Abstract
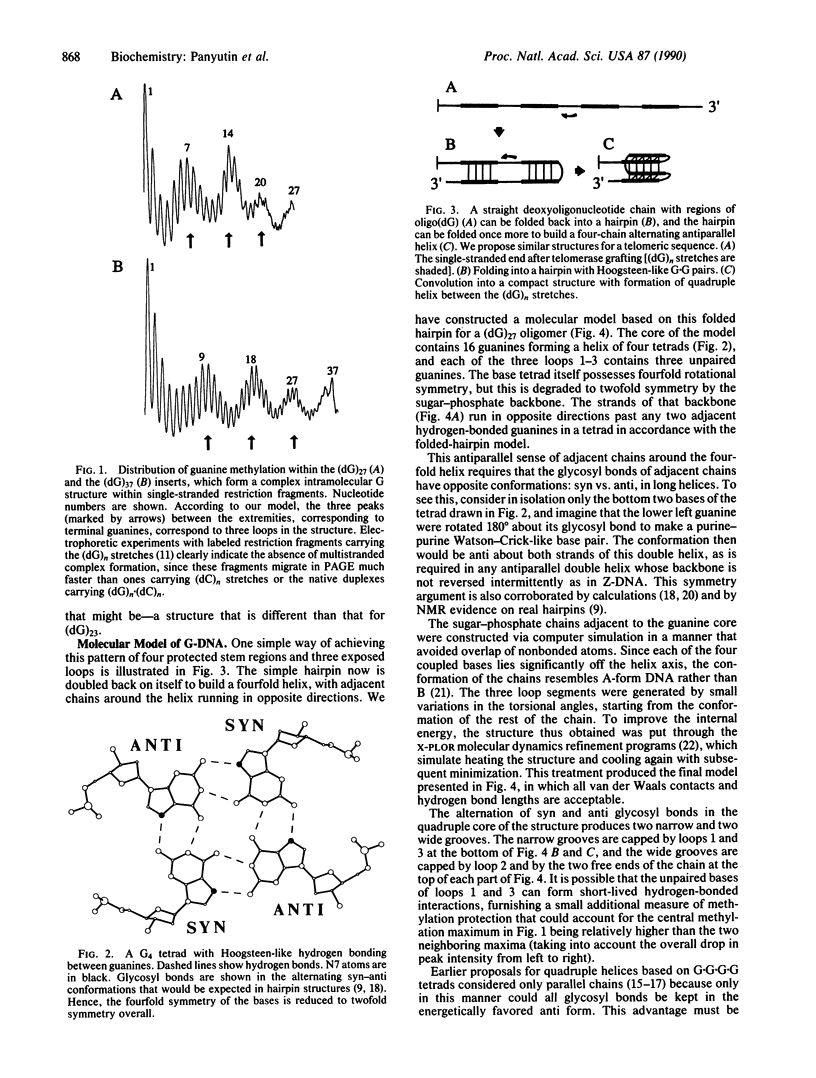
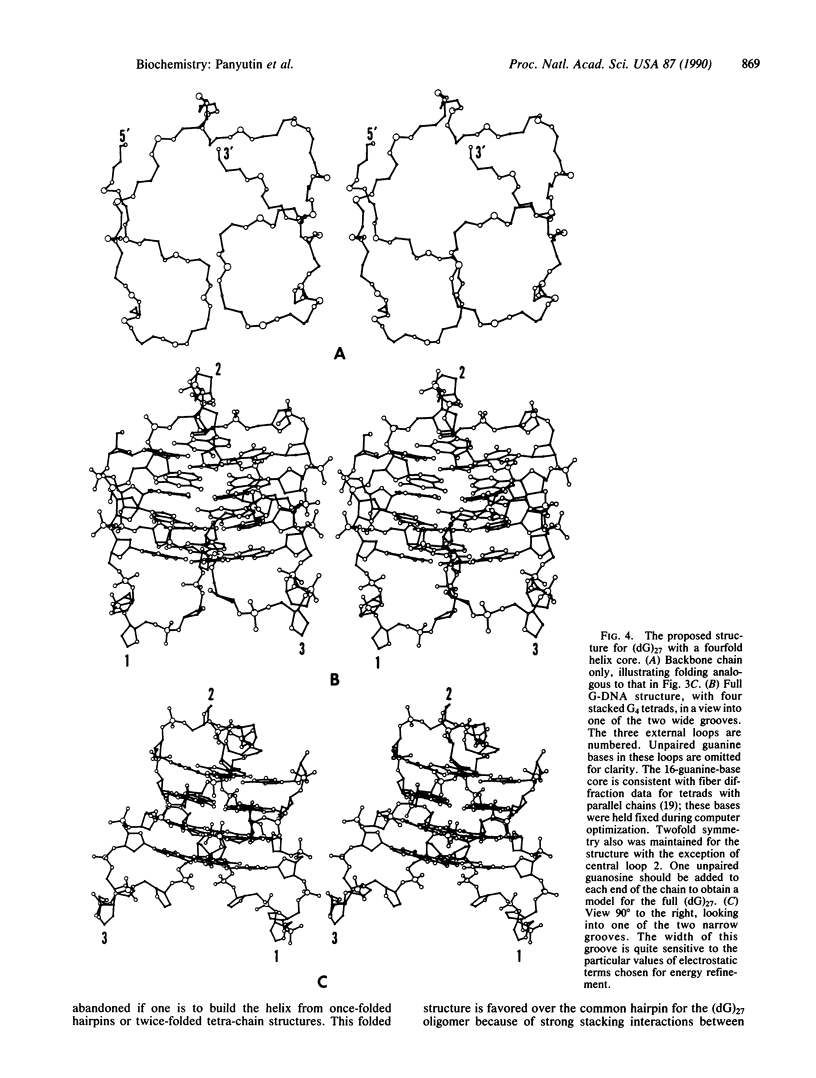
Our dimethyl sulfate modification experiments suggest that (dG)n stretches within single-stranded DNA fragments, which represent the simplest model for telomeric sequences, adopt a complex intrastrand structure other than a simple hairpin. We present a molecular model for the DNA structure that conforms to dimethyl sulfate methylation data. The principal element of this G-DNA structure is a quadruple helix formed by pairwise antiparallel segments of the twice-folded (dG)n stretch. This quadruple core has two wide and two narrow grooves connected by three loop-shaped segments. The strong stacking interactions of the neighboring guanine tetrads and the large number of hydrogen bonds formed can be the primary reasons that such structures are favored over a common hairpin for long (dG)n stretches. Such compact structures may be formed from (dG)n stretches of telomeric sequences.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Chandrasekaran R., Marttila C. M. Structures for polyinosinic acid and polyguanylic acid. Biochem J. 1974 Aug;141(2):537–543. doi: 10.1042/bj1410537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. H. The molecular structure of centromeres and telomeres. Annu Rev Biochem. 1984;53:163–194. doi: 10.1146/annurev.bi.53.070184.001115. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Cech T. R. G-strings at chromosome ends. Nature. 1988 Apr 28;332(6167):777–778. doi: 10.1038/332777a0. [DOI] [PubMed] [Google Scholar]
- Chou C. H., Thomas G. J., Jr, Arnott S., Smith P. J. Raman spectral studies of nucleic acids. XVII. Conformational structures of polyinosinic acid. Nucleic Acids Res. 1977 Jul;4(7):2407–2419. doi: 10.1093/nar/4.7.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuprina V. P., Poltev V. I. Alteration of the DNA double helix conformation upon incorporation of mispairs as revealed by energy computations and pathways of point mutations. Nucleic Acids Res. 1985 Jan 11;13(1):141–154. doi: 10.1093/nar/13.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner B. N., Yoon C., Dickerson J. L., Dickerson R. E. Helix geometry and hydration in an A-DNA tetramer: IC-C-G-G. J Mol Biol. 1984 Apr 25;174(4):663–695. doi: 10.1016/0022-2836(84)90089-5. [DOI] [PubMed] [Google Scholar]
- DONOHUE J., TRUEBLOOD K. N. Base pairing in DNA. J Mol Biol. 1960 Dec;2:363–371. doi: 10.1016/s0022-2836(60)80047-2. [DOI] [PubMed] [Google Scholar]
- Fowler R. F., Bonnewell V., Spann M. S., Skinner D. M. Sequences of three closely related variants of a complex satellite DNA diverge at specific domains. J Biol Chem. 1985 Jul 25;260(15):8964–8972. [PubMed] [Google Scholar]
- Frank-Kamenetskii M. D., Vologodskii A. V. Thermodynamics of the B-Z transition in superhelical DNA. Nature. 1984 Feb 2;307(5950):481–482. doi: 10.1038/307481a0. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989 Jan 26;337(6205):331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987 Dec 24;51(6):887–898. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- Henderson E., Hardin C. C., Walk S. K., Tinoco I., Jr, Blackburn E. H. Telomeric DNA oligonucleotides form novel intramolecular structures containing guanine-guanine base pairs. Cell. 1987 Dec 24;51(6):899–908. doi: 10.1016/0092-8674(87)90577-0. [DOI] [PubMed] [Google Scholar]
- Lyamichev V. I., Mirkin S. M., Frank-Kamenetskii M. D. Structures of homopurine-homopyrimidine tract in superhelical DNA. J Biomol Struct Dyn. 1986 Feb;3(4):667–669. doi: 10.1080/07391102.1986.10508454. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Moyzis R. K., Buckingham J. M., Cram L. S., Dani M., Deaven L. L., Jones M. D., Meyne J., Ratliff R. L., Wu J. R. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyutin I. G., Kovalsky O. I., Budowsky E. I. Magnesium-dependent supercoiling-induced transition in (dG)n.(dC)n stretches and formation of a new G-structure by (dG)n strand. Nucleic Acids Res. 1989 Oct 25;17(20):8257–8271. doi: 10.1093/nar/17.20.8257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L. J., Wang J. C. Energetics of B-to-Z transition in DNA. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6206–6210. doi: 10.1073/pnas.80.20.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Sen D., Gilbert W. Formation of parallel four-stranded complexes by guanine-rich motifs in DNA and its implications for meiosis. Nature. 1988 Jul 28;334(6180):364–366. doi: 10.1038/334364a0. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Collier D. A., Hanvey J. C., Shimizu M., Wohlrab F. The chemistry and biology of unusual DNA structures adopted by oligopurine.oligopyrimidine sequences. FASEB J. 1988 Nov;2(14):2939–2949. [PubMed] [Google Scholar]
- Wells R. D. Unusual DNA structures. J Biol Chem. 1988 Jan 25;263(3):1095–1098. [PubMed] [Google Scholar]
- Zimmerman S. B., Cohen G. H., Davies D. R. X-ray fiber diffraction and model-building study of polyguanylic acid and polyinosinic acid. J Mol Biol. 1975 Feb 25;92(2):181–192. doi: 10.1016/0022-2836(75)90222-3. [DOI] [PubMed] [Google Scholar]



