Abstract
Objective:
The aim of this study was to assess subclinical left (LV) and right ventricular (RV) dysfunction novel load-independent isovolumic myocardial acceleration (IVA) derived from tissue Doppler imaging (TDI) in patient with metabolic syndrome (MetS).
Methods:
This study had an observational case-control design. The study included 133 subjects which were divided into two groups: 75 patients with MetS and 58 controls without MetS. MetS was defined by the presence of ≥3 criteria according to ATP-NCEP III guidelines. All the subjects underwent laboratory blood tests and complete conventional echocardiography and TDI. Student’s t, Mann-Whitney U, Pearson’s, and multiple regression analysis were used for statistical analysis.
Results:
There were no significant difference between two groups in terms of traditional echocardiographic parameters. The diastolic and global functions of both ventricles were significantly impaired in MetS group. The TDI-derived IVA of the LV and the RV was significantly lower in patients with MetS (3.2±0.9 vs. 4.0±1.4, p<0.001 and 2.6±0.7 vs. 3.1±0.9, p=0.001, respectively). Whereas, TDI derived systolic velocity (Sa), and peak myocardial velocity during isovolumic contraction (IVV) of both ventricles were similar between the two groups. In the multiple regression analysis, waist circumference and diastolic blood pressure were found to be an independent determinant of IVA of LV (β=-.223, 95% CI=-.034 -.002, p=0.004) and RV (β=-.527, 95% CI=-.085 -.020, p=0.002) respectively.
Conclusion:
MetS affects global, diastolic, and systolic functions of two ventricles. This disruption lead to decreased function of heart was related with raised risk factors of MetS
Keywords: metabolic syndrome, isovolumic myocardial acceleration, systolic function, tissue Doppler imaging, regression analysis
Introduction
Metabolic syndrome (MetS) is a cluster of risk factors consisting of hyperglycemia, hypertriglyceridemia, lower high-density lipoprotein (HDL) cholesterol, hypertension, and abdominal obesity (1, 2). MetS is diagnosed with three or more of these metabolic abnormalities are present in the same person according to the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) (2). MetS is a risk factor for the development of type II diabetes and atherosclerotic cardiovascular disease. The association of Mets with left ventricular hypertrophy and left ventricular diastolic and systolic dysfunction was shown in clinical study (3). Ivanovic et al. (4) showed that diastolic and global functions of the left ventricle (LV) were significantly changed and that systolic functions of the LV were fully preserved in patients with MetS. The function of the right ventricle (RV) was also investigated in MetS groups. Tadic et al. (5) demonstrated the impairment of global functions of RV in MetS patients and this impairment was related to components of MetS. Also, Karakurt et al. (6) demonstrated the detoriation of both the systolic and diastolic functions of the RV by using the myocardial performance index (MPI), tricuspid annular plane systolic excursion (TAPSE) and some other parameters in MetS patients. Isovolumic myocardial acceleration (IVA) is a new tissue Doppler parameter which is used to assess the systolic function of both LVs and RVs. IVA is the ratio of tissue Doppler-derived peak myocardial velocity during isovolumic contraction (IVV) divided by the acceleration time (AT). This parameter has been validated in a variety of experimental (7, 8) and clinical (9, 10) settings. The IVA, which reflects an earlier isovolumic event and is more robust in terms of load dependency compared with peak dP/dt, is more sensitive to changes in contractile state than Emax (8).
The aim of this study was to assess left and right ventricular function in terms of novel load-independent IVA derived from TDI in patients with MetS.
Methods
Study design
This study is an observational case-control study.
Study population
The study was performed in Mehmet Akif Ersoy Thoracic and Cardiovascular Surgery Training and Research Hospital İstanbul-Turkey. Participants enrolled in the study were selected among patients admitted to the cardiology outpatient clinic from January 2012 to February 2013. Study population included 75 consecutive patients (mean age 47±10 years, 56% male) with MetS and 58 control subjects (mean age 44±8 years, 54.4% male) without MetS.
The exclusion criteria of the present study were defined as follows: angina, acute coronary syndromes, heart failure history, congenital, pericardial and valvular heart disease, atrial fibrillation or flutter, secondary hypertension, renal disease, thyroid disorders, malignancies, chronic obstructive pulmonary disease, pulmonary hypertension, atrio-ventricular conduction abnormality, left bundle branch block or any other intra-ventricular conduction delay, segmental wall motion abnormalities, LV ejection fraction (EF) <55%, pregnancy, and inflammatory diseases.
Written informed consent was obtained from all the patients following approval of the study by the institutional review board. The study was consistent with the Declaration of Helsinki.
Study protocol
Patients with MetS and control subjects without MetS included to the study. Anthropometric measurement obtained. Blood samples were drawn following overnight fasting period. Conventional echocardiography and tissue Doppler imaging were performed to all subjects.
Study variables
The baseline variables of the study were as following: age, sex, smoking status, body mass index (BMI), waist circumference, heart rate, blood pressure, fasting serum glucose, plasma lipids [i.e., triglyceride, high density lipoprotein (HDL) cholesterol, total cholesterol, and low-density lipoprotein (LDL) cholesterol concentrations] and echocardiographic measurements. In our study presence of MetS was a primary predictor variable, the outcome variables were RV and LV IVA.
Diagnosis and definitions
The diagnosis of MetS was based on the presence of three or more of the risk factors for MetS established by the NCEP ATP III 2005 guidelines: systolic blood pressure (SBP) and diastolic blood pressure (DBP) ≥130/≥85 mm Hg, fasting plasma glucose ≥100 mg/dL, waist circumference >102 cm for men and >88 cm for women, fasting triglycerides >150 mg/dL, and HDL cholesterol <40 mg/dL for men and <50 mg/dL for women (2). The diagnosis of diabetes was based on the criteria of the World Health Organization published in 2006 (11), and arterial hypertension was based on the recommendations of European Society of Cardiology Hypertension Guideline published in 2007 (12).
Conventional echocardiographic examination
All transthoracic echocardiographic examinations were performed with the GE vivid S6 Vingmed system 5 (Norway, Horten), which is equipped with 2.5-4 MHz transducers. All the patients were examined in the left lateral and supine positions with two-dimensional, M-mode, pulsed, and color flow Doppler echocardiography. Single lead electrocardiogram recordings were obtained continuously. For all the measurements, the average of at least five cardiac cycles was used.
The diameters of the LV, the thicknesses of the walls of the LV, and the left ventricular ejection fraction (modified Simpson’s rule) were measured according to published recommendations (13). The LV mass was calculated using the formula as previously described (14). LV mass index (LVMI) was indexed for the surface area. The right ventricular fractional area change (RV FAC) was measured from the apical four-chamber view. End-diastole was identified by the onset of the R-wave, and end-systole was identified as the smallest cavity size immediately before the opening of the tricuspid valve. The RV FAC was calculated using the formula: (end-diastolic area-end-systolic area)/end-diastolic area (15). TAPSE was used to assess the global systolic function of the RV. TAPSE was measured by M-mode using cursor in apical four-chamber view at tricuspid lateral annulus. Maximum displacement during systole was evaluated. Pulmonary artery systolic pressure (PAP) was estimated by continuous-wave Doppler imaging using the Bernoulli equation (15).
Tissue Doppler imaging
Doppler tissue echocardiography was performed using transducer frequencies between 3.5 and 4.0 MHz by adjusting the spectral-pulsed Doppler signal filters until a Nyquist limit of 15 to 20 cm/s was reached and then using the minimal optimal gain. Five consecutive cycles were recorded using a frame rate greater than 150 fps. The monitor sweep speed was set at 50 to 100 mm/s to optimize the spectral display of myocardial velocities. Every effort was made to align the pulsed-wave cursor to ensure that the Doppler angle of incidence was as close to 0 as possible to the direction of the walls. In the apical four-chamber view, the pulsed Doppler sample volume was placed at the level of the LV mitral annulus, and the RV tricuspid annulus at end-expiration (16).
The peak myocardial velocity during isovolumic contraction, acceleration time of peak myocardial velocity during isovolumic contraction (AT), peak myocardial systolic velocity (Sa), peak early and late diastolic velocities (E’ and A’), isovolumic contraction time (IVCT), isovolumic relaxation time (IVRT), and ejection time (ET) were measured. The MPI was calculated as the sum of the IVCT and the IVRT divided by the ET. The IVA was defined as the ratio of IVV divided by the AT (Fig. 1) (15).
Figure 1.
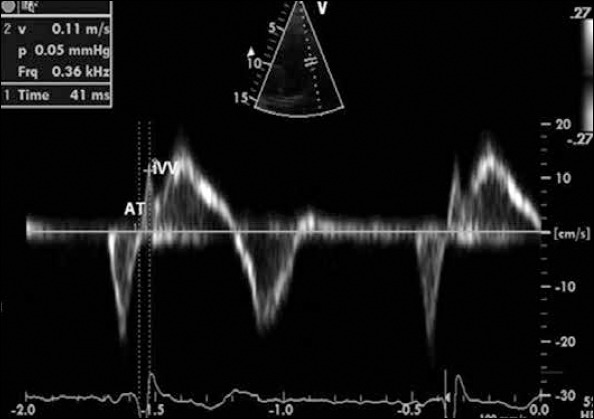
Tissue Doppler imaging obtained at lateral corner of tricuspid annulus
AT - time interval from the onset of the wave during isovolumic contraction to the time at peak velocity of this wave (accelaration time), IVV - isovolumic contraction velocity begins before the R - wave on electrocardiogram
All the measurements were obtained by a single observer who was blinded to the clinical details. To detect intraobserver variability, the same investigator repeated the echocardiographic measurements for pulsed-wave TDI-derived LV and RV Sa, IVV, IVA in 20 patients.
Statistical analyses
Statistical analyses were performed using the SPSS software version 17.0 for Windows (SPSS Inc., Chicago, Illinois, USA). The variables were investigated using visual (histograms, probability plots) and analytical methods (Kolmogorov-Smirnov/Shapiro-Wilk’s test) to determine the normal distribution. Descriptive analyses are presented using means and standard deviations or median and the interquartile range (IQR, range from the 25th to the 75th percentile). The categorical variables are expressed as numbers and percentages. A logarithmic transformation was applied to transform the numerical data (left ventricular IVA, right ventricular IVA, fasting plasma glucose, triglyceride and HDL-cholesterol) with a non-normal distribution into a normal distribution. Numerical variables were compared using the Student’s t-test, One-way ANOVA or Mann-Whitney U test. Tukey’s tests were performed to test the significance of pairwise differences by using Bonferroni correction to adjust for multiple comparisons. Categorical data were compared with the chi-square test. Pearson’s correlation coefficients were used to assess the relationship between continuous variables. A step-wise, multiple regression analysis was conducted to identify significant determinants of left ventricular and right ventricular IVA. The analysis included variables that showed a p value of less than 0.05 in Pearson’s correlation analysis. Intraobserver agreement was assessed with Pearson’s correlation coefficients, and a p value of less than 0.05 was considered significant.
Results
Basal characteristics
Demographic, clinic and laboratory parameters of patients and control groups were shown in Table 1. There were no significant difference between two groups in terms of age, gender, smoking, heart rate, serum creatinine and total and LDL cholesterol levels. As expected, those with MetS had significantly higher SBP, DBP, waist circumference, triglycerides and fasting plasma glucose level compared to controls (Table 1). Also, HDL cholesterol level was lower in MetS group than control group.
Table 1.
Demographic, clinic and laboratory parameters of patients and control groups
| MetS (n=75) | Control (n=58) | *P value | |
|---|---|---|---|
| Age, years | 47±10 | 44±8 | .081 |
| Sex, female, n, % | 33 (44) | 27 (46.6) | .769 |
| Smoking, n, % | 22 (29.3) | 17 (29.3) | .998 |
| Body mass index, kg/m2 | 30±4 | 27±4 | <.001 |
| Waist circumference, cm | 102±9 | 93±9 | <.001 |
| Diastolic blood pressure, mm Hg | 93±12 | 80±9 | <.001 |
| Systolic blood pressure, mm Hg | 149±19 | 129±13 | <.001 |
| Heart rate, beat/min | 78±13 | 78±13 | .852 |
| Fasting plasma glucose, mg/dL | 103±14 | 93±8 | <.001 |
| HDL-Cholesterol, mg/dL | 39±9 | 53±14 | <.001 |
| Triglyceride, mg/dL | 225±132 | 122±75 | <.001 |
| Total cholesterol, mg/dL | 215 (185-242) | 200 (187-224) | .232 |
| LDL-Cholesterol, mg/dL | 141 (117-170) | 117 (110-152) | .076 |
| Creatinine, mg/dL | 0.8 (0.7-0.9) | 0.7 (0.6-0.9) | .078 |
Value are presented as mean±SD, median (interquartile range) or number or percentage of patients.
Student’s t-test, Mann-Whitney U test, chi-square tests
HDL - high density lipoprotein; LDL - low density lipoprotein; MetS - metabolic syndrome
The numbers of risk factors for MetS were identified in patients respectively: three in 48% (n=36), four in 37.3% (n=28), and five in 14.7% (n=11) of patients. The presence of increased BP (clinical BP ≥130/85 mm Hg) was a common feature among the MetS patients (82.7%), followed by decreased HDL cholesterol (80%), increased levels of triglycerides (78.7%), increased waist circumference (68%), and high fasting plasma glucose (52%).
Left and right ventricular function
Although the LV mass index, interventricular septum (IVS) and posterior wall were higher in the patients with MetS, LV end-systolic and diastolic diameters and EF were similar between the two groups. RV function was completely preserved in patients with MetS in terms of TAPSE and RV FAC, and there was no significant difference in these parameters between the two groups. Also, there was no difference between two groups in terms of estimated PAP (Table 2).
Table 2.
Conventional and tissue Doppler imaging measurements of left and right ventricle
| MetS (n=75) | Control (n=58) | *P value | |
|---|---|---|---|
| Left ventricle | |||
| End-diastolic diameter, mm | 48.4±4.0 | 47.5±3.9 | .205 |
| End-systolic diameter, mm | 29.5±3.3 | 29.0±3.7 | .402 |
| Interventricular septum, mm | 12 (10-13) | 10 (8-11) | <.001 |
| Posterior wall, mm | 10 (9-11) | 9 (8-10) | .001 |
| Ejection fraction, % | 67 (64-70) | 68 (64-72) | .144 |
| Mass index, gr/m2 | 100.1±25.9 | 84.9±19.4 | <.001 |
| E’ velocity, cm/sec | 10.4±2.9 | 12.8±3.5 | <.001 |
| A’ velocity, cm/sec | 11.8±2.1 | 10.7±1.4 | <.001 |
| E’/A’ ratio | 0.8 (0.7-1.1) | 1.2 (0.9-1.4) | <.001 |
| Sa, cm/sec | 9.5 (8.0-10.5) | 10.0(8.5-11.0) | .145 |
| IVV, cm/sec | 7.0 (6.0-8.5) | 7.5 (6.5-9.5) | .135 |
| IVA, m/sec2 | 2.6±0.7 | 3.1±0.9 | .001 |
| MPI | 0.53±0.1 | 0.49±0.1 | .022 |
| Right ventricle | |||
| FAC, % | 64.1±8.2 | 64.8±7.8 | .684 |
| TAPSE, mm | 22.5±2.7 | 22.6±3.2 | .772 |
| Estimated PAP, mm Hg | 26.3±5.2 | 25.8±4.4 | .582 |
| Tricuspid annular E’ wave, cm/sec | 12.0±3.1 | 14.0±3.0 | <.001 |
| Tricuspid annular A’ wave, cm/sec | 16.0±3.4 | 14.3±3.9 | <.001 |
| E’/A’ ratio | 0.8±0.3 | 1.1±0.4 | <.001 |
| Sa, cm/sec | 14.0±2.9 | 14.3±3.0 | .587 |
| IVV, cm/sec | 13.9±3.5 | 14.0±3.0 | .490 |
| IVA, m/sec2 | 3.2±0.9 | 4.0±1.4 | <.001 |
| MPI | 0.44±0.1 | 0.39±0.1 | .011 |
E’, peak myocardial velocity during early diastole. A’ - Peak myocardial velocity during atrial contraction; FAC - fractional area change; IVA - myocardial acceleration during isovolumic contraction; IVV - peak myocardial velocity during isovolumic contraction; MetS - metabolic syndrome; MPI - myocardial performance index; Sa - peak myocardial velocity during systole; TAPSE - tricuspid annular plane systolic excursion; PAP - pulmonary artery pressure; Value are presented as mean±SD or median (interquartile range).
Student’s t-test, Mann-Whitney U test
In the comparison of right and left ventricular diastolic function, the tissue Doppler-derived parameters E’, A’, and E’/A’ ratio were significantly impaired in the MetS patients compared to the controls. Although Sa and IVV were similar between two groups, right and left ventricular systolic function IVA was significantly reduced in patients with MetS. The MPI of the systolic and diastolic function of both LV and RV was significantly higher in patients with MetS compared to controls (Table 2).
Left ventricular and right ventricular IVA in MetS subgroups
Both RV IVA and LV IVA were the same between in patients having increased or normal glucose level (p=0.483, p=0.283, respectively) and having normal or decreased HDL level (p=0.320, p=0.063, respectively). Also, RV IVA and LV IVA were found to be lower in increased TG group than normal group (p=0.001, p=0.033, respectively). While there was no difference between subgroups titled as normal and increased waist circumference in terms of RV IVA (p=0.620), LV IVA was found to be lower in increased waist circumference group (p=0.012). Moreover, there was no difference between hypertensive and normotansive groups regarding as LV IVA (p=0.459). However, RV IVA was significantly found to be lower in hypertensives than normotansives (p=0.012). Although the reverse relation between the number of risk factors of MetS and LV IVA was seen, this association was not significant between groups (all p values >0.05). Also, there was a significant difference between 2 and 3 criteria positive groups for MetS in terms of RV IVA (p=0.003) (Table 3).
Table 3.
Subgroup analysis of right and left ventricular IVA for each component of the metabolic syndrome
| Variables | Subgroups | RV IVA, m/sec2 | LV IVA, m/sec2 |
|---|---|---|---|
| Waist circumference, cm | Normal | 3.6±1.4 | 3.0±0.9 |
| Increased | 3.5±1.0 | 2.7±0.7 | |
| *p value | .620 | .012 | |
| Hypertension | Hypertensives | 3.3±1.0 | 2.8±0.8 |
| Non hypertensives | 4.1±1.5 | 2.9±0.9 | |
| *p value | <.001 | .459 | |
| Glucose, mg/dL | Increased | 3.4±1.1 | 2.7±0.7 |
| normal | 3.6±1.3 | 2.9±0.9 | |
| *p value | .483 | .283 | |
| Triglyceride, mg/dL | Normal | 3.9±1.4 | 3.0±0.9 |
| Increased | 3.2±1.0 | 2.7±0.8 | |
| *p value | .001 | .033 | |
| HDL, mg/dL | Normal | 3.7±1.3 | 2.9±0.8 |
| Decreased | 3.4±1.1 | 2.7±0.8 | |
| *p value | .320 | .063 | |
| Number of risk factors for MetS | 1 criteria positive | 3.9±1.1 | 3.1±0.9 |
| 2 criteria positive | 4.3±1.7# | 3.0±1.0 | |
| 3 criteria positive | 3.1±1.0 | 2.6±0.6 | |
| 4 criteria positive | 3.2±0.9 | 2.5±0.8 | |
| 5 criteria positive | 3.1±0.9 | 2.5±0.7 | |
| *p value | .001 | .023 |
Data are presented as mean±SD,
Student’s t-test, One-way ANOVA,
p=0.003 vs. 3 criteria positive
RV - right ventricle; LV - left ventricle; IVA - myocardial acceleration during isovolumic contraction; HDL - high density lipoprotein; MetS - metabolic syndrome
Correlation between isovolumic acceleration and metabolic syndrome parameters
The results of the correlation analysis were shown in Table 4. The IVA of the LV was significantly inversely correlated with the patient’s waist circumference, triglycerides levels, and number of risk factors. There was no correlation between the IVA of the LV and their fasting plasma glucose, HDL-cholesterol, DBP, and SBP. Although, the RV IVA was significantly inversely correlated with DBP, SBP, triglyceride levels, and numbers of risk factors, it was positively correlated with HDL cholesterol level. However, there was no correlation between RV IVA and fasting plasma glucose and waist circumference.
Table 4.
Correlation between right, left ventricular IVA and parameters of metabolic syndrome
| RV IVA | LV IVA | |||
|---|---|---|---|---|
| r | P | r | P | |
| Diastolic blood pressure | -.370 | <.001 | -.139 | .112 |
| Systolic blood pressure | -.255 | .003 | -.137 | .117 |
| Waist circumference | -164 | .059 | -.282 | .001 |
| Fasting plasma glucose | -.097 | .265 | -.050 | .566 |
| HDL | .240 | .005 | .142 | .103 |
| Triglyceride | -.289 | .001 | -.212 | .014 |
| Number of risk factors | -.280 | .001 | -.282 | .001 |
HDL - high density lipoprotein; IVA - myocardial acceleration during isovolumic contraction; LV - left ventricle; RV - right ventricle
Regression analysis
In univariate analysis, DBP, SBP, waist circumference, HDL cholesterol level, triglyceride levels and number of risk factors of MetS were found to be the parameters that were associated with RV IVA. Moreover, waist circumference, and number of risk factors of MetS were found to be the parameters that were associated with LV IVA. Although left ventricular mass index was considered to be a confounder in multiple regression analysis, waist circumference and DBP were found to be an independent determinant of IVA of LV (r2=104, ß=-.223, 95% CI=-.034 -.002, p=0.004) and RV (r2=197, ß=-.527, 95% CI=-.085 -.020, p=0.002) respectively (Table 5).
Table 5.
Regression analysis of right and left ventricular IVA for each component of the metabolic syndrome
| RV IVA | LV IVA | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| ß | P | ß | P | ß | P | ß | P | |
| Diastolic blood pressure | -.369 | <.001 | -.527 | .002 | -.141 | .106 | ||
| Systolic blood pressure | -.278 | .001 | .242 | .157 | -.144 | .097 | -.031 | .743 |
| Waist circumference | -.178 | .040 | -.084 | .381 | -.298 | .001 | -.223 | .024 |
| Fasting plasma glucose | -.117 | .181 | -.059 | .497 | ||||
| HDL | .233 | .007 | .127 | .206 | .161 | .064 | .017 | .864 |
| Triglyceride | -.239 | .006 | -.175 | .063 | -.112 | .201 | ||
| Number of risk factors | -.273 | .001 | -.027 | .816 | -.258 | .003 | -.133 | .231 |
HDL - high density lipoprotein; IVA - myocardial acceleration during isovolumic contraction; LV - left ventricle; RV - right ventricle
Reproducibility data
The intraobserver variability was low for TDI-derived velocities (LV Sa: r=0.93, p<0.001, LV IVV: r=0.92, p<0.001; LV IVA: r=0.94, p<0.001) (RV Sm: r=0.95, p<0.001; RV IVV: r=0.93, p<0.001; RV IVA: r=0.96, p<0.001).
Discussion
The main findings of the present study were as follows: 1) MetS affects global, diastolic, and systolic functions of two ventricles, 2) This disruption lead to decreased function of heart was related with raised risk factors of MetS, 3) In multiple regression analysis, waist circumference and DBP were found to be an independent determinant of IVA of LV and RV.
The effect of MetS on cardiac structure and function has been investigated previously in selected populations (3, 6). These studies evaluated the effects of MetS on cardiac functions (systolic, diastolic and global) both for RV and LV respectively. Structural and functional changes observed in the LV due to MetS would also be expected to be seen in RV. In this study, we found significant impairment in the function of both the RV and LV by using novel sensitive TDI indices.
The association of thicknesses of the walls of the LV with MetS was investigated, it was found significantly greater in patients with MetS compared to controls in our study. Yılmaz et al. (17) showed a significant increase in left ventricular wall thickness in MetS group similar to our results. Also, there are many clinical studies support this results are found in the literature (18-21). Left ventricular hypertrophy can be due to arterial hypertension or directly due to MetS. In the PAMELA trial, normotensive subjects with MetS also had an increased left ventricular mass index and an increased prevalence of left ventricular hypertrophy (21). Insulin resistance, which plays an important role in MetS, increases the accumulation of collagen in extracellular spaces, leading to thickening of the left ventricular wall (20).
The association of diastolic functions of the LV with MetS was investigated, we showed a significant decrease of E’ and E’/A’ with increase of A’ in MetS group as compared to controls. These findings were similar to those of previous studies (4, 6, 22, 23). MPI, which is detected by TDI, reflects both systolic and diastolic dysfunction. As some previous clinical studies, the MPI was significantly higher in the MetS groups than control subjects in our study (6, 22, 24, 25).
In our study, the left ventricular EF, Sa, and IVV measurements were not significantly different between two groups. Other studies also found no significant difference in terms of left ventricular systolic functions between MetS patients and controls based on the EF (5, 19, 21, 23). However, some investigators found reduced systolic functions as measured by fractional shortening (18, 26).
Using the TDI parameters Saseptal and Salateral, Ivanovic et al. (4) found no difference in left ventricular systolic functions between two groups. IVA is a reliable and load-independent measure of LV contraction (27). It can detect small changes in contractile function and shows a good correlation with invasive or noninvasive measures of LV dP/dt (7, 9). In our study, the left ventricular IVA was significantly reduced in the MetS patients as compared to controls. Crendal et al. (28) showed attenuated longitudinal strain and both diastolic and systolic strain rate while preserving left ventricular function measured by conventional methods. Also, they presented that an abdominal obesity lead to subclinic systolic dysfunction. In consistent with this study, we found a waist circumference was one of the independent determinant of left ventricular IVA. Tang et al. (29) reported that the increased risk of diastolic heart failure as higher the metabolic syndrome risk score. In contrast to this study, we showed that the number of risk factors of metabolic syndrome was associated with an increasingly compromised left ventricular function in our study. Subclinical systolic dysfunction can be related to lipotoxicity, increased cytokine activity and interstitial deposition of triacylglycerol, ventricular hypertrophy and fibrosis (16, 18-20, 29). The mechanisms underlying cardiac remodeling in MetS are multifactorial, but one of the pivotal contributors is thought to be myocardial fibrosis. Sciaretta et al. (30) demonstrated that cardiovascular damage is more frequent in hypertensive patients with MetS than in hypertensive’s without MetS and that hypertension is significantly related to increased levels of inflammation and fibrosis. Kosmala et al. (31) evaluated the effect of the aldosterone antagonist spironolactone added to standard angiotensin II inhibition and found increased myocardial abnormalities and decreased fibrotic markers in MetS patients.
In this study we investigated right ventricular diastolic and systolic function in MetS patients by using M-mode, two-dimensional, tissue Doppler imaging techniques, which are suggested to show different aspects of RV function. In accordance with previous studies, we demonstrated that TDI-derived right ventricular diastolic measurements and RV MPI are impaired in MetS patients (5, 6). Consistent with the literature, the RV TAPSE, FAC and TDI derived RV Sa, IVV indicating right ventricular global systolic functions were completely preserved in the MetS group (5, 6). On the other hand, decreased IVA of RV was shown in MetS patients in our study, it was associated with number of risk factors. In contrast to the findings of our study, Karakurt et al. (6) monstrated that not only RV Sa, but also TAPSE were worsened in patients with MetS compared with control subjects, as well as not significant associations between MetS components and echocardiographic parameters. The analysis of our results showed that DBP, SBP and triglyceride levels were inversely associated with the right ventricular IVA. The influence of MetS on the structure and function of the RV is not completely clarified. One of the possible reasons is that the increase in systemic vascular resistance in arterial hypertension leads to the increased vascular resistance in the pulmonary circulation, further causing the damage of the RV structure and function (32). However, the estimated PAP was not different between two groups in our study. Hypertension may cause IVS hypertrophy which has an essential role in systolic dysfunction of the RV (33). In our study, the patients with MetS showed thickening of the IVS in consistent with some other clinical studies (17, 34-37). Triglycerides may exert lipotoxic effects due to the accumulation of toxic lipid intermediaries or the generation of highly reactive oxygen species, thereby leading to dysfunction and/or apoptosis of cardiomyocytes (4). As the RV is relatively thinner than the LV, these effects may cause subclinical systolic dysfunction in the RV but not in the LV.
Study limitations
The main limitations of the study are 1) the absence of any comparison with the gold standard imaging modality, magnetic resonance imaging, in the evaluation of the functions of the RV and the LV and in the detection of fibrosis 2) Coronary artery disease was excluded based on history, electrocardiography, and echocardiography (wall motion abnormality), the lack of an evaluation of coronary arteries indirect and directly with exercise stress test and coronary angiography respectively.
Conclusion
In conclusion, MetS affects global, diastolic, and systolic functions of both the RV and LV. The number of risk factors of metabolic syndrome was related with increasingly compromised right and left ventricular functions. Further studies are needed to support the clinical utility of novel echocardiographic indices in detecting for subclinical systolic dysfunction in patients with MetS.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Author contributions: Concept - M.E., E.Ö.; Design - M.E.; Supervision - N.U.; Resource - M.E., A.K.K., E.Ö., Ö.A., S,Ö.; Materials - Ö.Ç.; Data collection&/or processing - M.E., A.K.K., H.D., S.Ö.; Analysis &/or interpretation - M.E., H.P.; Literature search - Ö.A., Ö.Ç., H.Ü.A.; Writing - İ.F.A., M.E., E.Ö.; Critical review - N.U., H.Ü.A., İ.F.A.
References
- 1.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–8. doi: 10.1016/S0140-6736(05)66378-7. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. American Heart Association;National Heart, Lung and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2. doi: 10.1161/CIRCULATIONAHA.105.169404. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 3.Chinali M, Devereux RB, Howard BV, Roman MJ, Bella JN, Liu JE, et al. Comparison of cardiac structure and function in American Indians with and without the metabolic syndrome (the Strong Heart Study) Am J Cardiol. 2004;93:40–4. doi: 10.1016/j.amjcard.2003.09.009. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 4.Ivanovic B, Tadic M, Simic D. Are all criteria of metabolic syndrome equally harmful? Acta Cardiol. 2011;66:189–96. doi: 10.1080/ac.66.2.2071250. [DOI] [PubMed] [Google Scholar]
- 5.Tadic M, Ivanovic B, Grozdic I. Metabolic syndrome impacts the right ventricle: true or false? Echocardiography. 2011;28:530–8. doi: 10.1111/j.1540-8175.2011.01390.x. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 6.Karakurt Ö, Öztekin S, Yazıhan N, Akdemir R. Impaired right ventricular functions in metabolic syndrome patients with preserved left ventricular ejection fraction. Arch Turk Soc Cardiol. 2011;39:549–56. doi: 10.5543/tkda.2011.01512. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 7.Vogel M, Cheung MM, Li J, Kristiansen SB, Scmidt MR, White PA, et al. Noninvasive assessment of left ventricular force-frequency relationships using tissue Doppler derived isovolumetric acceleration: validation in an animal model. Circulation. 2003;107:647–52. doi: 10.1161/01.CIR.0000058171.62847.90. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 8.Vogel M, Schmidt MR, Kristiansen SB, Cheung M, White PA, Sorensen K, et al. Validation of myocardial acceleration during isovolumic contraction as a novel noninvasive index of right ventricular contractility: comparison with ventricular pressure-volume relations in an animal model. Circulation. 2002;105:1693–9. doi: 10.1161/01.cir.0000013773.67850.ba. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 9.Toyono M, Harada K, Tamura M, Yamamoto F, Takada G. Myocardial acceleration during isovolumic contraction as a new index of right ventricular contractile function and it’s relation to pulmonary regurgitation in patients after repair of tetrology of Fallot. J Am Soc Echocardiogr. 2004;17:332–7. doi: 10.1016/j.echo.2003.12.022. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 10.Ertürk M, Aksu HU, Çelik O, Uzun F, Akgül O, Pusuroğlu H, et al. Evaluation of the effect of mitral stenosis severity on the left ventricular systolic function using isovolumic myocardial acceleration. Cardiol J. 2013 Nov 6; doi: 10.5603/CJ.a2013.0114. [Epub ahead of print] [CrossRef] [DOI] [PubMed] [Google Scholar]
- 11.Who Guideline Development Committee. Defination and diagnosis of diabetes mellitus and intermediate hyperglisemia. Geneve: Report of a WHO/IDF Consultation; 2006. [Google Scholar]
- 12.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 ESH-ESC Practice Guidelines for the Management of Arterial Hypertension: ESH-ESC Task Force on the Management of Arterial Hypertension. J Hypertens. 2007;25:1751–62. doi: 10.1097/HJH.0b013e3282f0580f. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 13.Lang RM, Biering M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: A report from the American society of echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European association of echocardiography, a branch of the European society of cardiology. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.echo.2005.10.005. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 14.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic asssessment of left ventricular hypertrophy: comparison to necropsy finding. Am J Cardiol. 1986;57:450–8. doi: 10.1016/0002-9149(86)90771-x. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 15.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 16.Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler Echocardiography: A report from the Doppler Quantification task force of the nomenclature and standards comittee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–84. doi: 10.1067/mje.2002.120202. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 17.Yılmaz M, Özlük OA, Akgümüş A, Peker T, Karaağaç K, Vatansever F, et al. Left atrial mechanical functions in patients with the metabolic syndrome. Acta Cardiol. 2013;68:133–7. doi: 10.1080/ac.68.2.2967269. [DOI] [PubMed] [Google Scholar]
- 18.Cuspidi C, Meani S, Fusi V, Severgnini B, Valerio C, Catini E, et al. Metabolic syndrome and target organ damage in untreated essential hypertensive. J Hypertens. 2004;22:1991–8. doi: 10.1097/00004872-200410000-00023. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 19.Ferrara LA, Cardoni O;Mancini M, Zanchetti A. Metabolic syndrome and left ventricular hypertrophy in a general population. Results from Gubbio Study. J Hum Hypertens. 2007;21:795–801. doi: 10.1038/sj.jhh.1002232. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 20.Sundström J, Arnlöv J, Stolare K, Linl L. Blood pressure-independent relations of left ventricular geometry to the metabolic syndrome and insulin resistance: a population-based study. Heart. 2008;94:874–8. doi: 10.1136/hrt.2007.121020. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 21.Mancia G, Bombelli M, Corrao G, Facchetti R, Madotto F, Giannattasio C, et al. Metabolic syndrome in the Pressioni Arteriose Monitorate E Loro Associazioni PAMELA study: Daily life blood pressure, cardiac damage, and prognosis. Hypertension. 2007;49:40–7. doi: 10.1161/01.HYP.0000251933.22091.24. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 22.de las Fuentes L, Brown A, Mathew S, Waggoner AD, Soto PF, Gropler RJ, et al. Metabolic syndome is associated with abnormal left, ventricular diastolic function independent of left ventricular mass. Eur Heart J. 2007;28:553–9. doi: 10.1093/eurheartj/ehl526. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 23.Ferrara L, Guida 1 L, Ferrara F, De Luca G, Staiano L, Celentano A, et al. Cardiac structure and function and arterial circulation in hypertensive patients with and without metabolic syndrome. J Hum Hypertens. 2007;21:729–35. doi: 10.1038/sj.jhh.1002222. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 24.Mahmud A, Almuntaser I, Brown A, King G, Crean P, Feely J. Left ventricular structural and functional changes in the metabolic syndrome. J Cardiometab Syndr. 2009;4:81–8. doi: 10.1111/j.1559-4572.2008.00043.x. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 25.Turhan H, Yaşar AS, Yağmur J, Kurtoğlu E, Yetkin E. The impact of metabolic syndrome on left ventricular function: evaluated by using the index of myocardial performance. Int J Cardiol. 2009;132:382–6. doi: 10.1016/j.ijcard.2007.12.007. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 26.Schillaci G, Pirro M, Pucci G, Mannarino MR, Gemelli F, Siepi D, et al. Different impact of the metabolic syndrome on left ventricular structure and function in hypertensive men and women. Hypertension. 2006;47:881–6. doi: 10.1161/01.HYP.0000216778.83626.39. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 27.Dalsgaard M, Synder EM, Kjaergaard J, Johnson BD, Hassager C, Oh JK. Isovolumic acceleration measured by tissue Doppler echocardiography is preload independent in healthy subjects. Echocardiography. 2007;24:572–9. doi: 10.1111/j.1540-8175.2007.00454.x. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 28.Crendal E, Walther G, Vinet A, Dutheil F, Naughton G, Lesourd B, et al. Myocardial deformation and twist mechanics in adults with metabolic syndrome: Impact of cumulative metabolic burden. Obesity (Silver Spring) 2013;21:679–86. doi: 10.1002/oby.20537. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 29.Tang Z, Zeng F, Li Z, Si Y, Zhou L. The association and predictive value analysis of metabolic syndrome on diastolic heart failure in patients at high risk for coronary artery disease. Diabetol Metab Syndr. 2013;5:30. doi: 10.1186/1758-5996-5-30. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sciarretta S, Ferrucci A, Ciavarella GM, De Paolis P, Venturelli V, Tocci G, et al. Markers of inflammation and fibrosis are related to cardiovascular damage in hypertensive patients with metabolic syndrome. Am J Hypertens. 2007;20:784–91. doi: 10.1016/j.amjhyper.2007.01.023. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 31.Kosmala W, Przewlocka-Kosmala M, Szczepanik-Osadnik H, Mysiak A, O’Moore-Sullivan T, Marwick TH. A randomized study of the beneficial effects of aldosterone antagonism on LV function, structure, and fibrosis markers in metabolic syndrome. JACC Cardiovasc Imag. 2011;4:1239–49. doi: 10.1016/j.jcmg.2011.08.014. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 32.Olivari MT, Fiorentini C, Polese A, Guazzi MD. Pulmonary hemodynamic and right ventricular function in hypertension. Circulation. 1978;58:1185–90. doi: 10.1161/01.cir.57.6.1185. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 33.Buckberg GD, RESTORE Group The ventricular septum: the lion of right ventricular function, and its impact on right ventricular restoration. Eur J Cardiothorac Surg. 2006;29:272–8. doi: 10.1016/j.ejcts.2006.02.011. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 34.Cicala S, Galderrisi M, Caso P, Petrocelli A, D’Errico A, de Divitiis O, et al. Right ventricular diastolic dysfunction in arterial systemic hypertension: Analysis by pulsed tissue Doppler. Eur J Echocardiogr. 2002;3:135–42. doi: 10.1053/euje.2001.0124. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 35.Cuspidi C, Vlerio C, Sala C, Negri F, Esposito A, Masaidi M, et al. Metabolic syndrome and biventricular hypertrophy in essential hypertension. J Hum Hypertens. 2009;23:168–75. doi: 10.1038/jhh.2008.119. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 36.Cuspidi C, Negri F, Giudici V, Valerio C, Meani S, Sala C, et al. Prevalence and clinical correlates of right ventricular hypertrophy in essential hypertension. J Hypertens. 2009;27:854–60. doi: 10.1097/HJH.0b013e328324eda0. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 37.Pedrinelli R, Canale ML, Giannini C, Talini E, Penno G, Dell’Omo G, et al. Right ventricular dysfunction in early systemic hypertension: A tissue Doppler imaging study in patients with high-normal and mildly increased arterial blood pressure. J Hypertens. 2010;28:615–21. doi: 10.1097/hjh.0b013e328334f181. [CrossRef] [DOI] [PubMed] [Google Scholar]


