Abstract
Objective:
This study aimed to investigate the predictive value of circumferential iliofemoral calcifications and current manufacturer recommendations, which are not evidence-based, in transfemoral (TF) transcatheter aortic valve implantation (TAVI)
Methods:
A patient cohort with a broad range of iliofemoral anatomies undergoing TF TAVI (n=132) were retrospectively divided as “suitable” (n=76, 58%) and “unsuitable” (n=56, 42%) candidates according to current recommendations. Iliofemoral angiography and reconstructed mul- tislice CT (MSCT) images were used for access screening in the majority of patients.
Results:
Vessel properties were significantly worse in the “unsuitable group.” The sheath-to-iliofemoral artery ratio (SIFAR) and calcium score were 1.35±0.2 and 1.7±0.8 in the unsuitable group, compared to 1.0±0.12 (p<0.0001) and 1.0±0.7 (p=0.0001) in the “suitable” patients. Major vascular complications (MVCs) occurred more frequently in the “unsuitable” group (10.7% vs. 2.6%, p=0.07) and were predicted by SIFAR [OR: 64, 95% CI: 1.4-2971, p=0.03] and circumferential iliofemoral calcifications [OR: 6, 95% CI: 1.2-26, p=0.025]. In the multivariate analysis, circumferential calcifications [HR: 3.6, 95% CI: 1-13.2, p=0.043] but not major vascular complications (MVCs) or manufacturer recommendations were associated with increased mortality.
Conclusion:
According to our results, manufacturer recommendations are safe but overly conservative. Circumferential iliofemoral calcifications may provide independent prognostic information in patients undergoing TAVI.
Keywords: circumferential iliofemoral calcifications, manufacturer recommendations, TAVI
Introduction
Transcatheter aortic valve implantation (TAVI) has been shown to be superior to conventional medical therapy in inoperable symptomatic patients with severe aortic valve stenosis (1). Although delivery systems have become smaller, peripheral vascular disease is still an important potential limitation of the transfemoral (TF) technique. Alternative access routes are feasible but also have their limitations.
Increased mortality after transapical (TA) TAVI has been observed in several registries and in the increased mortality after TA-TAVI has been reported frequently in the literature (2, 3). It has not been clarified yet if this finding is due to a higher incidence of co-morbidities in TA patients or the TA approach itself. In addition, subclavian access requires surgical cutdown, and the management of complications associated with this approach, like intrathoracic bleeding, is challenging. Therefore, TF access has become the preferred technique in many centers.
Manufacturers of TAVI delivery systems provide minimal artery diameter recommendations for their devices that are not evidence-based (2-4). In addition, since an association with increased mortality has been shown (5-9), predictors of vascular complications in TF-TAVI have been extensively published (10-12). In brief, the sheath-to-minimal artery diameter ratio and degree of calcification are important variables. However, the proposed calcification scores are operator-dependent and may not be reproducible. Multislice computed tomography (MSCT) for access evaluation has been recommended but may not be suitable in some patients with an increased risk of contrast- induced acute kidney injury (13).
The aim of this study was to investigate the predictive value of circumferential iliofemoral calcifications and current valve manufacturer recommendations in TF-TAVI. Based on these data, a workup algorithm was established to define patients who are most likely to benefit from MSCT evaluation. In contrast to previous studies, minimal vessel diameters were calculated from minimal lumen areas derived from reconstructed MSCT images, which may be more accurate.
Methods
Study population and design
In this retrospective, observational, single-center study, 139 patients who underwent TAVI at our institution between April 2010 and August 2012 were analyzed. All patients were discussed at a multidisciplinary heart team meeting, including cardiac surgeons, non-invasive cardiologists, interventional cardiologists, and anesthesiologists, before the procedure.
Careful screening to evaluate the suitability was performed in every patient. Tests included transthoracic and transesophageal echocardiography, coronary angiography, and angiography of the aortic root and iliofemoral arteries. Selective iliofemoral angiography was performed in a single plane and was available in 103 (78%) patients. Contrast-enhanced multislice CT (MSCT) of the aorta, including the iliofemoral vessels, was performed in 103 (78%) patients. Both imaging modalities (MSCT and angiography) were available in 76 (58%) patients.
Only candidates who had an attempt at the transfemoral (TF) approach (132 patients) were included in the final analysis. Seven patients underwent either a subclavian or transapical approach (Fig. 1) and were not included in this trial. No patient was turned down due to lack of access.
Figure 1.
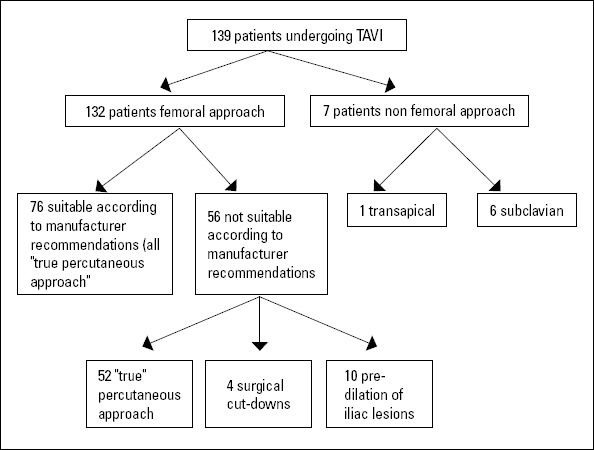
Study flow chart
Two subgroups of the included patients, based on the minimal lumen diameter (MLD) recommendations given by the valve manufacturers, were established: “suitable” and “not suitable” TF. Current recommendations (2-4) are a minimal lumen diameter (MLD) of 6.5 mm for the 19-F Novaflex introducer and 18-F e-Sheath (Edwards Lifesciences, USA) and 6 mm for the 16-F e-Sheath, 18-F Novaflex introducer (Edwards, USA), and 18-F Check-Flo Introducer system (Cook Medical, Bloomington, Indiana), used with the CoreValve (Medtronic). For the 20-F e-Sheath, which is used with the 29-mm Edwards valve, an MLD of 8 mm is required.
Procedures
All procedures were performed by an experienced TAVI team who had implanted more than 100 transcatheter aortic valves before the study period. Both of the commercially available percutaneous aortic valve bioprostheses-the Edwards valve (70%) (Edwards-SAPIEN XT, Edwards Sapien XT, Edwards Lifesciences, Irvine, CA, USA.) and the CoreValve Revalving system (30%) (Medtronic, Inc., Minneapolis, MN, USA)-were used. The technical details of the TAVI procedure with the currently available systems are described in detail elsewhere (7, 14, 15). If patients were not on dual antiplatelet therapy, a loading dose of clopidogrel (300 mg) and/or aspirin was administered. Both drugs were continued at a dosage of 75 mg per day. At the beginning of the procedure, a bolus of intravenous unfractionated heparin (80-100 IU/kg) was injected to achieve a target activated clotting time (ACT) of 250 s. Thereafter, the ACT was checked every 30 minutes.
Only patients with severely diseased iliofemoral vessels (identified on MSCT) underwent alternative approaches (subclavian or transapical). Planned femoral surgical cutdown, combined with surgical closure after valve delivery, was used in cases of severe calcification of the femoral artery at the level of the aimed puncture. In most patients, a “true percutaneous approach” using a “pre-closing technique” with the Prostar XL 10-F vascular closure system (Abbott Vascular, USA) or two Perclose/Proglide systems (Abbott Vascular, USA) were used. Both systems have been previously described (16, 17). In brief, direct puncture of the femoral artery between the femoral artery bifurcation and the inguinal ligament was performed. Correct position of the puncture site was confirmed by angiographic evaluation after introducing a 4-F sheath. A Prostar or two Proglide devices (the second one at 45° to the first one) were deployed. After deployment of the closure systems, the femoral artery sheath was upgraded stepwise over a stiff wire by using different dilators until the introducer sheath was carefully inserted. In some patients with severely diseased iliac vessels, predilation of significant lesions, as identified by MSCT, was performed using appropriately sized non-compliant balloons.
Following aortic valve deployment, the introducer sheath was retracted to the external iliac artery, and angiography was performed to assess for iliac artery complications. The femoral artery was subsequently closed by tying the sutures of the pre- deployed closing systems.
Assessment of vascular access
Wherever possible, iliofemoral vessel characterization was performed with contrast-enhanced MSCT. Only in patients where MSCT was not available were measurements taken from the fluoroscopic angiogram after calibration with a contrast- filled catheter. MSCT images were reconstructed, and the MLD was calculated from the smallest lumen area (MLA), measured in a perpendicular plane to the longitudinal axis of the vessel segment (MLD=[√(MLA/π)] x 2).
The MLD of the common femoral artery between the inguinal ligament and the profunda femoral artery, as well as the MLD of the iliac artery (common and external) proximal to the inguinal ligament, was recorded. Vessel tortuosity and calcification were evaluated as previously described for angiographic assessment (18). Calcification was defined as follows: 0- no calcification; 1- mild calcification; 2- moderate calcification; and 3- severe calcification. For tortuosity, the following grading system was used: 0- no tortuosity; 1- mild tortuosity (30° to 60°); 2- moderate tortuosity (60° to 90°); and 3- severe tortuosity (≥90°). In patients where an MSCT scan was performed, tortuosity was derived from 3D-reconstructed images. The presence of circumferential calcification (≥75% of vessel circumference) of the iliofemoral vessels was taken from the reconstructed CT images (perpendicular plane of the vessel segment).
Sheath-to-femoral artery ratio (SFAR) was defined as the ratio between the sheath outer diameter (in millimeters) and the femoral artery minimal lumen diameter (FMLD, in millimeters) (10). The sheath-to-iliac artery ratio (SIAR) was defined as the ratio between sheath outer diameter (in millimeters) and iliac minimal lumen diameter (IMLD, in millimeters). In addition, the minimal iliofemoral lumen diameter (IFMLD) and the sheath-to- IFMLD ratio were recorded. The outer diameters of the Edwards system introducer sheaths (Edwards Lifesciences, USA) for the 19- and 18-F Novaflex were 7.5 and 7.2 mm and 6.7, 7.2, and 8.0 mm for the 16-, 18-, and 20-F e-Sheaths, respectively. The 18-F Check-Flo Introducer system (Cook Medical, Bloomington, Indiana), used with the CoreValve (Medtronic), was 7.3 mm in outer diameter (3, 4, 10).
Treatment of vascular complications
Vascular complications were treated according to the operator's discretion. An endovascular technique was chosen wherever possible. Therefore, iliofemoral dissections were treated with balloon expandable stents or covered stents if necessary. Manual compression was used for femoral bleedings, and if unsuccessful, balloon angioplasty or surgical cutdown was performed. Vascular complications associated with hemodynamic instability were managed with balloon occlusion from the contralateral side, while covered stents were prepared for implantation.
Endpoint definitions
The aim of this study was to examine the current manufacturer-derived recommendations for transfemoral TAVI in patients with a wide range of iliofemoral anatomies. We also tried to establish an angiographic and MSCT-based algorithm to improve patient selection for TF-TAVI. The primary endpoint of this study was therefore the incidence of vascular complications as defined by VARC criteria (19). The incidence of iliofemoral major vascular complications was analyzed in the context of angiographic and MSCT-based screening. In brief, VARC divides vascular complications into major and minor complications. Major vascular complications are: 1) any thoracic aortic dissection; 2) access site or access-related vascular injury (dissection, stenosis, perforation, rupture, arterial-venous fistula, pseudo-aneu- rysm, hematoma, irreversible nerve injury, or compartment syndrome) leading to either death, significant blood transfusion (≥4 units), unplanned percutaneous or surgical intervention, or irreversible end-organ damage; or 3) distal embolization (non- cerebral) from a vascular source requiring surgery or resulting in amputation or irreversible end-organ damage. Minor vascular complications are defined as: 1) access site or access-related vascular injury not requiring unplanned percutaneous or surgical intervention and not resulting in irreversible end-organ damage; 2) distal embolization treated with embolectomy and/or thrombectomy and not resulting in amputation or irreversible end-organ damage; and 3) failure of percutaneous access site closure, resulting in interventional or surgical correction and not associated with death, significant blood transfusions, or irreversible end-organ damage.
Statistical analysis
Statistical analysis was performed using SPSS, version 19 for Windows (IBM, Chicago, IL, USA). Results of continuous variables are expressed as mean±standard error of the mean (SEM). Results of categorical data are reported as frequencies (%). Normality of the distribution of continuous variables was tested by means of the Kolmogorov-Smirnov goodness-of-fit test. Continuous variables were compared with the student t-test or the Mann-Whitney U test where appropriate. Categorical variables were compared with the chi-square test. Univariate and multiple regression analysis was performed to define predictors for major vascular complications. The SIFAR threshold associated with an increased incidence of major vascular complications was determined by calculation of the Youden index (sensitivity+specifity-1) after applying ROC analysis.
Cumulative survival was estimated with the Kaplan-Meier method and compared between the “suitable” and the “unsuitable” TF groups with the log-rank test. Multiple Cox regression analysis was performed to correct for log-EuroSCORE, age, and gender and to evaluate predictors of mortality. The level of significance was set at p<0.05. All reported p values are 2-sided.
Results
Baseline and procedural characteristics
During the study period, 139 patients underwent TAVI at the UCLH Heart Hospital. After appropriate screening, 76 (55%) patients fulfilled the current manufacturer recommendations for a transfemoral approach (“suitable” TF group). In addition, 58 patients (92%) of the remaining 63 patients underwent TAVI by transfemoral access, although the vessel diameters were smaller than recommended (“unsuitable” TF group). In 2 patients (1.5% of all TF implantations), an alternative access had to be used after an attempted approach from the femoral site failed- i.e., the delivery sheath could not be advanced into the abdomi-nal aorta. These patients were excluded from the final analysis. However, no vascular complication occurred in these 2 patients. In another 4 of the “unsuitable” TF patients, surgical cutdown to gain access to the common femoral artery was used. All other candidates underwent a “true” percutaneous transfemoral access. Finally, 7 patients (5%) underwent either subclavian or transapical access, and 132 patients (95%) underwent transfemoral TAVI (Fig. 1) using either the CoreValve (Medtronic, Inc., Minneapolis, MN, USA) (30.2%) or Edwards (Edwards Lifesciences, Irvine, CA, USA.) (69.8%) device. All patients underwent successful valve implantation.
Patient demographics and procedural characteristics are depicted in Table 1. There were no significant differences in gender, patient age, or log-EuroSCORE between the TF “suitable” and the TF “unsuitable” groups. Whereas vessel diameter and calcification were significantly worse in the “unsuitable” group, no differences were observed in vessel tortuosity (Table 1). The incidence of circumferential calcifications in the femoral and iliac vessels was highest in the “unsuitable” TF group, with no significant difference between the two groups.
Table 1.
Baseline characteristics
| All | TF unsuitable | TF suitable | P | |
|---|---|---|---|---|
| Number of patients | 132 | 56 (42%) | 76 (58%) | 0.342 |
| Male | 70 (53%) | 33 (59%) | 37 (49%) | 0.141 |
| Mean age, years | 82±8.6 | 80.5±8.4 | 83±8.6 | 0.090 |
| No CT scan | 29 (22%) | 13 (23%) | 16 (21%) | 0.644 |
| Log EuroSCORE | 19±10 | 19.1±10.1 | 18.8±10.1 | 0.910 |
| Edwards SAPIEN XT valve | 92 (70%) | 36 (64%) | 56 (74%) | 0.632 |
| Medtronic CoreValve | 40 (30%) | 20 (36%) | 20 (26%) | 0.224 |
| 16 F e-Sheath, inner diameter | 14 (11%) | 3 (5%) | 11 (14%) | 0.143 |
| 18 F e-Sheath, inner diameter | 51 (39%) | 23 (41%) | 28 (37%) | 0.527 |
| 20 F e-Sheath, inner diameter | 5 (4%) | 1 (2%) | 4 (5%) | 0.081 |
| 18 F Novoflex sheath, inner diameter | 10 (7.5%) | 5 (8.9%) | 5 (6.6%) | 0.641 |
| 19 F Novoflex sheath, inner diameter | 12 (9%) | 4 (7) | 8 (10.5%) | 0.522 |
| 18 F Cook sheath, inner diameter | 40 (30%) | 20 (36%) | 20 (26%) | 0.217 |
| Sheath outer diameter, mm | 7.24±0.3 | 7.3±0.3 | 7.2±0.3 | 0.112 |
| Planned open femoral cutdown | 4 (5%) | 4 (7%) | 0 | 0.041 |
| IFMLD all patients, mm* | 6.5±1.2 | 5.5±0.7 | 7.2±0.9 | <0.001 |
| IFMLD angio, mm | 6.4±0.3 | 4.9±0.3 | 7.09±0.1 | <0.001 |
| IFMLD MSCT, mm | 6.1±0.5 | 4.5±0.5 | 7.1±0.2 | <0.001 |
| FMLD all patients, mm* | 6.8±1.3 | 5.8±1.1 | 7.4±1 | <0.001 |
| FMLD angio, mm | 6.8±1.24 | 6.1±1 | 7.4±1 | <0.001 |
| FMLD MSCT, mm | 6.4±1.4 | 5.4±1.1 | 7.2±1.1 | <0.001 |
| IMLD all patients, mm* | 6.9±1.2 | 6.1±0.9 | 7.4±1 | <0.001 |
| IMLD angio, mm | 6.9±1.27 | 6.2±1.1 | 7.5±1 | <0.001 |
| IMLD MSCT, mm | 6.4±1.3 | 5.6±1 | 7.1±1.2 | <0.001 |
| SIFAR all patients | 1.15±0.2 | 1.35±0.2 | 1.0±0.12 | <0.001 |
| SIFAR angio | 1.14±0.25 | 1.28±0.27 | 1.02±0.13 | <0.001 |
| SIFAR MSCT | 1.19±0.36 | 1.33±0.26 | 1.02±0.1 | <0.001 |
| SFAR all patients* | 1.13±0.4 | 1.35±0.5 | 1.0±0.1 | <0.001 |
| SFAR angio | 1.1±0.21 | 1.23±0.2 | 0.99±0.13 | <0.001 |
| SFAR MSCT | 1.14±0.37 | 1.33±0.46 | 0.97±0.12 | <0.001 |
| SIAR all patients* | 1.1±0.2 | 1.2±0.2 | 1.0±0.13 | <0.001 |
| SIAR angio | 1.09±0.2 | 1.22±0.22 | 0.98±0.13 | <0.001 |
| SIAR MSCT | 1.09±0.2 | 1.23±0.19 | 0.97±0.11 | <0.001 |
| Calcification angio | 1.5±0.7 | 1.6±0.8 | 1.35±0.7 | 0.074 |
| Calcification MSCT | 1.6±0.8 | 1.9±0.8 | 1.36±0.6 | 0.001 |
| Iliofemoral circumferential calcification | 13 (12.6%) | 9 (21 %) | 4 (6.7 %) | 0.170 |
| Tortuosity angio | 1.21±0.8 | 1.16±0.8 | 1.24±0.7 | 0.138 |
| Tortuosity MSCT | 1.4±0.6 | 1.3±0.6 | 1.5±0.7 | 0.132 |
| Balloon predilatation | 10 (6%) | 10 (18%) | 0 | <0.001 |
The P values refer to the comparison of the two subgroups (“suitable” and “unsuitable” for TF approach). The percentages in brackets relate to the overall number of each particular group.
Angiographic data were used if MSCT was not available.
FMLD - femoral minimal lumen diameter; IFMLD - iliofemoral minimal lumen diameter; IMLD - iliac minimal lumen diameter; MSCT - multislice computer tomography; SFAR - sheath-to-minimal femoral lumen ratio; SIAR - sheath-to-minimal iliac lumen ratio; SIFAR - sheath-to-minimal iliofemoral lumen ratio
Predilatation of common or external iliac vessels with a non-compliant balloon due to significant narrowing was performed in 10 patients in the “unsuitable” TF group.
Vascular complications and clinical outcome
The characteristics and outcomes of vascular complications are reported in Tables 2 and 3. In brief, vascular complications occurred in 31 (23.5%) of all analyzed patients. According to the VARC definitions, these included 8 major (6%) and 23 (17.4%) minor complications (Table 2). Major complications occurred in 6 patients (11%) of the “unsuitable” TF group and in 2 patients (3%) of the “suitable” TF group and were mainly due to VARC major access site/access-related vascular injury (2 iliac dissections/ruptures, 2 dissections/ruptures of the abdominal aorta, 3 femoral dissections/ruptures). A hematoma of the ascending aortic wall that occurred during the valve implantation in 1 patient was classified as aortic dissection and managed conservatively.
Table 2.
Vascular complications and outcome
| All Patients TF | TF Unsuitable | TF Suitable | P | |
|---|---|---|---|---|
| Major complications | 8 (6%) | 6 (11%) | 2 (3%) | 0.071 |
| Dissection of ascending aorta | 1 (1%) | 1 (2%) | 0 | 0.162 |
| Severe access related vascular injury | 7 (5%) | 5 (9%) | 2 (3%) | 0.044 |
| Severe distal embolization | 0 | 0 | 0 | - |
| Minor complications | 23 (17%) | 15 (27%) | 8 (11%) | 0.021 |
| Mild access related vascular injury | 11 (8%) | 6 (11%) | 5 (7%) | 0.154 |
| Minor distal embolization | 0 | 0 | 0 | - |
| Failure access site closure | 12 (9%) | 9 (16%) | 3 (4%) | 0.032 |
| Ongoing disability due to complication | 0 | 0 | 0 | - |
| Transfused units of blood | 0.4±0.1 | 0.38±0.7 | 0.43±1.2 | 0.704 |
| Days of hospital admission | 9.7±10.5 | 9.6±11 | 9.9±10.4 | 0.839 |
| Major strokes | 4 (3%) | 2 (4%) | 2 (3%) | 0.685 |
| Need for post-procedural pacemaker | 21 (16%) | 8 (14.3%) | 13 (17%) | 0.644 |
| 30 day mortality | 5 (4%) | 2 (4%) | 3 (4%) | 0.911 |
| 1 year mortality | 25 (19%) | 12 (21%) | 13 (23%) | 0.564 |
The P value refers to the comparison of the two subgroups (“suitable” and “unsuitable” for TF approach). The percentages in brackets relate to the overall number of each particular group
Table 3.
Overview of VARC major vascular complications
| Vascular complication | Group | Complication description | Management | Death/death description | Death, after days | Death associated with complication |
|---|---|---|---|---|---|---|
| 1 | TF suitable | Rupture femoral artery | Surgical repair | No | - | No |
| 2 | TF suitable | Rupture abdominal aorta | Aorto iliac prosthesis + surgery | Yes/Stroke | 10 | No |
| 3 | TF unsuitable | Rupture femoral artery | Surgical repair | Yes/lymphoma | 442 | No |
| 4 | TF unsuitable | Iliac dissection | Aorto iliac prosthesis | No | - | No |
| 5 | TF unsuitable | Iliac dissection | Iliac stent | Yes/Heart failure | 91 | No |
| 6 | TF unsuitable | Rupture abdominal aorta | Aortic stent | Yes/Stroke | 197 | No |
| 7 | TF unsuitable | Haematoma ascending aorta | Conservative | Yes/Respiratory failure | 7 | No |
| 8 | TF unsuitable | Rupture femoral artery | Surgical repair | Yes/Heart failure | 552 | No |
Minor VARC vascular complications were significantly more frequent in the “unsuitable” TF group (15 vs. 8 patients, p=0.021) and were due to VARC minor access site or access-related vascular injury (11 patients) and VARC failure of percutaneous closure (12 patients). There was no difference in post-procedural red blood cell transfusions between the “suitable” and “unsuitable” TF groups (0.38±0.7 units versus 0.43±1.2, p=0.704). A significantly higher amount of red blood cell transfusions was found in patients with major vascular complications (1.62±2.7 versus 0.32±0.75, p=0.006).
No significant difference in 1-year survival (Fig. 2) was observed between both groups (log-rank test). During the 30-day follow-up, 5 patients died (3.8%): 2 in the “unsuitable” TF group (3.6%) and 3 in the “suitable” TF group (3.9%). In the multiple Cox regression analysis, only circumferential iliofemoral calcification [HR: 3.6, 95% CI: 1-13.2, p=0.043] but not major vascular complications [HR: 2.4, 95% CI: 0.6-9.6, p=0.209] or manufacturer recommendations [HR: 0.73, 95% CI: 0.24-2.2, p=0.565] predicted 1-year mortality. A Kaplan-Meier survival analysis comparing patients with and without circumferential iliofemoral calcifications is depicted in Figure 3. In the univariate analysis, major vascular complications were also significant predictors of mortality [HR: 2.97, 95% CI: 1.19-7.39, p=0.036]. No VARC vascular complication was the cause of death or ongoing disability, as depicted in Table 3.
Figure 2.
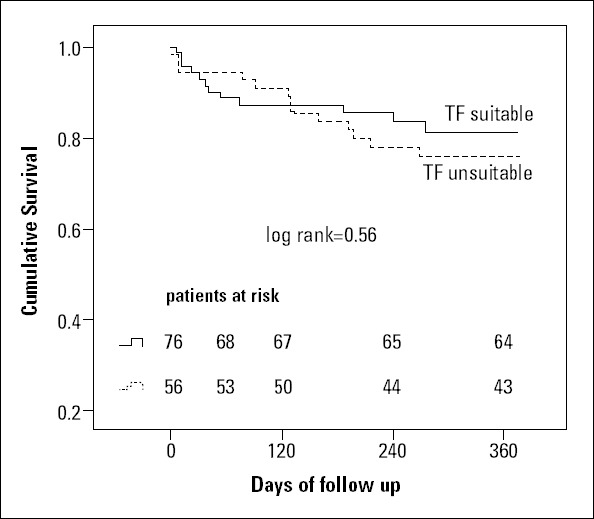
Kaplan-Meier survival analysis of TF “suitable” and TF “unsuitable” patients (unadjusted)
No significant differences in 1-year survival were found between the TF suitable and TF unsuitable patients (p=0.56).
TF - transfemoral
Figure 3.
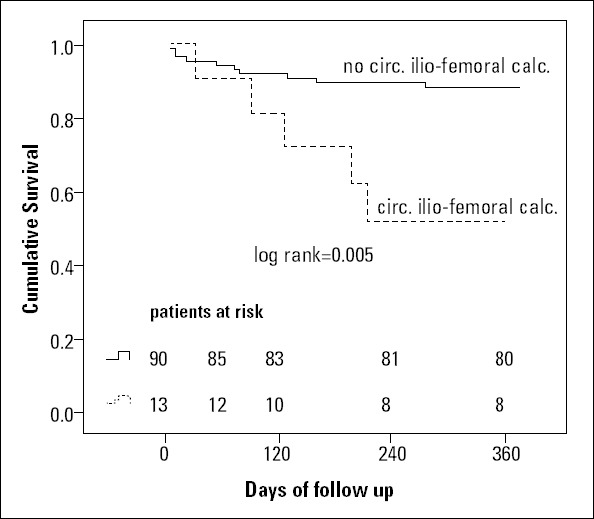
Kaplan-Meier survival analysis in patients with and without circumferential iliofemoral calcifications (unadjusted)
One-year mortality was significantly higher in patients with circumferential iliofemoral calcifications (p=0.005). Only patients who underwent an MSCT scan for TAVI workup were included in this analysis (n=103)
Treatment of vascular complications
Detailed information about major vascular complications, including treatment and outcome, is given in Table 3. All VARC major complications at the common femoral vessels were treated surgically, whereas major complications at the iliac site and abdominal aorta were managed by endovascular approach. A PTSX sizing balloon was inflated in the abdominal aorta to achieve immediate hemodynamic stabilization, followed by implantation of covered stents. However, one abdominal rupture (patient was in the “suitable” TF group) required surgical intervention due to occlusion of the right common iliac artery after implantation of an aorto-bi-iliac prosthesis. This patient died after 10 days following a massive stroke.
Predictors of major vascular complications
The univariate and multivariate analysis is depicted in Table 4. Only SIFAR measured by CT or angiogram and circumferential iliofemoral calcifications were significantly associated with the incidence of major vascular complications. The SIFAR threshold for increased incidence of major vascular complications in the entire study cohort was 1.14 (ROC area under the curve: 0.75, sensitivity: 0.88, specificity: 0.55); this screening threshold was associated with an odds ratio of 7.3 [95% CI: 1.5-35, p=0.012]. For MSCT and angiographic screening, thresholds of 1.19 (AUC 0.72, sensitivity 0.91, specificity 0.67) and 1.17 (AUC 0.79, sensitivity 0.8, specificity 0.64) were found. Angiographic and MSCT-derived measurements showed a significant correlation (Spearman rho: r=0.73, p<0.001, n=76).
Table 4.
Predictors for major vascular complications (univariate and multiple regression analysis)
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Parameter | OR (CI 95%) | P | Parameters | OR (CI 95%) | P | |
| SIFAR angio | 60 (1.9-1862) | 0.021 | Model 1 | SIFAR angio | 234 (1-54570) | 0.043 |
| Calcification angio | 1.4 (0.6-3.1) | 0.419 | Calcification angio | 0.96 (0.06-16.7) | 0.971 | |
| Tortuosity angio | 0.5 (0.1-1.5) | 0.244 | Tortuosity angio | 1.3 (0.2-8) | 0.727 | |
| SIFAR MSCT | 64 (1.4-2971) | 0.037 | Model 1 | SIFAR MSCT | 280 (0.9-90150) | 0.049 |
| Calcification MSCT | 0.4 (0.1-2) | 0.265 | Tortuosity MSCT | 0.9 (0.2-4.6) | 0.941 | |
| Tortuosity MSCT | 0.6 (0.2-1.7) | 0.311 | Circ. calcification | 5.4 (1-41) | 0.044 | |
| Circ. calcification | 6(1.2-26) | 0.020 | ||||
According to angiographic and MSCT measurements, 2 different models of predictors for major vascular complications were calculated (Model 1, Model 2). Circ - circumferential; SIFAR - sheath-to-minimal iliofemoral lumen ratio
Two VARC major complications occurred with the Novaflex delivery system (9% of the overall Novaflex sheaths used), 4 major complications occurred with the e-Sheaths (5.7%), and 2 (5%) major complications occurred with the 18 F Cook sheath (Corevalve introducer sheath).
Discussion
This study aimed to provide a standardized screening approach, including circumferential iliofemoral calcifications, to improve patient selection for TF-TAVI beyond current manufacturer recommendations. We found that these recommendations are safe but overly conservative (sensitivity 100%, specificity 50%). According to our data, the presence of circumferential iliofemoral calcifications is an important risk factor for vascular complications and also an independent predictor of increased mortality after TF-TAVI. Incorporating this MSCT-derived parameter in the workup algorithm (Fig. 4) of patients with an SIFAR ≥1 on angiographic screening improved the specificity for the prediction of major vascular complications to 62% without altering the sensitivity (100%).
Figure 4.
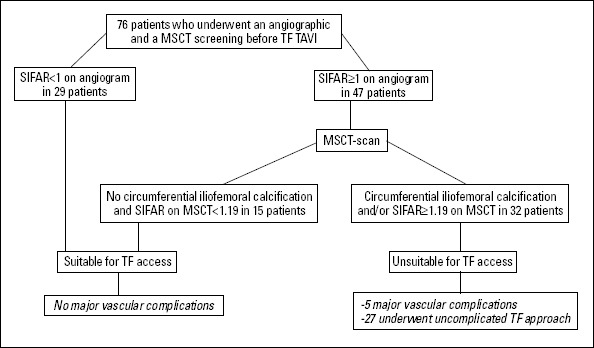
Screening approach for TF-TAVI
MSCT - multislice computer tomography; SIFAR - sheath to minimal iliofemoral lumen ratio; TF - transfemoral
Not surprisingly, a higher incidence of major (10.7% versus 2.6%) and minor (27% versus 11%) VARC vascular complications (VC) was found in our “unsuitable” TF access group. However, the overall number of major VCs was comparable to previous studies (10, 12). These complications were clearly associated with SIFARs derived from angiographic and MSCT imaging. Thresholds of 1.17 (angiography) and 1.19 (MSCT) in our study compare to 1.05 in a previous investigation, where mainly 22- and 24-F delivery sheaths were used (10). This finding may indicate the evolution of TAVI devices since smaller, expandable delivery systems with hydrophilic coating have become available.
Angiographic screening of access routes has been proposed as a simple and reliable screening method (18) for patients undergoing TAVI, and in fact, we also found a good correlation between diameters derived from reconstructed MSCT images and angiographic measurements. However, angiography does not allow 3-dimensional visualization of vessels. This is of particular interest in patients with a high calcium burden, as a significantly increased risk for major vascular complications was observed in our patients with circumferential iliofemoral calcifications. We therefore think that MSCT should be used liberally, especially when the sheath diameter exceeds the vessel diameter (SIFAR≥1). In this situation, a circumferential calcification, which can only be detected on MSCT, will most likely result in a complication.
Based on these assumptions, patients should undergo further investigation of iliofemoral vessels by MSCT in case of a SIFAR greater than or equal to 1 on angiogram. Alternative access routes should be considered in patients where reconstructed CT images reveal a SIFAR greater than 1.18 and/or the presence of circumferential vessel calcification.
Avoiding major vascular complications has become key for TAVI operators, since an association with increased mortality has been shown (5-9). Manufacturers of TAVI valves provide recommendations regarding peripheral vessel properties that are mainly based on vessel diameters (2-4). We found that these recommendations are safe but overly conservative. According to these recommendations, 42% of our cohort would have been considered unsuitable for a TF approach, although most of them (89%) underwent an uncomplicated procedure via the femoral route.
Beside these non-evidence-based recommendations, several risk factors for vascular complications have been established (10-12), and alternative access routes are preferred in cases of small iliofemoral vessel diameters and/or a high calcium burden (13). However, a worse outcome has also been reported for the TA approach, which is a frequently used alternative when TF TAVI is not feasible (11). Increased risk profiles of this pre-selected patient cohort have been discussed as a potential confounder. This hypothesis is supported by our finding that circumferential iliofemoral calcification, representative of severe peripheral vascular disease, was an independent predictor of mortality. In contrast, major vascular complications were only associated with a worse outcome in the univariate analysis, and no vascular complication was the cause of death in our study. Manufacturer recommendations did not predict mortality, as indicated by the similar survival rates in our “suitable” and “not suitable” TF groups. The duration of hospital admission was also comparable between both groups.
Based on these results, the question arises as to whether peripheral vascular disease may be considered an important risk factor, independent of the preferred access route.
Study limitations
This is a retrospective single-center experience with a limited number of patients. Due to the several devices and methods used to access the iliofemoral arteries, our patient cohort was highly heterogeneous. In addition, not all patients underwent MSCT screening, which significantly limited the deductions that could be made from our investigation. The results therefore need to be interpreted with caution. Although none of the deaths occurring in our study was directly related to a vascular complication, a strong association with mortality has been shown in other studies (5-8). Our intention was therefore not to promote the suitability of TF access in most patients but to safely expand the indications beyond current recommendations by including circumferential vascular calcifications in the screening routine.
Conclusion
Manufacturer recommendations and the presence of circumferential iliofemoral calcifications significantly predicted the incidence of vascular complications. Importantly, circumferential iliofemoral calcifications, but not major vascular complications, were associated with increased mortality in the multivariate analysis. This finding may support the predictive value of peripheral vascular disease, independent of the access route. MSCT may also therefore provide additional prognostic information beside details about vascular access.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept - M.R., M.J.M.; Design - M.R., M.J.M.; Supervision - M.J.M., P.M., U.L., J.Y.; Materials - S.K.A., M.R., R.D.P.; Data collection &/or processing - S.K.A., M.R.; Analysis &/or Interpretation - M.R., G.F., M.J.M.; Literature search - M.R., U.L., G.F.; Writing - M.R., S.K.A., P.M., U.L.; Critical review - R.D.P., J.Y., U.L., G.F., P.M.
References
- 1.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–607. doi: 10.1056/NEJMoa1008232. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 2.Zajarias A, Cribier AG. Outcomes and safety of percutaneous aortic valve replacement. J Am Coll Cardiol. 2009;53:1829–6. doi: 10.1016/j.jacc.2008.11.059. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 3.Mussardo M, Latib A, Chieffo A, Godino C, Ielasi A, Cioni M, et al. Periprocedural and short-term outcomes of transfemoral transcatheter aortic valve implantation with the Sapien XT as compared with the Edwards Sapien valve. JACC Cardiovasc Interv. 2011;4:743–50. doi: 10.1016/j.jcin.2011.05.004. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 4.Piazza N, Lange R, Martucci G, Serruys PW. Patient selection for transcatheter aortic valve implantation: patient risk profile and anatomical selection criteria. Arch Cardiovasc Dis. 2012;105:16573. doi: 10.1016/j.acvd.2012.02.007. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 5.Rodés-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Feindel CM, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk. Acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol. 2010;55:1080–90. doi: 10.1016/j.jacc.2009.12.014. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 6.Moat NE, Ludman P, de Belder MA, Bridgewater B, Cunningham AD, Young CP, et al. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis. The UK TAVI (United Kingdom Transcatheter Aortic Valve Implantation) registry. J Am Coll Cardiol. 2011;58:2130–8. doi: 10.1016/j.jacc.2011.08.050. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 7.Cribier A, Eltchaninoff H, Tron C, Bauer F, Agatiello C, Nercolini D, et al. Treatment of calcific aortic stenosis with the percutaneous heart valve: mid-term follow-up from the initial feasibility studies: the French experience. J Am Coll Cardiol. 2006;47:1214–3. doi: 10.1016/j.jacc.2006.01.049. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 8.Généreux P, Webb JG, Svensson LG, Kodali SK, Satler LF, Fearon WF, et al. PARTNER trial investigators. Vascular complications after transcatheter aortic valve replacement: insights from the PARTNER (Placement of AoRTicTraNscathetER Valve) trial. J Am Coll Cardiol. 2012;60:1043–52. doi: 10.1016/j.jacc.2012.07.003. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 9.Tchetche D, Van der Boon RM, Dumonteil N, Chieffo A, Van Mieghem NM, Farah B, et al. Adverse impact of bleeding and transfusion on the outcome post-transcatheter aortic valve implantation: insights from the Pooled-RotterdAm-Milano-Toulouse In Collaboration Plus (PRAGMATIC Plus) initiative. Am Heart J. 2012;164:402–9. doi: 10.1016/j.ahj.2012.07.003. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 10.Hayashida K, Lefèvre T, Chevalier B, Hovasse T, Romano M, Garot P, et al. Transfemoral Aortic valve implantation: new criteria to predict vascular complications. JACC - Cardiovasc Interv. 2011;4:851–8. doi: 10.1016/j.jcin.2011.03.019. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 11.Borz B, Durand E, Godin M, Tron C, Canville A, Litzler PY, et al. Incidence, predictors and impact of bleeding after transcatheter aortic valve implantation using the balloon-expandable Edwards prosthesis. Heart. 2013;99:860–5. doi: 10.1136/heartjnl-2012-303095. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 12.Toggweiler S, Gurvitch R, Leipsic J, Wood DA, Willson AB, Binder RK, et al. Percutaneous aortic valve replacement: vascular outcomes with a fully percutaneous procedure. J Am Coll Cardiol. 2012;59:113–8. doi: 10.1016/j.jacc.2011.08.069. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 13.Holmes DR, Jr, Mack MJ, Kaul S, Agnihotri A, Alexander KP, Bailey SR, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement: developed in collabra- tion with the American Heart Association, American Society of Echocardiography, European Association for Cardio-Thoracic Surgery, Heart Failure Society of America, Mended Hearts, Society of Cardiovascular Anesthesiologists, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. J Thorac Cardiovasc Surg. 2012;144:29–84. doi: 10.1016/j.jtcvs.2012.03.001. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 14.Cribier A, Eltchaninoff H, Tron C, Bauer F, Agatiello C, Nercolini D, et al. Outcomes after transcatheter aortic valve implantation with both Edwards-SAPIEN and CoreValve devices in a single center: the Milan experience. JACC Cardiovasc Interv. 2010;3:1110–21. doi: 10.1016/j.jcin.2010.09.012. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 15.Grube E, Schuler G, Buellesfeld L, Gerckens U, Linke A, Wenaweser P, et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third- generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J Am Coll Cardiol. 2007;50:69–76. doi: 10.1016/j.jacc.2007.04.047. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 16.Lee WA, Brown MP, Nelson PR, Huber TS, Seeger JM. Midterm outcomes of femoral arteries after percutaneous endovascular aortic repair using the Preclose technique. J Vasc Surg. 2008;47:919–23. doi: 10.1016/j.jvs.2007.12.029. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 17.Eisenack M, Umscheid T, Tessarek J, Torsello GF, Torsello GB. Percutaneous endovascular aortic aneurysm repair: a prospective evaluation of safety, efficiency, and risk factors. J Endovasc Ther. 2009;16:708–13. doi: 10.1583/08-2622.1. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 18.Eltchaninoff H, Kerkeni M, Zajarias A, Tron C, Godin M, Sanchez Giron C, et al. Aorto-iliac angiography as a screening tool in selecting patients for transfemoral aortic valve implantation with the Edwards SAPIEN bioprosthesis. EuroIntervention. 2009;5:438–42. doi: 10.4244/eijv5i4a69. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 19.Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. J Am Coll Cardiol. 2011;57:253–69. doi: 10.1016/j.jacc.2010.12.005. [CrossRef] [DOI] [PubMed] [Google Scholar]


