Abstract
Objective:
We sought to determine the role of mean platelet volume (MPV) for predicting long-term outcomes of elective percutaneous coronary intervention (PCI).
Methods:
On the basis of retrospective cohort study, we collected characteristics of 680 patients undergoing elective PCI from October 2005 to August 2010. The patients who had preoperative MPV were assessed for developing major adverse cardiac events (MACE) during 1-year follow-up. They were categorized into two groups including MPV <9.6 fL (n=89) and MPV ≥9.6 fL (n=92). Data were analyzed using t-test, chi-square test, Pearson correlation, receiver operating characteristic (ROC) curve and logistic regression.
Results:
One-hundred eighty one patients (26.6%) met inclusion criteria. The MACE was observed in 29 patients (16%); and its rate in low- and high-MPV groups was 11.2% and 20.7%, respectively (p=0.084). MPV was significantly higher in the patients with left ventricular ejection fraction (LVEF) <40% compared with that of ≥40% (p<0.001). There were a significant and negative correlation between MPV and platelet count (r=-0.305, p<0.001), and significant and positive correlations between MPV and platelet distribution width (PDW) and platelet large cell ratio (P-LCR) (r=0.615, p<0.001 and r=0.913, p<0.001; respectively). The best MPV cut-off point was 9.25 fL; the sensitivity and specificity were 79% and 38%, respectively. Elevated MPV was the best predictor of MACE at 1-year follow-up (OR=11.359, 95% CI 2.481-51.994, p=0.002).
Conclusion:
The results indicate that preoperative MPV is an independent predictor of the MACE at 1-year follow-up in the patients undergoing elective PCI. Moreover, it may be useful for risk stratification in such cases.
Keywords: mean platelet volume, percutaneous coronary intervention, major adverse cardiovascular events
Introduction
Since the percutaneous coronary intervention (PCI) has been introduced, restenosis and major adverse cardiac events (MACE) after performing PCI have been apparently reported (1, 2). By innovation in antiplatelet therapy, dual antiplatelet treatment, the rate of these complications has decreased; however, the number of these adverse events has been remained high leading to increase in concerns regarding this issue (2).
The platelets are derived from megakaryocytes in the bone marrow and completely are necessary for hemostasis. Also, some mediators produced by platelets are essential in coagulation, inflammation, atherothrombotic events as well as atherosclerosis which have been identified in the previous investigations (3, 4). Although the platelet volume is determined by the size of fragmented megakaryocyte, other factors including cytokines, growth factors and endothelial dysfunction may impact on its volume and subsequent reactivity. The larger platelets have more granules and consequent greater efficacious vasomotor, pro-inflammatory and haemostatic function that may lead to more cardiovascular events (5). Mean platelet volume (MPV) is an index for assessing the platelet activity measured as a component of the complete blood count. Recent studies have previously demonstrated correlation between MPV and cardiovascular morbidities and mortality (6). Notably, a high MPV value has been observed in patients with cardiovascular risk factors including diabetes mellitus, hypertension, hypercholesterolemia, smoking and obesity (6-8). Furthermore, it has been shown the diagnostic role of MPV, based on being inflammatory marker, in the patients with Behçet’s disease and malignancy (9, 10). In addition, the prognostic value of MPV for predicting long-term mortality after PCI has been demonstrated (11).
Some of the investigations have previously shown that a correlation exists between increased platelet indices before PCI and the rate of MACE (12-14). All the mentioned studies investigated on the cohort of patients undergoing elective and urgent PCI rather than elective one alone, and also some findings showed the lack of correlation between increased baseline MPV and PCI outcomes. Hence, we sought to determine the correlation between MPV value before purely elective PCI and the occurrence of defined MACE as a composite of endpoints including long-standing cardiac care unit (CCU) hospitalization postoperatively, target vessel revascularization (TVR), myocardial infarction (MI), stroke and death, and consequently, all patients were followed for a 1-year period in terms of the incidence of MACE.
Methods
Study design
This study was a retrospective cohort study. We collected the baseline clinical and PCI characteristics of six-hundred eighty patients who were intervened at Taleghani hospital catheterization laboratory from October 2005 until August 2010 in the West-Azerbaijan, Iran. This study was approved by our local Ethical Committee in the Urmia University of Medical Sciences.
The study cohort included the patients who had the history of previous acute coronary syndrome (ACS) and had already been scheduled to undergo purely elective PCI. After collecting data from registry, all patients who had the measurements of platelet indices including platelet count, MPV, platelet large cell ratio (P-LCR) and platelet distribution width (PDW) before intervention were assessed in terms of the occurring pre-defined MACE during 1-year follow-up period. The patients were excluded if: (1) had undergone primary PCI; (2) laboratory measurements were not available; (3) PCI had been failed; (4) renal insufficiency (creatinine ≥1.5 mg/dL); (5) having fever before intervention; (6) the history of malignancies; and (7) the history of rheumatic diseases.
Before intervention, patients pretreated with aspirin 325 mg, clopidogrel loading 300 mg and weight adjusted intravenous unfractionated heparin. The goal of stenting was full lesion coverage even with one or more stents. The selection of size and type of stent were based on operators’ decision. Patients were discharged on clopidogrel 75 mg/d/month for 1 year, aspirin 325 mg/d/month at least 3 months and 80 mg/d/month of aspirin for an indefinite period after PCI.
Based on our institution policy, the blood samples had been taken from all patients 6 to 24 hours before PCI performing to measure the routine complete blood count. The samples had been collected into tubes containing EDTA and were analyzed using automated cell analyzer, Sysmex KX21-N, Kobe, Japan.
Clinical outcomes and definitions
A total of 181 patients were accordingly fulfilled inclusion criteria. The endpoints of long-standing CCU admission, TVR, MI, stroke and death were considered as composite endpoints (MACE). All outcomes were provided by either telephone interviews or registry data at 1-year follow-up duration. If the patients had been hospitalized for a cardiac complaint, we were concisely obtained detailed clinical data concerning the diagnosis and revascularization of targeted vessel.
The MI was defined as ischemic symptoms including chest pain more than 20 minutes with ST-segments elevation in ≥2 contiguous leads and/or the elevation of cardiac biomarkers (creatine kinase MB isoenzyme and Troponin I) to >two-fold the upper limit of normal value. This definition had been used for detecting MI periprocedural and during the follow-up period. The long-standing CCU admission was defined as patients who were hospitalized more than 48 hours in the CCU ward following intervention due to three main reasons including recurrent resting chest pain, the dynamic alteration of electrocardiogram without elevation of the cardiac biomarkers and hemodynamic instability. Furthermore, TVR was accepted as repeated PCI or coronary artery bypass graft surgery (CABG) on the vessel intervened previously in our center. Because the patients had not been undergone control angiography at follow-up period, we could not differentiate between restenosis, stent thrombosis or new lesion developing.
The drug histories were considered as medications those had been consumed at least one day before admission for coronary intervention. All medical histories were obtained from patients folder. Hypertension was defined as those who had blood pressure above 140/90 mm Hg and/or taking antihypertensive drugs (15). Diabetes mellitus was diagnosed as those having one of these criteria; 1) The classic symptoms of diabetes plus plasma glucose concentration >200 mg/dL; 2) fasting plasma glucose ≥126 mg/dL; 3) 2-hour post load glucose ≥200 mg/dL during an OGTT; and/or 4) taking anti-diabetic medicines (16). The familial history of coronary artery disease (CAD) was diagnosed as patients had a first degree male relative or a female relative less than 55 and 65 years old with CAD, respectively (17).
Statistical analysis
Based on our hypothesis that the elevated MPV value is associated with poor prognosis, all patients were categorized into two groups. According to the median MPV value of entire cohort, the MPV groups included low-MPV (MPV <9.6 fL) and high-MPV (MPV ≥9.6 fL). Continues and categorical variables were analyzed using t-test and chi-square test, respectively. Correlation between two continues variables were calculated by the Pearson coefficient correlation. A receiver operating characteristic (ROC) curve was constructed to determine the area under the curve (AUC), sensitivity and specificity of baseline MPV value for predicting MACE incidence during 1-year follow-up.
Furthermore, a logistic regression model was used to identify the predictors of combined MACE. All measured outcomes including long-standing cardiac care unit CCU hospitalization, TVR, MI, stroke and death were defined as composite endpoints. The covariates included age; gender; BMI (body mass index); the history of diabetes mellitus, hypertension, smoking, the positive familial history of CAD and dyslipidemia; LVEF before procedure; intervened vessels; and laboratory measurements including PDW, P-LCR, platelet count, MPV, creatinine and hematocrit. The Hosmer-Lemeshow test was also used for goodness of fit for logistic regression.
All analyses were performed using SPSS version of 16.0 (SPSS Inc., Chicago, IL, USA). The p value of less than 0.05 was considered statistically significant and the odds ratios (ORs) were reported with the 95% confidence intervals (CIs).
Results
Relationship between MPV and clinical characteristics
Of 680 patients who underwent PCI from October 2005 to August 2010, 181 patients (26.6%) met inclusion criteria. The patients’ mean age was 58.2±10.9 years and 124 of those were male (p=0.148). The MPV was categorized as follows: (1) low-MPV, MPV <9.6 fL (n=89); (2) high-MPV, MPV ≥9.6 fL (n=92). Baseline clinical characteristics were depicted in Table 1.
Table 1.
Baseline clinical and procedural characteristics
| Total (n=181) | Low-MPV* (n=89) | High-MPV* (n=92) | P ** | |
|---|---|---|---|---|
| Age, years | 58.2±10.9 | 57.7±10.6 | 58.7±11.4 | 0.495 |
| Sex | 0.108 | |||
| Male | 124 (68.5) | 66 (74.2) | 58 (63.0) | |
| Female | 57 (31.5) | 23 (25.8) | 34 (37) | |
| Body mass index | 27.4±4.4 | 27.5±4.4 | 27.2±4.4 | 0.670 |
| MACE incidence | 29 (16) | 10 (11.2) | 19 (20.7) | 0.084 |
| Prior myocardial infarction | 71 (39.2) | 34 (38.2) | 37 (40.2) | 0.781 |
| Prior PCI | 33 (18.2) | 17 (19.1) | 16 (17.4) | 0.766 |
| Diabetes mellitus | 30 (16.6) | 13 (14.6) | 17 (18.5) | 0.484 |
| Dyslipidemia | 87 (48.1) | 46 (51.7) | 41 (44.6) | 0.338 |
| Hypertension | 89 (49.2) | 48 (53.9) | 41 (44.6) | 0.208 |
| Prior cerebrovascular events | 3 (1.66) | 0 (0) | 3 (3.3) | 0.086 |
| Smoking | 81 (44.8) | 35 (39.3) | 46 (50.0) | 0.149 |
| Familial history of CAD | 54 (29.8) | 21 (23.6) | 33 (35.9) | 0.071 |
| LVEF, % | 0.015 | |||
| ≥40% | 125 (69.1) | 69 (77.5) | 56 (60.9) | |
| <40% | 56 (30.1) | 20 (22.5) | 36 (39.1) | |
| Intervened vessel | ||||
| LAD | 101 (52.9) | 52 (51.5) | 49 (48.5) | 0.484 |
| RCA | 63 (31.6) | 28 (44.4) | 35 (55.6) | 0.353 |
| LCX | 35 (17.6) | 15 (42.9) | 20 (57.1) | 0.405 |
| Drug history | ||||
| β-blocker | 134 (74) | 68 (76.4) | 66 (71.7) | 0.474 |
| Statins | 103 (56.9) | 49 (55.1) | 54 (58.7) | 0.621 |
| Aspirin | 63 (34.8) | 32 (36.0) | 31 (33.7) | 0.094 |
| Dual antiplatelet | 72 (39.8) | 30 (33.7) | 42 (45.7) | 0.101 |
| Serum measurements | ||||
| Platelet count, 109/L | 209±56.9 | 225±56.4 | 194±53.1 | <0.001 |
| PDW, fL | 12.8±2.7 | 11.6±2.7 | 13.9±2.3 | <0.001 |
| P-LCR, % | 23.7±7.3 | 18.2±3.8 | 29.0±5.8 | <0.001 |
| Cholesterol, mg/dL | 157.5±34.2 | 160.6±34.3 | 154.4±34.0 | 0.218 |
| Triglyceride, mg/dL | 172.6±87.7 | 175.3±91.0 | 170.1±84.8 | 0.695 |
| LDL, mg/dL | 86.7±25.0 | 88.2±25.4 | 85.2±24.7 | 0.412 |
| HDL, mg/dL | 40.6±9.1 | 41.8±8.3 | 39.5±9.8 | 0.091 |
| Hematocrit, % | 39.3±5.6 | 38.8±5.8 | 39.7±5.4 | 0.299 |
| ALT, mg/dL | 31±36 | 28.9±21.9 | 33.2±45.9 | 0.297 |
| AST, mg/dL | 24.9±22.4 | 23.1±13.1 | 26.6±28.7 | 0.431 |
| Creatinine, mg/dL | 0.9±0.5 | 0.9±0.4 | 0.9±0.6 | 0.778 |
Values are presented as mean±SD and number (%)
MPV level was considered as low-MPV <9.6 fL and high-MPV ≥9.6 fL
Chi-square and t-test were used
ALT - alanine aminotransferase; AST - aspartate aminotransferase; CAD - coronary artery disease; HDL - high density lipoprotein; LAD - left anterior descending artery; LCX - left circumflex artery; LDL - low density lipoprotein; LVEF - left ventricular ejection fraction; MACE - major adverse cardiac event; MPV - mean platelet volume; PCI - percutaneous coronary intervention; PDW - platelet distribution width, P-LCR - platelet large-cell ratio, RCA - right coronary artery
Relationship between MPV and MACE
After division of patients based on MPV value, those characteristics and the incidence of MACE were analyzed. During 1-year follow-up period, the MACE incidence, as composite endpoints, were observed in 29 patients (16%); MI, long-standing CCU, TVR and death were occurred in 4, 18, 6 and 1 patients, respectively. Its rate in the low- and high-MPV groups was 10 (11.2%) and 19 (20.7%), respectively; and it was no significantly different (p=0.084), although in the high-MPV group it was nearly twice as many patients as the low-MPV group.
Relationship between MPV and subgroups by risk factors
A trend was seen for the patients in the high-MPV group to have more number of positive familial history of CAD than the other group (p=0.071). The number of patients with LVEF <40% in the high-MPV was more than that of low-MPV group (p=0.015). In terms of the mean of MPV level between subgroups stratified by gender, cardiovascular risk factors, drug histories, and LVEF; analyses only showed significant difference between LVEF subgroups (9.5±0.8 versus 10±0.9 fL), but others did not so (Fig. 1). Additionally, the rate of MACE in the above mentioned subgroups were not significantly different.
Figure 1.
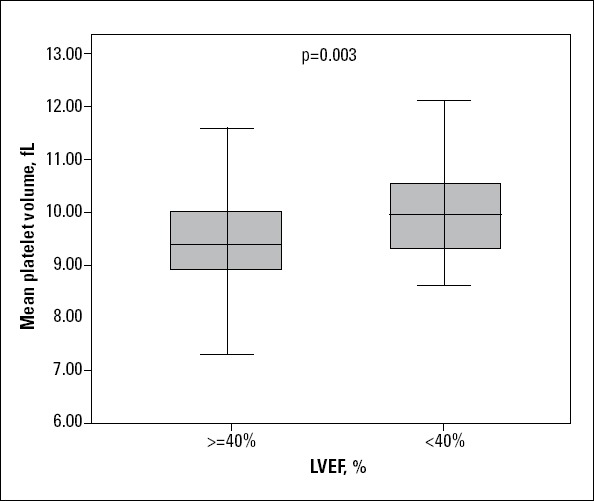
Mean platelet volume between groups by left ventricular ejection fraction (LVEF) was significantly different
Correlations between platelet indices
There was a significant and negative correlation between MPV and platelet count (r=0.305, p<0.001). Strong positive correlations were also observed between MPV and PDW and P-LCR that were statistically significant (r=0.615, p<0.001 and r=0.913, p<0.001; respectively). Any other correlations were not observed between MPV and laboratory measurements (Table 2).
Table 2.
Correlation analysis between mean platelet volume and other laboratory measurements
| r | P | |
|---|---|---|
| Platelet count | -.0305 | <0.001 |
| PDW | 0.615 | <0.001 |
| P-LCR | 0.913 | <0.001 |
| Cholesterol | 0.027 | 0.720 |
| Triglyceride | 0.053 | 0.484 |
| LDL | 0.073 | 0.329 |
| HDL | 0.122 | 0.103 |
| Hematocrit, % | 0.110 | 0.141 |
| ALT, mg/dL | 0.038 | 0.622 |
| AST, mg/dL | 0.060 | 0.430 |
| Creatinine, mg/dL | 0.102 | 0.172 |
ALT - alanine aminotransfe rase; AST - aspartate aminotransferase; HDL - high density lipoprotein; LDL - low density lipoprotein; PDW - platelet distribution width; P-LCR - platelet large-cell ratio
Diagnostic and prognostic values of MPV
ROC curve of MPV when predicting MACE was constructed. The AUC was 0.555 with 95% CI of 0.441-0.669 (p=0.347). The best MPV cut-off point for identifying MACE at follow-up was 9.25 fL; the sensitivity and specificity were 79% and 38%, respectively (Fig. 2).
Figure 2.
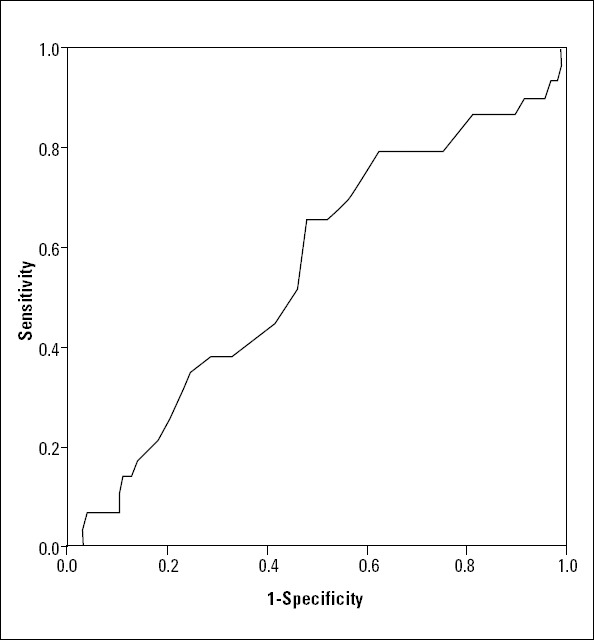
ROC curve showing diagnostic value of MPV for detecting PCI outcomes during 1-year follow-up period
The logistic regression model demonstrated that the high-MPV, MPV ≥9.6 fL, (OR=11.359, 95% CI 2.481-51.994, p=0.002) had the strongest association with the incidence of MACE at 1-year follow-up (Table 3). The Hosmer-Lemeshow test showed the high predictive value with p-value of 0.265.
Table 3.
Odds ratios (OR) for incidence of major adverse cardiac events (MACE) by logistic regression analysis
| OR | 95% CI | P* | |
|---|---|---|---|
| Age | 0.987 | 0.994-1.031 | 0.551 |
| Gender, male | 0.483 | 0.149-1.571 | 0.227 |
| Body mass index | 0.963 | 0.864-1.074 | 0.500 |
| Diabetes mellitus | 0.461 | 0.138-1.546 | 0.210 |
| Dyslipidemia | 1.906 | 0.725-5.013 | 0.191 |
| Hypertension | 0.723 | 0.266-1.961 | 0.524 |
| Smoking | 0.666 | 0.242-1.836 | 0.432 |
| Familial history of CAD | 1.671 | 0.594-4.700 | 0.331 |
| LVEF | 1.912 | 0.668-5.477 | 0.227 |
| LAD | 0.829 | 0.235-2.925 | 0.771 |
| RCA | 0.747 | 0.239-2.337 | 0.616 |
| LCX | 0.435 | 0.092-2.049 | 0.292 |
| Platelet count | 0.998 | 0.989-1.007 | 0.653 |
| MPV level** | 11.359 | 2.481-51.994 | 0.002 |
| PDW | 1.200 | 0.865-1.664 | 0.274 |
| P-LCR | 1.119 | 0.974-1.285 | 0.114 |
| Hematocrit | 1.126 | 0.976-1.299 | 0.104 |
| Creatinine | 4.325 | 0.455-41.103 | 0.202 |
Logistic regression analysis
MPV level was considered as low-MPV <9.6 fL and high-MPV .9.6 fL
CAD - coronary artery disease; CI - confidence interval; LAD - left anterior descending artery; LCX - left circumflex artery; LVEF - left ventricular ejection fraction; MPV - mean platelet volume; OR - odd ratio; PDW - platelet distribution width; P-LCR - platelet large-cell ratio; RCA - right coronary artery
Discussion
Based on the present investigation, the rate of MACE in high-MPV group was about two-fold of that in low-MPV group, and the pre-procedural elevated MPV was an independent predictor of combined MACE at 1-year follow-up period in the patients who underwent elective PCI. Furthermore, the MPV level was inversely associated with platelet count and directly with PDW and P-LCR values as other biomarkers indicating platelet activity.
Platelets are one of the blood cells that have an important role in atherothrombosis, thrombogenesis and atherosclerotic lesion rupture. MPV is a simple and easily available test evaluating platelet activity and can be used as a platelet monitoring tool (4). Patients undergoing PCI are at the higher risk of plaque rupture and arterial wall injuries. These can result in platelet activation and consequent increased procoagulant activity and inflammatory processes (18).
Many previous studies have shown that the pre-procedural elevated MPV is associated with the incidence of MACE and restenosis following PCI. Yang et al. (1) investigated the role of MPV as a prognostic factor for restenosis. They found that it may be considered as a potential marker of restenosis after PCI. Thus patients undergoing PCI with high pre-procedural MPV might benefit from a high-dose antiplatelet therapy after coronary intervention. Another study conducted by Huczek et al. (19) noted that MPV predicts angiographic reperfusion and 6-month mortality after primary PCI. Moreover, concerning correlation between MPV and cardiovascular risk factors, it was founded that it was at higher level in patients with history of cardiovascular risk factors including smoking and diabetes (7, 8).
Some investigations were conducted regarding the correlation between platelet size and PCI outcomes. Goncalves and Table 3. Odds ratios (OR) for incidence of major adverse cardiac events (MACE) by logistic regression analysis coworkers (14), in terms of MPV effect on PCI outcomes at 1-year follow-up, demonstrated that the pre-procedural MPV was the strong predictor of long-term adverse events. MPV value had prognostic value similar to troponin in the patients with ACS. Consequently, they concluded that MPV value can be considered as a marker for evaluating antiplatelet therapy after coronary intervention. The newly published investigation by Shah et al. (13) showed that there is not any correlation between baseline MPV and PCI outcomes. However, the mortality rate was greater in patients with increased MPV over the follow-up compared to those without MPV change or decrease in its value. They entered all patients who underwent elective or urgent PCI and did no differentiate between those in their analysis. Choi and coworkers (12) were also demonstrated that MPV was higher in the patients with adverse outcomes and cardiac death during follow-up as compared to those without those. Moreover, elevated MPV was more useful for predicting cardiac death in those with ACS undergoing PCI. These findings are similar to our results apart from the fact that our cohort was only the patients who underwent elective PCI rather than PCI following newly diagnosed with ACS, urgent PCI. It seems that there is a difference between the outcomes of coronary intervention after ACS and stable coronary artery disease. Due to these controversial findings and lack of sufficient investigations regarding this notion, it seems that further studies are needed to clarify it.
Prior studies’ findings are in accordance with our study showing the independent predictor role of elevated MPV value for the incidence of MACE after PCI at 1-year follow-up period. Although, none of the cardiovascular risk factors were associated with elevated MPV value except the LVEF subgroups which noted the more amount of LVEF <40% in the second compared with the first group.
Study limitations
This study suffered from some limitations based on its nature. First, our study was retrospective with a small sample size that might influence our findings for demonstrating more relations. Second, the blood samples analyzing was not the same for all patients and we could not definitely defined the duration of sample analyzing. Hence, probable delaying in measurements might cause abnormal MPV values (20). Third, our cohort included those who underwent only elective PCI rather than the PCI following ACS, and therefore the rate of MACE was lower compared with previous similar studies. These findings indicate that further and large-scale studies are required to elucidate the potential role of MPV in the patients undergoing PCI.
Conclusion
The result of this study suggests that MPV is an independent predictor of MACE at 1-year follow-up period in the patients undergoing elective PCI. Moreover, its value before PCI may be of benefit for risk stratification. Further prospective large-scale studies are needed to confirm our result.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Author contributions: Concept - M.H.S.M., R.E., Y.R.; Design - M.H.S.M., Y.R.; Supervision - M.H.S.M., R.E., K.K.; Resource - M.H.S.M., R.E.; Materials - M.M., A.R.; Data collection &/or processing - Y.R., A.Z.; Analysis &/or interpretation - Y.R.; Literature search - Y.R., A.Z., A.R.; Writing - Y.R.; Critical review - M.H.S.M., R.E., Y.R., M.M., K.K., A.R., A.Z.; Other - A.Z.
References
- 1.Yang A, Pizzulli L, Luderitz B. Mean platelet volume as marker of restenosis after percutaneous transluminal coronary angioplasty in patients with stable and unstable angina pectoris. Thromb Res. 2006;117:371–7. doi: 10.1016/j.thromres.2005.04.004. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 2.Bonello L, Paganelli F, Arpin-Bornet M, Auquier P, Sampol J, Dignat-George F, et al. Vasodilator-stimulated phosphoprotein phosphorylation analysis prior to percutaneous coronary intervention for exclusion of postprocedural major adverse cardiovascular events. J Thromb Haemost. 2007;5:1630–6. doi: 10.1111/j.1538-7836.2007.02609.x. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 3.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–94. doi: 10.1056/NEJMra071014. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 4.Kamath S, Blann AD, Lip GY. Platelet activation: assessment and quantification. Eur Heart J. 2001;22:1561–71. doi: 10.1053/euhj.2000.2515. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 5.Muscari A, De Pascalis S, Cenni A, Ludovico C, Castaldini N, Antonelli S, et al. Determinants of mean platelet volume (MPV) in an elderly population: relevance of body fat, blood glucose and ischaemic electrocardiographic changes. Thromb Haemost. 2008;99:1079–84. doi: 10.1160/TH07-12-0712. [DOI] [PubMed] [Google Scholar]
- 6.Chu SG, Becker RC, Berger PB, Bhatt DL, Eikelboom JW, Konkle B, et al. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost. 2010;8:148–56. doi: 10.1111/j.1538-7836.2009.03584.x. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papanas N, Symeonidis G, Maltezos E, Mavridis G, Karavageli E, Vosnakidis T, et al. Mean platelet volume in patients with type 2 diabetes mellitus. Platelets. 2004;15:475–8. doi: 10.1080/0953710042000267707. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 8.Kario K, Matsuo T, Nakao K. Cigarette smoking increases the mean platelet volume in elderly patients with risk factors for atherosclerosis. Clin Lab Haematol. 1992;14:281–7. doi: 10.1111/j.1365-2257.1992.tb00103.x. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 9.Ekiz O, Balta I, Şen BB, Rifaioğlu EN, Ergin C, Balta S, et al. Mean platelet volume in recurrent aphthous stomatitis and Behçet disease. Angiology. 2014;65:161–5. doi: 10.1177/0003319713492375. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 10.Demirkol S, Balta S, Küçük U, Çelik T. Mean platelet volume may indicate early diagnosed gastric cancer based on inflammation. Platelets. 2013 Jun 17; doi: 10.3109/09537104.2013.799646. [Epub ahead of print] [CrossRef] [DOI] [PubMed] [Google Scholar]
- 11.Balta S, Demirkol S, Çelik T, Akgül EO. Mean platelet volume as a surrogate marker of long-term mortality in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2013;112:142. doi: 10.1016/j.amjcard.2013.04.033. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 12.Choi SW, Choi DH, Kim HW, Ku YH, Ha SI, Park G. Clinical outcome prediction from mean platelet volume in patients undergoing percutaneous coronary intervention in Korean cohort: Implications of more simple and useful test than platelet function testing. Platelets. 2013 Aug 2; doi: 10.3109/09537104.2013.821606. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Shah B, Oberweis B, Tummala L, Amoroso NS, Lobach I, Sedlis SP, et al. Mean platelet volume and long-term mortality in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2013;111:185–9. doi: 10.1016/j.amjcard.2012.09.014. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goncalves SC, Labinaz M, Le May M, Glover C, Froeschl M, Marquis JF, et al. Usefulness of mean platelet volume as a biomarker for long-term outcomes after percutaneous coronary intervention. Am J Cardiol. 2011;107:204–9. doi: 10.1016/j.amjcard.2010.08.068. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 15.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29:43–8. [Google Scholar]
- 17.Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, et al. ACC/AHA 2002 guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction--summary article: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the Management of Patients With Unstable Angina) J Am Coll Cardiol. 2002;40:1366–74. doi: 10.1016/s0735-1097(02)02336-7. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 18.Tantry US, Gurbel PA. Platelet monitoring for PCI: is it really necessary? Hamostaseologie. 2009;29:368–75. [PubMed] [Google Scholar]
- 19.Huczek Z, Kochman J, Filipiak KJ, Horszczaruk GJ, Grabowski M, Piatkowski R, et al. Mean platelet volume on admission predicts impaired reperfusion and long-term mortality in acute myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol. 2005;46:284–90. doi: 10.1016/j.jacc.2005.03.065. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 20.Balta S, Demirkol S, Ünlü M, Çelik T. Other inflammatory markers should be kept in mind when assessing the mean platelet volume. Platelets. 2013 Apr 5; doi: 10.3109/09537104.2013.775643. Epub ahead of print. [CrossRef] [DOI] [PubMed] [Google Scholar]


