Abstract
Objective:
Because of the ongoing and recurring inflammatory state in familial Mediterranean fever (FMF), patients may experience a high risk of cardiovascular events. Our aim was to investigate the arterial stiffness and associated factors in patients with FMF.
Methods:
Sixty-nine consecutive FMF patients (including 11 females) and 35 controls (including 5 females) were enrolled in the study. The demographical, clinical, and laboratory data and genetic mutations of the patients were recorded. In the study, FMF patients according to the Tel-Hashomer criteria were included, whereas patients with other known inflammatory rheumatologic disease, atherosclerotic cardiovascular disease, hypertension, diabetes, those under the age of 18 years, or those refusing to participate in the study were excluded. Arterial stiffness measurements were performed using the TensioMed device (TensoMed Ltd, Budapest, Hungary).
Results:
The patient and control groups were similar in terms of the mean ages, BMIs, gender, systolic blood pressures, and smoking. FMF patients had a higher pulse wave velocity (PWV) (7.73±1.3 and 7.18±1.1 m/s; p=0.03) and lower brachial and aortic augmentation indexes (–64.6±14.6% and –54.6±25.9%, p=0.041 and 4.9±7.4% and 14.0±11.5%, p=0.025, respectively) compared with the controls. Thirty-one (45%) patients were in the “during-attack” state and had higher PWV (8.17±1.6 and 7.38±0.9 m/s; p=0.027) compared with the asymptomatic patients. PWV was correlated to serum CRP, WBC, ESR, fibrinogen, and neutrophil/lymphocyte ratios (r=0.666, 0.429, 0.441, 0.388, and 0.460, respectively). The genetic mutation and predominant attack type had no effect on arterial stiffness.
Conclusion:
FMF patients have increased arterial stiffness during attacks compared with asymptomatic patients and controls. The impaired arterial stiffness is correlated to the severity of the inflammatory state rather than to the attack type or genetic mutations.
Keywords: familial Mediterranean fever, arterial stiffness, inflammation, cardiovascular risk
Introduction
Familial Mediterranean fever (FMF) is an autosomal recessive disease characterized by recurrent inflammatory febrile attacks of the serosal and synovial membranes. Patients usually experience fever, peritonitis, pleuritis, arthritis, myalgia, and/or erysipelas-like skin lesions during the attacks (1). FMF is associated with the missense variations of the MEditerranean FeVer (MEFV) gene, which encodes a protein termed “pyrin.” Pyrin is expressed mainly in neutrophils and monocytes (2). Defective pyrin in FMF patients results in recurrent inflammatory events via an inappropriate and prolonged response to inflammatory stimuli and increased leukocyte migration to serosal sites (3). Elevation in the white blood cell (WBC) count, erythrocyte sedimentation rate (ESR), and serum inflammatory markers, such as C-reactive protein (CRP), serum amyloid A (SAA), and plasma fibrinogen are seen during attacks, which typically continue for 48–72 h. Colchicine therapy is effective in reducing the number and severity of attacks and the complications of FMF. Further medications, including steroids, thalidomide, anti-TNF agents, interferon-a, anakinra, and canakinumab, are, for a particular group of patients, resistant to colchicine therapy (4, 5).
Because of the ongoing and recurring inflammatory state, patients may experience a high risk of cardiovascular events. The ongoing chronic inflammation, increased vascular permeability, and NO synthesis due to the pro-inflammatory cytokines may finally result in endothelial dysfunction (ED). It is known that ED may have an important role in the development of atherosclerosis (6).
Arterial stiffness represents the viscoelastic properties of the vessel wall, and increased arterial stiffness is a common indicator for the atherosclerotic involvement of the vascular system by known atherosclerotic risk factors, such as hypertension, diabetes mellitus, smoking, hypercholesterolemia, and aging (7). Because of the current guidelines, it has a high cardiovascular predictive value, availability, reproducibility, and cost effectiveness in determining the future cardiovascular risk (8). It is available in many healthcare facilities. Increased arterial stiffness is also found to be associated with coronary artery disease, cerebrovascular disease, and peripheral arterial disease (9).
In this study, we aimed to study arterial stiffness and its association with clinical findings, attack types, genetic mutations, and serum markers of inflammation in patients with FMF.
Methods
Study design
A single center, cross-sectional, retrospective, case-control study was conducted in a rheumatology outpatient clinic of a tertiary referral center. The study protocol was approved by the Research and Ethics Committees of Gülhane Medical Faculty. Signed informed consent was obtained from each participant before any study-related procedures were performed.
Patients
A total of 104 subjects were consecutively enrolled in the study. Thirty-five (5 females, 14%) of them were healthy controls. Sixty-nine patients, of whom 11 (16%) were female, were patients with a diagnosis of FMF according to the Tel-Hashomer criteria (1). Patients with other known inflammatory rheumatologic disease, atherosclerotic cardiovascular disease, hypertension and diabetes, those under the age of 18, or those refusing to participate in the study were excluded.
Assessments
The demographic parameters, anthropometric measurements, current presence of any attack, previous attack types, genetic mutations, daily colchicine dose, blood pressures, medications, and laboratory results of the participants were obtained. The predominant attack type was recorded as peritonitis, pleuritis, arthritis, febrile myalgia, and erysipelas-like erythema. Patients with evidence of any target organ damage from high blood pressure (BP), diabetes mellitus, cardiac disease, thyroid dysfunction, liver disease, renal failure, malignancy, and other chronic, infectious, and inflammatory diseases were excluded. The patients were rested for 10 min before BP was measured. The mean of two BP measurements was calculated. The mean arterial blood pressure was calculated according to the following formula: MAP=DBP+[(SBP–DBP)/3]. The complete blood count, ESR, renal and hepatic function tests, serum CRP, fibrinogen, electrolytes, serum lipid levels, urine protein, and thyroid hormones of the patient and control groups were recorded. FMF patients had collected 24-h urine for protein measurement for routine control, and the results were recorded. Weight (kg) and height (cm) were obtained and BMI was calculated as body weight/height2 (kg/m2). Neutrophil/lymphocyte ratios were calculated by dividing the neutrophils by lymphocyte counts.
Arterial stiffness measurements
The TensioMed device (TensoMed Ltd., Budapest-Hungary) was used for the measurement of arterial stiffness. A rheumatologist examined all of the patients consecutively, and noted their demographic factors, physical findings, laboratory results, and the presence of attack. Another physician performed the stiffness measurements, and was blind to the patients’ and controls’ diagnosis, attack-status, and other factors. Systolic and diastolic blood pressures, arterial stiffness measurements, pulse wave velocity (PWV), aortic and brachial augmentation indexes (Aixao and Aixbr), central aortic pressure (CAP) analysis, and ankle-brachial index (ABI) measurements were made on the participants.
PWV, Aixao, Aixbr, and CAP analysis
The TensioMed arteriography measurement device reported the measurement results automatically. Measurements were made after a 5-minute rest of the patient, without smoking or taking caffeinated beverages for at least the last 30 minutes. They were in the sitting position in a reserved quiet room. The data of the distance between the jugular notch and the symphysis pubis of the patients were measured and recorded. During the measurement, an intentional brachial artery occlusion was executed (only 8–20 s) and the blood flow was stopped as a part of the process.
Ankle-brachial index (ABI) measurements
Lower extremity blood pressure was measured with a digital device (Omron Healthcare Co., Ltd, Japan) and entered into the arteriography device interface. This automatically gave the ankle-brachial index by calculating the division of the lower extremity blood pressure with the upper.
Statistical analyses
The SPSS 22.0 statistical package was used for statistical analysis. Independent samples t-test, chi-square test, correlation tests, univariate analysis, and linear regression analysis tests were used. Quantitative variables were expressed as the mean±standard deviation. The Kolmogorov–Smirnov and Shapiro–Wilkins tests were used to determine the distribution characteristics of variables and Levene’s test was used to determine the equality of variance. Differences between the groups were studied for significance by the independent samples t-test as appropriate. Categorical variables were compared by chi-square test. Pearson and Spearman correlation analysis were used to evaluate the relationships between variables. Results of the analysis were expressed as a percent for the qualitative variables and as the mean±standard deviation for continuous variables. The post hoc power of the study was calculated as 96.5%, when the mean of the stiffness measurements and patient numbers of the study groups were taken into account with an alpha error rate of 0.05. A two-sided p<0.05 was considered significant.
Results
The demographic, laboratory, and arterial stiffness measurements of the patient and control groups are shown in Table 1. The distribution of age, gender, smoking status, and means of BMI scores between the two groups were similar (p>0.05).
Table 1.
The demographic parameters, anthropometric measurements, arterial stiffness parameters, and laboratory results of the study groups. The P values indicate the significance of the compared values in the FMF patients and controls
| Patients n=69 | Controls n=35 | P** | |
|---|---|---|---|
| Gender, F (%) | 11(16) | 5(14) | NS |
| Smokers, n | 31 | 9 | NS |
| Age, years | 27.0±8.0 | 28.3±4.9 | NS |
| BMI, kg/m2 | 24.1±3.4 | 24.5±3.2 | NS |
| SBP, mm Hg | 123.9±10.8 | 121.9±13.0 | NS |
| DBP, mm Hg | 70.9±7.9 | 64.7±9.3 | 0.001 |
| MAP, mm Hg | 88.6±7.2 | 83.8±9.3 | 0.009 |
| PR, /min | 79.9±12.8 | 70.1±12.2 | <0.001 |
| Pulse wave velocity, m/s | 7.7±1.3 | 7.2±1.1 | 0.03 |
| Aortic augmentation index, % | 4.9±7.4 | 11.0±14.5 | 0.025 |
| Brachial augmentation index, % | -64.6±14.7 | -54.6±25.9 | 0.041 |
| Central aortic pressure, mm Hg | 108.2±9.8 | 108.4±13.2 | NS |
| Ankle-brachial index | 1.2±0.1 | 1.2±0.2 | NS |
| Serum C-reactive protein, mg/L | 43.7±84.3 | 3.6±6.7 | <0.001 |
| Erythrocyte sedimentation rate, mm/h | 24.3±19.5 | 10.2±4.3 | <0.001 |
| Plasma fibrinogen, mg/dL | 440.1±166.6 | NA | NA |
| WBC, ×1000/mm3 | 8.1±3.0 | 6.5±1.8 | 0.008 |
| Neutrophil/lymphocyte ratio | 2.5±1.6 | 1.9±0.6 | 0.037 |
| Hemoglobin, g/dL | 14.8±1.3 | 15.1±1.4 | NS |
| PLT, ×1000/mm3 | 268.3±67.3 | 286.4±73.2 | NS |
| Serum urea, mg/dL | 29.8±7.2 | 24.5±4.4 | 0.001 |
| Serum creatinine, mg/dL | 0.9±0.1 | 0.9±0.1 | NS |
| Serum uric acid, mg/dL | 5.7±1.3 | 10.1±13.9 | NS |
| LDL, mg/dL | 104.0±28.7 | 112.1±34.8 | NS |
| Creatinine kinase, U/L | 131.1±121.5 | 131.4±104.6 | NS |
| Serum magnesium, mg/dL | 2.0±0.2 | 2.1±0.1 | NS |
| Thyroid stimulating hormone, mIU/L | 1.6±0.9 | 1.5±0.7 | NS |
| 24-h urine protein, mg/day | 444±838 | NA* | NA |
| Colchicine dose, mg/day | 1.1±0.5 | NA | NA |
BMI - body mass index; CRP - C-reactive protein; DBP - diastolic blood pressure; F - female; LDL - low-density lipoprotein cholesterol; MAP - mean arterial pressure; NA - not available; NS - not significant; PLT - platelets; PR - pulse rate; SBP - systolic blood pressure; TSH - thyroid stimulating hormone; WBC - white blood cell.
- A 24-h urine protein test was not performed in the control group, but the urine dipstick test was negative for proteins in this group.
Chi-square test where appropriate, otherwise independent sample t-test
FMF patients had a significantly higher PWV (7.73±1.3 m/s and 7.18±1.1 m/s; p=0.03) and lower brachial and aortic augmentation indexes (–64.6±14.6% and –54.6±25.9%, p=0.041; 4.9±7.4% and 14.0±11.5%, p=0.025, respectively) compared with the controls. The central aortic pressure and ankle-brachial index measurements were statistically similar in the two groups. There was no association between PWV and Aix measurements.
Thirty-one (45%) of the FMF patients were examined during attacks and had significantly higher PWV measurements (8.17±1.6 m/s and 7.38±0.9 m/s; p=0.027) compared with asymptomatic patients (Table 2, Fig. 1). The PWV measurements had significant correlations to serum CRP, WBC, erythrocyte sedimentation rate, plasma fibrinogen, and neutrophil/lymphocyte ratios (NLR) (Fig. 2, Table 3). There was no association between the PWV and Aix measurements. The arterial stiffness measurements (PWV, Aixao, Aixbr, and CAP) had no association with serum inflammatory markers (ESR, CRP, fibrinogen, WBC, and NLR) in the control group. The serum magnesium levels were significantly correlated with ankle-brachial index measurements (Spearman rho=0.400, p=0.01).
Table 2.
The demographic parameters, anthropometric measurements, arterial stiffness parameters, and laboratory results of the during-attack and asymptomatic FMF patients. The P values indicate the significance of comparisons
| During-attack n=31 | Asymptomatic n=38 | P* | |
|---|---|---|---|
| Gender, F (%) | 3 | 8 | NS |
| Smokers, n | 15 | 16 | NS |
| Age, years | 26.4±8.1 | 27.6±8.1 | NS |
| BMI, kg/m2 | 24.1±3.4 | 24.1±3.4 | NS |
| SBP, mm Hg | 125.1±12.5 | 123.0±9.6 | NS |
| DBP, mm Hg | 70.5±8.4 | 71.3±7.5 | NS |
| MAP, mm Hg | 88.7±7.8 | 88.6±6.8 | NS |
| PR, /min | 83.9±15.5 | 76.8±9.1 | 0.028 |
| Pulse wave velocity, m/s | 8.2±1.6 | 7.4±0.9 | 0.027 |
| Aortic augmentation index, % | 3.0±7.3 | 6.4±7.3 | NS |
| Brachial augmentation index, % | -68.3±14.4 | -61.8±14.5 | NS |
| Central aortic pressure, mm Hg | 108.3±11.2 | 108.1±8.8 | NS |
| Ankle-brachial index | 1.2±0.1 | 1.2±0.1 | NS |
| RAPID-3 score | 15.7±6.9 | 9.5±7.9 | 0.019 |
| Serum C-reactive protein, mg/L | 66.9±112.6 | 23.9±41 | 0.039 |
| Erythrocyte sedimentation rate, mm/h | 30.35±19.8 | 19.1±18 | 0.018 |
| Plasma fibrinogen, mg/dL | 510.2±161.4 | 377.9±147 | 0.001 |
| WBC, ×1000/mm3 | 8.3±3.0 | 8.0±3.1 | NS |
| Neutrophil/lymphocyte ratio | 2.9±1.9 | 2.1±1.2 | 0.049 |
| Hemoglobin, g/dL | 14.8±1.1 | 14.7±1.5 | NS |
| PLT, ×1000/mm3 | 262.9±61.5 | 272.8±72.3 | NS |
| Serum urea, mg/dL | 29.2±7.7 | 30.3±6.9 | NS |
| Serum creatinine, mg/dL | 0.9±0.1 | 0.9±0.1 | NS |
| Serum uric acid, mg/dL | 6.0±1.5 | 5.5±1.1 | NS |
| LDL, mg/dL | 105.4±27.4 | 102.4±30.9 | NS |
| Creatinine kinase, U/L | 140.9±160 | 123.4±81.8 | NS |
| Serum magnesium, mg/dL | 2.1±0.2 | 2.0±0.2 | NS |
| Thyroid stimulating hormone, mIU/L | 1.7±0.8 | 1.5±1.1 | NS |
| 24-h urine protein, mg/day | 381±688 | 472±928 | NS |
| Colchicine dose, mg/day | 1.15±0.7 | 1.15±0.5 | NS |
BMI - body mass index; CRP - C-reactive protein; DBP - diastolic blood pressure; F - female; LDL - low-density lipoprotein cholesterol; MAP - mean arterial pressure; PLT - platelets; PR - pulse rate; SBP - systolic blood pressure; TSH - thyroid stimulating hormone; WBC - white blood cell. The bold P values refer to significant intergroup differences.
Chi-square test where appropriate, otherwise independent sample t-test
Figure 1.
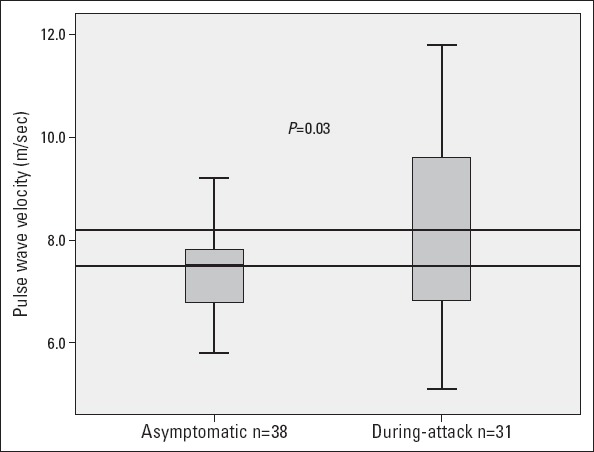
Difference in PWV measurements of asymptomatic and during-attack FMF patients. The horizontal lines refer to the medians of arterial stiffness measurements in the groups
Figure 2.
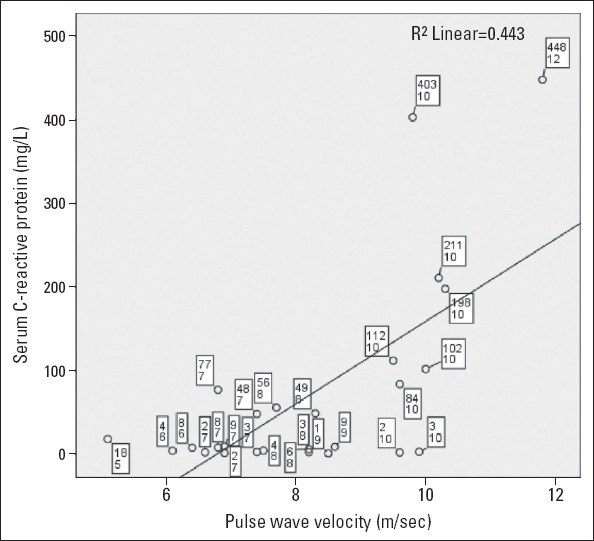
Chart representing the association between serum C-reactive protein (mg/dL) and pulse wave velocity (m/s) measurements in during-attack FMF patients. The mini-boxes refer to cases including CRP and PWV measurements, respectively
Table 3.
Correlations of arterial stiffness measurements to inflammatory markers in during-attack FMF patients (n=31)
| Pulse wave velocity m/s | |
|---|---|
| Serum C-reactive protein, mg/L | |
| r | 0.666 |
| P | <0.001 |
| White blood cell, /mm3 | |
| r | 0.429 |
| P | 0.025 |
| Erhytrocyte sedimentation rate, mm/h | |
| r | 0.441 |
| P | 0.021 |
| Plasma fibrinogen, mg/dL | |
| r | 0.388 |
| P | 0.045 |
| Neutrophil lymphocyte ratio | |
| r | 0.460 |
| P | 0.016 |
The mean disease duration from the first symptom was 11.3±8.4 (0–30) years in FMF patients. In the asymptomatic FMF patients, the PWV, Aixao, Aixbr, and CAP measurements were statistically similar to those of the controls. In these patients, the PWV, Aixao, Aixbr, and CAP measurements were significantly correlated with disease duration documented in years (Pearson r=–0.380, 0.360, 0.360, 0.390; p=0.035, 0.047, 0.046, 0.030, respectively).
The MEFV mutations of FMF patients are shown in Table 4. The main symptoms associated with attacks in FMF patients were peritonitis in 49 patients (71%), arthritis in 46 patients (66.7%), pleuritis in six patients (8.7%), myositis in five patients (7.2%), and erysipelas-like erythema in seven patients (10.1%). The mean colchicine dose of FMF patients was 1.12±0.5 mg/day, and the colchicine dose had no effect on arterial stiffness and inflammatory markers. The genetic mutation and attack symptoms had no association with the arterial stiffness measurements.
Table 4.
Genetic mutations of the study patients with FMF
| Homozygous | Heterozygous | |
|---|---|---|
| M694V (n. %) | 7 (10.1) | 19 (27.5) |
| R202Q (n. %) | 1 (1.4) | 11 (15.9) |
| M680I (n. %) | 1 (1.4) | 7 (10.1) |
| V726A (n. %) | – | 6 (8.7) |
| E148Q (n. %) | – | 3 (4.3) |
Discussion
In this study, we found that FMF patients have increased arterial stiffness measurements compared to healthy people, and that arterial stiffness measurements are higher in patients during the attack period. Our study is the first one to investigate arterial stiffness in FMF patients in the literature. Arterial stiffness in these patients is associated with the degree of inflammation, rather than the genetic mutation and the attack symptoms. Arterial stiffness measurements had no association with serum inflammatory markers in the controls. This finding raises the idea that FMF attacks may contribute to ED and organ damage through ongoing and recurring inflammation, rather than or in addition to the other factors that may be associated with organ damage in these patients.
FMF is characterized with an elevated inflammatory response and this state is associated with an imbalance in vasoconstriction, vasodilatation, smooth muscle cell proliferation, thrombogenesis, and fibrinolysis resulting in ED. The endothelial layer manages homeostasis, vascular permeability, vasoregulation, angiogenesis, and inflammatory response (10). It is a generally accepted hypothesis that ED is the preceding event for the development of atherosclerosis. ED and atherosclerosis play important roles in the end organ damage pathogenesis. Functional impairment of the endothelium is recognized at the early stages of the development of atherosclerosis and exists long before the occurrence of atherosclerotic cardiovascular disease. So, assessing the central role of the endothelium can provide a clinical opportunity to detect early disease, calculate cardiovascular risk, and evaluate the response to treatments. Previously, flow-mediated dilatation was studied as a gold standard indicator of endothelial damage and dysfunction (11). Nowadays, investigators focus on non-invasive determining methods of ED and atherosclerosis, such as arterial stiffness measurements. Arterial stiffness has a high cardiovascular predictive value, cost effectiveness, reproducibility, and availability in many healthcare facilities. It has been suggested as an additional test for the evaluation of hypertensive patients according to the current hypertension guidelines (8). Previous studies have suggested that arterial stiffness measurements correlate with serum biomarkers that represent ED (12, 13). Increased PWV and Aix are accepted to reflect increased cardiovascular risk. Aix is a ratio calculated from the blood pressure waveforms and represents the wave reflection and arterial stiffness. Aixao reflects the division of the augmentation pressure after the anacrotic notch during the heart beat by pulse pressure. Similarly, Aixbr is the ratio of brachial late to early systolic pressures. Aix values were found to be correlated with serum asymmetrical dimethylarginine (ADMA) levels in newly diagnosed hypertensive patients (14). Furthermore, it has been previously shown that prediabetes and newly diagnosed diabetes patients have increased arterial stiffness and markers of ED through rising blood glucose levels (15, 16). Increased PWV measurements in FMF patients may reflect increased cardiovascular risk. But increased Aix measurements could be expected in these patients, which is the opposite to the findings of our study. Change in hemodynamic factors in FMF patients may be associated with this finding, though we do not know the reason for this finding.
Neutrophils are considered to be key cells in the local and systemic inflammatory response in FMF, and are hyperactive during attacks (17). Sustained inflammation leads to functional alterations in microcirculation and may be a reason for increases in the cardiovascular problems in FMF (18, 19). The drug of choice for the prevention of attacks and the development of amilodosis in patients with FMF is colchicine, which has anti-neutrophilic, anti-inflammatory, anti-fibrotic, and anti-proliferative effects (20). Preliminary data propose that enhanced atherogenesis may accompany FMF in the absence of a sufficient suppression of inflammation by colchicine (21). In our study, we found that stiffness measurements get higher in the attack period. This may be due to inflammation, released cytokins during the attack period, or subsequent vasoconstiction. The asymptomatic patients represent similar arterial stiffness measurements with healthy controls, leading to the idea that FMF itself causes increased arterial stiffness in attacks by some local factors, rather than leading to an accelerated atherosclerotic disease process. The similarity of the arterial stiffness measurements between the healthy controls and asymptomatic patients may be because of the technical failure of the arterial stiffness measurements to represent the lower grade but ongoing inflammation persisting in non-attack periods. The exact nature and reasons for the higher stiffness measurements during the attack is yet poorly understood. Further studies are needed to comment on this subject.
The true cardiovascular disease prevalence in FMF is unknown. Preliminary observational studies report that cardiovascular features are much less focused than other features in FMF. When enough attention is paid, literature data looks to support higher ED in FMF patients. Akdoğan et al. (22) found impaired endothelial function and increased intima-media thickness in FMF patients in 2006. In a study by Yılmaz et al. (23), patients with FMF-related amyloidosis had increased cardiovascular event risk, probably related to the high ADMA levels, elevated inflammatory markers, and decreased flow-mediated dilatation measurements. Terekeci et al. (24) reported higher serum ADMA levels in FMF patients, indicating inflammation-related ED, and suggested the use of colchicine as effective in preventing the development of and reversing not only amyloidosis but also ED in patients with FMF (24). In another study, Pamuk et al. (25) reported that FMF patients receiving regular colchicine therapy during the inactive disease state had significantly lower levels of vascular injury parameters. In a cohort study by Salah et al. (26), cardiac involvement appeared to be common in children with FMF, and pericardial effusions were significantly related to the presence of mutation types E148Q, P369S, and V726A. The M694V mutation of the MEFV gene has been found to be associated with a complicated clinical course and increased sensitivity to inflammatory disorders and cardiac involvement (27). It has also been suggested that MEFV mutations in early coronary heart disease patients was significantly increased in contrast to coronary heart disease patients and healthy controls (28). However, our study lacked any association of arterial stiffness with genetic mutations. All of these data and our own results suggest that FMF might be a risk factor for subclinical atherosclerosis due to recurring inflammatory process.
A number of previous studies evaluated aortic stiffness echocardiographically. Yıldız et al. (29) demonstrated an increase in the carotid-femoral (aortic) PWV in patients with chronic inflammatory conditions, such as rheumatoid arthritis, systemic lupus erytematosus, FMF, granulomatous polyangiitis, sarcoidosis, psoriasis, and psoriatic arthritis. Sarı et al. (30) reported that subclinical myocardial involvement is present in a cohort of relatively young FMF patients free of classical cardiovascular risk factors and that pericardium and aorta seemed to be spared during the asymptomatic periods of FMF.
There are some studies lacking evidence of ED. In a study by Twig et al. (31), FMF was found to be associated with lower rates of most components of the metabolic syndrome compared with normal subjects, unlike other inflammatory conditions. Peru et al. (32) could not find any significant association between CRP, SAA, homocysteine, lipoprotein-a, and common carotid artery intima-media thickness.
Study limitations
Our study has some limitations. First, our study group is relatively small to represent the FMF patients and cardiovascular risk. Second, the cross-sectional design of our study may complicate our comments about long-term complications, and prospective trials should be considered. Third, female patients were fewer than males in our study. This lacks the appropriate investigation of gender differences of FMF patients in terms of arterial stiffness and cardiovascular risk. It would be better if appropriate number of females would have been recruited.
Conclusion
These results show that FMF patients have augmented arterial stiffness measurements that get worse during attacks compared with controls. The severity of the inflammatory state, rather than the attack type and genetic mutations seem to effect the augmented arterial stiffness measurements. Further studies are needed to explain the increased stiffness measurements and cardiovascular risk in during-attack FMF patients.
Acknowledgements:
The authors of this article report no conflict of interest. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – M.Ç., M.A.; Design – M.Ç., K.Ö., G.T.; Supervision – M.Ç., T.D., K.Ö., G.T.; Materials- M.Ç., M.A.; Data collection &/or processing – M.Ç., T.D., K.Ö., G.T., E.A., S.Y.; Analysis &/or interpretation – M.Ç., M.Çınar., M.A., T.D.; Literature search – K.Ö., T.D., M.A.; Writing – M.Ç., M.A., K.Ö.; Critical review – M.Ç., M.Çınar., E.A., S.Y.
References
- 1.Livneh A, Langevitz P. Diagnostic and treatment concerns in familial Mediterranean fever. Bailliere’s Best Pract Res Clin Rheumatol. 2000;14:477–98. doi: 10.1053/berh.2000.0089. [DOI] [PubMed] [Google Scholar]
- 2.Özen S. Familial mediterranean fever: revisiting an ancient disease. Eur J Pediatr. 2003;162:449–54. doi: 10.1007/s00431-003-1223-x. [DOI] [PubMed] [Google Scholar]
- 3.Bhat A, Naguwa SM, Gershwin ME. Genetics and new treatment modalities for familial Mediterranean fever. Ann N Y Acad Sci. 2007;1110:201–8. doi: 10.1196/annals.1423.022. [DOI] [PubMed] [Google Scholar]
- 4.Yılmaz S, Çınar M, Şimşek I, Erdem H, Pay S. Tocilizumab in the treatment of patients with AA amyloidosis secondary to familial Mediterranean fever. Rheumatology. 2015;54:564–5. doi: 10.1093/rheumatology/keu474. [DOI] [PubMed] [Google Scholar]
- 5.Portincasa P. Colchicine, biologic agents and more for the treatment of familial mediterranean fever. The old, the new, and the rare. Curr Med Chem. 2016;23:60–86. doi: 10.2174/0929867323666151117121706. [DOI] [PubMed] [Google Scholar]
- 6.Kasifoğlu T, Bilge SY, Sarı I, Solmaz D, Senel S, Emmungil H, et al. Amyloidosis and its related factors in Turkish patients with familial Mediterranean fever: a multicentre study. Rheumatology. 2014;53:741–5. doi: 10.1093/rheumatology/ket400. [DOI] [PubMed] [Google Scholar]
- 7.Kostis JB, Lawrence-Nelson J, Ranjan R, Wilson AC, Kostis WJ, Lacy CR. Association of increased pulse pressure with the development of heart failure in SHEP. Systolic Hypertension in the Elderly (SHEP) Cooperative Research Group. Am J Hypertens. 2001;14:798–803. doi: 10.1016/s0895-7061(01)02044-1. [DOI] [PubMed] [Google Scholar]
- 8.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34:2159–219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 9.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Münzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–8. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 10.Kharbanda RK, Deanfield JE. Functions of the healthy endothelium. Coron Artery Dis. 2001;12:485–91. doi: 10.1097/00019501-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Yılmaz MI, Sönmez A, Sağlam M, Güleç M, Kılıç S, Eyileten T, et al. Hemoglobin is inversely related to flow-mediated dilatation in chronic kidney disease. Kidney Int. 2009;75:1316–21. doi: 10.1038/ki.2009.63. [DOI] [PubMed] [Google Scholar]
- 12.Krantz MJ, Long CS, Hosokawa P, Karimkahani E, Dickinson M, Estacio RO, et al. Pulse wave velocity and carotid atherosclerosis in White and Latino patients with hypertension. BMC Cardiovascular Disorders. 2011;11:15. doi: 10.1186/1471-2261-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanındı A, Erkan AF, Alhan A, Töre HF. Arterial stiffness and central arterial wave reflection are associated with serum uric acid, total bilirubin, and neutrophil-to-lymphocyte ratio in patients with coronary artery disease. Anatol J Cardiol. 2015;15:396–403. doi: 10.5152/akd.2014.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Çakar M, Bulucu F, Karaman M, Ay SA, Kurt Ö, Balta Ş, et al. Asymmetric dimethylarginine and augmentation index in newly diagnosed patients with hypertension. Angiology. 2015;66:43–8. doi: 10.1177/0003319713513145. [DOI] [PubMed] [Google Scholar]
- 15.Çakar M, Balta Ş, Şarlak H, Akhan M, Demirkol S, Karaman M, et al. Arterial stiffness and endothelial inflammation in prediabetes and newly diagnosed diabetes patients. Arch Endocrinol Metab. 2015;59:407–13. doi: 10.1590/2359-3997000000061. [DOI] [PubMed] [Google Scholar]
- 16.Kalaycıoğlu E, Gökdeniz T, Aykan AÇ, Hatem E, Gürsoy OM, Çavuşoğlu G, et al. Ambulatory arterial stiffness index is associated with impaired left atrial mechanical functions in hypertensive diabetic patients: A speckle tracking study. Anatol J Cardiol. 2015;15:807–13. doi: 10.5152/akd.2014.5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Önen F. Familial Mediterranean fever. Rheumatol Int. 2006;26:489–96. doi: 10.1007/s00296-005-0074-3. [DOI] [PubMed] [Google Scholar]
- 18.Çalışkan M, Güllü H, Yılmaz S, Erdoğan D, Ünler GK, Çiftçi O, et al. Impaired coronary microvascular function in familial Mediterranean fever. Atherosclerosis. 2007;195:161–7. doi: 10.1016/j.atherosclerosis.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Tavil Y, Üreten K, Öztürk MA, Şen N, Kaya MG, Cemri M, et al. The detailed assessment of left and right ventricular functions by tissue Doppler imaging in patients with familial Mediterranean fever. Clin Rheumatol. 2008;27:189–94. doi: 10.1007/s10067-007-0676-0. [DOI] [PubMed] [Google Scholar]
- 20.Terkeltaub RA. Colchicine Update. 2008. Semin Arthritis Rheum. 2009;38:411–9. doi: 10.1016/j.semarthrit.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Gasparyan AY, Stavropoulos-Kalinoglou A, Mikhailidis DP, Toms TE, Douglas KM, Kitas GD. The rationale for comparative studies of accelerated atherosclerosis in rheumatic diseases. Curr Vasc Pharmacol. 2010;8:437–49. doi: 10.2174/157016110791330852. [DOI] [PubMed] [Google Scholar]
- 22.Akdoğan A, Calgüneri M, Yavuz B, Arslan EB, Kalyoncu U, Şahiner L, et al. Are Familial Mediterranean Fever patients at increased risk for atherosclerosis?Impaired endothelial function and ıncreased intima-media thickness are found in FMF. J Am Coll Cardiol. 2006;48:2351–3. doi: 10.1016/j.jacc.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Yılmaz MI, Demirkaya E, Açıkel C, Saldır M, Akar S, Çaycı T, et al. Endothelial function in patients with familial Mediterranean fever-related amyloidosis and association with cardiovascular events. Rheumatology (Oxford, England) 2014;53:2002–8. doi: 10.1093/rheumatology/keu231. [DOI] [PubMed] [Google Scholar]
- 24.Terekeci HM, Öktenli C, Özgürtaş T, Nalbant S, Top C, Çelik S, et al. Increased asymmetric dimethylarginine levels in young men with familial Mediterranean fever (FMF): is it early evidence of interaction between inflammation and endothelial dysfunction in FMF? J Rheumatol. 2008;35:2024–9. [PubMed] [Google Scholar]
- 25.Pamuk BO, Sarı I, Selçuk S, Gökce G, Kozacı DL. Evaluation of circulating endothelial biomarkers in familial Mediterranean fever. Rheumatol Int. 2013;33:1967–72. doi: 10.1007/s00296-013-2681-8. [DOI] [PubMed] [Google Scholar]
- 26.Salah S, Hegazy R, Ammar R, Sheba H, Abdelrahman L. MEFV gene mutations and cardiac phenotype in children with familial Mediterranean fever: a cohort study. Pediatr Rheumatol Online J. 2014;12:5. doi: 10.1186/1546-0096-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Çolak B, Gürlek B, Yegin ZA, Değer SM, Elbek S, Paşaoğlu H, et al. The relationship between the MEFV genotype, clinical features, and cytokine-inflammatory activities in patients with familial mediterranean fever. Ren Fail. 2008;30:187–91. doi: 10.1080/08860220701810364. [DOI] [PubMed] [Google Scholar]
- 28.Başar N, Kısacık B, Ercan S, Pehlivan Y, Yılmaz S, Şimsek I, et al. Familial Mediterranean fever gene mutations as a risk factor for early coronary artery disease. Int J Rheum Dis. 2014 Apr 7; doi: 10.1111/1756-185X.12356. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Yıldız M. Arterial distensibility in chronic inflammatory rheumatic disorders. Open Cardiovasc Med J. 2010;4:83–8. doi: 10.2174/1874192401004020083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarı I, Arıcan O, Can G, Akdeniz B, Akar S, Birlik M, et al. Assessment of aortic stiffness and ventricular functions in familial Mediterranean fever. Anatol J Cardiol. 2008;8:271–8. [PubMed] [Google Scholar]
- 31.Twig G, Livneh A, Vivante A, Afek A, Derazne E, Leiba A, et al. Cardiovascular and metabolic risk factors in inherited autoinflammation. J Clin Endocrinol Metab. 2014;99:E2123–8. doi: 10.1210/jc.2014-2096. [DOI] [PubMed] [Google Scholar]
- 32.Peru H, Altun B, Doğan M, Kara F, Elmacı AM, Oran B. The evaluation of carotid intima-media thickness in children with familial Mediterranean fever. Clin Rheumatol. 2008;27:689–94. doi: 10.1007/s10067-007-0764-1. [DOI] [PubMed] [Google Scholar]


