Abstract
Objective:
Cardiac autonomic dysfunction may develop in patients with polycystic ovary syndrome (PCOS). Heart rate variability (HRV) and heart rate turbulence (HRT) are used in assessing cardiac autonomic functions. The goal of this study was to compare the cardiac autonomic functions in patients with PCOS and healthy controls. To our knowledge, this is the first study evaluating cardiac autonomic functions in patients with PCOS with respect to both HRV and HRT.
Methods:
Twenty-three patients with PCOS (mean age 22.8±3.9 years) and 25 healthy female volunteers who were matched for age and body mass index (BMI) (mean age 23.5±6.2 years) were enrolled in this as case-control study. Twenty-four hour ambulatory electrocardiogram recordings of all participants were taken using Pathfinder software. The time domain parameters of HRV and HRT, including turbulence onset (TO) and turbulence slope, were calculated. Diagnosis of PCOS was made with physical and laboratory findings of hirsutism or biochemical hyperandrogenism and chronic anovulation. Diabetes mellitus, other hormon disorders or hormon therapy, pregnancy, atrial fibrilation, obesite, chronic diseases, disorders of the autonomic nervous system, a history of drug use affecting the autonomic nervous system were excluded.
Results:
There were no significant differences in HRV and HRT parameters between the two groups. Cardiovascular risk factors, such as BMI, blood pressure, fasting blood glucose, and lipid parameters, were also similar. Triangular index measure of HRV was negatively correlated with high density lipoprotein cholesterol levels (r=-0.47, p<0.05), while age and BMI were significantly correlated with TO (r=0.31 and 0.47, respectively; p<0.05 for all).
Conclusion:
Cardiac autonomic functions were not found to be altered in patients with PCOS in comparison with healthy controls. These results may be explained with the absence of concomitant cardiovascular risk factors with the patients being in the early stage of the disease.
Keywords: cardiac autonomic function, polycystic ovary syndrome, heart rate turbulence, heart rate variability
Introduction
Polycystic ovary syndrome (PCOS) is a metabolic and endocrine disorder that affects 6%-10% of the reproductive-age female population. This syndrome is characterized by polycystic ovary, hyperandrogenism, and menstrual irregularities (1). Cardiovascular risk factors, such as hypertension, lipid abnormalities, type 2 diabetes mellitus, metabolic syndrome, endothelial dysfunction, increased C-reactive protein and homocysteine levels are frequently accompanied by PCOS (2-8). Furthermore, decreased heart rate variability (HRV) and increased QT dispersion, which are risk factors for life-threatening ventricular arrhythmia, were observed in these patients (9-11).
Metabolic and cardiovascular disorders are known to be related to autonomic dysfunction that has a significant relationship with cardiovascular mortality (12-14). HRV and heart rate turbulence (HRT) provide important information regarding cardiac autonomic functions (15-17). HRV measures the oscillation in successive cardiac cycles as well as the oscillations between instantaneous heart rates (16). Previous studies have demonstrated reduced HRV to predict increased cardiac mortality (13). HRT is defined as the sinus rhythm cycle length fluctuation after isolated premature ventricular beats (VPB) (17). It has been deemed appropriate for risk estimation after acute myocardial infarction and as a prognostic evaluator of heart failure and other pathologies (18-20).
Baroreflex sensitivity (BRS) and HRV are believed to evaluate different aspects of autonomic control. There is a significant relationship between HRT and BRS. Therefore, it is suggested that HRT can be used instead of BRS (21, 22). Previous studies demonstrated autonomic dysfunction in PCOS as reduced HRV and exaggerated blood pressure response to exercise (9, 10, 23, 24). However, HRT has not been evaluated in PCOS yet. Therefore, we performed a study to evaluate the impact of PCOS on both HRT and HRV parameters.
Methods
Study design
The study was designed as case control study.
Study population
This study was performed in the Afyon Kocatepe University Hospital between February 1, 2013 and October 1, 2014 in the Departments of Cardiology and Gynecology. Study population comprised 33 recently diagnosed patients with PCOS and 38 healthy volunteers (women aged 18–35 years). Diagnosis of PCOS was made with physical and laboratory findings of hirsutism or biochemical hyperandrogenism and chronic anovulation. Age matched, regularly menstruating, healthy, female volunteers with normal ovulating cycles (28±2 days, blood progesterone levels >10 ng/mL in two consecutive cycles) and normal ultrasonographic appearance of the ovaries without any sign of hyperandrogenism were included as controls.
Women with menstrual irregularities, thyroid disorders, other hormonal disorders, pregnancy, diabetes mellitus, hypertension, ischemic heart disease, heart failure, atrial fibrillation, renal disease, chronic inflammatory diseases, disorders of the autonomic nervous system, a history of drug use affecting the autonomic nervous system, renal disease, smoking, lung disease, obesity, and women on any hormonal therapy or drugs were excluded.
Systolic and diastolic blood pressure of all patients and controls were measured from the right arm with a mercury manometer after resting of at least five min of sitting. Body weight and height were measured, and body mass index (BMI) was calculated. Venous blood samples for biochemical and hormonal tests were obtained in the morning between 08.00 and 08.30 after fasting for at least 12 h.
All subjects delivered their informed consent prior to inclusion. The study protocol was approved by the Ethics Committee of our institution.
HRT analysis
Twenty-four hour Holter monitoring (Reynolds Medical, Pathfinder Software Version V8.255, Hedford, England) was performed with all subjects. HRT measures, TO and TS, were automatically calculated by a computer program (HRT View, Version 0.60-0.1 Software Program, Munich, Germany). All Holter recordings were checked and artifacts that the program admitted as VPB were also excluded. TO reflects the early sinus acceleration after a VPB, whereas TS represents the late sinus deceleration following a VPB. TO is expressed as a percentage of change from the mean of two RR intervals preceding and two RR intervals following VPB. TO was separately calculated for all VPBs and then averaged. TS is the maximum positive slope of a regression line obtained over any sequence of five subsequent RR intervals within the first 20 sinus rhythm intervals after VPB. TO≥0% and TS≤2.5 ms/RR were considered abnormal (14).
HRV analysis
HRV measures, which were chosen according to the guidelines of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, were calculated for all subjects from 24 h of Holter recordings (10). The time domain HRV measures included standard deviations of all NN intervals (SDNN), mean of the standard deviation of all NN intervals for all 5-min segments of the entire recording (SDNNI), standard deviation of averages of NN intervals in all 5-min segments of the entire recording (SDANN), triangular index (TI), the square root of the mean of the sum of the squares of differences between adjacent NN intervals (RMSSD).
Statistical analysis
Statistical analysis was performed with SPSS for Windows version 15.0 (SPSS Inc., Chicago, IL, USA). Data were presented as mean±SD or median (interquartile range). Distribution of continuous variables was determined by the Kolmogorov–Smirnov test. Parametric data were compared using independent sample t-test and nonparametric data with Mann–Whitney U test. Correlations were performed using Spearman’s and Pearson tests. A p values <0.05 were considered statistically significant.
Results
Twenty-three subjects (10 patients and 13 controls) were excluded from the study because there was no detectable VPB on their 24 h Holter recordings enabling an accurate HRT analysis. Therefore, the study population comprised 23 untreated patients with PCOS (mean age 22.8±3.9 years) and 25 healthy controls (mean age 23.5±6.2 years). No significant differences in age, BMI, blood pressure, and minimum or maximum heart rate were defined between the patient and control groups (Table 1).
Table 1.
Demographic characteristics of patients and controls
| Variable | PCOS (n=23) | Controls (n=25) | P |
|---|---|---|---|
| Age, years | 22 (18-35) | 21.5 (18-35) | 0.6* |
| BMI, kg/m2 | 21.7±1.7 | 21.3±1.9 | 0.4† |
| DBP, mm Hg | 66.7±9.3 | 67±8.2 | 0.1† |
| SBP, mm Hg | 103.7±11.9 | 110.1±12.4 | 0.4† |
Data were shown as mean±SD, median (interquartile range).
Independent sample t test was used,
Mann-Whitney U test was used.
BMI - body mass index; DBP - diastolic blood pressure; SBP - systolic blood pressure
The hormonal and biochemical levels of the subjects are shown in Table 2. The groups were similar regarding fasting glucose, total cholesterol, high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), triglycerides, estradiol, and dehydroepiandrosterone sulfate (DHEAS) levels. However, in patients with PCOS, free testosterone and luteinizing hormone levels were significantly higher, and follicle-stimulating hormone levels were significantly lower compared with controls.
Table 2.
Hormonal and biochemical levels of the subjects
| Variable | PCOS (n=23) | Controls (n=25) | P |
|---|---|---|---|
| Fasting glucose, mg/dL | 78±10 | 81±9 | 0.28† |
| Total cholesterol, mg/dL | 190±26 | 182±14 | 0.18† |
| HDL, mg/dL | 62±15.61 | 57±8.31 | 0.19† |
| LDL, mg/dL | 102±15 | 103±14 | 0.59† |
| Triglycerides, mg/dL | 124 (45-156) | 122 (100-178) | 0.07* |
| FSH, mU/mL | 6.4±1.58 | 7.3±1.15 | <0.05† |
| LH, mU/mL | 8.2 (2.7-27.7) | 6.3 (3.98-7.8) | <0.05* |
| Free-testosterone, ng/dL | 3.7±1.93 | 1.6±0.7 | <0.05† |
| Estradiol, pq/mL | 40±13.5 | 38±9.5 | 0.57† |
| DHEAS, micg/dL | 246±68 | 230±44 | 0.36† |
Data were shown as mean±SD, median (interquartile range)
(Fasting glucose, total cholesterol, HDL, LDL, FSH, free testesterone, estradiol, DHEAS) Independent sample t test was used,
(trigliserides, LH) Mann-Whitney U test was used.
The HRV and HRT measures were not significantly different between the two groups (Table 3). In correlation analysis, there was no significant correlation between HRV and HRT parameters with sex hormones, total cholesterol, triglycerides, LDL-C, and fasting glucose levels. However, there was a significant negative correlation between TI and HDL-C levels (r=-0.33, p=0.032) and a significant positive correlation between BMI and age with TO (r=0.38, p= 0.008 and r=0.48, p<0.001, respectively) (Fig. 1-2).
Table 3.
Time domain HRV parameters and HRT parameters in patients and controls
| Variable | PCOS (n=23) | Controls (n=25) | P |
|---|---|---|---|
| TO (%) | -3.4±2.3 | -2.9±2.1 | 0.28† |
| TS (ms/RR) | 14.02±2 | 14.55±1.8 | 0.85† |
| SDNN (ms) | 126 (102-199) | 130 (70-203) | 0.8* |
| SDANN (ms) | 110 (70-198) | 108.5 (56-185) | 0.76* |
| SDNN index (ms) | 65.5 (36-125) | 58.5 (39-116) | 0.27* |
| RMSSD (ms) | 41 (16-78) | 38.5 (17-163) | 0.65* |
| TI (ms) | 37 (24-54) | 36 (22-56) | 0.74 |
| Maximum heart rate (BPM) | 133.9±25 | 129.5±17.9 | 0.7† |
| Minimum heart rate (BPM) | 55.2±5.6 | 58.70±8.7 | 0.06† |
| Mean heart rate (BPM) | 81 (66-100) | 84 (54-88) | 0.82* |
| Number of VPBs/24 h | 1 (1-6) | 1 (1-5) | 0.38* |
Data were shown as mean±SD, median (interquartile range)
(TO, TS, maximum heart rate, minimum hear rate) Independent sample t test was used,
(SDNN, SDANN, SDNN index, RMSSD, mean heart rate, Number of VPS) Mann-Whitney U test was used.
Figure 1.
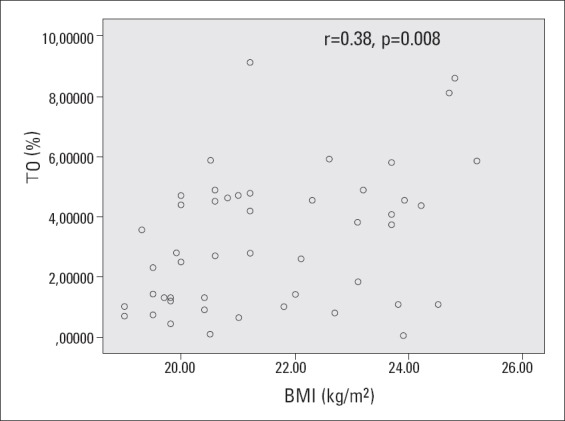
Relationship between BMI and TO
Pearson correlation test was used.
Figure 2.
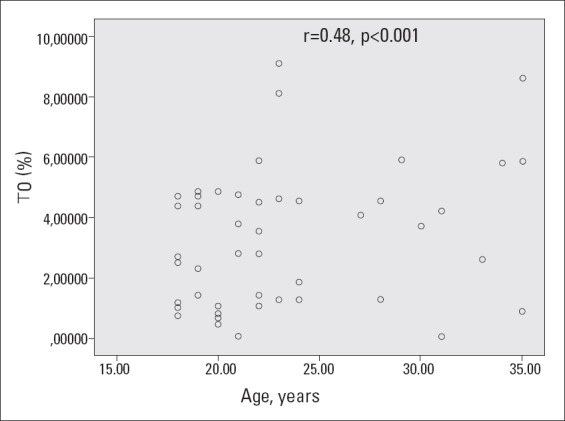
Relationship between age and TO
Spearman’s correlation test was used
Discussion
The main finding of our study is that HRT and HRV parameters do not alter in young patients with PCOS who do not have cardiovascular risk factors. PCOS occurs in women of reproductive age and is frequently accompanied by classic cardiovascular risk factors (2-8). Previous studies have demonstrated decreased HRV levels in patients with PCOS (9, 10, 23-26). However, it is not known how HRT measures are affected in this disease. In addition, it is not well understood whether the decreased HRV levels in patients with PCOS occur because of the disease itself or because of the effects of concomitant cardiovascular risk factors. Our investigation has novel findings as being the first study evaluating both HRV and HRT measures together in patients with PCOS who have no cardiovascular risk factors.
Domenico et al. (23) have assessed HRV in patients with PCOS at rest and during sympathetic stimulation and have investigated the impact of PCOS phenotype on HRV. No differences were observed in HRV of ovulatory PCOS, anovulatory PCOS, and healthy controls at rest. However, during sympathetic stimulation, a decrease was observed in HRV of the ovulatory group. The reduction in HRV measures was suggested to be associated with anovulation in PCOS. In addition, cardiovascular risk factors, such as BMI, waist circumference, blood pressure, and triglyceride levels, were found to be significantly higher in the anovulatory group. In our study, there was no such difference in cardiovascular risk factors between the patient and control groups.
Yıldırır et al. (9) have also evaluated the cardiac autonomic functions in women with PCOS and found a decrease in the parasympathetic component and an increase in the sympathetic component. However, in that study, serum HDL-C levels were observed to be low and serum triglycerides levels with total cholesterol/HDL-C ratio to be high. The relatively higher age of the patients compared with our study population and the presence of cardiovascular risk factors, such as dyslipidemia, may be responsible for these results differing from our study. The serum lipid profiles of our study population were within normal limits.
Obesity is a common finding of PCOS and is observed in more than half of the patients (27). The reduction in HRV was thought to be related with the weight gain in patients with PCOS in a study examining the relationship between HRV and cardiovascular risk factors in patients with PCOS (26). In addition to the incidence of obesity in the PCOS group, other cardiovascular risk factors, such as dyslipidemia, insulin resistance, and inflammation parameters, were also higher. In a recent study, HRV was observed to be significantly reduced in obese patients with PCOS compared with obese control group. However, no significant differences were observed between non-obese patients with PCOS compared with non-obese controls in the same study (25). Although the participants of our study had normal body weight, we found a relationship between BMI and TO. These findings together with our study suggest that obesity has an important role in the deterioration of cardiac autonomic functions in patients with PCOS.
Tekin et al. (24) have evaluated blood pressure and HRV response to exercise in young women with recently diagnosed PCOS. They have determined an exaggerated increase in blood pressure and a decrease in HRV in response to exercise. The absence of a significant difference between the control and patient groups in terms of cardiovascular risk factors suggested that the obtained results were directly associated with PCOS. However, in contrast to our study, the similarity of the hormone profiles of the patient and control groups were remarkable in that study.
Another study assessing the effects of sex steroids on HRV found a relationship between androgens and the parasympathetic component of HRV and also between estradiol and the sympathetic component of HRV. DHEAS was determined to be the most relevant sex steroid for cardiac autonomic tests (28). Several other studies have reported that both androgens and estrogens have a negative relationship with HRV (29, 30). A slight non-significant relationship was determined between sex steroids and some of the HRT and HRV measures in our study.
Study limitations
The main limitation of our study was the small sample size. Because HRT could not be calculated in participants who did not have any VPB in their Holter recordings, they were excluded from the study. Another important limitation of our study was the absence of spectral HRV analysis. Because of this limitation sympathetic tonus could not be evaluated; therefore, only parasympathetic tonus was assessed.
Conclusion
We did not find a significant difference in HRT and HRV measures of patients with PCOS in comparison with healthy controls. The absence of concomitant cardiovascular risk factors in our patient group who were still in the early stage of their disease process can be suggested as the reason for our findings. In PCOS, coexisting cardiovascular risk factors will appear to have a greater influence in the following years, causing autonomic dysfunction. Further studies with more participants are required to evaluate HRT together with HRV in patients with PCOS that have no cardiovascular risk factors.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept - G.Ö., E.O., A.A.; Design - G.Ö., B.S.Ü.; Supervision - E.O., Ö.A., A.A., G.K.; Materials - B.S.Ü., G.K.; Data collection &/or processing - G.Ö., B.S.Ü., G.K., H.D.; Analysis and/or interpretation - G.Ö., Ö.A., H.D.; Literature search - H.D., G.Ö.; Writing - G.Ö., H.D., Ö.A.; Critical review - G.K., E.O., A.A.
References
- 1.Carmina E, Lobo RA. Polycystic ovary syndrome (PCOS): Arguably the most common endocrinopathy is associated with significant morbidity in women. J Clin Endocrinol. 1999;84:1897–9. doi: 10.1210/jcem.84.6.5803. [DOI] [PubMed] [Google Scholar]
- 2.Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370:685–97. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- 3.Soares EM, Azevedo GD, Gadelha RG, Lemos TM, Maranhão TM. Prevalence of the metabolic syndrome and its components in Brazilian women with polycystic ovary syndrome. Fertil Steril. 2008;89:649–55. doi: 10.1016/j.fertnstert.2007.03.081. [DOI] [PubMed] [Google Scholar]
- 4.Weerakiet S, Srisombut C, Bunnag P, Sangtong S, Chuangsoongnoen N, Rojanasakul A. Prevalence of type 2 diabetes mellitus and impaired glucose tolerance in Asian women with polycystic ovary syndrome. Int J Gynecol Obstet. 2001;75:177–84. doi: 10.1016/s0020-7292(01)00477-5. [DOI] [PubMed] [Google Scholar]
- 5.Paradisi G, Steinberg HO, Hempfling A, Polycy Cronin J, Hook G, Shepard MK, et al. Polycystic ovary syndrome is associated with endothelial dysfunction. Circulation. 2001;103:1410–5. doi: 10.1161/01.cir.103.10.1410. [DOI] [PubMed] [Google Scholar]
- 6.Oliveira M, Costa L, Farias F, Viana A, Santos MP. Correlation of high sensitivity C-reactive protein levels and clinical and laboratory parameters in polycystic ovary syndrome patients. Rev Bras Ginecol Obstet. 2007;29:10–7. [Google Scholar]
- 7.Loverro G, Lorusso F, Mei L, Depalo R, Cormio G, Selvaggi L. The plasma homocysteine levels are increased in polycystic ovary syndrome. Gynecol Obstet Invest. 2002;53:157–62. doi: 10.1159/000058367. [DOI] [PubMed] [Google Scholar]
- 8.Gambineri A, Pelusi C, Vicennati V, Pagotto U, Pasquali R. Obesity and the polycystic ovary syndrome. Int J Obes. 2002;26:883–96. doi: 10.1038/sj.ijo.0801994. [DOI] [PubMed] [Google Scholar]
- 9.Yıldırır A, Aybar F, Kabakçı G, Yaralı H, Oto A. Heart rate variability in young women with polycystic ovary syndrome. Ann Noninvasive Electrocardiol. 2006;11:306–12. doi: 10.1111/j.1542-474X.2006.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saranya K, Pal GK, Habeebullah S, Pal P. Assessment of cardiovascular autonomic function in patients with polycystic ovary syndrome. J Obstet Gynaecol Res. 2014;40:192–9. doi: 10.1111/jog.12154. [DOI] [PubMed] [Google Scholar]
- 11.Gazi E, Gencer M, Hancı V, Temiz A, Altun B, Çakır Güngör AN, et al. Relationship of QT dispersion with sex hormones and insulin in young women with polycystic ovary syndrome: an observational study. Anatol J Cardiol. 2013;13:772–7. doi: 10.5152/akd.2013.264. [DOI] [PubMed] [Google Scholar]
- 12.Assoumou HG, Pichot V, Barthelemy JC, Dauphinot V, Celle S, Gosse P, et al. Metabolic syndrome and short-term and long-term heart rate variability in elderly free of clinical cardiovascular disease: the PROOF study. Rejuvenation Res. 2010;13:653–63. doi: 10.1089/rej.2010.1019. [DOI] [PubMed] [Google Scholar]
- 13.Tsuji H, Larson MG, Venditti FJ, Jr, Manders ES, Evans JC, Feldman CL, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94:2850–5. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 14.Lown B, Verrier RL. Neural activity and ventricular fibrillation. N Engl J Med. 1976;294:1165–70. doi: 10.1056/NEJM197605202942107. [DOI] [PubMed] [Google Scholar]
- 15.Kleiger RE, Stein PK, Bigger JT. Heart rate variability: Measurement and clinical utility. Ann Noninvasive Electrocardiol. 2005;10:88–101. doi: 10.1111/j.1542-474X.2005.10101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 17.Schmidt G, Malik M, Barthel P, Schneider R, Ulm K, Rolnitzky L, et al. Heart rate turbulance after ventricular premature beats as a predictor of mortality after myocardial infarction. Lancet. 1999;353:1390–6. doi: 10.1016/S0140-6736(98)08428-1. [DOI] [PubMed] [Google Scholar]
- 18.Bauer A, Malik M, Schmidt G, Barthel P, Bonnemeier H, Cygankiewicz I, et al. Heart rate turbulence: standards of measurement, physiological interpretation, and clinical use: International society for Holter and noninvasive electrophysiology consensus. J Am Coll Cardiol. 2008;52:1353–65. doi: 10.1016/j.jacc.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 19.Bauer A, Malik M, Barthel P, Schneider R, Watanabe MA, Camm AJ, et al. Turbulence dynamics: an independent predictor of late mortality after acute myocardial infarction. Int J Cardiol. 2006;107:42–7. doi: 10.1016/j.ijcard.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 20.Koyama J, Watanabe J, Yamada A, Koseki Y, Konno Y, Toda S, et al. Evaluation of heart-rate turbulence as a new prognostic marker in patients with chronic heart failure. Circ J. 2002;66:902–7. doi: 10.1253/circj.66.902. [DOI] [PubMed] [Google Scholar]
- 21.La Rovere MT, Pinna GD, Hohnloser SH. ATRAMI Investigators. Autonomic tone and reflexes after myocardial infarcton. Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias: Implications for clinical trials. Circulation. 2001;103:2072–7. doi: 10.1161/01.cir.103.16.2072. [DOI] [PubMed] [Google Scholar]
- 22.La Rovere MT, Maestri R, Pinna GD, Sleight P, Febo O, et al. Clinical and haemodynamic correlates of heart rate turbulence as a non-invasive index of baroreflex sensitivity in chronic heart failure. Clin Sci. 2011;121:279–84. doi: 10.1042/CS20110063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Domenico K, Wiltgen D, Nickel FJ, Magalhães JA, Moraes RS, Spritzer PM. Cardiac autonomic modulation in polycystic ovary syndrome: does the phenotype matter? Fertil Steril. 2013;99:286–92. doi: 10.1016/j.fertnstert.2012.08.049. [DOI] [PubMed] [Google Scholar]
- 24.Tekin G, Tekin A, Kılıçarslan EB, Haydardedeoğlu B, Katırcıbaşı T, Koçum T, et al. Altered autonomic neural control of the cardiovascular system in patients with polycystic ovary syndrome. Int J Cardiol. 2008;130:49–55. doi: 10.1016/j.ijcard.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 25.Hashim ZH, Hamdan FB, Al-Salihi AR. Autonomic dysfunction in women with polycystic ovary syndrome. Iran J Reprod Med. 2015;13:27–34. [PMC free article] [PubMed] [Google Scholar]
- 26.de SáJC, Costa EC, da Silva E, Zuttin RS, da Silva EP, Lemos TM, et al. Analysis of heart rate variability in polycystic ovary syndrome. Gynecol Endocrinol. 2011;27:443–7. doi: 10.3109/09513590.2010.501881. [DOI] [PubMed] [Google Scholar]
- 27.Zacur HA. Epidemiology, clinical manifestations and pathophysiology of polycystic ovary syndrome. Adv Stud Med. 2003;3:733–9. [Google Scholar]
- 28.Doğru MT, Başar MM, Yuvanç E, Şimşek V, Şahin O. The relationship between serum sex steroid levels and heart rate variability parameters in males and the effect of age. Türk Kardiyol Dern Ars. 2010;38:459–65. [PubMed] [Google Scholar]
- 29.Tsuji H, Ferdinand J, Venditti J, Evans JC, Larson MG, Feldman CL, et al. Determinants of heart rate variability. J Am Coll Cardiol. 1996;28:1539–46. doi: 10.1016/s0735-1097(96)00342-7. [DOI] [PubMed] [Google Scholar]
- 30.Liao D, Barnes RW, Chambless LE, Simpson RJ, Jr, Sorlie P, Heiss G. Age, race, and sex differences in autonomic cardiac function measured by spectral analysis of heart rate variability The ARIC study. Am J Cardiol. 1995;76:906–12. doi: 10.1016/s0002-9149(99)80260-4. [DOI] [PubMed] [Google Scholar]


