Abstract
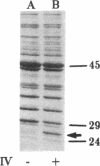
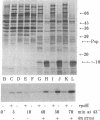
Filamentous phage infection induces the synthesis of large amounts of an Escherichia coli protein, phage shock protein (Psp), the product of a previously undescribed gene. This induction is due to the phage gene IV protein, pIV, an integral membrane protein. The uninduced level of Psp is undetectable, but when induced by prolonged synthesis of pIV, it can become one of the most abundant proteins in the cell. Psp is also synthesized transiently in response to several stresses (heat, ethanol, and osmotic shock). High-level synthesis occurs only after extreme treatment. Unlike the members of the heat shock regulon, Psp induction does not require the heat shock sigma factor, sigma 32; some stimuli that elicit sigma 32-dependent heat shock proteins do not induce Psp synthesis. The level of Psp induction after extreme stress is even higher in sigma 32 mutant cells, which are unable to mount a normal heat shock response, suggesting that these parallel stress responses are interrelated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ang D., Chandrasekhar G. N., Zylicz M., Georgopoulos C. Escherichia coli grpE gene codes for heat shock protein B25.3, essential for both lambda DNA replication at all temperatures and host growth at high temperature. J Bacteriol. 1986 Jul;167(1):25–29. doi: 10.1128/jb.167.1.25-29.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl H., Echols H., Straus D. B., Court D., Crowl R., Georgopoulos C. P. Induction of the heat shock response of E. coli through stabilization of sigma 32 by the phage lambda cIII protein. Genes Dev. 1987 Mar;1(1):57–64. doi: 10.1101/gad.1.1.57. [DOI] [PubMed] [Google Scholar]
- Baker T. A., Grossman A. D., Gross C. A. A gene regulating the heat shock response in Escherichia coli also affects proteolysis. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6779–6783. doi: 10.1073/pnas.81.21.6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassford P. J., Jr, Silhavy T. J., Beckwith J. R. Use of gene fusion to study secretion of maltose-binding protein into Escherichia coli periplasm. J Bacteriol. 1979 Jul;139(1):19–31. doi: 10.1128/jb.139.1.19-31.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., Model P., Zinder N. D. Effects of bacteriophage f1 gene III protein on the host cell membrane. Mol Gen Genet. 1982;186(2):185–192. doi: 10.1007/BF00331849. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Morphological and serological relationships of conjugative pili. Plasmid. 1980 Sep;4(2):155–169. doi: 10.1016/0147-619x(80)90005-0. [DOI] [PubMed] [Google Scholar]
- Brissette J. L., Russel M. Secretion and membrane integration of a filamentous phage-encoded morphogenetic protein. J Mol Biol. 1990 Feb 5;211(3):565–580. doi: 10.1016/0022-2836(90)90266-O. [DOI] [PubMed] [Google Scholar]
- Bukau B., Walker G. C. Cellular defects caused by deletion of the Escherichia coli dnaK gene indicate roles for heat shock protein in normal metabolism. J Bacteriol. 1989 May;171(5):2337–2346. doi: 10.1128/jb.171.5.2337-2346.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S., Ruettinger T. A temperature sensitive nonsense mutation affecting the synthesis of a major protein of Escherichia coli K12. Mol Gen Genet. 1975 Aug 5;139(2):167–176. doi: 10.1007/BF00264696. [DOI] [PubMed] [Google Scholar]
- Csonka L. N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989 Mar;53(1):121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drahos D. J., Hendrix R. W. Effect of bacteriophage lambda infection on synthesis of groE protein and other Escherichia coli proteins. J Bacteriol. 1982 Mar;149(3):1050–1063. doi: 10.1128/jb.149.3.1050-1063.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J. W., Gross C. A. Identification of the sigma E subunit of Escherichia coli RNA polymerase: a second alternate sigma factor involved in high-temperature gene expression. Genes Dev. 1989 Sep;3(9):1462–1471. doi: 10.1101/gad.3.9.1462. [DOI] [PubMed] [Google Scholar]
- Fayet O., Ziegelhoffer T., Georgopoulos C. The groES and groEL heat shock gene products of Escherichia coli are essential for bacterial growth at all temperatures. J Bacteriol. 1989 Mar;171(3):1379–1385. doi: 10.1128/jb.171.3.1379-1385.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein D., Horiuchi K. Interaction between the replication origin and the initiator protein of the filamentous phage f1. Binding occurs in two steps. J Mol Biol. 1987 Sep 20;197(2):157–174. doi: 10.1016/0022-2836(87)90115-x. [DOI] [PubMed] [Google Scholar]
- Grossman A. D., Erickson J. W., Gross C. A. The htpR gene product of E. coli is a sigma factor for heat-shock promoters. Cell. 1984 Sep;38(2):383–390. doi: 10.1016/0092-8674(84)90493-8. [DOI] [PubMed] [Google Scholar]
- Hill T. M., Kopp B. J., Kuempel P. L. Termination of DNA replication in Escherichia coli requires a trans-acting factor. J Bacteriol. 1988 Feb;170(2):662–668. doi: 10.1128/jb.170.2.662-668.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Akiyama Y., Yura T., Shiba K. Diverse effects of the MalE-LacZ hybrid protein on Escherichia coli cell physiology. J Bacteriol. 1986 Jul;167(1):201–204. doi: 10.1128/jb.167.1.201-204.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan J., Murialdo H. Stimulation of groE synthesis in Escherichia coli by bacteriophage lambda infection. J Bacteriol. 1982 Mar;149(3):1166–1170. doi: 10.1128/jb.149.3.1166-1170.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Lipinska B., King J., Ang D., Georgopoulos C. Sequence analysis and transcriptional regulation of the Escherichia coli grpE gene, encoding a heat shock protein. Nucleic Acids Res. 1988 Aug 11;16(15):7545–7562. doi: 10.1093/nar/16.15.7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinska B., Sharma S., Georgopoulos C. Sequence analysis and regulation of the htrA gene of Escherichia coli: a sigma 32-independent mechanism of heat-inducible transcription. Nucleic Acids Res. 1988 Nov 11;16(21):10053–10067. doi: 10.1093/nar/16.21.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Munson R. Separation of the inner (cytoplasmic) and outer membranes of Gram-negative bacteria. Methods Enzymol. 1974;31:642–653. doi: 10.1016/0076-6879(74)31070-1. [DOI] [PubMed] [Google Scholar]
- Peeters B. P., Peters R. M., Schoenmakers J. G., Konings R. N. Nucleotide sequence and genetic organization of the genome of the N-specific filamentous bacteriophage IKe. Comparison with the genome of the F-specific filamentous phages M13, fd and f1. J Mol Biol. 1985 Jan 5;181(1):27–39. doi: 10.1016/0022-2836(85)90322-5. [DOI] [PubMed] [Google Scholar]
- Polarek J. W., Walderhaug M. O., Epstein W. Genetics of Kdp, the K+-transport ATPase of Escherichia coli. Methods Enzymol. 1988;157:655–667. doi: 10.1016/0076-6879(88)57113-6. [DOI] [PubMed] [Google Scholar]
- Pratt D., Tzagoloff H., Erdahl W. S. Conditional lethal mutants of the small filamentous coliphage M13. I. Isolation, complementation, cell killing, time of cistron action. Virology. 1966 Nov;30(3):397–410. doi: 10.1016/0042-6822(66)90118-8. [DOI] [PubMed] [Google Scholar]
- Ravetch J. V., Ohsumi M., Model P., Vovis G. F., Fischhoff D., Zinder N. D. Organization of a hybrid between phage f1 and plasmid pSC101. Proc Natl Acad Sci U S A. 1979 May;76(5):2195–2198. doi: 10.1073/pnas.76.5.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel M., Model P. Filamentous phage pre-coat is an integral membrane protein: analysis by a new method of membrane preparation. Cell. 1982 Jan;28(1):177–184. doi: 10.1016/0092-8674(82)90387-7. [DOI] [PubMed] [Google Scholar]
- Schwartz F. M., Zinder N. D. Morphological changes in Escherichia coli infected with the DNA bacteriophage fl. Virology. 1968 Feb;34(2):352–355. doi: 10.1016/0042-6822(68)90246-8. [DOI] [PubMed] [Google Scholar]
- Serizawa H., Fukuda R. Structure of the gene for the stringent starvation protein of Escherichia coli. Nucleic Acids Res. 1987 Feb 11;15(3):1153–1163. doi: 10.1093/nar/15.3.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch K. L., Beckwith J. An Escherichia coli mutation preventing degradation of abnormal periplasmic proteins. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1576–1580. doi: 10.1073/pnas.85.5.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K., Erickson J., Sharma S., Georgopoulos C. Heat shock regulatory gene rpoH mRNA level increases after heat shock in Escherichia coli. J Bacteriol. 1986 Dec;168(3):1155–1158. doi: 10.1128/jb.168.3.1155-1158.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wang Q. P., Kaguni J. M. A novel sigma factor is involved in expression of the rpoH gene of Escherichia coli. J Bacteriol. 1989 Aug;171(8):4248–4253. doi: 10.1128/jb.171.8.4248-4253.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans S. C., Elledge S. J., Krueger J. H., Walker G. C. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J Bacteriol. 1985 Mar;161(3):1219–1221. doi: 10.1128/jb.161.3.1219-1221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolford J. L., Jr, Cashman J. S., Webster R. E. F1 Coat protein synthesis and altered phospholipid metabolism in f1 infected Escherichia coli. Virology. 1974 Apr;58(2):544–560. doi: 10.1016/0042-6822(74)90088-9. [DOI] [PubMed] [Google Scholar]
- Yamamori T., Yura T. Genetic control of heat-shock protein synthesis and its bearing on growth and thermal resistance in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1982 Feb;79(3):860–864. doi: 10.1073/pnas.79.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Fischman D. A., Steck T. L. Selective solubilization of proteins and phospholipids from red blood cell membranes by nonionic detergents. J Supramol Struct. 1973;1(3):233–248. doi: 10.1002/jss.400010308. [DOI] [PubMed] [Google Scholar]
- Yura T., Tobe T., Ito K., Osawa T. Heat shock regulatory gene (htpR) of Escherichia coli is required for growth at high temperature but is dispensable at low temperature. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6803–6807. doi: 10.1073/pnas.81.21.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]