Abstract
Acute heart failure (AHF) is a life threatening clinical syndrome with a progressively increasing incidence in general population. Turkey is a country with a high cardiovascular mortality and recent national statistics show that the population structure has turned to an ‘aged’ population. As a consequence, AHF has become one of the main reasons of admission to cardiology clinics. This consensus report summarizes clinical and prognostic classification of AHF, its worldwide and national epidemiology, diagnostic work-up, principles of approach in emergency department, intensive care unit and ward, treatment in different clinical scenarios and approach in special conditions and how to plan hospital discharge.
Keywords: acute heart failure, diagnosis, management
1. Introduction
Acute heart failure (AHF) is defined as a life threatening clinical syndrome with rapidly developing or worsening typical heart failure (HF) symptoms and signs requiring emergent treatment. Number of patients referring to emergency departments with AHF rise parallel to the increase of elderly individuals in population, in accordance with the increase of patients with asymptomatic left ventricular dysfunction and HF. Long and frequent hospitalizations, intensive medical treatment and expensive interventional methods for reducing the mortality bring considerably high costs in the treatment of AHF.
Turkey is a country with a high cardiovascular mortality rate- and recent national statistics show that the population structure has turned to an ‘aged’ population (1). As a consequence, AHF has become one of the main reasons of admission to cardiology clinics. Management of AHF in Turkey generally follows two international guidelines, either ESC Acute and Chronic Heart Failure Guidelines or ACCF/AHA Heart Failure Management Guidelines (2, 3). Novel specific AHF guidelines, like NICE (4) and the consensus paper of the Heart Failure Association of the ESC, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine (5) do also take attention of cardiologists. However, Turkish AHF patients show some epidemiological differences than European or American AHF patients and some pharmacological (e.g. toracemide, amrinone, nesiritide, etc.) and non-pharmacological treatments (e.g. left ventricular assist devices except in cardiac transplantation centers) are not available in the country. Therefore, a consensus report on the diagnosis and treatment of AHF highlighting easily accessible approaches seemed to be beneficial for clinical practice.
There are several national articles covering different clinical manifestations and their appropriate treatment approaches in AHF (6). However, number of randomized controlled clinical studies on AHF has increased over the recent years leading to new evidences and changes in recommendations on various topics. Therefore, an update was inevitable.
This consensus report on the Diagnosis and Treatment of AHF was developed by acknowledging these factors and focused specifically on the management of AHF in emergency departments and hospitals. It summarizes (a) clinical and prognostic classification of AHF on admission, (b) its epidemiology and prognosis, (c) initial diagnostic work-up, (d) principles of approach in emergency department, intensive care unit and ward, (e) treatment in different clinical scenarios and approach to special conditions and (f) how to plan hospital discharge. Two valuable authors (Dr. Kumudha Ramasubbu and Dr. Biykem Bozkurt) contributed to the report by drawing up arrangements during discharge.
The report does not aim to replace international guidelines or classical textbooks. Hence, classifications like ‘class of recommendation’ or ‘level of evidence’ were avoided. Treatment algorithms in the report were formed by the consensus of contributing authors.
Topics were elaborated in accordance with current guidelines and reflect the latest data. Treatment approaches which are not available in Turkey were briefly mentioned if there is adequate information about them. Nevertheless, it is inevitable to update management strategies within the next years following the termination of ongoing/future randomized controlled trials.
2. Classifications of acute heart failure
Despite having various clinical manifestations, AHF mostly presents with difficulty in breathing and/or signs of congestion. Thus, it can also be called a syndrome. AHF is classified into two groups according to the presence/absence of previous HF:
Worsening (decompensated) HF - Preexisting and stable HF that worsens suddenly or progressively is described as decompensated AHF.
New (de novo) HF - There is no known previous HF. Symptoms and findings appear suddenly after an acute event [e.g. acute myocardial infarction (AMI)] or gradually in the presence of asymptomatic left ventricular systolic and/or diastolic dysfunction.
Former ESC guidelines on Heart Failure (7) had classified patients into 6 categories on the basis of clinical presentation:
1-Acute decompensated congestive HF is the exacerbation of chronic HF characterized by gradual onset peripheral edema (often significant) and dyspnea (usually).
2-AHF with hypertension is defined as very rapid (often) onset of high systolic blood pressure (SBP) associated with pulmonary congestion and tachycardia due to sympathetic tonus increase, preserved left ventricular ejection fraction (LVEF), and relatively low mortality.
3-AHF with pulmonary edema is characterized with rapid or gradual onset of severe respiratory distress, diffuse rales in lungs with tachypnea and orthopnea and an arterial oxygen saturation (SaO2) <90%.
4-Cardiogenic shock is a highly fatal clinical syndrome with gradual or rapid onset organ/tissue hypoperfusion, oliguria/anuria associated with a SBP <90 mm Hg, cardiac index <2 L/min/m2 (or <1.8 L/min/m2 in severe cardiogenic shock), urine output <0.5 mL/kg/h.
5-AHF complicating acute coronary syndrome (ACS) is characterized by increase of left ventricular (LV) diastolic filling pressure and/or decrease of cardiac output due to myocardial ischemia or infarction.
6-Isolated right sided AHF is a clinical picture with rapid or gradual onset edema, jugular venous distention and hepatomegaly, often with clear lungs associated with hypotension, low LV filling pressure, and low cardiac output.
Different clinical manifestations of AHF can also be dealt in 5 clinical scenarios according to hemodynamic characteristics on admission as hypertensive, normotensive, hypotensive (with or without shock), developed on the course of ACS and acute right HF (Table 1). Each clinical scenario requires a specific treatment approach which is addressed in the following sections.
Table 1.
Demographical and clinical characteristics of 5 clinical scenarios of acute heart failure*
| Clinical scenario | Demographical characteristics | Clinical characteristics | Clinical presentation |
|---|---|---|---|
| CS-1 | Hypertensive AHF | Advanced age, women, DM, LVH, obesity, HT | Pulmonary edema is predominant |
| CS-2 | Normotensive progressive AHF | Other findings of dyspnea and/or congestion | Systemic edema is predominant |
| CS-3 | Hypotensive progressive AHF | Hypoperfusion and/or cardiogenic shock | Minimal systemic and pulmonary edema |
| CS-4 | Acute coronary syndrome | Symptoms and findings of ACS (high troponin alone is not enough) | |
| CS-5 | Acute right HF | Right ventricular dysfunction and systemic venous congestion findings No pulmonary edema |
AHF - acute heart failure; BP - blood pressure; CS - clinical scenario; DM - diabetes mellitus; HT - hypertension; LVH - left ventricular hypertrophy.
Adapted from reference 6
3. Epidemiology
Hospital admissions due to AHF have risen in recent years parallel to the increase of HF incidence and prevalence. This increase-probably a result of improved management of AMI- and chronic HF-mainly occurred between years 1980-2000 and hospital admission rate remained relatively at the same level between years 2000-2010 (8).
More than 80% of hospitalized AHF cases are over age 65 years (9). Also, HF is reported as the cause of hospitalization in 20% of cases older than 65 years (10). In wide-scale registry studies, 37-52% of cases admitted to hospital were female. Percentage of male patients was higher among younger patients but female patients predominated in advanced ages (11-15).
Most of the patients hospitalized due to AHF have decompensated HF and ratio of de novo HF is reported between 23-44% in different registry studies (11, 12). Underlying reason is ACS in approximately half of the latter cases. About 30-50% of the AHF patients have preserved LVEF Hypertension, left ventricular hypertrophy and diabetes are more likely to exist in AHF with preserved EF (HFpEF) compared to patients with reduced LVEF.
In-hospital mortality rate of AHF is similar to AMI and ranges between 4-7% in registry studies (12, 16, 17). Not only mortality, but also lengths of hospital stay and re-admission rates are higher in AHF. Average hospital stay was 9 days in EuroHeart Failure Survey II (EHFS II) (12). Nearly half of the cases were followed in intensive care unit (ICU) and mean follow-up period was 3 days. Duration of hospital stay was extended in cases who needed vasoactive medication and increased up to ~13 days (10-19 days) in patients with cardiogenic shock. Re-hospitalization rates in 30 days and in 6 months after hospital discharge were 20% and 50%, respectively (18, 19). Mortality is higher at second and third hospitalizations (20).
3.1. Prognostic classification
Three classifications are frequently used in prognostic evaluation of AHF patients. Two of them are developed for AHF patients presenting during an ACS [Killip (21) and Forrester (22)] and the third (Nohria-Stevenson) (23) is used for patients with cardiomyopathy. Therefore, first two can be used for new onset AHF and the third for worsening HFF Killip classification (Table 2) is based on clinical findings, whereas Forrester classification (Table 3) is formed on invasive hemodynamic findings. Mortality increases in accordance with the class in both classifications. Nohria-Stevenson classification-proposed for decompensated AHF-is a clinical classification made by evaluation of perfusion (cold-hot) and congestion (wet-dry) (see Figure 1 for further details). In this classification, short-term mortality is relatively low in A (hot and dry) and B (hot and wet) groups and higher in L (cold and dry) and C (cold and wet) groups (they respectively carry a 2-2.5 times higher risk according to group A) (23).
Table 2.
Killip classification (21)
| Class | Physical examination findings |
|---|---|
| I | No S3 and rales |
| II | Rales exists in less than half of the lungs |
| III | Rales exists in more than half of the lungs |
| IV | Cardiogenic shock |
Table 3.
Forrester classification (22)
| Class | Findings | PCWP (mm Hg) | CI (L/min/m2) |
|---|---|---|---|
| I | Normal | <18 | >2.2 |
| II | Pulmonary congestion | >18 | >2.2 |
| III | Low output | <18 | <2.2 |
| IV | Low output and pulmonary congestion (cardiogenic shock) | >18 | <2.2 |
CI - cardiac index; PCWP - pulmonary capillary wedge pressure
Figure 1.
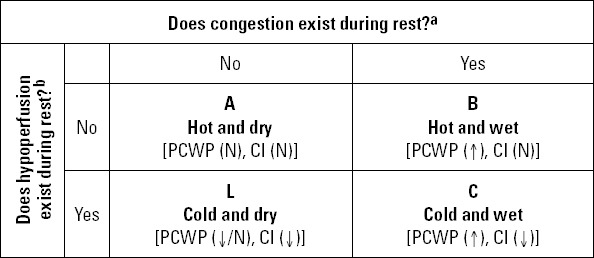
Nohria-Stevenson classification* (23)
aOrthopnea, paroxysmal nocturnal dyspnea, pulmonary rales, S3 gallop, increase in systolic pulmonary arterial pressure, increase in jugular venous pressure, hepatojugular reflux, hepatomegaly, edema, ascites
bNarrow pulse pressure, cold extremities, mental change, sleepiness, Cheyne-Stokes respiration, hypotension, renal dysfunction, decrease in diuresis, hyponatremia, acidosis. CI - cardiac index; PCWP - pulmonary capillary wedge pressure
*Adapted from reference 23
3.2. Acute heart failure in Turkey
HAPPY study (24) investigated epidemiology of HF in Turkey and estimated the prevalence HF and asymptomatic LV dysfunction in adults older than 35 years as 6.9% and 7.9%, respectively. These ratios were relatively higher than the prevalence in American (25) and European (26) countries, which have an older population compared to our country.
“Turkey Acute Heart Failure Diagnosis and Treatment Survey - TAKTIK Study” was conducted by the Working Group on Heart Failure of the Turkish Society of Cardiology to obtain data on AHF between 2007-2010 (27). Responses to questionnaires were collected via internet from 36 sites participating to the study and findings of 558 patients were compared with the European and American data in Table 4. TAKTIK study showed that AHF patients in our country were ~10 years younger than American and European patients and main etiology of HF was coronary artery disease (CAD). Most frequent factors accompanying/triggering decompensation were heart valve diseases (46%) and noncompliance to treatment (34%). The relatively lower frequencies of hypertensive AHF and AHF with preserved EF was remarkable. Low rate of optimal medical therapy was also a problem for our country as in other countries. Interestingly, ratio of evidence-based treatment did not increase significantly even after hospital discharge. Usage of ACE-I increased from 50% on admission to 54% at discharge, beta-blockers increased from 46% to 57%, and aldosterone antagonists from 40% to 52%. The only agent that was prescribed more than on admission was digoxin with an increase from 4% to 33%. OPTIMIZE-HF study (28), showed that discontinuation of beta-blockers in patients who have been using these drugs before hospitalization was associated with a higher mortality. In TAKTIK study, beta-blockers were discontinued in 13% of the patients, never started in 30%, continued in 33% and initiated in 24% of the patients. The corresponding ratios in OPTIMIZE-HF study were 3%, 13%, 57% and 27% respectively. All these findings suggest, that the ratio of patients at high risk is higher in our country (43% vs. 16%) compared to OPTIMIZE-HF population.
Table 4.
Data of patients on hospital admissions at TAKTIK and other registry studies
| TAKTIK27 | EHFS-II12 | ADHERE11 | OPTIMIZE-HF28 | |
|---|---|---|---|---|
| (n=558) | (n=3.580) | (n=105.388) | (n=48.612) | |
| Mean age (years) | 62±13 | 70±13 | 72±14 | 73±14 |
| Female (%) | 38 | 39 | 52 | 52 |
| New onset HF (%) | 24 | 37 | 23 | 12 |
| CAD (%) | 61 | 54 | 57 | 50 |
| Hypertension (%) | 53 | 63 | 73 | 71 |
| Diabetes (%) | 40 | 33 | 44 | 42 |
| Atrial fibrillation (%) | 32 | 39 | 31 | 31 |
| COPD(%) | 20 | 19 | 31 | 28 |
| CRF (%) | 16 | 17 | 30 | 20 |
| SBP (mm Hg) | 125±28 | - | 144±33 | 143+33 |
| SBP < 90 mm Hg (%) | 3 | 2 | 1 | 8 |
| SBP 90-140 mm Hg (%) | 78 | 48 | 70 | 44 |
| SBP >140 mm Hg (%) | 19 | 50 | 29 | 48 |
| Peripheral edema (%) | 65 | 23 | 66 | 85 |
| Cold extremities (%) | 34 | - | - | - |
| ACS (%) | 29 | 30 | - | 15 |
| Arrhythmias (%) | 30 | 32 | - | 14 |
| Valvular disease (%) | 46 | 27 | - | - |
| Infection (%) | 22 | 18 | - | 15 |
| NC to treatment (%) | 34 | 22 | - | 9 |
| Hemoglobin (g/dL) | 12.4±2.1 | - | 12.4±2.7 | 12.1+3.4 |
| Creatinine (mg/dL) | 1.4±0.9 | - | 1.8+1.6 | 1.8+1.8 |
| Troponin I (mg/dL) | 2.2±9 | - | - | 0.1 (median) |
| Left ventricular EF (%) | 33±13 | 38±15 | 34±16 | 39±18 |
| EF >%40 (%) | 20 | 34 (>%45) | 37 | 51 |
| Diuretic (%) | 62 | 71 | 41 | 66 |
| ACE-I (%) | 50 | 55 | 70 | 40 |
| Beta-blocker (%) | 46 | 43 | 48 | 53 |
| ARB (%) | 10 | 9 | 12 | 12 |
| MRA (%) | 40 | 28 | 9 | 7 |
| Digoxin (%) | 4 | 26 | 28 | 23 |
| In hospital mortality (%) | 3.4 | 6.7 | 4 | 3.8 |
ACE-I - angiotensin converting enzyme inhibitor; ADHERE - Acute Decompensated Heart Failure National Registry; ACS - acute coronary syndrome; ARB - angiotensin receptor blocker; EF - ejection fraction; EHFS-II - EuroHeart Failure Survey II; CAD - coronary artery disease; CRF - chronic renal failure; COPD - chronic obstructive pulmonary disease; HF - heart failure; MRA - mineralocorticoid receptor antagonist; NC - non-compliance; OPTIMIZE-HF - Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure; SBP - systolic blood pressure; TAKTİK; Turkey Acute Heart Failure Diagnosis and Treatment Survey
4. Clinical evaluation
4.1. Causes and precipitating factors of acute heart failure
The causes converting stable chronic HF to decompensated HF are called precipitating factors which can be divided into two groups as cardiac and non-cardiac (Table 5) (29). These factors are also observed as reasons leading to acute failure in de novo HF. Nevertheless, cause of decompensation cannot be exactly determined in one fourth of decompensated AHF patients.
Table 5.
Precipitating causes of acute decompensated or de novo heart failure
| Cardiac | Non-cardiac |
|---|---|
| Treatment non-compliance | Endocrinological diseases |
| 1. Sodium and fluid intake | Diabetes, thyrotoxicosis, hypothyroidism, etc. |
| 2. Non-compliance with drug treatment | |
| Pulmonary diseases | |
| Ischemic heart disease | Pulmonary emboli, asthma, COPD |
| 1. Acute coronary syndrome | |
| 2. Mechanical complications of AMI | Infections |
| Pneumonia, influenza, sepsis, etc. | |
| 3. Right ventricular MI | |
| Valvular heart disease |
Cases increasing blood volume |
| 1. Valvular stenosis | |
| 2. Valvular regurgitation | Anemia, shunts, beriberi, Paget disease |
| 3. Endocarditis | |
| 4. Aortic dissection |
Renal failure Drugs and addictions |
| Cardiomyopathies | |
| 1. Peripartum CMP | Drugs leading to sodium retention (e.g. steroids, tiazolidinediones, NSAI's), excessive alcohol or illegal drug addiction |
| 2. Acute myocarditis | |
| 3. Pericardial tamponade | |
| Hypertensive/arrhythmic | |
| 1. Hypertension | |
| 2. Acute arrhythmias (e.g. AF, tachyarrhythmias, serious bradycardia, etc.) |
Others
Cerebrovascular event, surgical intervention |
| Concomitant usage of negative inotropic drugs | |
| Verapamil, beta-blockers, diltiazem, nifedipine, etc. |
AF - atrial fibrillation; AMI - acute myocardial infarction; CMP - cardiomyopathy; COPD - chronic obstructive pulmonary disease; MI - myocardial infarction; NSAI - non-steroidal anti-inflammatory drugs
Main cardiac causes of decompensation are uncontrolled hypertension (10.7%), non-compliance to dietary (5.5%), and/or pharmaceutical recommendations (8.9%), pericardial tamponade, aortic dissection, arrhythmias (13.5%), ischemia and ACS (14.7%). Get With The Guidelines-Heart Failure Survey (GWTG-HF) examined the features of nonadherent patients to reduce rehospitalization for this population (30). Results of the study revealed that nonadherent patients had reduced EF, higher BNP levels and greater signs of congestion. Despite their higher risk profile, they had lower in-hospital mortality suggesting more stringent sodium and fluid restriction might be helpful for these patients.
Arrhythmias are one of the most common precipitating factors for acute HFF Among the arrhythmias, atrial fibrillation (AF) is the most common arrhythmia in patients presenting with acute decompensated HF. AF may lead to worsening of symptoms and even hemodynamic deterioration. Almost 40% of patients admitted to the hospital with the diagnosis of acute HF have AF. It also increases risk of thromboembolic complications (particularly stroke) and is associated with increased mortality. Therefore, ventricular rate control or rhythm control in presence of hemodynamic deterioration is very important. Also, anticoagulation should be given for the prevention of thromboembolic complications.
Leading non-cardiac causes are pulmonary diseases (15.3%), infections, worsening renal function (6.8%), anemia, endocrinological diseases and drug side effects, particularly nonsteroidal anti-inflammatory drugs.
Among the above mentioned factors, ACS is the major cause for de novo HF (42%), whereas valvular diseases, infections and treatment non-compliance more frequently lead to decompensated AHF. In patients with preserved LVEF, main causes of hospitalization are hypertension and non-cardiac factors (31).
Specialized HF clinics-currently few in numbers in Turkey-, raising patient awareness and post-discharge care at home may decrease rate of hospitalization. Main preventive measures for re-hospitalization are optimization of medical treatment, revascularization, device treatment and prophylactic influenza vaccination.
4.2. Symptoms and clinical findings
Clinical presentation in different clinical scenarios has been explained elsewhere in the text (See Section 2 and 6.1). Patients with AHF syndromes present with signs and symptoms of systemic and/or pulmonary congestion. Pulmonary congestion is associated with pulmonary venous hypertension often resulting in pulmonary interstitial and alveolar edema. Main clinical signs of pulmonary congestion include dyspnea, orthopnea, rales and a third heart sound. Systemic congestion manifests clinically by jugular venous distention with or without peripheral edema. Gradual increases in body weight are often observed. Elevated LV filling pressures (hemodynamic congestion) may be present days or weeks before the development of systemic and pulmonary congestion, which necessitate the hospital admission. This “hemodynamic congestion,” with or without clinical congestion, may have deleterious effects including ischemia and LV enlargement resulting in secondary mitral regurgitation.
4.3. Diagnostic methods
4.3.1 Electrocardiogram
12-lead ECG should be performed at initial evaluation in all AHF patients and cardiac rhythm should be monitored. ECG is almost always abnormal in patients admitted with AHF (32). It may provide information about the etiology (ischemia, infarction etc.) or precipitating factors of AHF if they exist (e.g. arrhythmia) and suitable treatment can be planned. Abnormalities like QRS prolongation or junctional rhythm in the ECG obtained on admission have also prognostic importance and are associated with higher in-hospital and follow-up mortality (33).
4.3.2 Chest X-ray
Chest X-ray is one of the routine diagnostic methods in patients hospitalized with suspected AHFF Cardiac enlargement and pulmonary congestion (vascular redistribution, interstitial, alveolar or pleural edema) or alternative causes of dyspnea like pulmonary disease can be determined. Nevertheless, a normal chest radiogram, which is observed in ~20% of cases, does not exclude AHF diagnosis.
4.3.3 Laboratory investigations
Routine biochemical examinations that should be performed during hospital admission include hemogram, blood glucose, urea, creatinine, BUN and estimated glomerular filtration rate (eGFR), electrolytes and transaminases, C-reactive protein, and thyroid stimulating hormone (TSH) level if available. Biochemical analysis can provide information on the precipitating factors of AHF (e.g. anemia, infection, hyperor hypothyroidism, renal failure etc.) and assist in deciding for suitable drug treatment.
Creatinine and electrolytes should be monitored at short intervals (daily during IV treatment, in 1-2 days after starting oral treatment) during AHF treatment. Renal functions worsen in 25% of patients during treatment and persistence of this deterioration is a sign of bad prognosis, especially if it is combined with ongoing signs of congestion (34). Approach to renal dysfunction developed in AHF patients is summarized in Section 6.2.
Liver function abnormalities are detected in about 75% of AHF patients and are closely related to the severity of disease and clinical findings (35). In bilateral and right sided AHF, cholestatic type (total bilirubin, gamma glutamyl transferase, alkaline phosphatase) liver dysfunction is detected in patients with moderate-to-severe tricuspid insufficiency, whereas in left sided AHF and hypotension (SBP <100 mm Hg) transaminase elevation is present. All liver function tests except for alkaline phosphatase may be abnormal in patients with poor NYHA functional class. Liver dysfunction almost always recovers after AHF treatment.
In suspected ACS, myocardial injury biomarkers should be obtained. However, elevation of these biomarkers alone does not confirm presence of myocardial infarction, because in 30-50% of HF cases, cardiac injury biomarkers can increase (even without myocardial infarction) and should be interpreted as an adverse prognostic sign in these patients. In suspected AMI, at least one of the following signs must be present to establish the diagnosis: significant rise and/or fall of the markers, accompanying ischemic symptoms, new ischemic ECG changes, loss of myocardial function on non-invasive testing. Oxygenation should routinely be assessed by pulse oximetry in emergency department and ICU. Arterial blood gas measurement should be reserved for patients with signs of dyspnea or hypoxia. It is beneficial in detecting respiratory failure and acidosis in AHF patients.
Oxygen saturation and partial oxygen pressure should also be evaluated while planning non-invasive/invasive ventilation. Arterial puncture may sometimes be difficult and venous samples may be helpful for evaluation of blood gases in these cases. The cut-off limits for interpretation of arterial acidosis and hypercapnia from venous samples are pH of blood <7.32 and pCO2 >51.3 mm Hg (36).
4.3.4 Natriuretic peptides
Natriuretic peptides have well-known diuretic, natriuretic, and vasodilatory properties. The cardiovascular actions actually belong to atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP). C-type natriuretic peptide affects mainly vascular endothelial system rather than the heart (37).
The most extensively studied member of the family is BNP which is synthesized in response to the ventricular wall tension. BNP is a hormone consisting of 32 amino acids including the 17 aminoacid ring form (single ring) which is specific to all natriuretic peptides. Inactive NT-proBNP and biologically active molecule BNP are secreted into blood in equimolar amounts, therefore both can be used to assess ventricular tension. ANP is stored in atrial granules and can be released in a significant amount into blood even by a slight stimulus. However, measuring the level of active ANP molecules is not practical in the clinical setting because it has a relatively short half-life. Attempts to measure its biologically inactive portion (NT-proANP) have also been unsuccessful (38). However, MR-proANP (mid-regional proANP the antigenic region in central of the precursor molecule) can be measured. BACH study (39) showed that a cut-off limit of 120 pg/mL is non-inferior to BNP for diagnosing AHFF
BNP or NT-proBNP should be measured for the initial evaluation of suspected AHF. Especially in the differential diagnosis of dyspnea, both BNP (<100 pg/mL) and NT-proBNP (<300 pg/mL) are valuable to exclude AHFF A clear consensus does not exist on repeated measurements of natriuretic peptides during hospitalization. Nevertheless, in patients with ongoing symptoms repeated measurements may be helpful in directing therapy. BNP/NT-proBNP levels measured prior to discharge can provide information on dry weight of the patient. Moreover, a significant reduction compared to baseline level is associated with favourable post-discharge outcome. However, it is not clear whether the percentage decrease from baseline (35-50%) or the absolute value at discharge (<350 pg/mL NT-proBNP) is more important for a good prognosis (40).
4.3.5 Echocardiography and pulmonary ultrasonography
Echocardiography is one of the recommended examinations for differential diagnosis and planning treatment of AHF. If performed in emergency conditions, it provides information about cardiac anatomy (e.g. volumes, geometry, mass, valves) and functions. It is beneficial in establishing diagnosis and cause (ischemia, pericardial tamponade, valvular disease etc.) of HF.
Thoracic ultrasonography or bedside echocardiography can provide information about pulmonary congestion. It can be performed with a wide range of frequencies (4 to 12 MHz) using a vascular or cardiac probe. High frequencies are used for the examination of the peripheral sites of lungs, whereas lower frequencies are used for imaging of deep lung tissues. In pulmonary ultrasonography, A lines indicate chronic obstructive pulmonary disease whereas B lines indicate presence of congestion (comet tails) (Figure 2a, b) (41).
Figure 2.
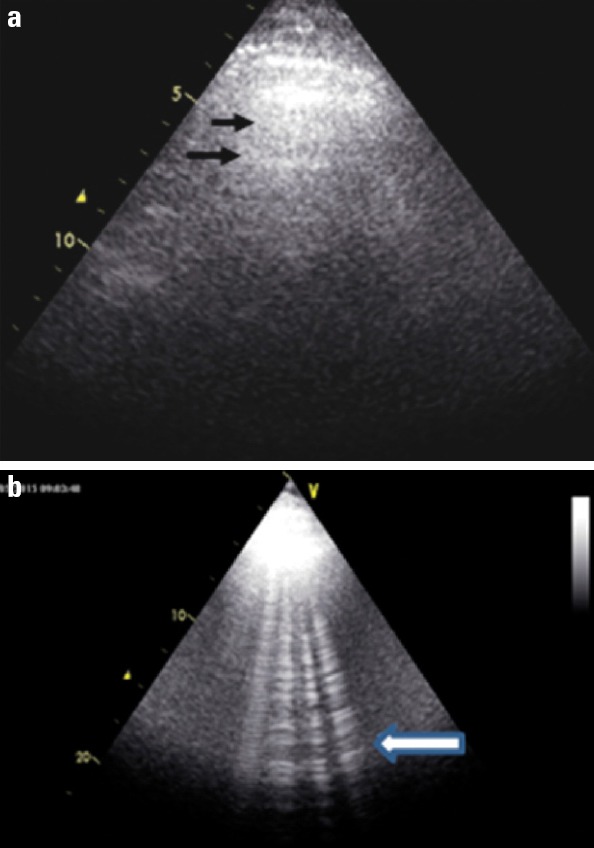
a, b. (a) Normal ultrasound. The ribs yield anechoic shadows (upper black arrow). Between the ribs there is a hyperechoing line, which is the pleural line. A lines are horizontal hyperechoing lines representing reverberations of the pleural line (black arrow). They are motionless and parallel to the pleural line. (b) Pulmonary edema. They are vertical narrow lines arising from the pleural line and end at the edge of the ultrasound screen. B-lines create a pattern called lung rockets that move in concert with lung sliding. The presence of B-lines (or comet tails) is an artifact that occurs with pulmonary odema (white arrow)
4.3.6 Hemodynamic monitorization
Non-invasive BP and urine output should be monitored in hospitalized AHF patients during acute management period. Other hemodynamic monitorizations are not used routinely, and become necessary depending on the clinical status of the patient. Central venous catheterization is performed in patients with low SBP and necessitating vasopressor treatment. Swan-Ganz (pulmonary artery) catheterization, which had been used more frequently in former years, is currently recommended only in selected cases, as it does not add more information to those obtained by non-invasive methods (42, 43).
Patients who would benefit from hemodynamic monitorization are: (i) patients with hypotension/cardiogenic shock who don’t respond to fluid treatment, (ii) ACS patients with mechanical complications, (iii) cases not responsive to standard treatment and measurement of intravascular volume, cardiac output and pulmonary capillary wedge pressure (PCWP) will be beneficial in planning the treatment, and (iv) cases who are candidates for heart transplantation or implantation of a left ventricular (LV) assist device.
Intra-arterial catheterization is used to monitor mean arterial pressure in patients with low SBP in whom signs of AHF don’t improve morbidity or mortality. It is also beneficial for patients who receive vasopressor treatment and in whom arterial blood gas analysis should be repeated frequently.
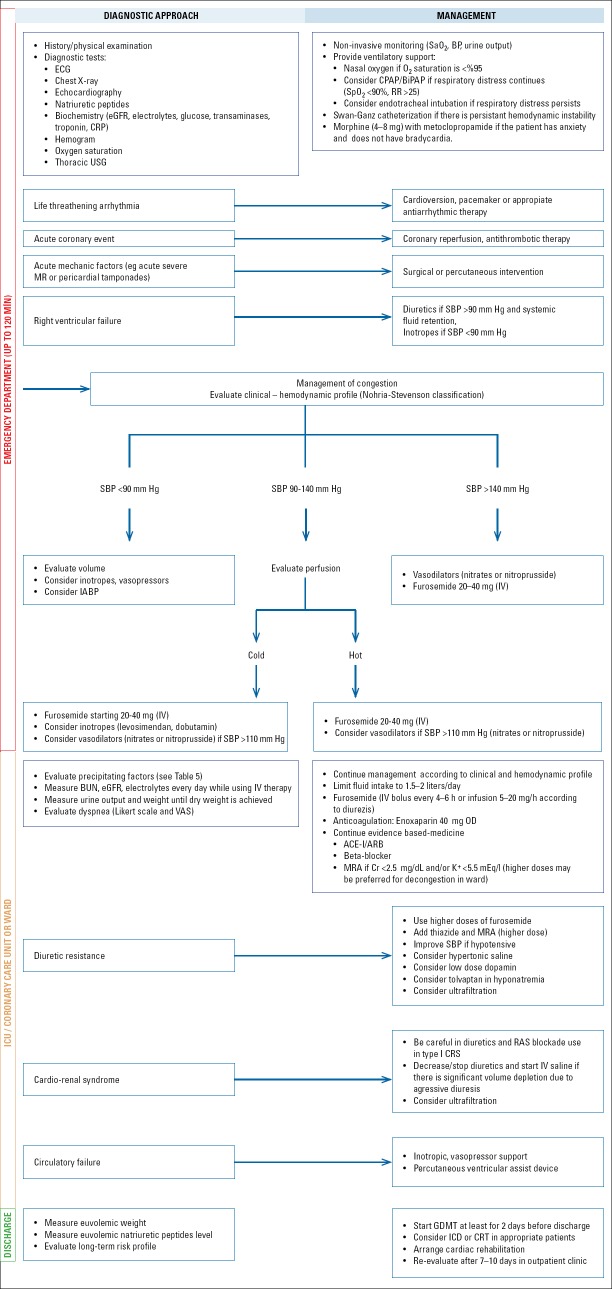
5. Management of acute heart failure
Main objectives of AHF treatment are symptomatic relief and hemodynamic recovery. Other initial treatment objectives are improving oxygenation to required levels (PaO2 >60 mm Hg, SpO2 >90%), restriction of organ damage and decreasing duration of stay in intensive care unit. Short-term objectives during the hospital stay include stabilization of clinical status by optimal treatment, starting appropriate oral pharmacological treatment, consideration of device treatment in selected cases and decreasing hospital stay.
5.1 Treatment approach in emergency department and intensive care units
AHF necessitates patients to admit to an emergency unit/hospital with symptoms and signs of HFF It has become a frequently encountered clinical problem that requires emergent management not only by cardiologists, but also by internal medicine, emergency medicine and intensive care specialists.
Due to the prognostic importance of early management, treatment should be started as soon as the patients reach to hospital, preferably in the ambulance (44, 45). Initial evaluation and management of patients in emergency department should ideally be accomplished in the first 2 hours of admission. Directing patients with suspected AHF to a cardiology unit or to a centre with coronary care (CCU)/intensive care (ICU) facilities is essential to improve the prognosis (46, 47).
The most critical step of emergent approach to AHF is determining the severity of dyspnea. Patients with tachypnea (respiratory rate >25/min), hypoxia (SpO2 <90% under oxygen therapy) and signs of increased respiratory overload (movement of accessory respiratory muscles, tripod position, difficulty in speaking) should be taken to a specific department where emergent ventilatory support can be provided (see Section 5.3).
Hemodynamics should be evaluated simultaneously with respiration. Hemodynamic monitorization is particularly important if heart rate is <60/min or >120/min, SBP <90 mm Hg or >180 mm Hg, proportional pulse pressure <25%, cold extremities are observed or changes in mental status occurs.
ECG, chest X-ray, natriuretic peptides, renal function tests, electrolytes, complete blood count, and troponins should be obtained in the emergency department (see Section 4.2). Routine echocardiography does not have a significant benefit in emergency diagnosis and treatment. However, thoracic ultrasonography and bed-side echocardiography are increasingly used by emergency physicians.
Oxygen therapy is currently applied to all patients. However, it is not beneficial in patients with a non-invasive oxygen saturation >95%. On the other hand, non-invasive ventilation (NIV) can improve prognosis of patients with respiratory distress by decreasing mechanical ventilation need.
Diuretic treatment can be started in emergency unit considering SBP and congestive findings of the patient on admission. Furosemide 40 mg IV bolus is adequate for most of the patients. Diuretic response (urine output >100 mL/h in first two hours, relief of dyspnea) should be waited after this initial dose (48). High dose diuretic administration may not be appropriate for a patient without congestion, even though AHF exists.
Vasoactive treatment should be started in all AHF patients as soon as possible. Time between first admission and initiation of intravenous treatment should not be more than 2 hours, because duration of hospital stay and in hospital mortality significantly decrease when treatment is started in the first 2 hours. Sublingual or oral nitrate treatment instead of parenteral forms of vasodilators can be preferred for patients with relatively less serious symptoms and findings in emergency unit (49). Parenteral vasodilator treatment may be started in more critical patients, but referral to an ICU or cardiology clinic should be planned simultaneously.
There is no strong evidence supporting benefits of routine opioid administration in emergency department (50). Morphine at a dose of 4-8 mg can be applied with metoclopropamide (morphine induces nausea) in patients with significant anxiety, then again respiration should be monitored carefully.
Vasopressor agents are not beneficial in the absence of hypoperfusion, contrarily they can be even harmful. Inappropriate inotrope usage in AHF seems to be a problem in our country (51).
Patients with a respiratory rate >25/min, SpO2 <90% or requiring intubation, SBP <90 mm Hg, hypoperfusion findings (existence of any: lactate >2 mmol/L, confusion, metabolic acidosis, oliguria, cold extremities in room temperature, mixed venous oxygen saturation <65%) should be directed into CCU/ICU. Patients with AHF related to ACS should be followed in CCU and should be revascularized as soon as possible. Patients without critical findings can be followed in ward and receive treatment including parenteral drugs.
Approximately half of AHF patients can be discharged from emergency department. Patients subjectively mentioning she/he has recovered, have a resting heart rate <100/min, a room air oxygen saturation >95%, urine output >30 cc/h, no orthostatic hypotension or end organ dysfunction are potential candidates for early discharge. These rules are not valid for de novo AHF patients who should always be hospitalized as their precipitating causes necessitate further evaluation and management.
5.2 Monitoring of hospitalized patients
Clinical status of the patient (e.g. symptoms, signs, weight, fluid balance, hemodynamics and biochemistry including eGFR, electrolytes, liver enzymes) should be monitored closely in ICU/CCU and in the ward daily (especially while on parental treatment).
Dyspnea of the patient should be assessed both by the physicians and patients themselves. Two different scales are used to evaluate the change in severity of self-assessment dyspnea in the recent trials (Table 6). In the Likert scale, the patient’s breathing is compared between admission and at the moment of examination (52). In the Visual Analog Scale (VAS), the patient is asked to draw a horizontal line on the scale between the number ‘0’ (which indicates the worst breathing the patient has ever felt) and the number ‘100’ (which indicates the best breathing ever) to show how he thinks his breathing is at that moment (52). These scales are not interchangeable and should be assessed simultaneously, because Likert measures of dyspnea initially improve rapidly with no significant improvement thereafter, whereas VAS measurements of dyspnea improve continually throughout hospital stay (52).
Table 6.
Scales for evaluation of dyspnea in AHF (52)
| 7-points Likert Scale | 100-mm Visual Analog Scale (VAS) |
|---|---|
| +3 Markedly better | jpg |
| +2 Moderately better | |
| +1 Mildly better | |
| 0 No change | |
| -1 Mildly worse | |
| -2 Moderately worse | |
| -3 Markedly worse |
Worsening HF is an important problem that needs more intensive therapy, longer stay in ICU or transfer from ward to ICU/CCU (Table 7). It occurs in approximately 10% to 15% of patients during the first 5 days of admission for AHF and is associated with higher risk for readmission and death (53).
Table 7.
Definitions of worsening heart, renal and liver failure in acute heart failure (53)
| Worsening heart failure | Failure to improve or worsening signs and symptoms of HF despite therapy that occurs •after 1-2 days (usually in the first 7 days) of hospitalization and •requires initiation or intensification of parenteral therapy (e.g. inotropes or vasoactive agents) or •implementation of mechanical cardiac or ventilatory support |
| Worsening renal failure | Increase in serum creatinine >0.3 mg/dL or decrease in estimated glomerular filtration rate >25% after admission |
| Ischemic hepatopatitis | Decreased blood supply (due to shock or low blood pressure) to liver resulting in liver injury and marked elevation of liver function tests |
| Congestive hepatopathy | Liver dysfunction due to venous congestion, usually right heart failure |
Impairment of renal and liver function is a frequent entity in treatment of AHF (See Section 4.3.3). Definitions for worsening renal function and hepatopathies are described in Table 7. Both of these conditions are usually managed with intensification of HF therapy.
5.3 General precautions in hospitalized patients
Fluid and sodium restriction - A fluid intake of 1.5-2 L/day is commonly recommended for AHF patients (especially for hyponatremic cases) to relieve symptoms and congestion during the initial management. However, this recommendation is not evidence based and the fluid intake should be individualized for every patient. Sodium restriction (to 2-3 g/day) may also help to control the symptoms and signs of congestion. Nonetheless, several studies have shown that a strict sodium restriction does not have an additional benefit, and even may be harmful for some patients (54).
Prophylaxis of venous thromboembolism - Hospitalization due to AHF carries a high risk for development of venous thromboembolism. Therefore prophylactic anticoagulation (enoxaparin 40 mg subcutaneously once daily or unfractionated heparin 5000 units 3 times/day subcutaneously) is usually recommended for patients during the hospital stay. However, evidence from randomized clinical trials are lacking for this recommendation and prophylaxis may not be necessary for not bedridden patients.
Oxygen therapy - In left sided AHF, pulmonary edema associated with hypoxemia requires oxygen supplementation. Similar to management in emergency department, oxygen therapy is recommended for patients with a SpO2 <95%. In the absence of hypoxemia, oxygen therapy may be harmful. Respiratory support for an AHF patient is performed to recover more severe hypoxemia, which is defined as a SpO2 <90% and a partial arterial oxygen pressure (PaO2) <60 mm Hg. A SpO2 <75% and a PaO2 <40 mm Hg indicates critical hypoxemia.
Oxygen therapy should be applied from simple to complicated and from non-invasive to invasive, starting with nasal cannula/mask, continuing with non-invasive ventilation (NIV) and finally invasive ventilation (IV) with endotracheal intubation. If hypoxemia of the patient is mild, nasal oxygen is given. However, in severe hypoxemia NIV can directly be started (Table 8).
Table 8.
Indications for noninvasive ventilation
| 1. Inadequate response to initial standard oxygen therapy |
| 2. High-risk of endotracheal intubation |
| 3. Persistent O2 saturation <90% or PaO2/FiO2 <200 mm Hg on >4 L/min oxygen |
| 4. Mild hypercapnia (PaCO2 >45 mm Hg) or acidosis (pH <7.3 but >7.1) |
| 5. Respiratory muscle fatigue |
| 6. Signs and symptoms of acute respiratory distress |
| 7. Respiratory rate >24 breaths/min |
FiO2 - fraction of inspired oxygen, PaO2 - partial pressure of arterial oxygen
Two types of NIV methods are used in the treatment of acute cardiopulmonary edema: 1) Continuous positive airway pressure (CPAP) providing positive airway pressure continuously through whole respiratory cycle, and 2) Bi-level positive airway pressure (BIPAP) providing positive pressure only through inspiration period and at the end of expiration. Ventilation settings according to type of NIV are shown in Table 9.
Table 9.
Settings of non-invasive ventilation
| A-CPAP settings |
| Start with 5-7.5 cm H2O |
| Increase in increments of 2 cm H2O, as tolerated and indicated |
| FiO2 >40% |
| B-BIPAP settings |
| Initial inspiratory pressure of 8-10 cm H2O |
| Increase in increments of 2-4 cm H2O (max ~20 cm H2O) aiming at tidal volume >7 mL/kg |
| Initial expiratory pressure of ~4-5 cm H2O |
| Maximum inspiratory pressure is 24 cm H2O and expiratory pressure 20 cm H2O |
| FiO2 >40% |
FiO2 - fraction of inspired oxygen; CPAP - continuous positive airway pressure, BIPAP - bilevel positive airway pressure
Objectives of NIV in acute cardiopulmonary edema are to improve oxygenation, to decrease respiratory effort and to increase cardiac output. Positive pressure given during expiration provides oxygenation, while positive pressure given during inspiration assists respiratory muscles. Besides, NIV decreases intrathoracic blood volume by decreasing preload of right ventricle and afterload of left ventricle, thereby improves cardiac functions. In a meta-analysis, NIV added to standard treatment decreased mortality (NNT value 14), need for invasive intubation (NNT value 8) and length of stay in ICU (approximately 1 day) (55). CPAP treatment also decreases mortality (NNT value 9) and intubation requirement (NNT value 7). However, BIPAP treatment does not decrease mortality when compared to standard or CPAP treatment. Thus, CPAP treatment is performed as the first choice due to its effectiveness and safety, as well as cost effectiveness and easier use compared to BIPAP BIPAP should be used in patients who are unresponsive (pressure requirement more than 12 cm H2O) or cannot tolerate CPAP treatment, in hypercapnic patients and in patients who develop respiratory muscle fatigue and hypoventilation.
Invasive endotracheal intubation is performed when NIV treatment is contraindicated (Table 10) or insufficient. Criteria for endotracheal intubation are listed in Table 11. Endotracheal intubation should be applied as short as possible because of its possible risks (trauma to the oro-pharynx and airway, excessive hypotension, arrhythmia, accumulation of respiratory debris due to inability to cough, especially nosocomial pneumonia, dysphonia, granuloma formation, increased hospital stay and costs and increased mortality).
Table 10.
Contraindications for noninvasive ventilation
| A - Absolute contraindications |
| 1. Coma |
| 2. Cardiac arrest |
| 3. Respiratory arrest |
| 4. Any condition requiring immediate intubation |
| B - Other contraindications |
| 1. Hemodynamic or cardiac instability |
| 2. Altered mental status (excluding cases secondary to hypercapnia) |
| 3. Inability to protect the airway or risk of aspiration |
| 4. Gastrointestinal bleeding - Intractable emesis and/or uncontrollable bleeding |
| 5. Facial surgery, trauma, deformity or burning |
| 6. Recent gastrointestinal or upper airway surgery (<7 days) |
| 7. Potential for upper airway obstruction |
| 8. Uncooperative and inability to tolerate the mask |
| 9. Lack of training |
Table 11.
The criteria for endotracheal intubation (55)
| A-Any one of the following |
| 1. pH less than 7.20 |
| 2. pH 7.20-7.25 on two occasions 1 hour apart |
| 3. Hypercapnic coma (Glasgow Coma Scale score <8 and PaCO2 >60 mm Hg) |
| 4. PaO2 less than 45 mm Hg |
| 5. Cardiopulmonary arrest |
| B-Two or more of the following in the context of respiratory distress |
| 1. Respiratory rate greater than 35 breaths/minute or less than 6 breaths/minute |
| 2. Tidal volume less than 5 mL/kg |
| 3. Blood pressure changes, with SBP <90 mm Hg |
| 4. Oxygen desaturation to <90% despite adequate supplemental oxygen |
| 5. Hypercapnia (PaCO2 >10 mm increase) or acidosis (pH decline >0.08) from baseline |
| 6. Obtundation |
| 7. Diaphoresis |
| 8. Abdominal paradox |
SBP - systolic blood pressure; PaO2 - partial pressure of arterial oxygen; PaCO2 - partial pressure of arterial carbon dioxide
5.4 Pharmacological treatment
5.4.1. Treatment approach according to systolic blood pressure
The clinical scenario should be well defined in each case to determine appropriate management approach (see also Section 2 and 6.1). SBP is the leading determinant of the clinical scenario and treatment approach according to each clinical scenario on admission is assessed specially in following sections (Table 12) (56).
Table 12.
Clinical presentation of acute heart failure according to the SBP on admission (56)
| High SBP ‘Vascular Insufficiency’ | Normal or low SBP ‘Cardiac Insufficiency’ |
|---|---|
| Rapidly worsening (minutes, hours) | Gradually worsening (days) |
| Pulmonary congestion | Systemic congestion |
| Fluid redistribution | Fluid accumulation |
| Acute increase in PCWP | Chronically high PCWP |
| Radiographic congestion +++ | Radiographic congestion + |
| Weight gain/edema + | Weight gain/edema +++ |
| Preserved LVEF | Low LVEF |
| Rapid response to treatment | Relatively slow response to treatment |
LVEF - left ventricular ejection fraction; PCWP - pulmonary capillary wedge pressure; SBP - systolic blood pressure
In patients presenting with a high SBP (>140 mm Hg), sudden and abrupt increase in BP is associated with sympathetic hyperactivity. Rapid increase of LV filling pressure and fluid redistribution leads to pulmonary congestion and dyspnea. Pulmonary congestion is more marked than systemic congestion. This presentation of AHF may also be defined as ‘vascular insufficiency’.
SBP on admission is among normal limits (100-140 mm Hg) in nearly half of the cases. These patients usually have previously known HF with reduced EF. Symptoms worsen slowly but progressively within days. They present with systemic congestion. Signs of pulmonary congestion are not marked despite high LV filling pressure. This type of AHF is defined as ‘cardiac insufficiency’.
Low SBP (<90 mm Hg) is observed in 2-8% of cases and is associated with low cardiac output and organ hypoperfusion. Cardiogenic shock is present in 1-2% of AHF cases.
Evaluation of clinical congestion is important to determine treatment approach to AHF. Pulmonary congestion that develops due to sudden increase of ventricular filling pressure without increase in systemic volume overload is called ‘hemodynamic congestion’. Patients with this clinical presentation are generally euvolemic and do not have signs related to systemic fluid accumulation. Examples include hypertensive AHF, severe LV dysfunction due to ACS or AHF due to acute mitral insufficiency. Fluid redistribution rather than systemic fluid overload is characteristic for these cases.
Acute decompensated HF-as an exacerbation of chronic HF- is a typical example for systemic congestion associated with peripheral edema and weight gain reflecting increase in total fluid overload.
In most cases hospitalized due to AHF, clinical findings are related to systemic and/or pulmonary congestion rather than low cardiac output. Main treatment approach is vasodilators administered with diuretics. Vasodilators are the essential part of treatment in hemodynamic congestion and diuretics are used in lower doses in this condition. Whereas in systemic congestion associated with volume overload, diuretics constitute the cornerstone of the treatment and vasodilator treatment is given to decrease hemodynamic congestion. Inotropic treatment is required in cases not responsive to diuretic and/or vasodilator treatment, findings of hypotension and organ perfusion.
In hypertensive AHF, vasodilator treatment is crucial as high BP and pulmonary congestion are related to volume redistribution rather than hypervolemia. Low dose diuretics may be added to vasodilator treatment, however high dose diuretic treatment should be avoided.
Initial treatment of wet and hot HF accompanied by tissue congestion due to hypervolemia comprises of diuretics and vasodilators. In baseline treatment of dry and cold HF accompanied by hypotension and peripheral hypoperfusion due to low output, inotropic and vasodilator agents are used as first-line treatments (see Figure 1). Balanced administration of diuretics, vasodilators and inotropes should be considered in clinical presentation of combined congestion and perfusion disorder.
Dopamine should be started in cases presenting with cardiogenic shock, and in inadequate response to dopamine; norepinephrine should be initiated. Invasive ventilation, intra-aortic balloon pump and LV assist devices should be considered if necessary.
5.4.2 Diuretic strategies
One of the main objectives of AHF treatment is resolving congestion of the patient. The goal of diuretic therapy administered for this purpose is to provide euvolemia (dry weight) with the lowest possible dose and not to harm hemodynamics of the patient while achieving diuresis. For removing congestion and relieving symptoms, urine output should be increased to >40 mL/h and a weight loss of 1-1.5 kg/day should be achieved. Patient’s BP fluid balance, weight at the same hour of each day (preferably in the morning), daily renal functions and electrolytes should be monitored as long as parenteral treatment continues.
To remove volume overload in AHF, loop diuretics are administered via parenteral route. Main loop diuretics are furosemide, bumetanide and torasemide; however, furosemide is the most commonly used one, both in our country and in the whole world. Initial dose of intravenous furosemide is 20-40 mg. Total daily dose of furosemide should at least be equal to the total daily dose that the patient was taking before hospitalization and can generally be safely increased up to 2.5 times of the prehospitalization doses.
As half-lifes of diuretics are relatively short, doses should be repeated in periods or infusion treatment should be applied. If bolus treatment is preferred, dose can be repeated in 4-6 hours according to volume overload of the patient. In continuous infusion treatment, furosemide is started with 10 mg/hour dose and continued with 5-20 mg/hour according to response of the patient. The most appropriate treatment dose and route of administration (bolus or continuous infusion) is not clear. In DOSE trial (Diuretic Optimization Strategies Evaluation Trial) (57) that aimed to answer this question, continuous infusion was compared with bolus infusion in every 12 hours and low dose (equal to previously taken oral dose) was compared with high dose (2.5 times of previous oral dose) by a 2x2 factorial design. No significant difference was observed between the two applications; however, symptoms such as shortness of breath improved more effectively in the high dose group (although temporary renal dysfunction was more frequently observed in this group). Generally, limited numbers of patients were randomized in trials comparing infusion treatment vs. bolus treatment. Meta-analysis of these trials show that there is no significant difference between continuous and bolus infusion; however, more effective diuresis is provided by continuous infusion (58, 59).
If an adequate diuresis cannot be provided by loop diuretics, diuretic dose is increased or a second diuretic (e.g. thiazides or spironolactone) is added. Generally, thiazides are added in daily practice; however, evidence regarding benefits of high dose spironolactone (50-100 mg) is also increasing (60).
Hypertonic saline treatment can increase the effect of diuretic treatment by drawing fluid from interstitial area into intravascular area. A meta-analysis (61) of 5 randomized controlled trials comparing clinical outcome of patients who received only IV furosemide vs. hypertonic saline added to IV furosemide reported that hypertonic saline administration achieved better weight loss, preserved renal functions, shortened duration of hospital stay and decreased re-hospitalization after discharge and all-cause mortality. Hypertonic saline is infused as 100-150 mL NaCl with different concentrations (between 2.4-7.5%) 1-2 times/day depending on the Na level of the patient. Until more data is obtained, hypertonic saline is suggested to be administrated in patients with a creatinine level <3 mg/dL, who have fluid accumulation in interstitial area, depleted intravascular volume and no response to standard treatment.
Another option for patients with inadequate diuresis on standard treatment is low dose dopamine (1-5 pg/kg/min). However, two randomized clinical trials comparing low dose dopamine with standard treatment in AHF patients [DAD-HF II (62) and ROSE (63)] could not show any additional benefit of dopamine for increasing diuresis and protecting renal functions. Therefore, low dose dopamine administration should be reserved for patients who do not respond to standard treatment and have relatively lower blood pressure.
One of the current diuretic options in AHF patients who have hyponatremia and risk for cognitive dysfunction is tolvaptan, which is a selective vasopressin 2 receptor antagonist. Tolvaptan induces water diuresis (aquaresis) instead of salt diuresis with conventional diuretics. In EVEREST trial (Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan) (64), tolvaptan did not affect mortality and hospitalization in AHF patients, but increased urine amount and serum sodium, and improved congestive signs like dyspnea and edema. There was a statistically, but not clinically significant, greater increase in serum creatinine with tolvaptan (0.08 mg/dL versus 0.03 mg/dL) compared to placebo.
Parenteral diuretic treatment should be continued until congestive findings (rales, jugular venous distention, peripheral edema, ascites) disappear or decrease to a reasonable degree. Thereafter, treatment should be continued with the lowest dose of oral diuretic sufficient to keep the patient in dry weight.
5.4.3 Ultrafiltration
Ultrafiltration (UF) is an alternative method to diuretic treatment for removing congestion in hypervolemic patients. It is based on removing fluid and molecules with low molecular weight (<20 kDa) from circulation via a semi-permeable membrane. Contrary to diuretic treatment, fluid removed by UF is isoosmotic or iso-natremic. An UF rate of 200-300 mL/h is usually adequate but can be speed up to 500 mL/h.
Advantages of UF compared to diuretic treatment are to remove more sodium (and less potassium), to control amount and rate of fluid to be removed and to cause less neuro-hormonal and electrolyte changes (65). Beyond these advantages, existence of diuretic resistance in one fourth of HF patients makes veno-venous UF an important treatment option for isolated hypervolemic HF (66).
Efficacy of UF was examined in the Relief for Acutely fluid-overloaded Patients with decompensated CHF (RAPID-CHF) trial (67). Patients were randomized to a single 8 h UF session in addition to usual care or to usual care alone. In the end of the study, no significant difference both in efficacy and safety was detected. In the Ultrafiltration Versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated HF (UNLOAD) trial (68) which was the first large trial on the efficacy of UF, weight loss at 48 h (5.0±3.1 kg vs. 3.1±3.5; p=0.001) and net fluid loss (4.6 L vs. 3.3 L; p=0.001) were higher in the UF group. At 90 days, the UF group had lower hospitalization rate compared to usual care group (18% vs. 32%; p=0.037). Though to these positive results favoring UF, methodological limitations like unblinded trial design, suboptimal doses of diuretics, restriction of fluid removal with only 2 L, exclusion of patients with renal dysfunction and leaving duration and rate of UF to the decision of physicians were criticized.
Renal failure, which is an important co-morbidity in HF patients, is present at various degrees in 30% of AHF patients. CARRESS-HF trial (69) included 188 patients with cardiorenal syndrome or AHF with renal dysfunction (mean serum creatinine was 1.9 mg/dL in UF group and 2.09 mg/dL in diuretics group). Contrary to UNLOAD trial, data of CARESS-HF showed that stepwise diuretic regimen was superior to UF. No difference was detected in weight loss and mortality in two treatment arms and difference between serum creatinine was in favor of pharmacologic treatment (p=0.03). Side effects including renal failure, bleeding and complications due to catheter were detected significantly more in UF group (57% versus 72%, p=0.03).
These data was evaluated in ESC and ACCF/AHA Heart Failure Guidelines and ESC 2012 guidelines listed UF among controversial subjects due to insufficient evidence or consensus, and ACCF/AHA 2013 guidelines positioned UF in treatments with a class IIb recommendation to relieve of congestive symptoms in patients with significant volume overload (2, 3). Nonetheless, side effects brought out by high dose diuretics treatment, worsening of renal function and increase of mortality make UF irrevocable for patients in whom all diuretics strategies had failed.
Isolated UF application should be restricted in patients refractory to diuretic treatment and renal dysfunction due to volume overload more than structural renal damage. Other procedures (hemodialysis, peritoneal dialysis, hemofiltration etc.) should be considered in patients who have hyperuremia syndrome (azotemia, metabolic acidosis, hyperkalemia) accompanying HF.
A nephrologists’ opinion should be taken before starting UF in candidate patients, and the procedure should be supervised by an experienced team. Another problem restricting routine use of UF is its high cost. UF cost is affected by several issues such as duration of hospital stay, re-hospitalization frequency and its cost, applied number of UF and cost of filters. When single use filters are used, cost increases considerably. However, when rate and duration of hospital stay are considered, UF becomes more economic in terms of national social insurance.
5.4.4 Vasodilators
Vasoactive drugs are the most important treatment alternatives to improve hemodynamic preload-afterload mismatch present on the basis of AHF. They can be classified in 3 groups as traditional nitro-vasodilators, natriuretic peptide analogues and other vasodilators.
Nitro-vasodilators (nitroglycerine, nitroprusside) are recommended to improve hemodynamics (decrease in PCWP and LV filling pressure) and for symptomatic relief in patients with a SBP >110 mm Hg provided close follow-up is maintained. Nitroglycerine treatment is started with 10-20 pg/min and can be increased in a stepwise fashion observing hemodynamic response of the patient (Table 13). Nitroprusside has not been widely studied in AHF, but in patients with marked increase in SBP it may be given with careful hemodynamic monitorization. However, at least 10 mm Hg decrease in mean arterial pressure is an acceptable target in clinical practice (49). Duration of nitrovasodilator therapy is 24-48 hours. Beyond this time, tachyphylaxia or tolerance to nitroglycerin or intoxication with nitroprusside may occur.
Table 13.
| Initial dose | Infusion dose | Precautions | |
|---|---|---|---|
| Nitroglycerine | 10-20 pg/min | 5-200 pg/min | Tolerance and tachyphylaxis on continuous use |
| Isosorbide dinitrate | 1 mg/h | 1-10 mg/h | |
| Nitroprusside | 0.3 p/kg/min | 0.3-10 pg/kg/min | Invasive hemodynamic monitoring is required; marked hypotension may occur; longer infusions may cause thiocyanate toxicity. |
| Nesiritide | 2 pg/kg (bolus) | 0.01 pg/kg/min | Hypotension |
| Ularitide | 15 ng/kg/min | Increased sweating, dizziness, nausea and hypotension | |
| Serelaxin | 30 pcg/kg/day | Hypotension |
Modified from ESC 2012 heart failure guidelines (2)
Natriuretic peptide analogues include at least 2 agents: nesiritide and ularitide. Both of these drugs are not available in Turkey, however there are several studies conducted with this group of agents. In ASCEND-HF study (70), nesiritide improved dyspnea faster than usual care, however did not change composite endpoint defined as rehospitalization for heart failure and 30-days all-cause mortality, and was relatively expensive. Main symptomatic benefit with nesiritide is achieved when the drug is applied in first 15.5 hours after admission (71). This observation may seem strange for the ones who practice in AHF management. In real life, diuretics + nitrate treatment combination is started in first 30 minutes nearly in all patients (72). Moreover, this treatment provides a highly effective symptomatic recovery in 75% of patients. However, time factor had been ignored in clinical trials testing new agents in AHF until recent years. Testing many agents at wrong time periods may have resulted in missing the most beneficial time of many agents. Ularitide, another agent of the group is an isoform of ANP resistant to breakdown by neutral endopeptidases. Currently, it is being tested in Phase III clinical trials like the ongoing Trial of Ularitide’s Efficacy and safety in patients with Acute Heart Failure (TRUE-AHF).
The prototype agent for other vasodilators group is Serelaxin. Serelaxin is recombinant human relaxin -2, which is a natural peptide that regulates maternal adaptations to pregnancy (73). It acts through specific G-protein coupled relaxin receptors (RXFP1 and 2) and endothelin B receptors. Activation of these receptors results in the activation of NO synthase in endothelial cells and subsequently in vasodilation. Hemodynamic effects of serelaxin include increase in arterial compliance, cardiac output, renal blood flow and creatinine clearance. Unlike the nitrates, it has some inotropic effect and does not appear to reduce venous tone. Clinical effects of serelaxin was studied in Pre-RELAXAHF (74) and RELAX trials (75). In both of them, treatment with serelaxin was associated with relief of dyspnea and reduction in 180-day mortality [RELAX trial (n=1161): placebo 65 deaths vs. serelaxin 42 deaths; HR 0.63, 95% CI 0.42-0.93; p=0.019]. It is one of the agents currently being investigated in phase III clinical trials (RELAX-2).
Clevidipine, a calcium channel blocker agent, was investigated in PRONTO trial (76), and was found superior to traditional nitrovasodilator treatment to relieve dyspnea in hypertensive AHF patients.
One of basic principles of vasodilator treatment is to avoid symptomatic hypotensive response that may occur and probably be harmful. Recently, it was reported that HF-rEF and HF-pEF patients respond differently to treatment, and HF-pEF patients respond to vasodilator with exaggerated blood pressure decrease (77). Symptomatic hypotension is also more frequently observed in de novo AHF patients. Vasodilators may be harmful in patients with inadequate preload. On the other hand, vasodilator treatment is safe in normotensive patients with HF-rEF who have congestion and dilated jugular veins. In any case, monitorization and careful titration is required for vasodilators.
5.4.5 Positive inotropes
Inotropic agents constitute one of the 3 basic pharmacologic treatment groups in AHF management, although they are not used as frequently as diuretics and vasodilators. Their main indication is hypotension and cardiogenic shock accompanying AHF but they may also be used in cases who are resistant to initial vasodilator and diuretic treatment.
Inotropic agents increase myocardial contractility and cardiac output, decrease ventricular filling pressure and PCWP and thereby provide symptomatic and hemodynamic recovery (78). However, data exists that they also provoke ischemia and serious arrhythmias by increasing intracellular calcium level, oxygen consumption and myocardial oxygen requirement and may have a direct toxic effect on myocardium. Although not based on randomized controlled, double blind trials, data exists on their adverse effects for long-term mortality. Therefore their use was restricted to cases who have low output presenting with hypotension. Low cardiac output due to systolic dysfunction exists in 5-10% of AHF cases. Inotropic treatment alone or with vasodilator treatment is required in these cases to improve clinical picture in short time (79). In general, hypotension and/or hypoperfusion are determinants for inotropic treatment decision. If indicated, inotropic treatment should be started in early phase, be given at minimum required dose and discontinued in the shortest time possible. Inotropic treatment does not have a role in AHF due to diastolic dysfunction.
Frequently used agents in clinical practice are adrenergic receptor agonists dopamine and dobutamine, calcium sensitizing agent levosimendan and phospodiesterase III inhibitors amrinone and milrinone. Dopamine and dobutamine have mainly inotropic effects, whereas amrinone, milrinone and levosimendan have also vasodilatator properties accompanying their inotropic effects (Table 14) (80).
Table 14.
| Loading dose | Infusion dose | |
|---|---|---|
| Dopamine | None | <3 pgr/kg/min: renal diuretic effect |
| 3-5 pgr/kg/min: inotropic effect | ||
| >5 pgr/kg/min: inotropic + vasopressor effect | ||
| Dobutamine | None | 2-20 pgr/kg/min |
| Levosimendan* | Optional (6-12 pgr/kg, >10 min time) | 0.1 pgr/kg/min (can be increased 0.2 pgr/kg/min or decreased 0.05 pgr/kg/min according to SBP) |
| Milrinone* | Optional (25-75 pgr/kg) | 0.375-0.75 pgr/kg/min |
| Norepinephrine | None | 0.2-1.0 pgr/kg/min |
| Epinephrine | During resuscitation 1 mg IV, (can be repeated every 3-5 min) | 0.05-0.5 pgr/kg/min |
Has also vasodilator property. If SBP <90 mm Hg loading dose is not given. IV - intravenous; SBP - systolic blood pressure
Modified from ESC 2012 heart failure guidelines
Dopamine
Dopamine in low doses (<2-3 pgr/kg/min) causes renal, coronary and cerebral vasodilatation by effecting only dopaminergic receptors. In higher doses (>3 pgr/kg/min), it increases myocardial contractility as a result of beta-1 receptor stimulation. A dose of 3-5 pgr/kg/min is recommended for inotropic effectiveness. Higher doses (>5 pgr/kg/min) increase systemic vascular resistance and hence BP by affecting alpha-adrenergic receptors (Table 14). It can be called as a ‘vasopressor inotrop’ due to its effects at higher doses. Dopamine is an appropriate agent to increase cardiac output and achieve a BP level to preserve peripheral perfusion in HF with serious hypotension (<90 mm Hg) or cardiogenic shock.
Dobutamine
Dobutamine is an inotropic agent increasing cardiac output by dose dependent inotropic effect via beta-1 receptors (81). It increases cardiac output more than dopamine. Dobutamine should be preferred as the initial treatment in AHF cases with normal or near normal BP and low cardiac output and dopamine should be chosen in cases with significant hypotension. Infusion rate can be titrated up to 15-20 pgr/kg/min according to recovery of symptoms, hemodynamic response and diuresis (Table 14). While increasing doses, care must be taken for development of tachycardia and arrhythmias. Its most important disadvantage is development of tolerance after 24-48 hours of administration and decrease of its effectiveness when using beta-blockers. Dobutamine may induce ischemia, increase residual ischemia and enlarge the infarct area in cases with CAD.
Levosimendan
Levosimendan is an ‘inodilator agent’ showing inotropic effect by increasing calcium sensitivity of contractile proteins in myocardium, leading to vasodilation in vascular smooth muscles with opening ATP-dependent potassium channels and thereby decreasing peripheral vascular resistance and cardiac pre- and afterload (82). Increasing cardiac contractility without increasing intracellular calcium level sets levosimendan apart from traditional inotropics. It does not lead to myocardial oxygen consumption and ischemia. Levosimendan provides more hemodynamic benefit in increasing cardiac output and decreasing PCWP compared to dobutamine (83). A recent meta-analysis suggested that levosimendan may reduce mortality in various cardiac settings in adult patients (84). It is a preferable inotropic agent in patients with CAD and ACS. In AHF secondary to an AMI, levosimendan has been shown to be a safe inotropic agent. Contrary to dobutamine, the efficiency of levosimendan is not affected by beta-blocker usage. Furthermore, when compared to dobutamine, levosimendan reduces short-term mortality in patients with previous heart failure or who were on beta-blockers previously. Current ESC guidelines on heart failure recommend levosimendan as a class IIb indication to reverse the effect of beta-blockade if beta-blockade is thought to be contributing to hypoperfusion in AHF and in AHA/ACC 2013 heart failure guidelines there is no recommendation about levosimendan (2, 3).
Levosimendan is administered as 24-hour IV infusion at a dose of 0.05-0.2 pgr/kg/min following a loading dose of 6-12 pgr/kg/min in 10 minutes. Hypotension may develop due to vasodilatation, therefore loading dose can be omitted in many cases if the initial SBP is <100 mm Hg. Levosimendan is not recommended in patients with a SBP <85 mm Hg.
Phosphodiesterase-!!! enzyme inhibitors
Phosphodiesterase (PDE) III inhibitors increase contractility by increasing intracellular calcium and decreasing intracellular cAMP degradation via selective inhibition of PDE-III enzyme. They also cause arterial and venous vasodilatation by PDE inhibition. Therefore, they have ‘inodilator’ effects. Inotropic effectiveness is less than dobutamine and vasodilator effects are less than nitroprusside which is a strong vasodilator. The effectiveness of PDE III inhibitors does not decrease under the treatment with beta-blockers (85). Negative data exists about their safety in HF with CAD.
Milrinone is a strong PDE-III inhibitor, that also increases beta-adrenergic receptor sensitivity by inhibiting guanine nucleotide binding protein which inhibits beta-receptors. Therefore, synergic interaction exists between milrinone and beta-agonists. It decreases ventricular filling pressures more than dobutamine due to its vasodilator effects. Combination with dobutamine may be considered for cases with near normal BP level. Hemodynamic effectiveness reaches to highest level in 10-15 minutes following IV bolus administration. Thrombocytopenic effect is less than amrinone. However, care should be taken for abnormalities related to liver tests, hypotension, atrial and ventricular arrhythmias. Milrinone is available in Turkey, but results of placebo controlled trials relating to an increased mortality with milrinone restricted its widely use (85).
Amrinone and enoximone are not available in our country. Amrinone is not widely used due to its thrombocytopenic effect and rapid development of drug tolerance (86). Enoximone is a selective PDE-III inhibitor which is 10 times less potent than milrinone. Bolus dose is given to cases with normal SBP at baseline and administrated as continuous infusion. It is mainly metabolized by liver and active sulfoxide metabolites are eliminated via kidney. As in milrinone, dose should be decreased in case of renal failure. It rarely causes thrombocytopenia.
Combined use of inotropic agents
Efficacy and safety of inotropic agents are dose dependent. Cardiac output increases dose dependently; however undesirable effects also increase concomitantly. When inotropics with different mechanisms of action are combined, inotropic effect and cardiac output increase more powerfully. Low dose dopamine and dobutamine combination is a frequently used combination in daily practice to increase diuresis. In advanced HF resistant to dobutamine, levosimendan can be added to dobutamine infusion to improve clinical and hemodynamic results (87). Combination of dobutamine that affects via beta-1 adrenergic activity with milrinone or amrinone that effect via decreasing degradation of postreceptor cAMP provides additive inotropic effectiveness (88).
Vasopressor agents
Vasopressor agents are required to provide or preserve organ perfusion in patients with life threatening hypotension such as cardiogenic shock and in patients when cardiac output can not be recovered and appropriate SBP level (>90 mm Hg) cannot be achieved by inotropic agents and/or fluid treatment. However, their use should be restricted with the minimum possible dose and shortest time as cardiac afterload will increase due to increased peripheral vascular resistance and accordingly organ perfusion will decrease by vasopressor agents. Serious arrhythmias, worsening of renal function and myocardial ischemia can be observed during management.
Epinephrine is a catecholamine with equal and high affinity to beta-1, beta-2 and alpha 1 receptors. Therefore, it is accepted as a relatively balanced vasopressor agent in terms of vasodilator and vasoconstrictor effects. Its chronotropic affect becomes more significant in HFF At low doses, cardiac output is increased by inotropic and chronotropic affect due to beta-1 activity and vasoconstriction due to alpha-1 activity is relatively balanced with beta-2 receptor activity. At high doses, alpha-1 affect becomes prominent and systemic vascular resistance significantly increases in addition to cardiac output. It is generally used at a dose of 0.05-0.5 pgr/kg/min. In clinical practice, epinephrine is used if hypotension cannot be controlled with inotropic agents. Unless serious hypotension, it is not recommended in decompensated HFF Epinephrine is not recommended as an inotropic and/or vasopressor agent for cardiogenic shock. Its use is especially recommended in cardiac arrest and asystole as a rescue agent at 1 mg IV bolus.
Norepinephrine is a catecholamine with a very high alpha-1 receptor and lower beta-1 and beta-2 receptor affinity (72). Thus it is a strong vasoconstrictor but a weak inotropic agent. In general, norepinephrine is used to increase BP It is not recommended for decompensated HF, and should be reserved for hypotension resistant to dopamine or for increasing BP and coronary perfusion in cardiogenic shock. As it is a weak inotropic agent, its effect on cardiac output is not significant. When compared with epinephrine it does not lead to a significant increase in heart rate. Norepinephrine is generally used at doses of 0.2-1.0 pgr/kg/min.
5.5 Mechanical assist devices
Mechanical circulatory assist devices and/or heart transplantation are the only remaining treatment options in patients with end-stage HF despite optimal pharmacological and device therapy like CRT/ICD. Progressive improvement and successful results of LV assist technology lead to widespread use of these devices as a bridge to heart transplant, bridge to decision, bridge to recovery or long-term destination therapy in end-stage HFF Nevertheless, the exact role of mechanical circulatory support in the management of AHF is not clear, as these patients represent a heterogeneous group. Generally, short-term mechanical support devices are preferred in AHF and long-term assist devices are reserved for end-stage chronic HFF Intermediate or short-term percutaneous assist devices may also be used for refractory cardiogenic shock patients with multi-organ failure on the purpose of bridge to heart transplant or destination therapy. Occasionally, long-term assist devices are used in AHF patients who are at immediate risk of death until a thorough clinical evaluation can be completed and therapeutic options are decided (89, 90). Probability of recovery, estimated recovery time and suitability of the patient for heart transplantation should be considered before deciding for mechanical circulatory support in decompensated AHFF Patients with a reversible pathology like acute fulminant myocarditis or postpartum cardiomyopathy and patients with idiopathic dilated cardiomyopathies response to mechanical assist devices better than HF cases due to ischemic reasons (91).
LV assist devices vary from big extracorporeal systems to small size devices applied percutaneously. Main advantage of surgically implanted devices is their ability to provide complete support to cardiac stroke volume, but replacing these devices requires re-operation. Permanent anticoagulation is needed for both surgically or percutaneously implanted devices. Detailed information on surgically implanted assist devices, which are available in very limited number of centers in Turkey, is beyond the scope of this report.
Percutaneous ventricular assist device systems
Percutaneous devices are primarily designed for LV assist and frequently used for treatment of refractory cardiogenic shock developed after AMI or acute cardiac decompensation due to other reasons. Implantation of percutaneous ventricular assist devices is less invasive and starts to support patient’s circulation earlier.
Intraaortic balloon pump (IABP) is the most commonly used percutaneous circulatory assist device, as it can be implanted easily and rapidly. It provides better blood supply to coronary arteries and myocardium by increasing aortic diastolic pressure and decreases LV afterload by decreasing aortic pressure during systole which results in reduced myocardial oxygen consumption. Cardiac stroke volume increases approximately 10-20% with IABP support (92). As cardiac circulatory support provided by IABP is maximum 1.5 L/min, appropriate candidates for IABP are patients who require low level support. In patients with severe myocardial damage, cardiac stroke volume cannot be increased adequately to fulfill the demands of body and other circulatory support systems are required in these patients. A meta-analysis of 7 randomized controlled trials, suggests that IABP may have a beneficial effect on some hemodynamic parameters, but does not have a survival benefit (93). Therefore recent guidelines do not strongly recommend IABP support for treatment of AMI patients with cardiogenic shock (2-4). In current ESC guidelines (2), IABP is recommended to support circulation before surgical correction of specific acute mechanical problems (e.g. interventricular septal rupture and acute mitral regurgitation), during severe acute myocarditis and in selected patients with acute myocardial ischemia or infarction before, during, and after percutaneous or surgical revascularization.
There are two kinds of widely used percutaneous LVADs: TandemHeart (Cardiac Assist, Pittsburgh, PA, USA) and Impella LP 2.5 (Abiomed Europe GmbH, Aachen, Germany). Main advantages of percutaneous LVADs over surgically implanted LVAD’s are their lower cost and easier and faster implantation and removal (generally implanted under fluoroscopy in catheter laboratory). Disadvantages are vascular entrance complications and limited flow (94). Despite the fact that percutaneous VADs are applied successfully in cardiogenic shock after AMI and early survival rate is around 70%, superiority of percutaneous VADs over IABP was not supported adequately by randomized trials (95, 96).
ECMO (Extracorperal Membrane Oxygenation)
The principle of ECMO is to remove part of blood from body and transfuse it back to the bloodstream after oxygenisation. There two forms of ECMO: veno-venous and veno-arterial. Veno-venous way is preferred in respiratory insufficiencies without cardiac dysfunction and veno-arterial way is preferred in HF (cardiac ECMO). Veno-arterial ECMO has a rapid onset of action. It decreases cardiac preload and oxygen consumption, fulfills oxygen demand of body and supplies blood to vital organs (97). Either due to its effectiveness or rapid and easy application, ECMO has become a frequently preferred mechanical support device for AHF in all age groups including newborn babies, cardiogenic shock, HF developed following cardiac surgery, pulmonary emboli, fatal arrhythmias and cardiac arrest. ECMO indications are same as other LV assist devices. Decision is based on INTERMACS category of the patient (98). ECMO indication exists at INTERMACS profile 1 and profile 2 [Profile 1: Hemodynamic instability in spite of increasing doses of catecholamines and/or mechanical circulatory support with critical hypoperfusion of target organs (severe cardiogenic shock) and Profile 2. Intravenous inotropic support with acceptable blood pressure but rapid deterioration of kidney function, nutritional state, or signs of congestion]. Recently patients with profile 3 and 4 may also undergo ECMO implantation because of beneficial results obtained in these patients (Profile 3. Hemodynamic stability with low or intermediate, but necessary due to hypotension, doses of inotropics, worsening of symptoms, or progressive kidney failure and Profile 4. Temporary cessation of inotropic treatment is possible, but the patient presents frequent symptom recurrences and typically with fluid overload). Major contraindications to ECMO are uncontrolled bleeding, unrecoverable neurologic and end organ damage, and untreatable malignity, patients with end-stage HF who are not candidates for heart transplant or persistent LVAD treatment, absence of appropriate vascular entrance for ECMO cannulation and aortic dissection or serious aortic insufficiency.
Survival with cardiac ECMO was reported at varying rates between 23-71% (99). Highest mortality was observed in cases with cardiac arrest and lowest mortality was observed in cases with HF developed following myocarditis (100, 101).
Weaning time from ECMO changes according to the patient. Minimum time required for a damaged ventricle to recover itself should not be less than 12-24 hours. Generally, ECMO is removed in most of the patients in less than 1 week. During weaning process venous saturation, acid-base balance and urination parameters and cardiac functions should be monitored. ECMO flow should be decreased to the level of 1 L/min/m2 gradually. At this step, pharmacological inotropics may be necessary. If cardiac functions do not recover during this time, long term LVAD should be started.
6. Special groups
6.1 Treatment algorithms according to clinical scenarios
Major determinants of the clinical scenario are SBP systemic or pulmonary congestion, peripheral perfusion, volume overload or redistribution, precipitating factors, underlying etiology and rate of appearance of symptoms and findings.
Clinical scenario 1: Acute heart failure presenting with
hypertension accompanied by dyspnea and/or congestion
SBP is >140 mm Hg in approximately half of AHF patients. This clinical scenario mostly appears as the first attack of AHF and symptoms start suddenly. Predominant finding is pulmonary (radiographic/clinical) rather than systemic congestion due to rapid fluid redistribution from systemic to pulmonary circulation (Table 1) (101-103). Tachycardia and vasoconstriction are observed due to increase of sympathetic and neurohormonal activities.
This group of patients responds well to vasodilator therapy and usually do not need high diuretic doses as their main mechanism of dyspnea is fluid redistribution to the lungs rather than fluid accumulation.
Clinical scenario 2: Acute heart failure presenting with
normal SBP accompanied by dyspnea and/or congestion
SBP is in normal limits in ~40% of the cases. In this second scenario, symptoms and signs develop gradually in days or weeks. There is significant systemic congestion, which results in weight gain and peripheral edema. Pulmonary congestion may also be present; but it is milder than in the first clinical scenario. Generally, the second scenario develops as acute decompensation of chronic HFF LV systolic dysfunction and dilatation are detected on echocardiography. Chronic ventricular high pressure increases further with the deterioration of the clinical symptoms. Multi-organ failure may be observed with progressive deterioration of the clinical condition. Renal and/or liver dysfunction or worsening may appear.
Parenteral vasodilators are the cornerstone of the treatment, but care must be taken not to decrease SBP below 110 mm Hg. Low dose IV diuretics (furosemide 20 mg IV bolus) can also be added. Vasodilator and diuretic dosage can be increased according to the clinical and hemodynamic response. If pulmonary congestion is the predominant presentation and systemic congestion is absent, starting treatment with high dose potent diuretics may lead to hypovolemia and hypoperfusion that result in worsening renal function. In cases with dyspnea accompanied by weight gain, peripheral edema and venous distention, IV diuretics (furosemide 20-40 mg bolus) and vasodilators should be given together and their dosage should be titrated according to the diuretic and clinical response. If adequate diuresis cannot be achieved, continuous diuretic infusion should be considered.
Vasodilatator drugs such as nesiritide may be considered in the presence of high systemic vascular resistance accompanied by low bioactive BNP levels. However, data of wide scale clinical trials indicated that patients using nesiritide have the risk of hypotension and additionally there is no decrease in mortality and/or hospitalizations (104, 105).
Studies with the novel vasodilator serelaxin were promising for patients in this clinical scenario (see Section 5.4.4). Ongoing trials with this agent will show its exact role in AHF.
Clinical scenario 3: Acute heart failure presenting with
hypotension, dyspnea and other congestion symptoms
This is the rarest AHF scenario (<%10) characterized by low SBP and low cardiac output together with findings of organ hypoperfusion (106). Two different subtypes are defined: 1) Cardiogenic shock with marked hypoperfusion and 2) Low SBP without evidence of hypoperfusion/cardiogenic shock. Cardiogenic shock is characterized by low cardiac output accompanied by signs of organ hypoperfusion (hypotension, changes in mental status, cold and cyanotic skin) and evidence of volume overload (dyspnea, rales). Hemodynamic findings include prolonged and resistant hypotension, decrease in cardiac index (<2,2 L/min/m2) and increase in PCWP (>18 mm Hg).
Patients with cardiogenic shock should undergo echocardiography examination in order to detect and manage acute pathologies that deteriorate hemodynamic condition. Swan-Ganz catheterization is frequently performed to confirm the diagnosis, to ensure that filling pressures are adequate, and to guide changes in therapy. Pharmacological support includes inotropic and vasopressor agents which should be used in the lowest possible doses. Higher inotropic and vasopressor doses are associated with poorer outcomes. In patients with persistent hemodynamic impairment despite inotropic and vasopressor treatment, IABP or temporary mechanic support devices should be considered.
Clinical scenario 4: Acute heart failure complicating acute coronary syndromes
AHF complicating ACS includes patients with de novo HF that developed during an ACS or patients with worsening of preexisting HF. ACS was the triggering factor of HF in 30% of patients included in EuroHeart Survey II (12). New onset HF was detected in 37% of the patients enrolled in same study and 42% of these cases were due to ACS. HF was present in two thirds of patients on admission, in the remaining patients it developed during hospital stay. AHF is observed at similar rates in ST segment elevated and non-ST segment elevated MI and at lower rates in unstable angina patients. LVEF was preserved in approximately half of the patients. ACS patients with prior LV dysfunction, transmural anterior MI, advanced age, markedly high cardiac injury biomarkers and no reperfusion treatment have higher risk of new onset AHFF Patients with HF complicating ACS have a poorer short- and long-term prognosis compared to ACS patients without HF (107, 108). Those who developed HF during hospital stay and have advanced HF have worse prognosis (109). HF recurrence rate is also high at recovered patients (110).
In ACS patients, LV diastolic filling pressure increases and/or cardiac output decreases due to ischemia (decreasing ventricular compliance, stunning or hibernating) or infarction (extensive myocardial loss, free wall rupture, ventricular septal rupture and papillary muscle dysfunction). Subsequent pulmonary congestion or tissue perfusion damage lead to HFF Preexisting heart disease, arrhythmias, anemia, hypertension, hypovolemia, acidosis, hypoxia and negative inotropic or inappropriate use of vasodilator drugs trigger or contribute to develop of HFF
Cases with AHF complicating ACS should be taken into CCU as soon as possible. Antiplatelet agents, heparin, nitrates (if SBP is >90 mm Hg) and nasal oxygen should be given as the initial therapy immediately after establishing the diagnosis. IV morphine may be considered in patients with ongoing or recurrent angina despite nitrates treatment. However, as data of CRUSADE trial (111) showed that morphine is associated with an increased mortality in patients with unstable angina and non ST-segment elevated MI, this treatment should not be given unless necessary.
The cornerstone of treatment consists of revascularization (thrombolytic, percutaneous coronary intervention or coronary bypass surgery) (112). If revascularization cannot be performed immediately or performed revascularization is inadequate, care should be taken that pharmacological treatment does not impair coronary perfusion (hypotension should be avoided and LV end-diastolic pressure should be kept at optimal level) and not increase oxygen demand of myocardium (heart rate and contractility should not increase).
SBP is the most important determinant of in-hospital, short- and long-term mortality (13). In cases with SBP <90 mm Hg, serious hypotension may be the result of hypovolemia, arrhythmias, left and right ventricular dysfunction, mechanical complications due to MI, use of vasodilators or fibrinolytics, concomitant sepsis or pulmonary emboli. Generally, hypovolemia is considered if LVEF is >35%. If EF is <35%, pump failure due to myocardial damage is the main reason of hypotension, but hypovolemia may also be an accompanying factor. Hypovolemia can be determined by clinical findings or right heart catheterization when necessary. In the absence of overt pulmonary congestion, fluid administration may be helpful for differential diagnosis. Indicator of an optimal preload achieved by fluid administration is PCWP which should be ~18 (15-20) mm Hg. If hemodynamic improvement cannot be established IABP or short-term vasopressor agents should be considered. If mean arterial pressure cannot be increased over 65 mm Hg, heart transplantation or long-term assist device implantation as bridge to transplantation should be planned according to the patients’ status. For directing treatment of mechanical complications in ACS Table 15 maybe helpful.
Table 15.
Echocardiographic findings and treatments of mechanical complications which may develop in patients with acute coronary syndromes
| Diagnosis | Echocardiographic findings | Initial treatment | Advanced treatment |
|---|---|---|---|
| Right ventricular myocardial infarction | •Supports ECG and clinical findings •Right ventricular dilatation •Movement disorder on right ventricular free wall |
•Stop nitrates •Dont give diuretics •Load fluid •Correct bradycardia |
•PCI/Thrombolytic •IABP •Inotropic treatment (levosimendan) |
| Free wall rupture | •Pericardial effusion •Tamponade •Echogenic particules in effusion |
•Pericardiosynthesis •Load fluid •IABP |
• Emergency surgery |
| Ventricular septal rupture | •Location, size, Qp/Qs •Right heart catheterisation if findings are uncertain |
If stable with medical treatment | •Coronary angiography •Immediate surgery |
| If hemodynamic unstability •IABP •Intubation •Right heart catheterisation |
•Coronary angiography •Emergency surgery |
||
| Acute mitral regurgitation | •Acute mitral regurgitation •Papillary muscle rupture •TEE if findings are uncertain •If TEE is not adequate: Right heart catheterisation (to exclude ventricular septal rupture) |
If stable with medical treatment | •Coronary angiography •Immediate surgery |
| If hemodynamic unstability •IABP •Intubation •Right heart catheterisation |
•Coronary angiography •Emergency surgery |
||
| Dynamic LV outflow tract obstruction | •Akinetic apex •Hyperdynamic basal IVS •Systolic anterior motion (SAM) |
Drugs to be discontinued •Inotropics •Nitrates •IABP |
Drugs to be given: Beta-blocker |
ECG - electrocardiography; IABP - intra-aortic balloon pump; IVS - interventricular septum; PCI - percutaneous coronary intervention; Qp/Qs - ratio of pulmonary flow (Qp) to systemic flow (Qs); TEE - transesophageal echocardiography
The cornerstone of the management is to provide coronary reperfusion. Vasodilators and inotropic or vasopressor treatments for appropriate cases combined with ACS-specific therapy should be given. High dose diuretic treatment should be avoided in AHF due to ACS, as these patients are usually euvolemic.
Clinical scenario 5: Acute right heart failure
Right heart failure effects morbidity and mortality negatively in both chronic and acute HF. Right HF and pulmonary hypertension due to left HF cause excessive increase in right ventricular filling pressure and lead to increased venous pressure in splanchnic bed. This situation results in edema and endotoxemia on intestinal wall with permeability problems and in decrease of absolute glomerular pressure (renal arterial pressure-renal venous pressure) in kidneys. Renal venous pressure, in other words central venous pressure is accepted as the most important reason for renal perfusion decrease and renal dysfunction. In these patients, it is important to decrease right side pressures by appropriate vasodilator and diuretic treatment combination. Diuretic treatment may remain ineffective in cases with persistently high venous pressure.
In right sided AHF, fluid loading is usually not effective. Inotropic treatment is recommended in case of hypotension and hypoperfusion. It should be kept in mind, that mechanical ventilation may further deteriorate clinical findings by increasing pulmonary resistance. Etiology-specific treatments, like revascularization and intra-aortic balloon pump for right ventricular MI or thrombolytic and anticoagulant treatment for right sided HF due to pulmonary embolism, should be considered.
6.2 Cardiorenal syndrome
The interaction between cardiac and renal diseases is bidirectional. Acute or chronic HF affects renal functions as acute and chronic renal diseases effect heart. The term “cardiorenal syndrome” (CRS) is used to express this interaction. CRS has 5 types (113):
Type 1 - Acute renal damage developed due to AHF
Type 2 - Progressive chronic renal disease developed due to chronic cardiac dysfunction (e.g. chronic HF)
Type 3 - Acute cardiac dysfunction developed due to sudden primary renal failure (e.g. renal ischemia or glomerulonephritis)
Type 4 - Cardiac dysfunction such as arrhythmias and CAD or HF developed due to primary chronic renal disease (renocardiac syndrome).
Type 5 (secondary) - Development of both cardiac and renal dysfunction as a result of acute or chronic systemic diseases (e.g. sepsis, diabetes).
Frequency of moderate to severe renal failure (eGFR<60 mL/min/1.73 m2) is approximately 30-60% in HF patients. In Acute Decompensated Heart Failure National Registry (ADHERE) study (114), chronic kidney disease (serum creatinine >2.0 mg/dL) was detected in 30% of the patients hospitalized due to HF. Average eGFR was 55 mL/min/m2 and normal values (>90 mL/min/1.73 m2) were detected in only 9% of the patients. Type 1 or type 2 CRS may develop during the treatment of HF, even if the initial renal functions are normal. An increase of 0.3 mg/dL in serum creatinine, which occurs generally in first 3-5 days of hospitalization, was reported in ~20-30% of patients in different series (115, 116). Risk factors enhancing development of renal dysfunction in HF patients include previous HF, history of diabetes, uncontrolled hypertension and a baseline creatinine >1.5 mg/dL.
6.2.1 Pathophysiology and clinical findings
There is a complicated pathophysiologic relation between two insufficient organs and each of them may affect the other by similar hemodynamic, neurohormonal and immunologic/biochemical feedback pathways. A common opinion about renal dysfunction developed during AHF is that; renal dysfunction is directly a result of decrease in renal blood flow in patients with decreased LV systolic functions. However, recently obtained data indicate that neurohormonal activation, central venous congestion, anemia and oxidative stress play a complex role in CRS development.
There is no linear relationship between cardiac output, renal blood flow and renal dysfunction. If contractility, heart rate and cardiac index increase with clinical inotropic treatment, a short term increase in urine volume is also observed. Nevertheless, inotropic treatment is not associated with an improved prognosis in CRS. In Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial (117), increase of cardiac index was not associated with a recovery in renal functions.
Another factor that decreases GFR independent from renal blood flow is hypotension, which is reported in 2% of patients hospitalized with decompensated AHF. The most important determinant of cardiovascular circulation is pressure gradient in capillary network system. As long as an adequate capillary arterio-venous pressure gradient is maintained, blood stream increases (Poiseuille law). If arterial pressure decreases or venous pressure increases, capillary gradient and therefore blood stream decreases. In cases with increased central venous pressure (CVP) like in HF, glomerular pressure gradient which is a capillary network also decreases. Renal blood flow is affected from pressure increase in efferent arterioles more than pressure decrease in afferent arterioles. Thus, it is suggested that decrease in both urine volume and renal blood flow is associated with renal venous pressure increase rather than the decrease in arterial pressure (118).
A higher CVP was detected in hospitalized AHF patients with renal dysfunction and relationship between CVP and renal dysfunction was reported to be independent from SBP PCWP and cardiac index. Another finding that supports the role of increased CVP (and renal venous pressure) is the association of cervical venous distention with high serum creatinine, hospitalization due to HF and mortality risk (119).
It is important to differentiate CRS from underlying renal disease in HF patients with increased serum creatinine and/or decreased eGFR. Making this differentiation is not always possible and in some patients both can be possible. Significant proteinuria (generally >1000 mg/day), hematuria together with urinary sediments, small size of kidneys in radiologic evaluation suggest underlying renal disease. A normal urine test is typical for CRS; however, it should be remembered that urine tests may also be normal in nephrosclerosis and obstructive nephropathy. Blood urine nitrogen/creatinine ratio (BUN/Cre) is frequently used to differentiate pre-renal from renal azotemia. Increased BUN/Cre ratio (>20) typically indicates pre-renal etiology (if there is no increase in urine production). Diuretic treatment should not be withheld in HF cases with increased BUN/Cre ratio if marked congestion exists. Urine sodium excretion may be helpful while making up this decision. As RAAS and sympathetic nervous system are activated in HF, sodium retention increases and urine sodium concentration is expected to be <25 mEq/L. However, this value may increase a little during diuretic administration.
6.2.2. Treatment
Diuretics
Effect of diuretic treatment on GFR is considerably variable in HF patients. In some patients, diuretics increase serum creatinine by decreasing cardiac filling pressure and cardiac output, whereas others do not experience any change in LV enddiastolic pressure, cardiac output and serum creatinine. In some patients, a decrease in serum creatinine is observed with diuretic treatment as a result of recovery of LV filling and functions by a decrease in intra-abdominal, renal venous and right ventricular dilatation (reverse Bernheim phenomenon).
There is no randomized controlled trial investigating the effect of loop diuretics on cardiovascular endpoints in type 1 CRS patients. Nevertheless, it is unacceptable to leave AHF patients with congestion. If clinical congestion exists, a high creatinine level does not impede diuretic administration; moreover, renal functions may recover by diuresis in patients with serious congestion and renal dysfunction. However, it is not possible to predict which patient will recover by diuretic treatment prior to treatment.
Best clinical results in decompensated AHF were obtained in patients in whom fluid was aggressively removed despite causing low-moderate intensity damage to renal functions. Hemoconcentration (described as increase in at least two of hematocrit, serum albumin or serum total protein values) which is the indicator of fluid loss in trials was found to be related with renal dysfunction and mortality decrease (120). Renal function in these patients generally return to normal within four weeks after hospital discharge. Hemoconcentration developed in late rather than early phase of hospitalization is related to high dose diuretic administration, more weight loss and survival improvement (121). Patients who developed hypotension or renal dysfunction during diuretic treatment should receive diuretics at decreased doses.
Renin-angiotensin-aldosterone-system antagonists
From HF or renal insufficiency whichever is the primary one, renin angiotensin aldosterone system (RAAS) causes progression of other disease by activating angiotensin II and reactive oxygen products (122). Oxidative damage is considered among the factors in CRS development.
GFR decreases in most of the AHF patients following initiation of RAAS antagonists, but a moderate increase may also be observed in some patients. In analyses investigating effects of RAAS antagonists in HF patients with chronic renal diseases, it was determined that benefits of these drugs are not effected by renal dysfunction; but hyperkalemia and deterioration of renal dysfunction may be observed at a higher level (123, 124). Increase in serum creatinine is expected to be much higher in patients with low BP who take diuretics concomitantly. In these patients, mild creatinine elevation should be monitored, and in further increases diuretic dose should be diminished as the first measure. If they are considered to cause significant renal dysfunction, they should be discontinued. RAAS blockers are usually not prescribed in AHF patients during discharge, because of concern of renal dysfunction. However, they are definitely beneficial in HF and morbidity and mortality increase in patients who have discontinued of these drugs. RAAS blocker titration should be closely followed particularly in patients with chronic renal disease.
Role of mineralocorticoid receptor antagonists (MRA) is unknown in CRS treatment. Data regarding use of spironolactone in HF patients at a dose of diuretic effect (at least 50 mg/day) are limited. Sodium retention is prevented completely and atrial natriuretic peptide was decreased significantly by giving 200 mg spironolactone two times daily to severe HF patients with diuretic resistance (125). Today, natriuretic doses of MRA’s and effects of these doses in AHF and CRS patients are not adequately known and more extensive trials are required.
Vasodilators
The beneficial effects of intravenous vasodilators on renal functions and mortality could not be confirmed. In ADHERE registries including approximately 100.000 AHF patients, renal dysfunction developed more frequently in patients who received vasodilators in addition to intravenous diuretic treatment (126). However, a causal relationship cannot be established, because HF patients who received combination treatment were in advanced HF stage.
Effects of nesiritide; a synthetic natriuretic peptide, on renal functions were much more debated. In a meta-analysis of seven multi-center randomized controlled trials, significant renal dysfunction was detected in AHF patients who were given nesiritide treatment (127). Conversely, in two recent randomized controlled trials, nesiritide did not disturb renal functions despite not providing clinical benefit (63, 104).
Inotropic drugs
Inotropics are not routinely used except for selected AHF patients or cardiogenic shock. Their role in CRS treatment is unclear. Inotropic drugs are expected to improve renal functions by increasing renal blood flow and probably decreasing renal venous pressure. However, findings that support this expectation were only observed in patients receiving dopamine. Dopamine doses which increase blood flow are <3 pgr/kg/min and this effect is considered to be dependent on dilatation dopamine exerts in both large and small resistant renal vessels (128). Nevertheless, clinical effectiveness and safety of dopamine in CRS patients could not be proven.
Ultrafiltration
Ultrafiltration is usually applied in patients with diuretic resistance and/or renal dysfunctionin AHF (See Section 4.3.3). Current international guidelines indicate that ultrafiltration can be applied if urine output is <20 mL/h despite standard treatment in acute pulmonary edema; however, its effectiveness and safety is not adequately known.
Vasopressine antagonists (Aquauretics)
In Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) study (64), urine output was increased in patients receiving tolvaptane and serum creatinine increase (0.03 mg/dL versus 0.08 mg/dL) was statistically significant compared to placebo, but it remained clinically insignificant. Trials investigating role of tolvaptane in CRS are still continuing.
Adenosine-AI receptor antagonist
PROTECT study (129) investigated effects of rolofylline, an adenosine-A1 receptor antagonist in hospitalized AHF patients with renal dysfunction. Frequency of cardiovascular endpoints and renal dysfunction were similar with selective adenosine A1 receptor antagonist to placebo, moreover neurologic adverse events (stroke and seizure) were frequent in patients receiving rolofyllin.
Serelaxin
In studies performed in AHF patients with serelaxin, there was no increase in new onset renal failure and besides, adverse events related to renal dysfunction developed less compared to placebo. Patients required less intravenous diuretic and vasoactive drugs (74, 75). Ongoing phase 3 RELAX-2 study is expected to give more detailed information regarding serelaxin use in CRS patients.
7. Discharge from hospital and long-term objectives and strategies after hospitalization
For patients admitted with decompensated AHF, determining the optimal time of discharge is important for further morbidity and HF hospitalizations. Although there is an impetus to shorten hospital stay, discharging these patients too early when they are not adequately compensated can lead to persistent and recurrent HF decompensations and HF hospitalizations. At the time of discharge, the patient should be hemodynamically stable. That is, the patient should not be hypotensive, and hypertension should be well controlled. Any active rhythm problems such as bradycardia and rate control for atrial fibrillation should be addressed and no significant ventricular arrhythmias should be present. Levels of serum electrolytes and other major laboratory markers must be stable and the patient must be at least near to euvolemia. Other than clinical judgment, no other surrogate markers are currently required in deciding or targeting for discharge readiness. Although high BNP concentrations are associated with a poor prognosis, and a drop in BNP levels correlates with a better prognosis, a BNP-guided treatment in decompensated AHF has demonstrated conflicting results. Thus, it is still recommended to clinically assess the patient’s readiness for discharge rather than basing the decision on the level of BNP as an inpatient. However, the ACC/AHA guidelines do state that clinical risk-prediction tools and/or biomarkers can be used to identify patients at higher risk for post-discharge clinical events (3, 130).
At the time of discharge, the patient must be switched from IV diuretic therapy to oral diuretic therapy and should be stable on the oral regimen. Furthermore, guideline directed medical therapy must be started and if not, the reason for not starting should be documented. Initiation and continuation of evidence-based heart failure medications has been demonstrated to significantly improve clinical outcomes for patients with systolic HF (Table 16) (2, 3, 131). However, caution is advised in certain patient groups such as the patients who were on inotropes during hospitalization and beta-blockers will be initiated and the patients with renal insufficiency or at risk for hyperkalemia for which therapy with angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARB) or aldosterone antagonists is planned. Ideally, the plan for medical therapy in the outpatient setting should be outlined in the discharge summary, that is, plan for up titration or addition of medications. If the patient does not have a device, the need for device therapy should be assessed (implantable defibrillator and cardiac resynchronization therapy) and plan for implantation should be outlined (2, 3).
Table 16.
| Daily starting dose | Daily target dose | |
|---|---|---|
| Angiotensin converting enzyme (ACE) inhibitor | ||
| Captopril | 6.25 mg t.i.d | 50 mg t.i.d |
| Enalapril | 2.5 mg b.i.d | 10-20 mg b.i.d |
| Lisinopril | 2.5-5 mg o.d | 40 mg o.d |
| Ramipril | 1.25-2.5 mg o.d | 5 twice or 10 mg o.d |
| Trandolapril | 0.5-1 mg o.d | 4 mg o.d |
| Beta-blocker | ||
| Bisoprolol | 1.25 mg o.d | 10 mg o.d |
| Carvedilol | 3.125 mg b.i.d | 25-50 mg b.i.d |
| Metoprolol succinate (CR/XL) | 12.5-20 mg o.d | 200 mg o.d |
| Nebivolol* | 1.25 mg o.d | 10 mg o.d |
| Angiotensin receptor blocker | ||
| Candesartan | 4-8 mg o.d | 32 mg o.d |
| Valsartan | 40 mg b.i.d | 160 mg b.i.d |
| Losartan | 50 mg o.d | 150 mg o.d |
| Aldosterone antagonists | ||
| Eplerenone | 25 mg o.d | 50 mg o.d |
| Spironolactone | 25 mg o.d | 25-50 mg o.d |
| Hydralazine and isosorbide dinitrate | ||
| Hydralazine | 25 to 50 mg t.i.d or four times | 300 mg daily in divided doses |
| Isosorbide dinitrate | 20 to 30 mg t.i.d or four times | 120 mg daily in divided doses |
Modified from ESC 2012 and ACCF/AHA heart failure guidelines
Recommended by ESC but not by ACCF/AHA guidelines
Prior to discharge, comprehensive HF education should be completed with the patient and caregivers. This includes educating the patient on the nature of the disease, medications to be taken, diet, fluid intake, factors that may cause HF decompensation, signs and symptoms of HF decompensation, what to do if symptoms worsen, monitoring of weight and vitals (blood pressure and heart rate) and recommended activity level (132). Written instructions and educational material should be given to the patient and family to review at home (Table 17). Several national societies provide resources for patients and many centers have put together their own education pamphlets (133, 134). Thorough communication of discharge instructions together with close outpatient follow-up has demonstrated improved outcomes in these patients (135). Moreover, an additional 1 hour teaching session with a nurse at the time of hospital discharge has been shown to improve compliance, decrease risk of rehospitalization or death at lower costs of care (136).
Table 17.
Patient discharge instructions
| Dispostion/ | • Home with home health care |
|---|---|
| discharge to | •Inpatient rehabilitation facility •Palliative care (home or inpatient) |
| Activity | • Restricted, as tolerated, rehabilitation •Return to work •Return to driving |
| Diet/fluid restriction | • Fluid intake 1.5-2 L/day • Sodium restriction (2-3 g/day |
| Medications | •Anticoagulation medications •Antiarrhythmic agents •HF medications |
| Follow-up | •Follow-up appointments •Laboratory tests •Imaging tests •Referral to electrophysiologist (device therapy?) •Cardiology (preferably HF) clinic visit |
| Weight log | •Take log book to doctor's visit •Call provider if you gain 1.5-2 kg over 2-3 days •Weigh every day at same time and write it in log book |
| Vitals log | •Heart rate •Blood pressure |
| Smoking | • Assess the status, |
| cessation | •Advice and assist to quit, •Arrange a smoking cessation programme |
| Alcohol limitation/elimination | • 2 units per day in men or 1 unit per day in women. 1 unit is 10 mL of pure alcohol (e.g. 1 glass of wine, 1/2 pint of beer, 1 measure of spirit). |
| Discuss symptom recognition and management | •Inform patients on dyspnea and signs/symptoms of congestion •Educate regarding self-adjusting their diuretic doses by monitoring their congestive symptoms and signs as well as daily body weights |
| Contact numbers for during office hours and after hours questions/concerns | • Stay connected with the patients by telephone monitoring programs |
As with any other cardiovascular admission, cardiovascular risk factors and other co-morbidities should be addressed during the hospitalization and patients should be counseled with respect to better controlling these risk factors, eg. dietary counseling for diabetics, diet and exercise, weight loss, sleep apnea, smoking cessation and alcohol moderation.
A smooth transition from inpatient to outpatient care can be challenging in HF patients due to the complex nature of the disease, associated co-morbid conditions, multiple medications and various specialties involved in the care of the patients but is critical for maintaining patients in an optimized state and improving long-term outcomes. Key to ensuring optimal outpatient care and preventing rehospitalization is deciding on the most advantageous disposition for the patient, that is, home with or without home health care, nursing home, rehabilitation facility or palliative care. The patient’s functional capacity and ability to ambulate should be assessed (if possible with the help of physiotherapists) prior to discharge and respective arrangements made depending on the patient’s ambulatory status. If available, it is helpful to use case management and social work services to aid in the decision making and organizing. An outpatient disposition strategy should be delineated for each patient individually. In general, a follow-up clinic visit should be scheduled at 7-10 days after discharge with plan to check laboratory studies for monitoring of renal function and electrolytes (3, 137, 138).
A little more stringency is advised for patients with advanced HF and recurrent HF decompensations/hospitalizations. Prior to discharge, these patients should be stable on an oral medication regimen for at least 24 hours, that is, they should be stable on oral diuretics and intravenous vasodilators and inotropes (unless they are discharged with home inotropic therapy). For these high risk patients, plans for close follow-up should be made by ensuring monitoring devices are available at home (such as scales and blood pressure monitors) and home health care/visiting nurse arranged for certain patients. There should be a follow-up by a visiting nurse or telephone call within 3 days of discharge (3, 132, 133). The ACC/AHA guidelines, recommend that these patients at high risk for hospital readmission be referred to multidisciplinary HF disease-management programs to reduce the risk of rehospitalization for HF (3, 130, 139, 140). A systematic review of several trials has demonstrated that specialized multiprofessional outpatient care reduced mortality by 25%, HF hospitalizations by 26%, and all-cause hospitalizations by 19% (130).
A comprehensive all-around discharge preparation of the ADHF patient and family is vital for a good patient outcome in the long run (Table 18). Individual hospitals have devised their own discharge checklists for the physicians to ensure that all aspects of care have been addressed. In a small study of 96 patients, a checklist was used prior to discharge by physicians in the care of 48 patients. The remaining 48 patients were discharged in a conventional manner. The patients who were discharged using the checklist, were more likely to be on ACE inhibitors or ARBs, had a higher rate of dose uptitration for ß-blockers and/or ACE inhibitors/ARBs and demonstrated a lower 30-day (19% to 6%) and 6-month (42% to 23%) readmission rate (141). The results of this small study are promising but will have to be replicated in a randomized fashion and on a larger scale.
Table 18.
Physician discharge checklist
| Disposition: assess with help of case manager, social worker, physiotherapist | • Home with physical therapy or arranged cardiac rehabilitation • Inpatient rehabilitation • Palliative care |
| Precipitating factors for HF decompensation identified and addressed | • Arrhythmias • Myocardial ischemia • Non-adherence to medication • Non-adherence to diet •Hypertension •Infections |
| Medications: Yes, No, Reasons for not prescribing/contraindications | • Medications for AF: antiarrhythmics, anticoagulation • Medications for CAD,: ASA, statins •Antidiabetics •Influenza and pneumococcal vaccination •HF medications (including digoxin, ivabradin, nitrates, LCZ 696, etc.) |
| Device therapy: | •Assess indication •Electrophysiology/arrhythmia consult if indicated |
| Counseling | •Fluid intake •Diet •Daily weights +/- vitals •Educate on HF symptoms and what to do •Education on medications, importance of compliance, potential side effects •Physical activity •Address risk factors: smoking, alcohol, weight loss •Preferred if available: Enhanced teaching by HF nurse, teaching by dietitian/nutritionist |
| Follow-up | •Dietitian •Laboratory tests •Testing: chest x-ray, echocardiogram, stress test, ECG, etc. •Physicians (primary care physician, cardiologist, electrophysiologist, other specialists) |
AF - atrial fibrillation; ASA - acetylsalicylic acid; CAD - coronary artery disease; ECG - electrocardiogram; HF - heart failure
An algorithm summarizing the approach to the diagnosis and management of AHF from emergency department until discharge is presented in Figure 3.
Figure 3.
Algorithm for diagnosis and management of acute heart failure
ACEI - angiotensin converting enzyme inhibitor; ARB - angiotensin receptor blocker; BIPAP - bilevel positive airway pressure; CPAP - continuous positive airway pressure; CRS - cardiorenal syndrome; CRT - cardiac resynchronization therapy; ECG - electrocardiogram; IABP - intra aortic balloon pump; ICD - implantable cardioverter defibrillator; MRA - mineralocorticoid receptor antagonist; RAS - renin-angiotensin aldosteron system; SBP - systolic blood pressure; SpO2 - partial pressure of arterial oxygen; USG - ultrasonography
7.1 Following discharge
Following discharge, patients should be seen in the outpatient clinic within 7-10 days (Table 19). During the outpatient clinic visit, a thorough history with focus on symptoms of HF decompensation should be obtained. The activity level of the patient should be documented and which symptoms limit the activity level. The patient should be questioned on adherence to dietary instructions (fluid restriction, low salt) and medications. It should be noted if the patient is having any side effects to the medications or any difficulty taking them. The home weight, blood pressure and heart rate log should be reviewed and discussed with the patient. A thorough examination should be performed with focus on the cardiovascular system. Laboratory tests, specifically electrolytes and renal function may also be obtained on the first post-discharge visit.
Table 19.
Parameters to assess on follow-up clinic visits
| History | •Signs and symptoms of HF decompensation •Confirming patient's understanding of disease process and symptoms to watch out for •Any hospitalizations, admission to emergency department, visits with other physicians •Review with patient and family weight, blood pressure, heart rate log |
| Life style | •Confirming patient's understanding and adherence to diet and fluid restriction •Activity level |
| Medications | •Any changes in medications since last visit/discharge •Compliance, medication reconciliation •Any difficulty taking medications: side effects, affordability |
| Testing | •Laboratory tests (eGFR, electrolytes, BNP etc.) •Other (as needed): ECG, chest X-ray, echocardiography, stress testing, etc. |
| Medication uptitration or addition as tolerated | • Aim for target doses of beta-blockers, ACE-inhibitors • Adding ivabradine, LCZ696 etc. |
ACE - angiotensin converting enzyme; BNP - B-type natriuretic peptide; ECG - electrocardiogram; eGFR - estimated glomerular filtration rate HF - heart failure
If there are no contraindications, heart failure medications should be uptitrated gradually to the recommended goal dose. If the patient was not initiated on a beta-blocker in the hospital, addition of a beta-blocker should be considered if the patient is euvolemic and hemodynamically stable. During each clinic visit, it is important to reiterate the importance of compliance with diet, medications and daily weights and educate the patient on the expectations. The time of the next follow-up visit will depend on the patient’s disease severity, level of compliance, etc. In high risk patients, regular telephone support in between clinic visits should be considered. Although, individual RCTs did not show a robust benefit, a meta-analysis of RCTs did demonstrate a decrease in risk of HF hospitalization with this strategy (142, 143). Other than clinical follow-up, no other surrogate markers have been shown to reliably predict or recognize early worsening heart failure. Serial BNP monitoring has not been shown to be superior to clinical assessment and is not indicated other than to confirm clinical deterioration (144, 145). Moreover, monitoring thoracic impedance (non-invasive measure of intrathoracic fluid) and treatment decisions based on this has not beenshown to improve outcomes (139, 146). More recently, several implantable devices have been developed to measure pressures invasively in the outpatient setting. Although one trial did demonstrate a reduction in HF admissions when treatment was guided using the device, this strategy still has to be studied more closely before accepting it as a guideline recommendation (147).
Several National Societies offer resources to educate physicians and patients on heart failure and outline strategies to improved quality of care in these patients, reduce rehospitalizations and improve long-term outcomes. The American Heart Association, for example, has promoted the campaign “Get with the Guidelines”, a hospital-based performance improvement tool to help comply with evidence-based management of HF patients (148). The strategies in this campaign have been shown to reduce 30-day mortality and readmission rates in patients admitted with ADHFF A further AHA initiative, Target: HF, supplies health care providers with resources and materials to improve HF awareness, prevention and treatment. This initiative focuses on outpatient strategies such as medication optimization, early follow-up care and coordination, care transitions and enhanced patient education to improve HF quality of care, reduce readmission and improve long-term survival (149).
Although we have an armamentarium of medications and devices available for the treatment of HF, the outcomes in patients admitted with ADHF are still grim and leave a lot of room, for improvement. Apart from implementing the use of medications and devices, there has been an effort to raise awareness of this public health problem. The focus has been on educating, disseminating and implementing strategies that will help improve outcomes in these patients such as repeated education, rigorous follow-up and easing transition of care from hospital to home. There is still a lot to be done in this respect, but the discussed strategies are a step in the right direction.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept - D.U., Y.Ç., M.B.Y., M.E., M.Z., A. T.; Design - D.U., Y.Ç., M.B.Y., M.E., M.Z., A. T.; Supervision - D.U., Y.Ç., M.B.Y.; Data collection and/or processing - D.U., Y.Ç., M.B.Y., M.E., M.Z., A. T., K.K., K.R., B.B.; Literature review - D.U., Y.Ç., M.B.Y., M.E., M.Z., A. T.; Writer - D.U., Y.Ç., M.B.Y., M.E., M.Z., A. T., K.K., K.R., B.B.; Critical review - D.U., Y.Ç., M.B.Y., M.E., M.Z., A. T.
References
- 1.Onat A, Murat SN, Ciçek G, Ayhan E, Ornek E, Kaya H, et al. Regional distribution of all-cause mortality and coronary disease incidence in Turkey: findings of Turkish Adult Risk Factor survey 2010. [Article in Turkish] Turk Kardiyol Dern Ars. 2011;39:263–8. doi: 10.5543/tkda.2011.01446. [DOI] [PubMed] [Google Scholar]
- 2.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–69. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 4.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 5.National Clinical Guideline Centre (UK) Acute Heart Failure: Diagnosing and Managing Acute Heart Failure in Adults. London: National Institute for Health and Care Excellence (UK); 2014. Oct, [PubMed] [Google Scholar]
- 6.Mebazaa A, Yilmaz MB, Levy P, Ponikowski P, Peacock WF, Laribi S, et al. Recommendations on pre-hospital & early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine. Eur J Heart Fail. 2015;17:544–58. doi: 10.1002/ejhf.289. [DOI] [PubMed] [Google Scholar]
- 7.Zoghi M, Cavuşoğlu Y, Yilmaz MB, Nalbantgil S, Eren M, Mebazaa A. Practical approach to acute heart failure with algorithms. [Article in Turkish] Anadolu Kardiyol Derg. 2009;9:436–46. [PubMed] [Google Scholar]
- 8.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ES-ICM) Eur Heart J. 2008;29:2388–442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 9.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics-2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang J, Mensah GA, Croft JB, Keenan NL. Heart failure-related hospitalization in the U.S., 1979 to 2004. J Am Coll Cardiol. 2008;52:428–34. doi: 10.1016/j.jacc.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 11.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:200718. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 12.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–16. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, et al. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27:2725–36. doi: 10.1093/eurheartj/ehl193. [DOI] [PubMed] [Google Scholar]
- 14.Gheorghiade M, Abraham WT, Albert NM, Greenberg BH, O’Connor CM, She L, et al. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006;296:2217–26. doi: 10.1001/jama.296.18.2217. [DOI] [PubMed] [Google Scholar]
- 15.Maggioni AP, Anker SD, Dahlström U, Filippatos G, Ponikowski P, Zannad F, et al. Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12,440 patients of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail. 2013;15:1173–84. doi: 10.1093/eurjhf/hft134. [DOI] [PubMed] [Google Scholar]
- 16.Zannad F, Mebazaa A, Juillière Y, Cohen-Solal A, Guize L, Alla F, et al. Clinical profile, contemporary management and one-year mortality in patients with severe acute heart failure syndromes: The EFICA study. Eur J Heart Fail. 2006;8:697–705. doi: 10.1016/j.ejheart.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) Am J Cardiol. 2008;101:1016–22. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 18.Maggioni AP, Dahlström U, Filippatos G, Chioncel O, Crespo Leiro M, Drozdz J, et al. EURObservational Research Programme: regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot) Eur J Heart Fail. 2013;15:808–17. doi: 10.1093/eurjhf/hft050. [DOI] [PubMed] [Google Scholar]
- 19.Jong P, Vowinckel E, Liu PP, Gong Y, Tu JV. Prognosis and determinants of survival in patients newly hospitalized for heart failure: a population-based study. Arch Intern Med. 2002;162:1689–94. doi: 10.1001/archinte.162.15.1689. [DOI] [PubMed] [Google Scholar]
- 20.Aghababian RV. Acutely decompensated heart failure: opportunities to improve care and outcomes in the emergency department. Rev Cardiovasc Med. 2002;3(Suppl 4):3–9. [PubMed] [Google Scholar]
- 21.Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJ, Granger CB, et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116:1482–7. doi: 10.1161/CIRCULATIONAHA.107.696906. [DOI] [PubMed] [Google Scholar]
- 22.Killip T, 3rd, Kimball JT. Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol. 1967;20:457–64. doi: 10.1016/0002-9149(67)90023-9. [DOI] [PubMed] [Google Scholar]
- 23.Forrester JS, Diamond GA, Swan HJ. Correlative classification of clinical and hemodynamic function after acute myocardial infarction. Am J Cardiol. 1977;39:137–45. doi: 10.1016/s0002-9149(77)80182-3. [DOI] [PubMed] [Google Scholar]
- 24.Nohria A, Tsang SW, Fang JC, Lewis EF, Jarcho JA, Mudge GH, Stevenson LW. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol. 2003;41:1797–804. doi: 10.1016/s0735-1097(03)00309-7. [DOI] [PubMed] [Google Scholar]
- 25.Değertekin M, Erol C, Ergene O, Tokgözoğlu L, Aksoy M, Erol MK, et al. Heart failure prevalence and predictors in Turkey: HAPPY study. [Article in Turkish] Turk Kardiyol Dern Ars. 2012;40:298–308. doi: 10.5543/tkda.2012.65031. [DOI] [PubMed] [Google Scholar]
- 26.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33:1948–55. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 27.Cowie MR, Mosterd A, Wood DA, Deckers JW, Poole-Wilson PA, Sutton GC, Grobbee DE. The epidemiology of heart failure. Eur Heart J. 1997;18:208–25. doi: 10.1093/oxfordjournals.eurheartj.a015223. [DOI] [PubMed] [Google Scholar]
- 28.Eren M, Zoghi M, Çavuşoğlu Y, Orhan AL, TAKTIK Study Investigators Turkish Registry on hospitalized acute heart failure patients: TAKTIK. Cardiovascular Therapeutics. 2012;30(Suppl 1):31. OP66. [Google Scholar]
- 29.Fonarow GC, Abraham WT, Albert NM, Gattis WA, Gheorghiade M, Greenberg B, et al. Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF): rationale and design. Am Heart J. 2004;148:43–51. doi: 10.1016/j.ahj.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Opasich C, Rapezzi C, Lucci D, Gorini M, Pozzar F, Zanelli E, et al. Precipitating factors and decision-making processes of short-term worsening heart failure despite “optimal” treatment (from the IN-CHF Registry) Am J Cardiol. 2001;88:382–7. doi: 10.1016/s0002-9149(01)01683-6. [DOI] [PubMed] [Google Scholar]
- 31.Ambardekar AV, Fonarow GC, Hernandez AF, Pan W, Yancy CW, Krantz MJ, Get With the Guidelines Steering Committee and Hospitals Characteristics and in-hospital outcomes for nonadherent patients with heart failure: findings from Get With The Guidelines-Heart Failure (GWTG-HF) Am Heart J. 2009;158:644–52. doi: 10.1016/j.ahj.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 32.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–9. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 33.Dzudie A, Milo O, Edwards C, Cotter G, Davison BA, Damasceno A, et al. Prognostic significance of ECG abnormalities for mortality risk in acute heart failure: insight from the Sub-Saharan Africa Survey of Heart Failure (THESUS-HF) J Card Fail. 2014;20:45–52. doi: 10.1016/j.cardfail.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Václavík J, Spinar J, Vindis D, Vítovec J, Widimsky B, Cíhalík C, et al. ECG in patients with acute heart failure can predict in-hospital and long-term mortality. Intern Emerg Med. 2014;9:283–91. doi: 10.1007/s11739-012-0862-1. [DOI] [PubMed] [Google Scholar]
- 35.Aronson D, Burger AJ. The relationship between transient and persistent worsening renal function and mortality in patients with acute decompensated heart failure. J Card Fail. 2010;16:541–7. doi: 10.1016/j.cardfail.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Vyskocilova K, Spinarova L, Spinar J, Mikusova T, Vitovec J, Malek J, et al. Prevalence and clinical significance of liver function abnormalities in patients with acute heart failure. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014 doi: 10.5507/bp.2014.014. [DOI] [PubMed] [Google Scholar]
- 37.Masip J, De Mendoza D, Planas K, Paez J, Sanchez B, Cancio B. Peripheral venous blood gases and pulse-oximetry in acute cardiogenic pulmonary oedema. Eur Heart J Acute Cardiovasc Care. 2012;1:275–80. doi: 10.1177/2048872612457087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lok DJ, Klip IT, Voors AA, Lok SI, Bruggink-Andréde la Porte PW, Hillege HL, et al. Prognostic value of N-terminal pro C-type natriuretic peptide in heart failure patients with preserved and reduced ejection fraction. Eur J Heart Fail. 2014;16:958–66. doi: 10.1002/ejhf.140. [DOI] [PubMed] [Google Scholar]
- 39.McKie PM, Cataliotti A, Sangaralingham SJ, Ichiki T, Cannone V, Bailey KR, et al. Predictive utility of atrial, N-terminal pro-atrial, and N-terminal pro-B-type natriuretic peptides for mortality and cardiovascular events in the general community: a 9-year follow-up study. Mayo Clin Proc. 2011;86:1154–60. doi: 10.4065/mcp.2011.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daniels LB, Clopton P, Potocki M, Mueller C, McCord J, Richards M, et al. Influence of age, race, sex, and body mass index on interpretation of midregional pro atrial natriuretic peptide for the diagnosis of acute heart failure: results from the BACH multinational study. Eur J Heart Fail. 2012;14:22–31. doi: 10.1093/eurjhf/hfr157. [DOI] [PubMed] [Google Scholar]
- 41.Logeart D, Thabut G, Jourdain P, Chavelas C, Beyne P, Beauvais F, et al. Predischarge B-type natriuretic peptide assay for identifying patients at high risk of re-admission after decompensated heart failure. J Am Coll Cardiol. 2004;43:635–41. doi: 10.1016/j.jacc.2003.09.044. [DOI] [PubMed] [Google Scholar]
- 42.Volpicelli G, Melniker LA, Cardinale L, Lamorte A, Frascisco MF. Lung ultrasound in diagnosing and monitoring pulmonary interstitial fluid. Radiol Med. 2013;118:196–205. doi: 10.1007/s11547-012-0852-4. [DOI] [PubMed] [Google Scholar]
- 43.Vernon C, Phillips CR. Pulmonary artery catheters in acute heart failure: end of an era? Crit Care. 2009;13:1003. doi: 10.1186/cc8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nohria A, Mielniczuk LM, Stevenson LW. Evaluation and monitoring of patients with acute heart failure syndromes. Am J Cardiol. 2005;96:32G–40G. doi: 10.1016/j.amjcard.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 45.Wuerz RC, Meador SA. Effects of prehospital medications on mortality and length of stay in congestive heart failure. Ann Emerg Med. 1992;21:669–74. doi: 10.1016/s0196-0644(05)82777-5. [DOI] [PubMed] [Google Scholar]
- 46.Peacock WF, Emerman C, Costanzo MR, Diercks DB, Lopatin M, Fonarow GC. Early vasoactive drugs improve heart failure outcomes. Congest Heart Fail. 2009;15:256–64. doi: 10.1111/j.1751-7133.2009.00112.x. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi M, Kohsaka S, Miyata H, Yoshikawa T, Takagi A, Harada K, et al. Association between prehospital time interval and short-term outcome in acute heart failure patients. J Card Fail. 2011;17:742–7. doi: 10.1016/j.cardfail.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 48.Collins SP, Lindsell CJ, Naftilan AJ, Peacock WF, Diercks D, Hiestand B, et al. Low-risk acute heart failure patients: external validation of the Society of Chest Pain Center’s recommendations. Crit Pathw Cardiol. 2009;8:99–103. doi: 10.1097/HPC.0b013e3181b5a534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.ter Maaten JM, Valente MA, Damman K, Hillege HL, Navis G, Voors AA. Diuretic response in acute heart failure-pathophysiology, evaluation, and therapy. Nat Rev Cardiol. 2015;12:184–92. doi: 10.1038/nrcardio.2014.215. [DOI] [PubMed] [Google Scholar]
- 50.Nieminen MS, Böhm M, Cowie MR, Drexler H, Filippatos GS, Jondeau G, et al. Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:384–416. doi: 10.1093/eurheartj/ehi044. [DOI] [PubMed] [Google Scholar]
- 51.Peacock WF, Hollander JE, Diercks DB, Lopatin M, Fonarow G, Emerman CL. Morphine and outcomes in acute decompensated heart failure: an ADHERE analysis. Emerg Med J. 2008;25:205–9. doi: 10.1136/emj.2007.050419. [DOI] [PubMed] [Google Scholar]
- 52.Follath F, Yilmaz MB, Delgado JF, Parissis JT, Porcher R, Gayat E, et al. Clinical presentation, management and outcomes in the Acute Heart Failure Global Survey of Standard Treatment (ALARM-HF) Intensive Care Med. 2011;37:619–26. doi: 10.1007/s00134-010-2113-0. [DOI] [PubMed] [Google Scholar]
- 53.Allen LA, Metra M, Milo-Cotter O, Filippatos G, Reisin LH, Bensimhon DR, et al. Improvements in signs and symptoms during hospitalization for acute heart failure follow different patterns and depend on the measurement scales used: an international, prospective registry to evaluate the evolution of measures of disease severity in acute heart failure (MEASURE-AHF) J Card Fail. 2008;14:777–84. doi: 10.1016/j.cardfail.2008.07.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davison BA, Metra M, Cotter G, Massie BM, Cleland JG, Dittrich HC, et al. Worsening Heart Failure Following Admission for Acute Heart Failure: A Pooled Analysis of the PROTECT and RELAX-AHF Studies. JACC Heart Fail. 2015;3:395–403. doi: 10.1016/j.jchf.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 55.Song EK, Moser DK, Dunbar SB, Pressler SJ, Lennie TA. Dietary sodium restriction below 2 g per day predicted shorter event-free survival in patients with mild heart failure. Eur J Cardiovasc Nurs. 2014;13:541–8. doi: 10.1177/1474515113517574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vital FM, Ladeira MT, Atallah AN. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary oedema. Cochrane Database Syst Rev. 2013;5:CD005351. doi: 10.1002/14651858.CD005351.pub3. [DOI] [PubMed] [Google Scholar]
- 57.Gheorghiade M, De Luca L, Fonarow GC, Filippatos G, Metra M, Francis GS. Pathophysiologic targets in the early phase of acute heart failure syndromes. Am J Cardiol. 2005;96:11G–17G. doi: 10.1016/j.amjcard.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 58.Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu MY, Chang NC, Su CL, Hsu YH, Chen TW, Lin YF, et al. Loop diuretic strategies in patients with acute decompensated heart failure: a meta-analysis of randomized controlled trials. J Crit Care. 2014;29:2–9. doi: 10.1016/j.jcrc.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 60.Alqahtani F, Koulouridis I, Susantitaphong P, Dahal K, Jaber BL. A meta-analysis of continuous vs intermittent infusion of loop diuretics in hospitalized patients. J Crit Care. 2014;29:10–7. doi: 10.1016/j.jcrc.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 61.Ferreira JP, Santos M, Almeida S, Marques I, Bettencourt P, Carvalho H. Mineralocorticoid receptor antagonism in acutely decompensated chronic heart failure. Eur J Intern Med. 2014;25:67–72. doi: 10.1016/j.ejim.2013.08.711. [DOI] [PubMed] [Google Scholar]
- 62.De Vecchis R, Esposito C, Ariano C, Cantatrione S. Hypertonic saline plus i.v furosemide improve renal safety profile and clinical outcomes in acute decompensated heart failure: A meta-analysis of the literature. Herz. 2015;40:423–35. doi: 10.1007/s00059-013-4041-6. [DOI] [PubMed] [Google Scholar]
- 63.Triposkiadis FK, Butler J, Karayannis G, Starling RC, Filippatos G, Wolski K, et al. Efficacy and safety of high dose versus low dose furosemide with or without dopamine infusion: the Dopamine in Acute Decompensated Heart Failure II (DAD-HF II) trial. Int J Cardiol. 2014;172:115–21. doi: 10.1016/j.ijcard.2013.12.276. [DOI] [PubMed] [Google Scholar]
- 64.Chen HH, Anstrom KJ, Givertz MM, Stevenson LW, Semigran MJ, Goldsmith SR, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA. 2013;310:2533–43. doi: 10.1001/jama.2013.282190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Konstam MA, Gheorghiade M, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297:1319–31. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 66.Kahveci A, Tuğlular S. Kalp Yetmezliğinde İleri Ultrafiltrasyon Seçeneği. Klinik Gelişim. 2011;24:63–6. [Google Scholar]
- 67.Publication Committee for the VMAC Investigators (Vasodilatation in the Management of Acute CHF) Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287:1531–40. doi: 10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]
- 68.Bart BA, Boyle A, Bank AJ, Anand I, Olivari MT, Kraemer M, et al. Ultrafiltration versus usual care for hospitalized patients with heart failure: the Relief for Acutely Fluid-Overloaded Patients With Decompensated Congestive Heart Failure (RAPID-CHF) trial. J Am Coll Cardiol. 2005;46:2043–6. doi: 10.1016/j.jacc.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 70.Costanzo MR, Guglin ME, Saltzberg MT, Jessup ML, Bart BA, Teerlink JR, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2007;49:675–83. doi: 10.1016/j.jacc.2006.07.073. [DOI] [PubMed] [Google Scholar]
- 71.Bart BA, Goldsmith SR, Lee KL, Givertz MM, O’Connor CM, Bull DA, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367:2296–304. doi: 10.1056/NEJMoa1210357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dandamudi S, Chen HH. The ASCEND-HF trial: an acute study of clinical effectiveness of nesiritide and decompensated heart failure. Expert Rev Cardiovasc Ther. 2012;10:557–63. doi: 10.1586/erc.12.31. [DOI] [PubMed] [Google Scholar]
- 73.Stiles S. ‘How Soon’ Might Trump ‘How’ for ADHF Treatment: ASCEND-HF. http://www.medscape.com/viewarticle/743415 .
- 74.Mebazaa A, Parissis J, Porcher R, Gayat E, Nikolaou M, Boas FV, et al. Short-term survival by treatment among patients hospitalized with acute heart failure: the global ALARM-HF registry using propensity scoring methods. Intensive Care Med. 2011;37:290–301. doi: 10.1007/s00134-010-2073-4. [DOI] [PubMed] [Google Scholar]
- 75.Teichman SL, Unemori E, Dschietzig T, Conrad K, Voors AA, Teerlink JR, et al. Relaxin, a pleiotropic vasodilator for the treatment of heart failure. Heart Fail Rev. 2009;14:321–9. doi: 10.1007/s10741-008-9129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Teerlink JR, Metra M, Felker GM, Ponikowski P, Voors AA, Weatherley BD, et al. Relaxin for the treatment of patients with acute heart failure (Pre-RELAX-AHF): a multicentre, randomised, placebo-controlled, parallel-group, dose-finding phase IIb study. Lancet. 2009;373:1429–39. doi: 10.1016/S0140-6736(09)60622-X. [DOI] [PubMed] [Google Scholar]
- 77.Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381:29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 78.Peacock WF, Chandra A, Char D, Collins S, Der Sahakian G, Ding L, et al. Clevidipine in acute heart failure: Results of the A Study of Blood Pressure Control in Acute Heart Failure-A Pilot Study (PRONTO) Am Heart J. 2014;167:529–36. doi: 10.1016/j.ahj.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 79.Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol. 2012;59:442–51. doi: 10.1016/j.jacc.2011.09.062. [DOI] [PubMed] [Google Scholar]
- 80.Hastillo A, Taylor DO, Hess ML. Specific positive inotropic agents. In: Messerli FH, editor. Cardiovascular Drug Therapy. 2nd ed. Philadelphia: W.B. Saunders Company; 1996. pp. 1151–61. [Google Scholar]
- 81.Hashim T, Sanam K, Revilla-Martinez M, Morgan CJ, Tallaj JA, Pamboukian SV, et al. Clinical Characteristics and Outcomes of Intravenous Inotropic Therapy in Advanced Heart Failure. Circ Heart Fail. 2015;8:880–6. doi: 10.1161/CIRCHEARTFAILURE.114.001778. [DOI] [PubMed] [Google Scholar]
- 82.Guglin M, Kaufman M. Inotropes do not increase mortality in advanced heart failure. Int J Gen Med. 2014;7:237–51. doi: 10.2147/IJGM.S62549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murphy MB, Vaughan CJ. Dopamine. In: Messerli FH, editor. Cardiovascular Drug Therapy. 2nd ed. Philadelphia: W.B. Saunders Company; 1996. pp. 1161–66. [Google Scholar]
- 84.Cavusoglu Y. The use of levosimendan in comparison and in combination with dobutamine in the treatment of decompensated heart failure. Expert Opin Pharmacother. 2007;8:665–77. doi: 10.1517/14656566.8.5.665. [DOI] [PubMed] [Google Scholar]
- 85.Gruhn N, Nielsen-Kudsk JE, Theilgaard S, Bang L, Olesen SR, Aldershvile J. Coronary vasorelaxant effect of levosimendan, a new inodilator with calcium-sensitizing properties. J Cardiovasc Pharmacol. 1998;31:741–9. doi: 10.1097/00005344-199805000-00013. [DOI] [PubMed] [Google Scholar]
- 86.Landoni G, Biondi-Zoccai G, Greco M, Greco T, Bignami E, Morelli A, et al. Effects of levosimendan on mortality and hospitalization. A meta-analysis of randomized controlled studies. Crit Care Med. 2012;40:634–46. doi: 10.1097/CCM.0b013e318232962a. [DOI] [PubMed] [Google Scholar]
- 87.Felker GM, Benza RL, Chandler AB, Leimberger JD, Cuffe MS, Califf RM, et al. Heart failure etiology and response to milrinone in decompensated heart failure: results from the OPTIME-CHF study. J Am Coll Cardiol. 2003;41:997–1003. doi: 10.1016/s0735-1097(02)02968-6. [DOI] [PubMed] [Google Scholar]
- 88.Maisel AS, Wright CM, Carter SM, Ziegler M, Motulsky HJ. Tachyphylaxis with amrinone therapy: association with sequestration and down-regulation of lymphocyte beta-adrenergic receptors. Ann Intern Med. 1989;110:195–201. doi: 10.7326/0003-4819-110-3-195. [DOI] [PubMed] [Google Scholar]
- 89.Nanas JN, Papazoglou PIP, Terrovitis JV, Kanakakis J, Dalianis A, Tsolakis E, et al. Hemodynamic effects of levosimendan added to dobutamine in patients with decompensated advanced heart failure refractory to dobutamine alone. Am J Cardiol. 2004;94:1329–32. doi: 10.1016/j.amjcard.2004.07.128. [DOI] [PubMed] [Google Scholar]
- 90.Gage J, Rutman H, Lucido D, LeJemtel TH. Additive effects of dobutamine and amrinone on myocardial contractility and ventricular performance in patients with severe heart failure. Circulation. 1986;74:367–73. doi: 10.1161/01.cir.74.2.367. [DOI] [PubMed] [Google Scholar]
- 91.Chen JM, DeRose JJ, Slater JP, Spanier TB, Dewey TM, Catanese KA, et al. Improved survival rates support left ventricular assist device implantation early after myocardial infarction. J Am Coll Cardiol. 1999;33:1903–8. doi: 10.1016/s0735-1097(99)00132-1. [DOI] [PubMed] [Google Scholar]
- 92.Selzman CH, Chang PP, Vernon-Platt T, Bowen A, Kowalczyk S, Sheridan BC. Use of the Jarvik 2000 continuous flow left ventricular assist device for acute myocardial infarction and cardiogenic shock. J Heart Lung Transplant. 2007;26:756–8. doi: 10.1016/j.healun.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 93.Maybaum S, Mancini D, Xydas S, Starling RC, Aaronson K, Pagani FD, et al. Cardiac improvement during mechanical circulatory support: a prospective multicenter study of the LVAD Working Group. Circulation. 2007;115:2497–505. doi: 10.1161/CIRCULATIONAHA.106.633180. [DOI] [PubMed] [Google Scholar]
- 94.Hedayati N, Sherwood JT, Schomisch SJ, Carino JL, Cmolik BL. Circulatory benefits of diastolic counterpulsation in an ischemic heart failure model after aortomyoplasty. J Thorac Cardiovasc Surg. 2002;123:1067–73. doi: 10.1067/mtc.2002.121682. [DOI] [PubMed] [Google Scholar]
- 95.Unverzagt S, Buerke M, de Waha A, Haerting J, Pietzner D, Seyfarth M, et al. Intra-aortic balloon pump counterpulsation (IABP) for myocardial infarction complicated by cardiogenic shock. Cochrane Database Syst Rev. 2015;3:CD007398. doi: 10.1002/14651858.CD007398.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thiele H, Smalling RW, Schuler GC. Percutaneous left ventricular assist devices in acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2007;28:2057–63. doi: 10.1093/eurheartj/ehm191. [DOI] [PubMed] [Google Scholar]
- 97.Thiele H, Lauer B, Hambrecht R, Boudriot E, Cohen HA, Schuler G. Reversal of cardiogenic shock by percutaneous left atrial-to-femoral arterial bypass assistance. Circulation. 2001;104:2917–22. doi: 10.1161/hc4901.100361. [DOI] [PubMed] [Google Scholar]
- 98.Seyfarth M, Sibbing D, Bauer I, Fröhlich G, Bott-Flügel L, Byrne R, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008;52:1584–8. doi: 10.1016/j.jacc.2008.05.065. [DOI] [PubMed] [Google Scholar]
- 99.Ziemba EA, John R. Mechanical circulatory support for bridge to decision: which device and when to decide. J Card Surg. 2010;25:425–33. doi: 10.1111/j.1540-8191.2010.01038.x. [DOI] [PubMed] [Google Scholar]
- 100.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, et al. The Fourth INTERMACS Annual Report:4,000 implants and counting. J Heart Lung Transplant. 2012;31:117–26. doi: 10.1016/j.healun.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 101.Short BL, Williams L. ECMO Specialist Training Manual. 3rd ed. Michigan: ELSO; 2010. p. 155. [Google Scholar]
- 102.Kawahito K, Murata S, Yasu T, Adachi H, Ino T, Saito M, et al. Usefulness of extracorporeal membrane oxygenation for treatment of fulminant myocarditis and circulatory collapse. Am J Cardiol. 1998;82:910–1. doi: 10.1016/s0002-9149(98)00503-7. [DOI] [PubMed] [Google Scholar]
- 103.McCarthy RE, 3rd, Boehmer JP, Hruban RH, Hutchins GM, Kasper EK, Hare JM, Baughman KL. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N Engl J Med. 2000;342:690–5. doi: 10.1056/NEJM200003093421003. [DOI] [PubMed] [Google Scholar]
- 105.Chatti R, Fradj NB, Trabelsi W, Kechiche H, Tavares M, Mebazaa A. Algorithm for therapeutic management of acute heart failure syndromes. Heart Fail Rev. 2007;12:113–7. doi: 10.1007/s10741-007-9013-6. [DOI] [PubMed] [Google Scholar]
- 106.Mebazaa A, Gheorghiade M, Pina IL, Harjola VP, Hollenberg SM, Follath F, et al. Practical recommendations for prehospital and early in-hospital management of patients presenting with acute heart failure syndromes. Crit Care Med. 2008;36(1 Suppl):129–39. doi: 10.1097/01.CCM.0000296274.51933.4C. [DOI] [PubMed] [Google Scholar]
- 107.O’Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 108.Arora RR, Venkatesh PK, Molnar J. Short and long-term mortality with nesiritide. Am Heart J. 2006;152:1084–90. doi: 10.1016/j.ahj.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 109.Gheorghiade M, Zannad F, Sopko G, Klein L, Pina IL, Konstam MA, et al. Acute heart failure syndromes: current state and framework for future research. Circulation. 2005;112:3958–68. doi: 10.1161/CIRCULATIONAHA.105.590091. [DOI] [PubMed] [Google Scholar]
- 110.Spencer FA, Meyer TE, Goldberg RJ, Yarzebski J, Hatton M, Lessard D, et al. Twenty year trends (1975-1995) in the incidence, in-hospital and long-term death rates associated with heart failure complicating acute myocardial infarction: a community-wide perspective. J Am Coll Cardiol. 1999;34:1378–87. doi: 10.1016/s0735-1097(99)00390-3. [DOI] [PubMed] [Google Scholar]
- 111.Roe MT, Chen AY, Riba AL, Goswami RG, Peacock WF, Pollack CV, Jr, et al. Impact of congestive heart failure in patients with non-ST-segment elevation acute coronary syndromes. Am J Cardiol. 2006;97:1707–12. doi: 10.1016/j.amjcard.2005.12.068. [DOI] [PubMed] [Google Scholar]
- 112.Khot UN, Jia G, Moliterno DJ, Lincoff AM, Khot MB, Harrington RA, Topol EJ. Prognostic importance of physical examination for heart failure in non-ST-elevation acute coronary syndromes: the enduring value of Killip classification. JAMA. 2003;290:2174–81. doi: 10.1001/jama.290.16.2174. [DOI] [PubMed] [Google Scholar]
- 113.Torabi A, Cleland JG, Khan NK, Loh PH, Clark AL, Alamgir F, et al. The timing of development and subsequent clinical course of heart failure after a myocardial infarction. Eur Heart J. 2008;29:859–70. doi: 10.1093/eurheartj/ehn096. [DOI] [PubMed] [Google Scholar]
- 114.Meine TJ, Roe MT, Chen AY, Patel MR, Washam JB, Ohman EM, et al. Association of intravenous morphine use and outcomes in acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative. Am Heart J. 2005;149:1043–9. doi: 10.1016/j.ahj.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 115.Rott D, Behar S, Leor J, Hod H, Boyko V, Mandelzweig L, et al. Effect on survival of acute myocardial infarction in Killip classes II or III patients undergoing invasive coronary procedures. Am J Cardiol. 2001;88:618–23. doi: 10.1016/s0002-9149(01)01802-1. [DOI] [PubMed] [Google Scholar]
- 116.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–39. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 117.Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J, ADHERE Scientific Advisory Committee and Investigators High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13:422–30. doi: 10.1016/j.cardfail.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 118.Forman DE, Butler J, Wang Y, Abraham WT, O’Connor CM, Gottlieb SS, et al. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004;43:61–7. doi: 10.1016/j.jacc.2003.07.031. [DOI] [PubMed] [Google Scholar]
- 119.Damman K, Navis G, Voors AA, Asselbergs FW, Smilde TD, Cleland JG, et al. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail. 2007;13:599–608. doi: 10.1016/j.cardfail.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 120.Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, et al. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol. 2008;51:1268–74. doi: 10.1016/j.jacc.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 121.Blake WD, Wegria R, et al. Effect of increased renal venous pressure on renal function. Am J Physiol. 1949;157:1–13. doi: 10.1152/ajplegacy.1949.157.1.1. [DOI] [PubMed] [Google Scholar]
- 122.Drazner MH, Rame JE, Stevenson LW, Dries DL. Prognostic importance of elevated jugular venous pressure and a third heart sound in patients with heart failure. N Engl J Med. 2001;345:574–81. doi: 10.1056/NEJMoa010641. [DOI] [PubMed] [Google Scholar]
- 123.Greene SJ, Gheorghiade M, Vaduganathan M, Ambrosy AP, Mentz RJ, Subacius H, et al. Haemoconcentration, renal function, and post-discharge outcomes among patients hospitalized for heart failure with reduced ejection fraction: insights from the EVEREST trial. Eur J Heart Fail. 2013;15:1401–11. doi: 10.1093/eurjhf/hft110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Testani JM, Brisco MA, Chen J, McCauley BD, Parikh CR, Tang WH. Timing of hemoconcentration during treatment of acute decompensated heart failure and subsequent survival: importance of sustained decongestion. J Am Coll Cardiol. 2013;62:516–24. doi: 10.1016/j.jacc.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tojo A, Onozato ML, Kobayashi N, Goto A, Matsuoka H, Fujita T. Angiotensin II and oxidative stress in Dahl Salt-sensitive rat with heart failure. Hypertension. 2002;40:834–9. doi: 10.1161/01.hyp.0000039506.43589.d5. [DOI] [PubMed] [Google Scholar]
- 126.Lesogor A, Cohn JN, Latini R, Tognoni G, Krum H, Massie B, et al. Interaction between baseline and early worsening of renal function and efficacy of renin-angiotensin-aldosterone system blockade in patients with heart failure: insights from the Val-HeFT study. Eur J Heart Fail. 2013;15:1236–44. doi: 10.1093/eurjhf/hft089. [DOI] [PubMed] [Google Scholar]
- 127.Vardeny O, Wu DH, Desai A, Rossignol P, Zannad F, Pitt B, Solomon SD, RALES Investigators Influence of baseline and worsening renal function on efficacy of spironolactone in patients With severe heart failure: insights from RALES (Randomized Aldactone Evaluation Study) J Am Coll Cardiol. 2012;60:2082–9. doi: 10.1016/j.jacc.2012.07.048. [DOI] [PubMed] [Google Scholar]
- 128.Hensen J, Abraham WT, Dürr JA, Schrier RW. Aldosterone in congestive heart failure: analysis of determinants and role in sodium retention. Am J Nephrol. 1991;11:441–6. doi: 10.1159/000168356. [DOI] [PubMed] [Google Scholar]
- 129.Costanzo MR, Johannes RS, Pine M, Gupta V, Saltzberg M, Hay J, et al. The safety of intravenous diuretics alone versus diuretics plus parenteral vasoactive therapies in hospitalized patients with acutely decompensated heart failure: a propensity score and instrumental variable analysis using the Acutely Decompensated Heart Failure National Registry (ADHERE) database. Am Heart J. 2007;154:267–77. doi: 10.1016/j.ahj.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 130.Sackner-Bernstein JD1, Skopicki HA, Aaronson KD. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111:1487–91. doi: 10.1161/01.CIR.0000159340.93220.E4. [DOI] [PubMed] [Google Scholar]
- 131.Elkayam U, Ng TM, Hatamizadeh P, Janmohamed M, Mehra A. Renal Vasodilatory Action of Dopamine in Patients With Heart Failure: Magnitude of Effect and Site of Action. Circulation. 2008;117:200–5. doi: 10.1161/CIRCULATIONAHA.107.737106. [DOI] [PubMed] [Google Scholar]
- 132.Massie BM, O’Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, et al. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med. 2010;363:1419–28. doi: 10.1056/NEJMoa0912613. [DOI] [PubMed] [Google Scholar]
- 133.Kociol RD, Horton JR, Fonarow GC, Reyes EM, Shaw LK, O’Connor CM, et al. Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: data from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) linked to Medicare claims. Circ Heart Fail. 2011;4:628–36. doi: 10.1161/CIRCHEARTFAILURE.111.962290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Heart Failure Society of America. Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1–194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 135.Bonow RO, Bennett S, Casey DE, Jr, Ganiats TG, Hlatky MA, Konstam MA, et al. ACC/AHA clinical performance measures for adults with chronic heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Heart Failure Clinical Performance Measures) endorsed by the Heart Failure Society of America. J Am Coll Cardiol. 2005;46:1144–78. doi: 10.1016/j.jacc.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 136. http://www.hfsa.org/heart_failure_education_modules.asp .
- 137. http://www.heart.org/idc/groups/heartpublic/0wcm/Qhcm/documents/downloadable/ucm_300316.pdf .
- 138.McAlister FA, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol. 2004;44:810–9. doi: 10.1016/j.jacc.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 139.Koelling TM, Johnson ML, Cody RJ, Aaronson KD. Discharge education improves clinical outcomes in patients with chronic heart failure. Circulation. 2005;111:179–85. doi: 10.1161/01.CIR.0000151811.53450.B8. [DOI] [PubMed] [Google Scholar]
- 140.Krumholz HM, Chen YT, Wang Y, Vaccarino V, Radford MJ, Horwitz RI. Predictors of readmission among elderly survivors of admission with heart failure. Am Heart J. 2000;139(1 Pt 1):72–7. doi: 10.1016/s0002-8703(00)90311-9. [DOI] [PubMed] [Google Scholar]
- 141.Hernandez AF, Greiner MA, Fonarow GC, Hammill BG, Heidenreich PA, Yancy CW, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303:1716–22. doi: 10.1001/jama.2010.533. [DOI] [PubMed] [Google Scholar]
- 142.Fonarow GC, Albert NM, Curtis AB, Stough WG, Gheorghiade M, Heywood JT, et al. Improving evidence-based care for heart failure in outpatient cardiology practices: primary results of the Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF) Circulation. 2010;122:585–96. doi: 10.1161/CIRCULATIONAHA.109.934471. [DOI] [PubMed] [Google Scholar]
- 143.Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, et al. Association between performance measures and clinical outcomes for patients hospitalized with heart failure. JAMA. 2007;297:61–70. doi: 10.1001/jama.297.1.61. [DOI] [PubMed] [Google Scholar]
- 144.Basoor A, Doshi NC, Cotant JF, Saleh T, Todorov M, Choksi N, et al. Decreased readmissions and improved quality of care with the use of an inexpensive checklist in heart failure. Congest Heart Fail. 2013;19:200–6. doi: 10.1111/chf.12031. [DOI] [PubMed] [Google Scholar]
- 145.Anker SD, Koehler F, Abraham WT. Telemedicine and remote management of patients with heart failure. Lancet. 2011;378:731–9. doi: 10.1016/S0140-6736(11)61229-4. [DOI] [PubMed] [Google Scholar]
- 146.Inglis SC, Clark RA, McAlister FA, Stewart S, Cleland JG. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: Abridged Cochrane Review. Eur J Heart Fail. 2011;13:1028–40. doi: 10.1093/eurjhf/hfr039. [DOI] [PubMed] [Google Scholar]
- 147.Troughton RW, Frampton CM, Yandle TG, Espiner EA, Nicholls MG, Richards AM. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. Lancet. 2000;355:1126–30. doi: 10.1016/s0140-6736(00)02060-2. [DOI] [PubMed] [Google Scholar]
- 148.Jourdain P, Jondeau G, Funck F, Gueffet P, Le Helloco A, Donal E, et al. Plasma brain natriuretic peptide-guided therapy to improve outcome in heart failure: the STARS-BNP Multicenter Study. J Am Coll Cardiol. 2007;49:1733–9. doi: 10.1016/j.jacc.2006.10.081. [DOI] [PubMed] [Google Scholar]
- 149.van Veldhuisen DJ, Braunschweig F, Conraads V, Ford I, Cowie MR, Jondeau G, et al. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation. 2011;124:1719–26. doi: 10.1161/CIRCULATIONAHA.111.043042. [DOI] [PubMed] [Google Scholar]
- 150.Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–66. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 151. http://www.heart.org/HEARTORG/HealthcareResearch/GetWithTheGuidelines/GetWithTheGuidelines-HF/Get-With-The-Guidelines-Heart-Failure-Home-Page_UCM_306087_SubHomePage.jsp .
- 152. http://www.heart.org/HEARTORG/HealthcareResearch/TargetHF-Stroke/TargetHF/Target-HF_UCM_307433_SubHomePage.jsp .