Abstract
Objective:
It is unclear whether carvedilol and nebivolol produce different effects on short-term left ventricle (LV) systolic function in heart failure (HF). These drugs could improve systolic and diastolic functions of the LV. Thus, we aimed to compare their effects on LV systolic functions in patients with non-ischemic HF.
Methods:
This study included 61 symptomatic non-ischemic HF patients with low ejection fraction (EF) (EF≤40%) between September 2008 and November 2010. The patients were randomized to carvedilol (n=31, 16 males) or nebivolol (n=30, 19 male). They were evaluated clinically and echocardiographically at baseline and 3 and 6 months after target dose; 42% of patients in the carvedilol group and 47% in the nebivolol group achieved the target dose before randomization. LV systolic functions were evaluated with ventricle diameters, EF, ejection time (ET), isovolumic contraction time (IVCT), isovolumic relaxation time (IVRT), and myocardial performance index (MPI).
Results:
At 6 months, carvedilol and nebivolol similarly improved EF (from 33±4% to 36±5%, p<0.01 and from 34±5% to 37±5%, p<0.01, inter-group p=0.30, respectively) and MPI (from 0.71±0.10 to 0.53±0.07, p<0.01 and from 0.69±0.13 to 0.52±0.08, p<0.01, intergroup p=0.45, respectively). LV diameter was reduced by a similar extent in both groups. In each group, IVCT and IVRT were significantly shortened and ET was prolonged, but there was no inter-group difference. Functional capacity improved similarly (from NYHA Class II-III to Class I-0) in both groups, as did heart rate and blood pressure. Reduction of pro-B-type natriuretic peptide levels was also comparable in both groups (p=0.41).
Conclusion:
Carvedilol and nebivolol can similarly improve LV systolic functions in non-ischemic HF patients.
Keywords: carvedilol, nebivolol, systolic function, heart failure, myocardial performance index
Introduction
Heart failure (HF) is a complex clinical syndrome with increasing prevalence and high hospitalization and mortality rates despite its therapeutic advances (1). Activation of the sympathetic nervous system is one of the major pathophysiological abnormalities in the HF setting (1, 2). This activation leads to ventricular remodeling and progression of cardiac dysfunction (2). Symptoms and prognosis are usually associated with reduced systolic function of the left ventricle (LV) (3).
Clinical studies have shown that carvedilol and nebivolol reduce mortality and improve event-free survival in HF patients (4-6). Their benefits are largely attributed to improvement in LV ejection fraction (EF), volume, and diameter (7-12). These two agents have favorable properties, such as vasodilatory, antioxidant, and anti-proliferative effects, in addition to other beta-blockers (13, 14).
On the other hand, it is a disputable subject as to which beta-blocker is more effective in HF treatment and whether carvedilol or nebivolol should be a first-choice agent (15). This issue is important for clinicians, since beta-blockers have different properties. Preclinical studies point out a trend toward carvedilol or nebivolol due to their effects on cardiac remodeling and their pleiotropic properties (15, 16).
Up to now, only three studies have compared the effects of carvedilol and nebivolol on LV functions, exercise capacity, and clinical outcomes (10-12). They provided divergent results. However, two of them showed a trend in favor of carvedilol on EF and functional class (10, 11). The last study, with a larger size, reported that carvedilol and nebivolol provided similar effects in HF patients with low EF at 24 months (12). Thus, we aimed to compare the effects of carvedilol and nebivolol on LV systolic function and pro-B-type natriuretic peptide (proBNP) level in non-ischemic HF patients.
Methods
Patients and dose titration of study drugs
This study included 68 consecutive patients prospectively with symptomatic moderate or severe non-ischemic HF [New York Heart Association (NYHA) class II to III] who had undergone coronary angiography to define the etiology of HF, with normal coronary arteries or non-significant stenosis (stenosis <40%). However, 7 patients were excluded from the study because of recent atrial fibrillation (n=2), beta-blocker intolerance (n=2), NYHA class IV HF (n=1), and refusal to control echocardiography (n=2). Finally, the remaining 61 patients were randomly assigned to receive carvedilol (n=31) or nebivolol (n=30) in a single-blind and open-label fashion. Other exclusion criteria were heart failure with significant coronary stenosis, history of myocardial infarction, moderate or severe valvular heart disease, resting heart rate <60 beats/min, systolic blood pressure (BP) <100 mm Hg, previous intolerance to beta-blocker therapy, history of asthma or use of bronchodilators, hypo-or hyperthyroidism, hepatic or renal failure (serum creatinine >2.0 mg/dL), rhythm disturbances including second-or third-degree heart block, sick sinus syndrome, atrial fibrillation, and complete bundle brunch block.
Some findings of this study on the effects of both agents on diastolic LV function were previously published elsewhere (17). Thus, the patient characteristics and laboratory and echocardiography findings were similar to those of the previously published study. The study was approved by our institutional ethics committee. All participants gave written informed consent.
In patients who were on beta-blocker therapy before the study, beta-blocker therapy was stopped at least 1 week for drug elimination. Thereafter, carvedilol or nebivolol was randomly started, and they were up-titrated as previously reported elsewhere (17). After the target or maximum tolerable dose, patients were evaluated in the outpatient clinic at the first, third, and sixth months. Complete blood count, biochemical analysis, and echocardiographic measurements were made in patients at baseline and 3 and 6 month after the target dose. Heart rate, BP, and body weight were evaluated at each visit. Also, the functional status of patients was assessed according to NYHA. Other medications were given according to current chronic HF guidelines (1). All patients received angiotensin-converting enzyme inhibitor (ACEI, lisinopril) and diuretics in appropriate dosages. Candesartan, an angiotensin receptor blocker (ARB), was given when ACEI intolerance occurred.
Blood sampling and assays
Blood samples were drawn for hematological and routine biochemical analyses. Biochemical analyses were performed with an Olympus AU-640 (Olympus Diagnostica, Hamburg,
Germany). For NT-pro-BNP measurements, a 5-mL blood sample was collected into a plastic tube containing potassium EDTA. Plasma level of NT-proBNP was measured using an electroche-miluminescence immune assay with the Elecsys 2010 analyzer (Roche Diagnostics, Mannheim, Germany).
Echocardiographic evaluation
Echocardiographic examinations were performed by the same investigator (MK), who was blinded to the patients’ data, at baseline and 3 and 6 months. Measurements were acquired at the end of expiration during normal breathing in the left lateral decubitus position. Two-dimensional, M-mode, and Doppler echocardiographic measurements were obtained according to the recommendations of the American Society of Echocardiography (18) with a System 5 echocardiography device (GE Vingmed Ultrasound) with a 2.5 MHz FPA transducer. The mean of 3 cardiac cycles with the ECG record was considered the final measurement. The left atrial size, LV diameter, and wall thickness were measured using M-Mode echocardiography. LVEF was calculated by Simpson's method.
For transmitral flow, pulsed-wave Doppler sample volume was positioned at the mitral leaflet tips in the apical four-chamber view. Early diastolic peak flow velocity (E) and late diastolic peak flow velocity (A) were measured by transmitral Doppler imaging, and then the mitral E/A ratio was estimated. Isovolumic relaxation time (IVRT) was measured as the interval from the end of LV outflow to the onset of mitral diastolic flow in the apical five-chamber view by recording both flows with pulsed-wave Doppler imaging (18).
As previously defined (19), myocardial performance index (MPI or Tei index) was calculated as the sum of isovolumic times (contraction and relaxation times) divided by the ejection time (ET) of LV outflow. The sum of isovolumic times was obtained by subtracting ET from the interval between the onset and cessation of the mitral inflow Doppler signal in the apical four-chamber view. The ET was measured as the interval between the onset and cessation of the LV outflow signal in the apical five-chamber view with pulsed-wave Doppler imaging. Isovolumic contraction time (IVCT) was estimated by subtracting IVRT from the sum of isovolumic times. The final measurements were considered the mean of three measurements. The intra-observer variabilities were 4.1%, 5.2%, 6.7%, and 3.2% for EF, MPI, IVRT, and ET, respectively.
Statistical analyses
All analyses were performed with a commercially available statistical program (SPSS Version 13.0, SPSS Inc., Chicago, IL, USA). All data were tested for normal distribution with the Kolmogorov-Smirnov test. For data showing an abnormal distribution, median and interquartile ranges were displayed. Continuous variables were presented as mean±standard deviation, and categorical ones were presented as percentage (%). The two study groups were compared using student t-test or Mann-Whitney U and chi-square or Fisher exact test as appropriate. In each group, follow-up comparisons (baseline and 6 months) were performed using paired t-test and Wilcoxon rank tests as appropriate. A two-tailed p value of <0.05 was considered statistically significant.
Results
Baseline demographic and clinical features, except for use of aspirin, were comparable in both groups (Table 1). Laboratory tests and initial echocardiographic variables were also comparable in both groups (Table 2). Target dose of the study drugs was reached in 42% and 47% of patients in the carvedilol and nebivolol groups, respectively (p=0.85).
Table 1.
Demographic, clinical, and laboratory characteristics of the therapy groups
| Carvedilol group, n= 31 | Nebivolol group, n=30 | P | |
|---|---|---|---|
| Mean age, year | 61±11 | 60±14 | 0.73 |
| Male/Female | 16/15 | 19/11 | 0.36 |
| Body weight, kg | 78±18 | 75±12 | 0.48 |
| NYHA class, II/III | 52%/48% | 60%/40% | 0.61 |
| Smoking | 8 (26%) | 11 (37%) | 0.42 |
| Hypertension | 15 (45%) | 15 (48%) | 0.90 |
| Diabetes mellitus | 5 (16%) | 5 (17%) | 0.99 |
| Hyperlipidemia | 7 (22%) | 10 (33%) | 0.40 |
| Obesity | 11 (35%) | 8 (26%) | 0.46 |
| Systolic BP, mm Hg | 143±17 | 141±13 | 0.57 |
| Diastolic BP mm Hg | 91±15 | 90±10 | 0.60 |
| Heart rate, bpm | 81±9 | 82±9 | 0.72 |
| Medications | |||
| ACE inhibitor/ARB | 28 (90%)/3 (9%) | 27 (90%)/4 (13%) | 0.96/0.66 |
| Spironolactone | 9 (29%) | 5 (17%) | 0.25 |
| Other diuretics | 31 (100%) | 30 (100%) | 0.99 |
| Statins | 4 (13%) | 2 (7%) | 0.67 |
| Digoxin | 2 (6%) | 2 (7%) | 0.97 |
| Aspirin | 20 (64%) | 11 (36%) | 0.03 |
| Creatinine, mg/dL | 0.97±0.2 | 1.0±0.3 | 0.27 |
| Sodium, mEq/L | 140±3.3 | 141±3.6 | 0.27 |
| Potassium, mEq/L | 4.4±0.4 | 4.4±0.4 | 0.84 |
| Hematocrit, % | 42.±4.7 | 42.4.8 | 0.72 |
| NT-proBNP, pg/mL | 666 (442-1350) | 661 (455-1013) | 0.61 |
Obesity was defined as body mass index ≥30 kg/m2.
ACE -angiotensin-converting enzyme; ARB - angiotensin-1 receptor blocker; BP - blood pressure; NT-proBNP - n-terminal pro-B-type natriuretic peptide; NYHA - New York Heart Association
Table 2.
Baseline echocardiographic parameters of carvedilol and nebivolol groups
| Carvedilol, n= 31 | Nebivolol, n=30 | P | |
|---|---|---|---|
| LV diastolic diameter, mm | 58±7 | 57±6 | 0.65 |
| LV systolic diameter, mm | 46±7 | 44±6 | 0.22 |
| Septal thickness, mm | 11±1.2 | 11±1.5 | 0.48 |
| Posterior wall thickness, mm | 11±1.1 | 10±1.1 | 0.08 |
| LV ejection fraction, % | 33±4.2 | 34±4.9 | 0.22 |
| LA diameter, mm | 43.4±4.2 | 41.5±4.1 | 0.68 |
| IVRT, ms | 108±13 | 107±22 | 0.83 |
| IVCT, ms | 70±9 | 67±13 | 0.40 |
| ET, ms | 248±23 | 250±23 | 0.78 |
| MPI | 0.71±0.10 | 0.69±0.13 | 0.40 |
ET - ejection time; IVCT - isovolumic contraction time; IVRT - isovolumic relaxation time; LA - left atrium; LV - left ventricle; MPI - myocardial performance index
Table 3 shows the temporal changes in clinical and echocardiographic variables in the carvedilol and nebivolol groups at 3 and 6 months. Heart rate, blood pressure, and body weight were significantly reduced in each group at 3 and 6 months. However, there were no differences in inter-groups at any point (Table 3). Similarly, functional class significantly improved in each group (p<0.01), but there was no difference in inter-groups. In addition, concomitant medications did not differ in either group. Four patients received ARB (candesartan) due to intolerance to ACEI (2 patients in both groups).
Table 3.
Temporal changes in clinical and echocardiographic variables of the groups at 3 and 6 months
| Carvedilol, n= 31 | Nebivolol, n=30 | P | ||||
|---|---|---|---|---|---|---|
| 3 months | 6 months | 3 months | 6 months | P 1 | P 2 | |
| NYHA class I | 81% | 96%* | 86% | 93%* | 0.45 | 0.54 |
| HR, bpm | 75±8 | 67±7* | 74±9 | 66±6* | 0.78 | 0.52 |
| SBP mm Hg | 137±14 | 122±14* | 132±12 | 118±15* | 0.10 | 0.27 |
| DBF, mm Hg | 85±10 | 75±12* | 82±8 | 71±9* | 0.14 | 0.10 |
| Weight, kg | 76±17 | 76±18* | 73±11 | 73±12* | 0.43 | 0.44 |
| LVEDD, mm | 58±7 | 57±7* | 56±6 | 55±5* | 0.16 | 0.16 |
| LVESD, mm | 46±7 | 44±7* | 42±7 | 41 ±7* | 0.07 | 0.07 |
| LVEF, % | 33±5 | 36±5* | 36±5 | 37±5* | 0.09 | 0.30 |
| LA diameter, mm | 43.4±4.4 | 42.4±4.7 | 41.0±3.9 | 40.6±3.8 | 0.26 | 0.19 |
| IVRT, ms | 101±12 | 94±10* | 98±15 | 92±10* | 0.43 | 0.25 |
| IVCT, ms | 64±9 | 53±8* | 65±12 | 54±12* | 0.89 | 0.83 |
| ET, ms | 253±24 | 277±20* | 258±18 | 273±20* | 0.36 | 0.45 |
| MPI | 0.64±0.01 | 0.53±0.07* | 0.62±0.01 | 0.52±0.08* | 0.49 | 0.45 |
| ProBNP pg/mL | 445 (226-845) | 137 (113-216)* | 395 (299-661) | 123 (105-186)* | 0.87 | 0.41 |
p≤0.01 versus baseline in each group.
P1-comparisons of two groups at 3 months; P2-comparisons of two groups at 6 months. DBP - diastolic blood pressure; HR - heart rate; LVEDD - left ventricular end-diastolic diameter; LVESD - left ventricular end-systolic diameter; LVEF- LV ejection fraction; SBP - systolic blood pressure. Other abbreviations are as in Tables 1 and 2.
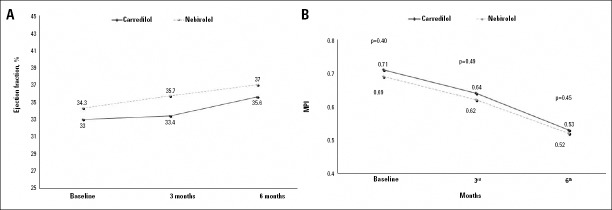
The LV systolic and diastolic diameters were significantly reduced in each group (each p<0.01, Table 3). Accordingly, EF was significantly elevated in each group at 6 months but was comparable in both groups, though it was slightly higher in the nebivolol than carvedilol group at 3 months (from 33±4.2 to 33±5% and 36±5%, from 34±4.9% to 36±5% and 37±5%, respectively) (Table 3, Fig. 1a).
Figure 1.
Temporal changes in ejection fraction and myocardial performance index (MPI) of the left ventricle after carvedilol and nebivolol treatment. There was no significant difference in ejection fraction (A) and MPI (B) between the two groups at the follow-up. However, ejection fraction was slightly higher in the nebivolol group at 3 months
Carvedilol and nebivolol significantly shortened IVCT and IVRT but prolonged ET. However, there was no difference with regard to these variables in both groups (Table 3). Similarly, both agents significantly reduced MPI in each group (from 0.71±0.10 to 0.53±0.07 and from 0.69±0.13 to 0.52±0.08, each p<0.01, respectively) but was comparable in both groups (Fig. 1b).
During the 6 months, median NT-proBNP levels were significantly reduced with carvedilol and nebivolol therapy (p<0.001, Table 3). However, the reductions did not differ in either therapy group at 3 and 6 months.
At follow-up, there was no death, hospitalization for heart failure, or discontinuation of study drugs for adverse effects. The drugs were well tolerated.
Discussion
At the 6-month follow-up, carvedilol and nebivolol produced a similar improvement in functional capacity and LV functions, including EF, MPI, isovolumic times, and ET, in patients with nonischemic HF in the present study. Also, mitral E/A ratio and LV and LA diameters were similarly reduced with both agents.
Beta-blockers are the mainstay therapy for HF patients (1). They attenuate the activation of the sympathetic nervous system in the development of HF, prevent ventricular remodeling, and improve cardiac function (7-12, 16). As a result, they improve symptoms and clinical outcomes, such as death and hospitalization (1, 4-6, 10-12). However, it is a subject of debate whether all beta-blockers accepted for HF are similarly effective for HF treatment, because carvedilol and nebivolol have additional favorable properties (15, 16).
Carvedilol blocks not only beta-1 and beta-2 adrenoreceptors but also alpha-1 receptor, producing additional vasodilatory effects. Furthermore, it has antioxidant, anti-proliferative, anti-endotelin, and anti-apoptotic properties (13). Similarly, nebivolol also has vasodilatory effects derived from the L-arginine/nitric oxide pathway, as well as antioxidant and anti-proliferative properties (14, 20). On the other hand, it is unclear whether these favorable properties translate into clinical benefit, though carvedilol produced more clinical benefits in the COMET (Carvedilol Or Metoprolol European Trial) study compared with metoprolol, with some criticisms on metoprolol dose and salt (21).
Previous studies have demonstrated that carvedilol (7, 10-13) and nebivolol (8-12) individually not only reduce LV systolic and diastolic size but also increase EF and exercise capacity in HF patients with reduced EF. However, it is uncertain whether the two agents have similar effects on LV function and clinical outcomes (10-12).
Up to now, three studies have compared the effects of carvedilol and nebivolol on LV function and clinical outcomes in HF patients with low EF (10-12). Their results are different. Two of them showed a trend in favor of carvedilol, although there was no clear different between the two agents (10, 11). However, a larger-sized study (n=160) recently reported that carvedilol and nebivolol similarly improve LV systolic function, exercise capacity, and survival in hypertensive HF patients at the 24-month follow-up (12). In our study, functional capacity, LV dimensions, and EF were also similarly improved with carvedilol and nebivolol after a 6-month therapy. Furthermore, MPI was also reduced to a similar extent in both therapy groups. But, in our study, we evaluated only non-ischemic HF patients, and only 45%-48% of them were hypertensive.
Patrianakos et al. (10) showed that carvedilol-treated patients had higher EF than nebivolol-treated ones after 12-month treatment, unlike our findings. The reason for this can be the use of a lower dose (5 mg) as the target dose for nebivolol (10 mg in our study), with a slightly lower initial EF in the nebivolol group. Similarly, Lombardo et al. (11) showed a similar elevation in EF in carvedilol- and nebivolol-treated patients with ischemic or nonischemic HF, though they used a target dose of 5 mg for nebivolol, whereas we and Marazzi et al. (12) used a target dose of 10 mg for nebivolol, as recommended in HF treatment.
The Tei index or MPI is a reliable marker reflecting both LV systolic and diastolic function. It is less dependent on heart rate, loading states, and LV geometry, compared with EF (19). The MPI can increase with severity of HF, and its high value has been reported to be associated with mortality and the need for heart transplantation in patients with severe systolic dysfunction (EF <30%) (22). Palloshi et al. (23) reported that compared with EF, MPI was improved earlier in HF patients on carvedilol therapy and was more sensitive to the improvement in LV function.
In our study, carvedilol or nebivolol therapy significantly reduced MPI (from 0.71±0.10 to 0.53±0.07, p<0.01 and from 0.69±0.13 to 0.52±0.08, p<0.01, respectively) at 6 months, but both agents produced a similar improvement at 3 and 6 months. To our knowledge, there is no study investigating the comparative effects of carvedilol and nebivolol on MPI in HF patients. However, it has been reported that MPI is significantly reduced in HF patients with carvedilol treatment (23, 24).
Based on the improvement in LV function, beta-blocker therapy is likely to lower NT-proBNP levels in HF patients over time, but previous results are divergent (11, 25-28). This might be due to small sample size, variable follow-up times, and differences between study populations. Both carvedilol and nebivolol therapy reduced NT-proBNP levels similarly in our study at 3 and 6 months, but there was no difference in both therapy groups, whereas, Lombardo et al. (11) reported no reduction in NT-proBNP levels in carvedilol and nebivolol groups at the 6-month follow-up.
Parallel to the echocardiographic and neurohormonal improvement, we observed that both drugs significantly improved functional capacity, blood pressure, and heart rate to a similar extent at 3 and 6 months. These findings are concordant with results from previous studies (4-8, 10-12).
Study limitations
There are several limitations to this study. Firstly, our study population is small, since we used strict exclusion criteria. Accordingly, it limits the statistical power of the study. Secondly, we did not perform exercise or 6-minute walk test for the evaluation of functional capacity. However, functional capacity according to NYHA classification has been commonly used for HF patients. Also, we did not use a scoring system to determine the life quality. Finally, our findings reflect the situation in only patients with non-ischemic HF. It may be possible that carvedilol and nebivolol might have different effects on LV function in ischemic HF patients.
Conclusion
Our findings suggest that carvedilol and nebivolol have similar beneficial effects on LV systolic function, MPI, and functional capacity in patients with non-ischemic HF. In addition, each drug is well tolerated.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept - M.K.; Design - M.K. Supervision - A.D.; Resource - M.K.; Materials - Ş.T.; Data collection and/or processing - Ş.T.; Analysis and/or interpretation - M.K.; Literature search - M.O.; Writing - Ş.T.; Critical review - D.E.
References
- 1.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–69. doi: 10.1093/eurjhf/hfs105. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 2.Francis GS, Benedict C, Johnston DE, Kirlin PC, Nicklas J, Liang CS, et al. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure: a substudy of the Studies of Left Ventricular Dysfunction (SOLVD) Circulation. 1990;82:1724–9. doi: 10.1161/01.cir.82.5.1724. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 3.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33:1948–55. doi: 10.1016/s0735-1097(99)00118-7. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 4.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–55. doi: 10.1056/NEJM199605233342101. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 5.Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, et al. Carvedilol Prospective Randomized Cumulative Survival Study Group (COPERNICUS). Effect of carvedilol on survival in severe chronic heart failure. New Engl J Med. 2001;344:1651–8. doi: 10.1056/NEJM200105313442201. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 6.Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, Borbola J, et al. SENIORS Investigators. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS) Eur Heart J. 2005;26:215–25. doi: 10.1093/eurheartj/ehi115. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 7.Doughty RN, Whalley GA, Gamble G, MacMahon S, Sharpe N. Left ventricular remodeling with carvedilol in patients with congestive heart failure due to ischemic heart disease. Australia-New Zealand Heart Failure Research Collaborative Group. J Am Coll Cardiol. 1997;29:1060–6. doi: 10.1016/s0735-1097(97)00012-0. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 8.Patrianakos AP, Parthenakis FI, Mavrakis HE, Saatsaki M, Diakakis GF, Chlouverakis GI, et al. Effects of nebivolol on left ventricular function and exercise capacity in patients with non-ischaemic dilated cardiomyopathy. A randomised placebo-controlled study. Hellenic J Cardiol. 2005;46:199–207. [PubMed] [Google Scholar]
- 9.Ghio S, Magrini G, Serio A, Klersy C, Fucili A, Ronaszèki A, et al. SENIORS investigators. Effects of nebivolol in elderly heart failure patients with or without systolic left ventricular dysfunction: results of the SENIORS echocardiographic substudy. Eur Heart J. 2006;27:562–8. doi: 10.1093/eurheartj/ehi735. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 10.Patrianakos AP, Parthenakis FI, Mavrakis HE, Diakakis GF, Chlouverakis GI, Vardas PE. Comparative efficacy of nebivolol versus carvedilol on left ventricular function and exercise capacity in patients with nonischemic dilated cardiomyopathy. A 12-month study. Am Heart J. 2005;150:985, e9–18. doi: 10.1016/j.ahj.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 11.Lombardo RM, Reina C, Abrignani MG, Rizzo PA, Braschi A, De Castro S. Effects of nebivolol versus carvedilol on left ventricular function in patients with chronic heart failure and reduced left ventricular systolic function. Am J Cardiovasc Drugs. 2006;6:259–63. doi: 10.2165/00129784-200606040-00006. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 12.Marazzi G, Volterrani M, Caminiti G, Iaia L, Massaro R, Vitale C, et al. Comparative long term effects of nebivolol and carvedilol in hypertensive heart failure patients. J Card Fail. 2011;17:703–9. doi: 10.1016/j.cardfail.2011.05.001. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 13.Doughty RN, White HD. Carvedilol: use in chronic heart failure. Expert Rev Cardiovasc Ther. 2007;5:21–31. doi: 10.1586/14779072.5.1.21. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 14.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–14. doi: 10.1161/CIRCULATIONAHA.105.602532. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 15.Remme WJ. Which beta-blocker is most effective in heart failure? Cardiovasc Drugs Ther. 2010;24:351–8. doi: 10.1007/s10557-010-6247-7. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 16.Rehsia NS, Dhalla NS. Mechanisms of the beneficial effects of beta-adrenoceptor antagonists in congestive heart failure. Exp Clin Cardiol. 2010;15:e86–95. [PMC free article] [PubMed] [Google Scholar]
- 17.Doğan A, Karabacak M, Tayyar S, Erdogan D, Özaydın M. Comparison of the effects of carvedilol and nebivolol on diastolic functions of the left ventricle in patients with non-ischemic heart failure. Cardiol J. 2014;21:76–82. doi: 10.5603/CJ.a2013.0062. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 18.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. American Society Echocardiography Committee on Standards, Subcommittee on Quantitation of Two- Dimensional Echocardiograms. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. J Am Soc Echocardiogr. 1989;2:358–67. doi: 10.1016/s0894-7317(89)80014-8. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 19.Tei C, Ling LH, Hodge DO, Bailey KR, Oh JK, Rodeheffer RJ, et al. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac functional study in normals and dilated cardiomyopathy. J Cardiol. 1995;26:357–66. [PubMed] [Google Scholar]
- 20.Ignarro LJ, Byrns RE, Trinh K, Sisodia M, Buga GM. Nebivolol: a selective beta-1 adrenergic receptor antagonist that relaxes vascular smooth muscle by nitric oxide- and cyclic GMP-dependent mechanisms. Nitric Oxide. 2002;7:75–82. doi: 10.1016/s1089-8603(02)00112-x. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 21.Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, et al. Carvedilol or Metoprolol European Trial Investigators. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure. Results of the Carvedilol or Metoprolol European Trial (COMET) Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 22.Harjai KJ, Scott L, Vivekananthan K, Nunez E, Edupuganti R. The Tei index: a new prognostic index for patients with symptomatic heart failure. J Am Soc Echocardiogr. 2002;15:864–8. doi: 10.1067/mje.2002.120892. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 23.Palloshi A, Fragasso G, Silipigni C, Locatelli M, Cristell N, Pala MG, et al. Early detection by the Tei index of carvedilol-induced improved left ventricular function in patients with heart failure. Am J Cardiol. 2004;94:1456–9. doi: 10.1016/j.amjcard.2004.08.020. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 24.Rhodes J, Margossian R, Darras BT, Colan SD, Jenkins KJ, Geva T, et al. Safety and efficacy of carvedilol therapy for patients with dilated cardiomyopathy secondary to muscular dystrophy. Pediatr Cardiol. 2008;29:343–51. doi: 10.1007/s00246-007-9113-z. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 25.Hartmann F, Packer M, Coats AJ, Fowler MB, Krum H, Mohacsi P, et al. Prognostic impact of plasma N-terminal pro-brain natriuretic peptide in severe chronic congestive heart failure. A Substudy of the study of the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Trial. Circulation. 2004;110:1780–6. doi: 10.1161/01.CIR.0000143059.68996.A7. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 26.Li N, Li Y, Wang F, Jiang W, Huang J, Xu Z, et al. Does NT-proBNP remain a sensitive biomarker for chronic heart failure after administration of a beta-blocker? Clin Cardiol. 2007;30:469–74. doi: 10.1002/clc.20150. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenberg J, Gustafsson F, Remme WJ, Riegger GAJ, Hildebrandt PR. Effect of beta-blockade and ACE Inhibition on B-type natriuretic peptides in stable patients with systolic heart failure. Cardiovasc Drug Ther. 2008;22:305–11. doi: 10.1007/s10557-008-6099-6. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 28.Conraads VM, Metra M, Kamp O, De Keulenaer GW, Pieske B, Zamorano J, et al. Effects of the long-term administration of nebivolol on the clinical symptoms, exercise capacity, and left ventricular function of patients with diastolic dysfunction: results of the ELANDD study. Eur J Heart Fail. 2012;14:219–25. doi: 10.1093/eurjhf/hfr161. [CrossRef] [DOI] [PubMed] [Google Scholar]