |
| E/A ratio | 1.00±0.31 | 0.99±0.23 | 0.959 | 0.89±0.25 | 0.99±0.30 | 0.006 | 0.110 |
| Lat-sep e’, cm/s | 8.9±1.8 | 10.7±1.8 | <0.001 | 8.3±2.3 | 10.0±2.6 | <0.001 | 0.564 |
| Lateral IVRT, ms | 96±11 | 90±9 | <0.001 | 99±9 | 91±10 | <0.001 | 0.692 |
| Septal IVRT, ms | 95±9 | 88±7 | <0.001 | 100±12 | 90±7 | <0.001 | 0.785 |
| Lateral s’, cm/s | 11.5±0.5 | 11.8±0.6 | 0.286 | 11.1±0.8 | 11.6±0.7 | 0.156 | 0.324 |
| Septal s’, cm/s | 10.9±0.6 | 11.3±0.5 | 0.212 | 10.8±0.7 | 11.1±0.7 | 0.243 | 0.355 |
| E/e’ ratio | 7.8±1.5 | 6.3±1.1 | <0.001 | 8.6±2.2 | 6.9±1.9 | <0.001 | 0.516 |
| LAVI, mL/m2 | 21.0±3.6 | 18.9±2.7 | <0.001 | 21.1±3.0 | 19.0±2.7 | <0.001 | 0.959 |
| Lateral MPI | 0.64±0.16 | 0.61±0.23 | 0.021 | 0.64±0.11 | 0.58±0.07 | <0.001 | 0.270 |
| Septal MPI | 0.61±0.10 | 0.58±0.08 | 0.006 | 0.63±0.08 | 0.59±0.07 | <0.001 | 0.837 |
Values are expressed as mean±standard deviation. E wave and A wave-peak early filling (E) and atrial filling (A) velocities; IVRT-isovolumic relaxation time; Lat/sep e’-mean of lateral and septal peak early diastolic velocities; LAVI-left atrial volume index; MDT-deceleration time of peak E wave; MPI-myocardial performance index; s’-peak systolic annular velocity.
P value of paired samples test;
P value of comparison of groups in terms of after treatment values corrected for baseline values
Table 5.
Correlation of proportional change in apelin concentration and proportional change in echocardiographic parameters and blood pressure after 1 month of therapy
| n=77 | Correlation coefficient | |
|---|---|---|
| r | P | |
| E wave | 0.094 | 0.416 |
| A wave | 0.163 | 0.157 |
| E/A | 0.065 | 0.572 |
| MDT | 0.056 | 0.629 |
| IVRT | 0.027 | 0.813 |
| LAVI | 0.011 | 0.923 |
| Lat-sep e’ | -0.033 | 0.778 |
| Lateral IVRT | 0.117 | 0.456 |
| Septal IVRT | 0.059 | 0.349 |
| Lateral s’ | -0.078 | 0.292 |
| Septal s’ | -0.014 | 0.543 |
| E/e’ | 0.032 | 0.781 |
| Lateral MPI | -0.233 | 0.041 |
| Septal MPI | -0.098 | 0.395 |
| SBP | 0.073 | 0.526 |
| DBP | 0.075 | 0.516 |
Data obtained from Spearman’s correlation analysis are shown in the table. A wave-peak atrial filling (A) velocity; DBP - diastolic blood pressure; E wave - peak early filling velocity; IVRT - isovolumic relaxation time; Lat/sep e’ - mean of lateral and septal peak early diastolic velocities; LAVI - left atrial volume index; MDT - deceleration time of E wave; MPI - myocardial performance index; s’ - peak systolic annular velocity; SBP - systolic blood pressure
Discussion
The main findings of the present study are that plasma apelin concentrations increased significantly in newly diagnosed patients with primary hypertension after 1 month of effective treatment with either telmisartan or amlodipine. This is the first study demonstrating an increase in the apelin concentration to a similar extent with two anti-hypertensive drug regimens with different mechanisms of action. The change in the apelin concentration was not associated with the observed improvements in left ventricular diastolic function as well as the decrease in blood pressure.
It has previously been reported that apelin may play a role in the pathophysiology of hypertension (4–6, 16–21). In animal models and clinical studies, decreased apelin concentrations have been documented in hypertension compared with participants with normal blood pressure (4, 5, 20, 21). Papadopoulos et al. (21) measured plasma apelin concentration in 130 patients who underwent 24-hour ambulatory blood pressure monitoring; 24 were found to have masked hypertension. They found that apelin concentration was significantly lower in patients with masked hypertension compared with normotensive controls. In a recent study, even subjects with high normal blood pressure had significantly lower apelin levels than subjects with optimal blood pressure (22).
Przewlocka-Kosmala et al. (5) enrolled 232 patients with hypertension, 56 of whom had never been treated. Their control group comprised 76 healthy volunteers, and patients with hypertension were found to have lower plasma apelin concentration than controls. In that study apelin concentration was reported to be in the range of 120–480 pg/mL, which are in a similar range as our findings (reported as ng/mL in our study). Interestingly, patients with hypertension who had been taking antihypertensive drugs (discontinued 2 weeks before entering the study) and those who had never been treated had similar plasma apelin concentrations, however, they also had similar SBP and DBP (in the entire cohort, mean SBP was 155±13 mm Hg and the mean DBP was 94±10 mm Hg). The finding that the comparably low plasma apelin concentrations in treatment naive patients with hypertension and those who had discontinued antihypertensive treatment had in the study by Przewlocka-Kosmala et al.(5), informed our decision to examine the change in apelin concentration in patients newly diagnosed with hypertension after being established on an effective treatment regime, as well as to compare the baseline plasma apelin concentration of patients with healthy controls. In our cohort, treatment brought about a significant decrease in blood pressure and a significant increase in plasma apelin concentration; however, there was no correlation between the magnitudes of these changes. These findings are in agreement with Przewlocka-Kosmala et al. (5), who also found no significant correlation between the extent of reduction in circulating apelin concentration and blood pressure in 232 patients with essential hypertension. They suggested that ambulatory blood pressure monitoring might be a more accurate technique to illuminate a possible association; we used ambulatory blood pressure monitoring to evaluate the efficiency of therapy 1 month after the initiation of treatment, but made the diagnosis of hypertension on the basis of recordings taken in our outpatients department. Most of the patients in our cohort had stage 1 hypertension requiring monotherapy, and patients requiring combination therapy were excluded from the study. The lack of a statistically significant relationship between the change in plasma apelin concentration and the change in blood pressure concurs with other investigations but might also be explained by our diagnostic methods, the lack of a wide variation in blood pressure in the treatment groups, and a relatively small sample size.
Apelin is thought to act centrally and peripherally to reduce blood pressure. In the peripheries, apelin causes arterial vasodilatation via the endothelial nitric oxide pathway (19, 23, 24); however, it has no effect on venous tone (25). An increase in intracerebral apelin causes a hypertensive response (23, 24); thus it appears that the central and peripheral components of the apelinergic system seem to have opposing effects on arterial blood pressure. It is difficult to explain the mechanism by which plasma apelin concentration might increase after control over blood pressure has been restored, as the activity and pharmacology of the apelin/APJ pathway is not fully understood; our study was not designed to identify the underlying mechanism. It has been reported that apelin opposes angiotensin in the cardiovascular system, and that angiotensin converting enzyme 2 (ACE 2) acts to eliminate apelin (26). In experimental animals, lack of activity in the APJ pathway reportedly suppressed expression of the angiotensin II type 1 receptor, which increased when APJ was activated (27). In a rat model of hypertensive heart failure, down regulation of apelin and APJ expression was significantly increased in animals treated with olmesartan (28). These observations support our findings that plasma apelin concentration is elevated in patients treated with telmisartan; nonetheless amlodipine treatment elevated circulating apelin concentration to a similar extent. It is plausible; therefore, that any increase in plasma apelin concentration occurs through one or more mechanisms triggered by reduction in blood pressure, and are independent of the drug used. Further studies are needed to test this hypothesis.
We observed that diastolic function had improved after 1 month of therapy with telmisartan or amlodipine, and that the magnitude of the improvement with each drug was comparable. Zaliunas et al. (29) have also reported a measurable benefit of amlodipine on LV diastolic function in patients with arterial hypertension and stable angina after 4 weeks of treatment. In the longer term, Mattioli et al. (30) observed significant improvements in diastolic filling parameters, LA volume and LV hypertrophy after 3, 6, 9, and 12 months of telmisartan monotherapy. We used these findings to inform our decision that 1 month of treatment was enough to control hypertension, and for improvement in diastolic function and changes apelin concentration to be evident. Lower levels of apelin is reportedly independently associated with more profound impairment of LV diastolic function (5), but despite the beneficial effects of treatment on diastolic function and apelin concentration, we did not find any association between the change in circulating apelin concentration and indices of diastolic function. The pathophysiological mechanism underpinning the relationship between apelin and diastolic function is not understood. Przewlocka-Kosmala et al. (5) suggested that the deleterious effect of decreased apelin concentration on LV diastolic function could be a consequence of diminished endothelial nitric oxide synthesis; however, further studies will be needed to examine this hypothesis. The absence of a relationship between follow-up apelin concentration and improvement in diastolic function in our cohort may be due to the relatively short duration of our study.
Study limitations
Our study has several limitations. First, our study sample size might not have been large enough to detect significant associations between change in apelin concentration and the echocardiographic parameters used to determine LV diastolic function. Second, using clinic measurements to diagnose hypertension may have influenced the composition of the study cohort, although we used ambulatory monitoring to measure blood pressure at the end of the treatment period, and the protocol was the same for all participants. Third, the study design was open label. Fourth, 13 patients were excluded from the analysis for a variety of reasons, and although this is a relatively high proportion of the study cohort, exclusions were evenly distributed between the groups. We did not use more sensitive echocardiographic methods of evaluating cardiac function, such as myocardial strain and strain rate. These methods are able to detect subtle changes in myocardial mechanics and a relationship with change in apelin concentration could have been observed; however, we elected to use well-established parameters to evaluate LV diastolic function recommended in international guidelines. Finally, even though we were able to show significant differences in apelin concentration, blood pressure measurements, and echocardiographic indices for diastolic function, relatively short study period is another limitation and longer duration follow-up studies may strengthen the accuracy of data.
Conclusions
Plasma apelin concentration was significantly lower in patients with hypertension than the control group, who had normal blood pressure. A significant increase in apelin concentration was observed after 1 month of effective treatment with either telmisartan or amlodipine. The magnitude of the change in apelin concentration was associated neither with the extent of the improvement in diastolic function nor with the degree of change in blood pressure. Effective blood pressure control, rather than the drug choice, seems to increase apelin concentration.
Acknowledgements:
There is no conflict of interest regarding any of the authors. All support for this study came from institutional and departmental resources.
Footnotes
Conflict of interest: None declared.
Funding: This research received no grant from any funding agency in the public, commercial, or not-for-profit sectors.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – B.P.; Design – B.P., K.O.; Supervision – B.P., K.O., U.A.B., M.Z.U., D.Ö., H.M.; Materials – B.P., K.O., U.A.B., M.Z.U., D.Ö., H.M.; Data collection &/or processing – B.P., K.O., U.A.B., M.Z.U., D.Ö., H.M.; Analysis &/or interpretation – B.P., D.Ö.; Literature search – B.P., K.O.; Writing – S.S.B., B.P., K.O.; Critical review – B.P., K.O.; Other – D.Ö.
References
- 1.Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakewaga T, Zou MX. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–6. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 2.Lee DK, Cheng R, Nguyen T, Fan T, Kariyawasam AP, Liu Y. Characterization of apelin, the ligand for the APJ receptor. J Neurochem. 2000;74:34–41. doi: 10.1046/j.1471-4159.2000.0740034.x. [DOI] [PubMed] [Google Scholar]
- 3.Barnes G, Japp AG, Newby DE. Translational promise of the apelin/APJ system. Heart. 2010;96:1011–6. doi: 10.1136/hrt.2009.191122. [DOI] [PubMed] [Google Scholar]
- 4.Sönmez A, Çelebi G, Erdem G, Tapan S, Genç H, Taşcı I, et al. Plasma apelin and ADMA levels in patients with essential hypertension. Clin Exp Hypertens. 2010;32:179–83. doi: 10.3109/10641960903254505. [DOI] [PubMed] [Google Scholar]
- 5.Przewlocka-Kosmala M, Kotwica T, Mysiak A, Kosmala W. Reduced circulating apelin in essential hypertension and its association with cardiac dysfunction. J Hypertens. 2011;29:971–9. doi: 10.1097/HJH.0b013e328344da76. [DOI] [PubMed] [Google Scholar]
- 6.Charles CJ. Putative role for apelin in pressure/volume homeostasis and cardiovascular disease. Cardiovasc Hematol Agents Med Chem. 2007;5:1–10. doi: 10.2174/187152507779315804. [DOI] [PubMed] [Google Scholar]
- 7.Mancia G, Fagard R, Narkiewicz K, Redán J, Zanchetti A, Böhm M, et al. 2013 Practice guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;34:1925–38. doi: 10.1097/HJH.0b013e328364ca4c. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Y, Wang Y, Qiao S. Apelin: a potential marker of coronary artery stenosis and atherosclerotic plaque stability in ACS patients. Int Heart J. 2014;55:204–12. doi: 10.1536/ihj.13-234. [DOI] [PubMed] [Google Scholar]
- 9.Cebeci AN, Güven A, Kuru LI. Perinephric adipose tissue thickness in relation to blood pressure, plasma apelin and C -reactive protein levels in obese adolescents. Minerva Endocrinol. 2016;41:166–74. [PubMed] [Google Scholar]
- 10.Habchi M, Duvillard L, Cottet V, Brindisi MC, Bouillet B, Beacco M, et al. Circulating apelin is increased in patients with type 1 or type 2 diabetes and is associated with better glycaemic control. Clin Endocrinol (Oxf) 2014;81:696–701. doi: 10.1111/cen.12404. [DOI] [PubMed] [Google Scholar]
- 11.Peltonen T, Näpänkangas J, Vuolteenaho O, Ohtonen P, Soini Y, Juvonen T, et al. Apelin and its receptor APJ in human aortic valve stenosis. J Heart Valve Dis. 2009;18:644–52. [PubMed] [Google Scholar]
- 12.Zhang DL, Liao H, Wei YY, Zhang Y, Zhang QD, Wang ZG. Elevation of serum apelin-13 is positively correlated with ADMA in patients on maintenance hemodialysis. Clin Nephrol. 2009;71:405–12. doi: 10.5414/cnp71405. [DOI] [PubMed] [Google Scholar]
- 13.Dalzell JR, Rocchiccioli JP, Weir RA, Jackson CE, Padmanabhan N, Gardner RS, et al. The Emerging Potential of the Apelin-APJ System in Heart Failure. J Card Fail. 2015;21:489–98. doi: 10.1016/j.cardfail.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Chapman CB, Ewer SM, Kelly AF, Jacobson KM, Leal MA, Rahko PS. Classification of left ventricular diastolic function using American Society of Echocardiography Guidelines: agreement among echocardiographers. Echocardiography. 2013;30:1022–31. doi: 10.1111/echo.12185. [DOI] [PubMed] [Google Scholar]
- 15.Tei C, Nishimura RA, Seward JB, Tajik AJ. Noninvasive Doppler-derived myocardial performance index: correlation with simultaneous measurements of cardiac catheterization measurements. J Am Soc Echocardiogr. 1997;10:169–78. doi: 10.1016/s0894-7317(97)70090-7. [DOI] [PubMed] [Google Scholar]
- 16.Tatemoto K, Takayama K, Zou MX, Kumaki I, Zhang W, Fujimiya M. The novel peptide apelin lowers blood pressure via nitric oxide-dependent mechanism. Regul Pept. 2001;99:87–92. doi: 10.1016/s0167-0115(01)00236-1. [DOI] [PubMed] [Google Scholar]
- 17.Lee DK, Saldivia VR, Nguyen T, Cheng R, George SR, O’Dowd BF. Modification of the terminal residue of apelin-13 antagonizes its hypotensive action. Endocrinology. 2005;146:231–6. doi: 10.1210/en.2004-0359. [DOI] [PubMed] [Google Scholar]
- 18.Ishida J, Hashimoto T, Hashimoto Y, Nishiwaki S, Iguchi T, Harada S. Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivo. J Biol Chem. 2004;279:26274–9. doi: 10.1074/jbc.M404149200. [DOI] [PubMed] [Google Scholar]
- 19.Kagiyama S, Fukuhara M, Matsumura K, Lin Y, Fujii K, Iida M. Central and peripheral cardiovascular actions of apelin in conscious rats. Regul Pept. 2005;125:55–9. doi: 10.1016/j.regpep.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Ren CX, Qi YF, Lou LX, Chen L, Zhang LK, et al. Exercise training promotes expression of apelin and APJ of cardiovascular tissues in spontaneously hypertensive rats. Life Sci. 2006;79:1153–9. doi: 10.1016/j.lfs.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 21.Papadopoulos DP, Mourouzis I, Faselis C, Perrea D, Makris T, Tsioufis C. Masked hypertension and atherogenesis: the impact of apelin and relaxin plasma levels. J Clin Hypertens (Greenwich) 2013;15:333–6. doi: 10.1111/jch.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liakos CI, Sanidas EA, Perrea DN, Grassos CA, Chantziara V, Viniou NA, et al. Apelin and visfatin plasma levels in healthy individuals with high normal blood pressure. Am J Hypertens. 2016;29:549–52. doi: 10.1093/ajh/hpv136. [DOI] [PubMed] [Google Scholar]
- 23.Seyedabadi M, Goodchild AK, Pilowsky PM. Site-specific effects of apelin-13 in the rat medulla oblongata on arterial pressure and respiration. Auton Neurosci. 2002;101:32–8. doi: 10.1016/s1566-0702(02)00178-9. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Q, Yao F, Raizada MK, O’Rourke ST, Sun C. Apelin gene transfer into the rostral ventrolateral medulla induces chronic blood pressure elevation in normotensive rats. Circ Res. 2009;104:1421–8. doi: 10.1161/CIRCRESAHA.108.192302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Japp AG, Cruden NL, Amer DA, Li VK, Goudie EB, Johnston NR, et al. Vascular effects of apelin in vivo in man. J Am Coll Cardiol. 2008;52:908–13. doi: 10.1016/j.jacc.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Iwanaga Y, Kihara Y, Takenaka H, Kita T. Down-regulation of cardiac apelin system in hypertrophied and failing hearts: possible role of angiotensin II-angiotensin type 1 receptor system. J Mol Cell Cardiol. 2006;41:798–806. doi: 10.1016/j.yjmcc.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Sun X, Iida S, Yoshikawa A, Senbonmatsu R, Imanaka K, Maruyama K, et al. Non-activated APJ suppresses the angiotensin II type 1 receptor, whereas apelin-activated APJ acts conversely. Hypertens Res. 2011;34:701–6. doi: 10.1038/hr.2011.19. [DOI] [PubMed] [Google Scholar]
- 28.Fukushima H, Kobayashi N, Takeshima H, Koguchi W, Ishimitsu T. Effects of olmesartan on Apelin/APJ and Akt/endothelial nitric oxide synthase pathway in Dahl rats with end-stage heart failure. J Cardiovasc Pharmacol. 2010;55:83–8. doi: 10.1097/FJC.0b013e3181c87a82. [DOI] [PubMed] [Google Scholar]
- 29.Zaliunas R, Jurkevicus R, Zabiela V, Brazdzionyte J. Effect of amlodipine and lacidipine on left ventricular diastolic and long axis functions in arterial hypertension and stable angina pectoris. Acta Cardiol. 2005;60:239–46. doi: 10.2143/AC.60.3.2004999. [DOI] [PubMed] [Google Scholar]
- 30.Mattioli A, Zennaro M, Bonatti S, Bonetti L, Mattioli G. Regression of left ventricular hypertrophy and improvement of diastolic function in hypertensive patients treated with telmisartan. Int J Cardiol. 2004;97:383–8. doi: 10.1016/j.ijcard.2003.10.018. [DOI] [PubMed] [Google Scholar]


