Abstract
Objective:
Renal dysfunction is associated with increased cardiovascular morbidity and mortality. The alteration in renal function as a marker of mortality in pulmonary thromboembolism (PTE) has not been studied extensively.
Methods:
Four hundred four consecutive patients diagnosed with non-high-risk PTE (without cardiogenic shock or blood pressure <90 mm Hg) were prospectively enrolled in the study between 2005-2010. Kidney function, based on glomerular filtration rate (GFR), calculated by the simplified modification in diet in renal disease (MDRD) equation (sMDRD); troponin I; B-type natriuretic peptide (BNP); and echocardiographic markers of right ventricular (RV) function were determined in survivors versus non-survivors after a 2-year follow-up.
Results:
GFR was significantly lower in non-survivors than in survivors: 51.85±19.08 mL/min/1.73 m2 and 71.65±23.21 mL/min/1.73 m2, respectively (p=0.000). The highest 2-year mortality rate (20%) was recorded in patients with moderate renal dysfunction associated with RV dysfunction. Using multivariate analysis, we found that GFR is an independent predictor of 2-year mortality (OR 0.973, 95% CI: 0.959-0.987, p=0.000), besides troponin I, dyslipidemia, acceleration time of pulmonary ejection, pericardial effusion, and BNP
Conclusion:
The association of renal dysfunction with right ventricular dysfunction in patients with non-fatal pulmonary thromboembolism resulted in high mortality. Renal dysfunction, assessed by glomerular filtration rate, may be used in the risk stratification of patients with non-high-risk pulmonary thromboembolism, besides troponin I, BNP and right ventricle echocardiographic dysfunction markers.
Keywords: creatinine clearance, filtration rate, pulmonary thromboembolism, mortality, MDRD
Introduction
Pulmonary thromboembolism (PTE) is the third most common cause of hospital admission after acute myocardial infarction and stroke (1, 2). Chronic kidney disease (CKD) is associated with increased cardiovascular morbidity and mortality. The high incidence of venous thromboembolism in acute end-stage renal disease, nephrotic syndrome, or stages 3 and 4 CKD is well known (3-7). The relationship between venous thromboembolism-related mortality and renal dysfunction, assessed by glomerular filtration rate (GFR), has not been fully elucidated.
Methods
Four hundred four patients diagnosed with PTE between 2005 and 2010 were prospectively enrolled in this study. The diagnosis of pulmonary thromboembolism was made by: angio-CT scan, pulmonary ventilation-perfusion scintigraphy (high or intermediate probability of PTE, and compression ultrasonography (presence of deep vein thrombosis of the lower limbs). The main goal of this study was to determine mortality of all causes in our study group. The inclusion criteria were: patients aged >18 with low and intermediate risk (non-high-risk) PTE, without previous oral anticoagulant therapy. The exclusion criteria were: patients with high-risk PTE [associated with cardiogenic shock or blood pressure <90 mm Hg (8)], patients with end-stage diseases and life expectancy below 1 year, patients at high risk of bleeding during anticoagulant therapy [previous gastrointestinal bleeding of irreversible cause, advanced chronic kidney (stage 5 CKD) or liver hepatic disease, previous hemorrhagic stroke, poor anticoagulant control, and suboptimal monitoring of anticoagulant therapy], and patients enrolled in other studies. Low-risk PTE was defined by the absence of RV dysfunction or signs of myocardial injury. Intermediate-risk PTE was defined by increased serum levels of troponin and/or pro-BNP and/or RV dysfunction with blood pressure >90 mm Hg. All patients received standard anticoagulant therapy with intravenous unfractionated heparin or a subcutaneous weight-adjusted dose of low-molecular-weight heparin, followed by oral anticoagulants (acenocoumarol).
Urea and creatinine were determined in blood samples collected within 24 hours of admission in standard tubes, maintained at room temperature, using the modified kinetic Jaffe reaction. In addition, we performed a quantitative immunological assay for the detection of pro-BNP/NT pro-BNP in heparinized venous blood (Roche CARDIAC pro-BNP test) and a quantitative measurement of troponin I using the PATHFAST cTnI test (chemi-luminescence immunoassay and MAGTRATION technology, Mitsubishi Chemical Europe GmbH, Gauting, Germany). The normal reference values for pro-BNP and troponin I were 0-125 pg/mL and 0-0.02 ng/mL, respectively. GFR was calculated using the sMDRD (simplified Modification in Diet in Renal Disease) formula. Kidney function according to GFR values was defined as normal (>90 mL/min/1.73 m2), stage 2 CKD (60-89 mL/min/1.73 m2), stage 3 CKD (30-59 mL/min/1.73 tf), stage 4 CKD (15-29 mL/min/1.73 tf), and stage 5 CKD (<15 mL/min/1.73 m2). RV function parameters were assessed by transthoracic echocardiography within the first 24 hours of admission using a SonoScape SSI-8000 ultrasound system (Boncaler Ltd, Danroves, Germany) and standard echocardiographic views. RV dysfunction was defined as: TAPSE <16 mm, acceleration time of pulmonary ejection <90 ms, and systolic pulmonary artery pressure (sPAP) >30 mm Hg. The study protocol was approved by the hospital ethics committee. All patients included in the study provided written informed consent.
Statistical analysis
Statistical analysis was performed using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA) for Windows. The quantitative data were expressed as mean and standard deviation, while the qualitative data were expressed as median and range. The normal distribution of quantitative data was assessed by Kolmogorov-Smirnov fit test; to compare two groups, we used chi-square test for qualitative data, student t-test for normally distributed quantitative data, and Mann-Whitney U test for abnormally distributed quantitative data. The comparisons between more than two groups were made using ANOVA (for normally distributed quantitative data) and Kruskal-Wallis (for non-normally distributed quantitative data) tests. The parameters that were significant in the univariate analysis were brought into a forward stepwise bivariate logistic regression model in order to better investigate their influence. The Hosmer-Lemeshow goodness-of-fit test was used to check if the model fit the real data well. The parameters included into the model were also checked against multi-collinearity. A p value >0.05 was considered statistically significant.
Results
Enrolled in this study were 438 patients; 34 (7.76%) patients were lost to follow-up (up to 2 years), and therefore, the study group included 404 patients. Mean age was 62.32±14.26 years; 205 (50.7%) patients were men. Only 14 (3.8%) patients were low-risk; 357 (96.2%) patients were assigned to the intermediate-risk group. Thirty-three deaths were recorded, all in the intermediate-risk group. The clinical characteristics of the patients are shown in Table 1. GFR was significantly lower in non-survivors than in survivors: 51.85±19.08 mL/min/1.73 m2 versus 71.65±23.21 mL/min/L.73 m2 (p=0.000).
Table 1.
Demographic, clinical, and laboratory characteristics of patients according to clinical course
| Parameters | All patients (n=404) | Non-survivors (n=33) | Survivors (n=371) | P |
|---|---|---|---|---|
| Clinical | ||||
| Age, years | 62.32±14.26 | 69.03+11.17 | 61.73+14.37 | 0.005* |
| Men n (%) | 205 (50.7) | 14 (42.4) | 191 (51.5) | 0.319 |
| SBPmm Hg | 132.21±21.60 | 131.21+28.14 | 132.30+20.96 | 0.305 |
| DBPmm Hg | 80.63±12.38 | 81.21+11.39 | 80.58+12.47 | 0.791 |
| Heart rate >90/bpm | 174 (43.1) | 16 (48.5) | 158 (42.6) | 0.512 |
| AF, n (%) | 153 (37.9) | 15 (45.5) | 138 (37.2) | 0.349 |
| SaO2 | 89.71±8.121 | 87.28+7.92 | 90.03+8.11 | 0.102 |
| COPD, n (%) | 99 (24.5) | 10 (30.3) | 89 (24.0) | 0.419 |
| Hypertension, n (%) | 171 (42.3) | 13 (39.4) | 158 (42.6) | 0.722 |
| DM, n (%) | 75 (18.6) | 8 (24.2) | 67 (18.1) | 0.381 |
| CHD, n (%) | 217 (53.7) | 21 (63.6) | 196 (52.8) | 0.233 |
| Cancer, n (%) | 16 (4.0) | 0 (0.0) | 16 (4.3) | 0.223 |
| Dyslipidemia, n (%) | 99 (24.5) | 1 (3.0) | 98 (26.4) | 0.003* |
| Biological | ||||
| BNP pg/mL | 336.70±535.116 | 913.82+907.65 | 285.23+456.14 | 0.000* |
| Troponin I, ng/mL | 0.03±0.20 | 0.15+0.69 | 0.01+0.04 | 0.000* |
| GFR, mL/min/1.73 m2 | 70.04±23.52 | 51.85+19.08 | 71.65+23.21 | 0.000* |
| Echocardiographic | ||||
| PE, n (%) | 92 (22.8) | 13 (39.4) | 79 (21.3) | 0.018* |
| Severe TR, n (%) | 177 (43.8) | 11 (33.3) | 166 (44.7) | 0.206 |
| TAPSE, mm | 17.91±4.58 | 17.45+3.84 | 17.95+4.64 | 0.577 |
| AT, ms | 99.99±18.62 | 94.00+18.86 | 100.52+18.54 | 0.054* |
| sPAfP mm Hg | 70.19±22.82 | 73.03+19.18 | 69.94+23.12 | 0.708 |
| RVTDD, mm | 37.08±9.15 | 37.94+7.49 | 37.01+9.28 | 0.575 |
AF - atrial fibrillation; AT - acceleration time; CHD - coronary heart disease; COPD - chronic obstructive bronchopneumopathy; DBP - diastolic blood pressure; DM - diabetes mellitus; GFR - glomerular filtration rate; IVS - interventricular septum; PE - pericardial effusion; RV - right ventricle; RVTDD - right ventricular telediastolic diameter; SaO2 - oxygen saturation in room air; SBP - systolic blood pressure; sPAP - systolic pulmonary arterial pressure; TR - tricuspid regurgitation;
means p<0.05
Renal dysfunction and clinical parameters
Compared to patients without renal dysfunction, patients with PTE and low GFR (<90 mL/min/1.73 m2) are more likely to be older and female and have a higher body mass index (Table 2). In addition, they had more comorbidities, such as diabetes mellitus, coronary heart disease, previous deep thrombophlebitis or varicose veins, COPD, and/or heart failure.
Table 2.
Characteristics of patients with pulmonary embolism according to glomerular filtration rate
| Parameters | GFR <30 mL/min/1.73 m2(n=15) | GFR 30-59 mL/min/1.73 m2(n=133) | GFR >60 mL/min/1.73 m2(n=256) | P |
|---|---|---|---|---|
| Clinical | ||||
| Age, years | 71.60+11.38 | 68.56+11.53 | 58.54+14.35 | 0.000* |
| Men, n (%) | 4(26.7) | 51 (38.3) | 150 (58.6) | 0.000* |
| SBPmm Hg | 143.67±28.93 | 129.39+19.77 | 133.01+21.83 | 0.167 |
| DBPmm Hg | 81.33+19.22 | 79.06+11.84 | 81.41+12.14 | 0.224 |
| Heart rate >90/bpm | 5 (33.3) | 64 (48.1) | 105 (41.0) | 0.301 |
| AF, n (%) | 7 (46.7) | 58 (43.6) | 88 (34.4) | 0.158 |
| SaO2 | 84.40+6.73 | 88.86+8.58 | 90.43+7.80 | 0.156 |
| COPD, n (%) | 4 (26.7) | 39 (29.3) | 56 (21.9) | 0.264 |
| Hypertension, n (%) | 9 (60.0) | 57 (42.9) | 105 (41.0) | 0.347 |
| DM, n (%) | 5 (33.3) | 32 (24.1) | 38 (14.8) | 0.028* |
| CHD, n (%) | 9 (60.0) | 78 (58.6) | 130 (50.8) | 0.297 |
| Cancer, n (%) | 0 (0.0) | 9 (6.8) | 7 (2.7) | 0.112 |
| Dyslipidemia, n (%) | 3 (20.0) | 27 (20.3) | 69 (27.0) | 0.322 |
| Biological | ||||
| BNP pg/mL | 351.87+400.43 | 404.61+477.01 | 300.40+567.76 | 0.001* |
| Troponin I, ng/mL | 0.02+0.03 | 0.06+0.34 | 0.01+0.02 | 0.001* |
| Echocardiographic | ||||
| PE, n (%) | 2 (13.3) | 33 (24.8) | 57 (22.3) | 0.574 |
| Severe TR, n (%) | 8 (53.3) | 67 (50.4) | 102 (9.8) | 0.104 |
| TAPSE, mm | 16.87+4.06 | 17.36+4.34 | 18.25+4.70 | 0.132 |
| AT, ms | 89.93+16.84 | 97.82+18.64 | 101.71+18.48 | 0.015* |
| sPAP mm Hg | 79.20+26.63 | 70.41+20.85 | 69.55+23.54 | 0.688 |
| RVTDD, mm | 40.47+10.81 | 37.08+8.91 | 36.88+9.16 | 0.338 |
AF - atrial fibrillation; AT - acceleration time; COPD - chronic obstructive bronchopneumopathy; DBP - diastolic blood pressure; DM - diabetes mellitus; GFR - glomerular filtration rate; CHD - coronary heart disease; IVS - interventricular septum; PE - pericardial effusion; RV - right ventricle; RVTDD - right ventricular telediastolic diameter; SaO2 - oxygen saturation in room air; SBP - systolic blood pressure; sPAP - systolic pulmonary arterial pressure; TR - tricuspid regurgitation;
means p<0.005
In patients with stage 3 or 4 CKD, the probability of survival decreased to 70%-80% after the first year (Fig. 1).
Figure 1.
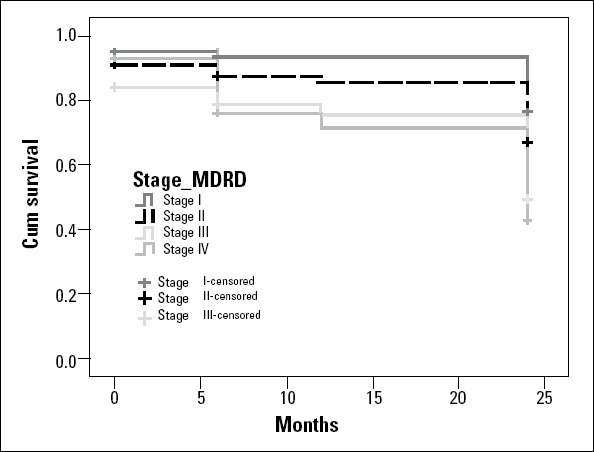
Kaplan-Meier survival curve according to GFR, assessed by sMDRD
The area under the curve (AUC) of the GFR, assessed by the sMDRD ROC curve, for predicting 2-year mortality was 0.598 (Fig. 2). The cut-off value of GFR for predicting 2-year mortality in our study was 70 mL/min/1.73 m2.
Figure 2.
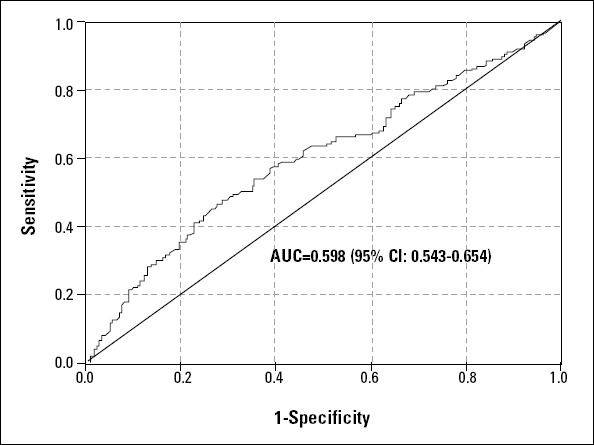
The ROC curve of GFR, assessed by sMDRD
Renal dysfunction and myocardial injury markers
The mean value of troponin was significantly higher in non-survivors than in survivors (0.15±0.69 ng/mL versus 0.01±0.04 ng/mL; p=0.000). GFR and troponin were statistically significant negatively correlated in both non-survivors (r=-0.291; p=0.045) and survivors (r=-0.275; p=0.049). The mean BNP value was significantly higher in non-survivors compared to survivors (913.82±907.65 pg/mL and 285.23±456.14 pg/mL, respectively; p=0.000). BNP was significantly associated with GFR only in non-survivors (r=-0.552; p=0.003); in survivors, the correlation was statistically insignificant (r=-0.039; p=0.386).
Renal dysfunction and right ventricular dysfunction
GFR and TAPSE were significantly correlated in survivors (r=+0.064; p=0.012) and not significantly correlated in non-survivors (r=+0.124; p=0.210). The same was true for GFR and sPAP [non-survivors (r=-0.161; p=0.372) and survivors (r=-0.188; p=0.001)]. Acceleration time was statistically significant in both non-survivors (r=+0.356; p=0.001) and survivors (r=+0.131; p=0.001).
The 2-year survival probability in patients with stage 4CKD and RV dysfunction was about 60%; in the absence of severe renal impairment but with RV dysfunction, this probability was approximately 80%, showing the additive prognostic value of GFR (Fig. 3).
Figure 3.
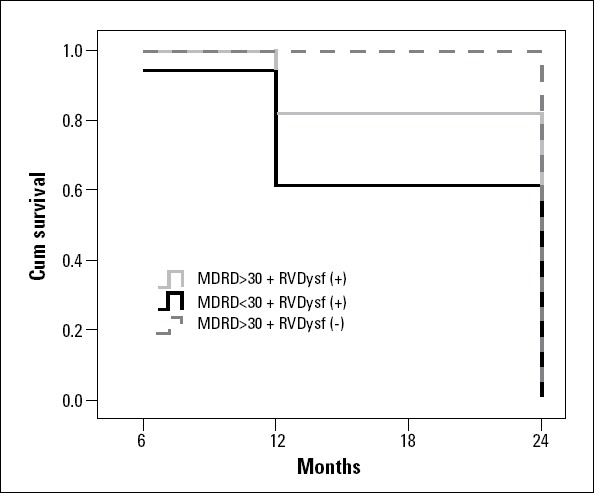
Survival curve depending on GFR, assessed by sMDRD, and right ventricular dysfunction
Using multivariate analysis, we found GFR to be an independent predictor of 2-year mortality (OR 0.973, 95% CI: 0.959-0.987, p=0.000), besides troponin I, dyslipidemia, acceleration time of pulmonary ejection, pericardial effusion, and BNP (Table 3).
Table 3.
Predictors of 2-year mortality in nonfatal pulmonary thromboembolism
| Parameters | OR | 95% CI | P |
|---|---|---|---|
| BNP | 1.010 | 1.010-1010 | 0.000 |
| Troponin I | 31.65 | 47.2-212.0 | 0.000 |
| GFR | 0.973 | 0.959-0.987 | 0.000 |
| Pericardial effusion | 1.304 | 1.161-1.571 | 0.000 |
| Acceleration time | 0.979 | 0.963-0.996 | 0.016 |
| Dyslipidemia | 5.089 | 1.199-21.595 | 0.027 |
BNP - B-type natriuretic peptide; GFR - glomerular filtration rate; OR: odds ratio
Discussion
The main finding in this study was that renal dysfunction increases 2-year mortality in patients with non-high-risk PTE. The association between renal dysfunction and PTE was initially suspected in patients undergoing dialysis and in kidney transplant patients due to the increasing of incidence of venous thromboembolism (9, 10). Moderate renal dysfunction is associated with an increased risk for venous thromboembolism, the same being true for obesity, bed rest, and prolonged immobility (11).
Renal dysfunction is known as an independent risk factor for morbidity and mortality in various other conditions, such as acute coronary syndromes and heart failure (12-15). A recent study found correlations between pulmonary hypertension and worsening renal function in patients with mild-to-moderate mitral stenosis (16).
Renal function is often estimated using creatinine-based formulas. Accurate quantitative determination of GFR requires the determination of urinary clearance of exogenous markers, such as inulin or 125I-iothalamate. sMDRD has a higher accuracy in predicting morbidity and mortality, being correlated with 125I-iothalamate clearance in patients with heart failure (17). Similarly, in our patients at risk for fatal PTE (non-survivors), sMDRD offered much lower values compared to patients at risk of nonfatal PTE.
In our study, only 15 patients (3.71%) had a baseline GFR below 30 mL/min/1.73 m2; at 2 years, this percentage increased to 9.9% (40 patients). Compared to the RIETE study (18), the prevalence of renal dysfunction at a baseline GFR below 30 mL/min/1.73 m2 was approximately 1.5-fold lower (3.71% versus 5.6%). Risk assessment in hemodynamically stable patients remains controversial; a risk assessment algorithm would be extremely useful for clinicians. In hemodynamically stable PTE patients, the echocardiographic signs of RV dysfunction are strongly correlated with troponin (19). A troponin level >0.01 ng/mL predicts in-hospital adverse events (20), while a normal troponin level predicts a favorable outcome (21). Markers of myocardial injury, such as cardiac troponin (22, 23), or markers of RV dysfunction, such as BNP (24) and echocardiographic RV evaluation (25), have been found to be adequate indicators of morbidity and mortality and consequently were included in recent guidelines. The same correlations were found in our study group, troponin being significantly higher in patients progressing to death (0.15±0.69 versus 0.01±0.04 ng/mL, p=0.000) as compared to survivors, and having at the same time a strong statistical correlation with sMDRD in both groups. High troponin I levels were found in 35.1% of non-survivors compared with 24.9% of survivors. A troponin value >0.04 ng/mL was the cut-off value for predicting mortality in our patients (OR 1.937, 95% CI 1.0943.428).
Besides troponin, elevated BNP levels in patients with PTE are significantly associated with echocardiographic parameters of RV dysfunction (26). A BNP level below 85 pg/ml has a high negative prognostic value, excluding with great accuracy the echocardiographic changes in normotensive patients; patients with BNP over 527 pg/mL (all with RV dysfunction on echocardiography) have the highest mortality and complication rates (27). In our study, BNP had a significantly higher mean value in non-survivors than in survivors (913 pg/mL vs. 285 pg/mL, p=0.000). GFR was not significantly correlated with BNP in survivors. The BNP cut-off value of >500 pg/mL was almost similar to that in previous studies and was associated with a mortality of approximately 50% within the first 6 months; after 1 year, the survival probability dropped to approximately 20%. In patients with BNP levels ranging between 250-499 pg/mL, the 1-year and 2-year survival probability dropped to 50%. BNP levels <250 pg/mL were not associated with any fatal risk. Both troponin I and BNP besides GFR, were found to be independent predictors of 2-year mortality.
As mentioned in the GRACE score and other clinical rules (LR-PED), atrial fibrillation may be an independent predictor of 6-month mortality in patients with acute pulmonary embolism; however, these data should be tested and validated in prospective studies using larger cohorts (28, 29). In our study, although the prevalence of atrial fibrillation was 37.9%, the difference between the survivor and non-survivor groups was not statistically significant. The multivariate analysis did not validate this parameter as an independent predictor of 2-year mortality, possibly due to the high prevalence of cardiovascular diseases in our group.
Low GFR is associated with telediastolic RV diameter, maximum tricuspid regurgitation gradient, acceleration time of pulmonary ejection, and the presence of paradoxical movements of the interventricular septum (30). In our study, except for the telediastolic RV diameter, all echocardiographic parameters of RV dysfunction were highly significantly correlated with GFR, even though the correlation was weak; acceleration time of pulmonary ejection showed the best statistical correlation in patients with nonfatal risk. In non-survivors, acceleration time was the only statistically significant parameter. In addition, of the echocardiographic parameters, it was the only one that was found to be an independent predictor of mortality. Acceleration time of pulmonary ejection proved to be a predictive marker of mortality in patients suspected of PTE and an independent marker of survival in patients with nonfatal PTE confirmed by scintigraphy (31). In addition, this parameter is inversely correlated with RV dysfunction and has diagnostic importance in PTE, especially in the presence of proximal thrombi (32-34).
In our study, metabolic syndrome seems to increase mortality risk in patients with renal dysfunction. Dyslipidemia was found to be an independent predictor of mortality. This may occur through the effects of circulating lipid molecules on the vascular endothelium, platelet function, and coagulation factors (35). Diabetes mellitus and obesity are statistically significantly more frequently associated with lower GFR. All of these factors increase the cardiovascular risk and subsequently the risk of kidney damage.
Study limitations
The small number of non-survivors is due to the fact that hemodynamically unstable patients with PTE were excluded from the study, with the aim of this study being the assessment of patients at risk for nonfatal PTE. Few autopsies have been performed; therefore, possible recurrences of fatal venous thromboembolism were not diagnosed. The sMDRD formula might show several limitations in patients with GFR over 60 mL/min/1.73 m2 (32). The initial group also failed to include patients with a GFR below 15 mL/min/1.73 m2, with a single patient being included in this group at the end. This could influence the identification of more significant results in the case of severe renal dysfunction. Data on the history of CKD before PTE could not be obtained from all patients. The diverse etiologies of PTE determined different therapeutic approaches in terms of oral anticoagulation treatment duration. This topic will be further investigated.
Conclusion
Concurrence of renal dysfunction and right ventricular dysfunction in patients with a risk for nonfatal pulmonary thromboembolism is associated with high mortality. Renal dysfunction, assessed by glomerular filtration rate, may be used in the risk stratification of patients with non-high-risk pulmonary thromboembolism, besides troponin, BNP and echocardiographic markers of right ventricular dysfunction.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept - A.Q., C. A.G.; Design - A.Q., C. A.G.; Supervision - A.Q., C. A.G.; Resource - A.Q.; Materials - A.Q.; Data collection &/or processing - A.Q., D.M. T., S.D. I., V. A.; Analysis &/or interpretation - A.Q., S.D. I., M.F; Literature search - A.Q., D. T.; Writing - A.Q., M.F, D.M. T.; Critical review - A.Q., S.D. I., M.F, V. A.
References
- 1.De Monaco NA, Dang Q, Kapoor WN, Ragni MV. Pulmonary embolism incidence is increasing with use of spiral computed tomography. Am J Med. 2008;121:611–7. doi: 10.1016/j.amjmed.2008.02.035. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burge AJ, Freeman KD, Klapper PJ, Haramati LB. Increased diagnosis of pulmonary embolism without a corresponding decline in mortality during the CT era. Clin Radiol. 2008;63:381–6. doi: 10.1016/j.crad.2007.10.004. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 3.Mahmoodi BK, ten Kate MK, Waanders F, Veeger NJ, Brouwer JL, Vogt L, et al. High absolute risks and predictors of venous and arterial thromboembolic events in patients with nephrotic syndrome: results from a large retrospective cohort study. Circulation. 2008;117:224–30. doi: 10.1161/CIRCULATIONAHA.107.716951. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 4.Stein PD, Henry JW. Prevalence of acute pulmonary embolism among patients in a general hospital and at autopsy. Chest. 1995;108:978–81. doi: 10.1378/chest.108.4.978. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 5.Abbott KC, Cruess DF, Agodoa LY, Sawyers ES, Tveit DP. Early renal insufficiency and late venous thromboembolism after renal transplantation in the United States. Am J Kidney Dis. 2004;43:120–30. doi: 10.1053/j.ajkd.2003.08.047. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 6.Kayali F, Najjar R, Aswad F, Matta F, Stein PD. Venous thromboembolism in patients hospitalized with nephrotic syndrome. Am J Med. 2008;121:226–30. doi: 10.1016/j.amjmed.2007.08.042. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 7.Wattanakit K, Cushman M, Stehman-Breen C, Heckbert SR, Folsom AR. Chronic kidney disease increases risk for venous thromboembolism. J Am Soc Nephrol. 2008;19:135–40. doi: 10.1681/ASN.2007030308. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galie N, Pruszczyk P, et al. ESC Committee for Practice Guidelines (CPG). Guidelines on the diagnosis and management of acute pulmonary embolism. The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) Eur Heart J. 2008:2276–315. doi: 10.1093/eurheartj/ehn310. [DOI] [PubMed] [Google Scholar]
- 9.Abbott KC, Cruess DF, Agodoa LY, Sawyers ES, Tveit DP. Early renal insufficiency and late venous thromboembolism after renal transplantation in the United States. Am J Kidney Dis. 2004;43:120–30. doi: 10.1053/j.ajkd.2003.08.047. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 10.Tveit DP, Hypolite IO, Hshieh P, Cruess D, Agodoa LY, Welch PG, et al. Chronic dialysis patients have high risk for pulmonary embolism. Am J Kidney Dis. 2002;39:1011–7. doi: 10.1053/ajkd.2002.32774. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 11.Anderson FA, Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107:I9–16. doi: 10.1161/01.CIR.0000078469.07362.E6. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 12.Al Suwaidi J, Reddan DN, Williams K, Pieper KS, Harrington RA, Califf RM, et al. Prognostic implications of abnormalities in renal function in patients with acute coronary syndromes. Circulation. 2002;106:974–80. doi: 10.1161/01.cir.0000027560.41358.b3. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 13.Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–95. doi: 10.1056/NEJMoa041365. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 14.Smith GL, Lichtman JH, Bracken MB, Shlipak MG, Phillips CO, DiCapua P, et al. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol. 2006;47:1987–96. doi: 10.1016/j.jacc.2005.11.084. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 15.Smith GL, Masoudi FA, Shlipak MG, Krumholz HM, Parikh CR. Renal impairment predicts long-term mortality risk after acute myocardial infarction. J Am Soc Nephrol. 2008;19:141–50. doi: 10.1681/ASN.2007050554. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zorkun C, Amioğlu G, Bektaşoğlu G, Zorlu A, Ekinözü I, Turgut OO, et al. Elevated mean pulmonary artery pressure in patients with mild-to-moderate mitral stenosis: a useful predictor of worsening renal functions? Anatol J Cardiol. 2013;13:457–64. doi: 10.5152/akd.2013.144. [DOI] [PubMed] [Google Scholar]
- 17.Smilde TD, van Veldhuisen DJ, Navis G, Voors AA, Hillege HL. Drawbacks and prognostic value of formulas estimating renal function in patients with chronic heart failure and systolic dysfunction. Circulation. 2006;114:1572–80. doi: 10.1161/CIRCULATIONAHA.105.610642. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 18.Monreal M, Falgá C, Falgá C, Valle R, Barba R, Bosco J, Beato JL, et al. RIETE Investigators Venous thromboembolism in patients with renal insufficiency: findings from the RIETE Registry. Am J Med. 2006;119:1073–9. doi: 10.1016/j.amjmed.2006.04.028. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 19.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353:1386–9. doi: 10.1016/s0140-6736(98)07534-5. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 20.Pruszczyk P, Bochowicz A, Torbicki A, Szulc M, Kurzyna M, Fijalkowska A, et al. Cardiac troponin T monitoring identifies high-risk group of normotensive patients with acute pulmonary embolism. Chest. 2003;123:1947–52. doi: 10.1378/chest.123.6.1947. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 21.Janata K, Holzer M, Laggner AN, Mullner M. Cardiac troponin T in the severity assessment of patients with pulmonary embolism: cohort study. BMJ. 2003;326:312–3. doi: 10.1136/bmj.326.7384.312. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Becattini C, Vedovati MC, Agnelli G. Prognostic value of troponins in acute pulmonary embolism: a meta-analysis. Circulation. 2007;116:427–33. doi: 10.1161/CIRCULATIONAHA.106.680421. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 23.Jimenez D, Uresandi F, Otero R, Lobo JL, Monreal M, Martí D, et al. Troponin-based risk stratification of patients with acute nonmassive pulmonary embolism: systematic review and metaanalysis. Chest. 2009;136:974–82. doi: 10.1378/chest.09-0608. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 24.Klok FA, Mos IC, Huisman MV. Brain-type natriuretic peptide levels in the prediction of adverse outcome in patients with pulmonary embolism: a systematic review and meta-analysis. Am J Respir Crit Care Med. 2008;178:425–30. doi: 10.1164/rccm.200803-459OC. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 25.Sanchez O, Trinquart L, Colombet I, Durieux P, Huisman MV, Chatellier G, et al. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J. 2008;29:1569–77. doi: 10.1093/eurheartj/ehn208. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 26.Tulevski II, Hirsch A, Sanson BJ, Romkes H, van der Wall EE, van Veldhuisen DJ, et al. Increased brain natriuretic peptide as a marker for right ventricular dysfunction in acute pulmonary embolism. Thromb Haemost. 2001;86:1193–6. [PubMed] [Google Scholar]
- 27.Pieralli F, Olivotto I, Vanni S, Conti A, Camaiti A, Targioni G, et al. Usefulness of bedside testing for brain natriuretic peptide to identify right ventricular dysfunction and outcome in normotensive patients with acute pulmonary embolism. Am J Cardiol. 2006;97:1386–90. doi: 10.1016/j.amjcard.2005.11.075. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 28.Paiva LV, Providencia RC, Barra SN, Faustino AC, Botelho AM, Marques AL. Cardiovascular risk assessment of pulmonary embolism with the GRACE risk score. Am J Cardiol. 2013;111:425–31. doi: 10.1016/j.amjcard.2012.10.020. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 29.Barra SN, Paiva LV, Providência R, Fernandes A, Leitao Marques A. Atrial fibrillation in acute pulmonary embolism: prognostic considerations. Emerg Med J. 2014;31:308–12. doi: 10.1136/emermed-2012-202089. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 30.Kostrubiec M, Labyk A, Pedowska-Wloszek J, Pacho S, Wojciechowski A, Jankowski K, et al. Assessment of renal dysfunction improves troponin-based short-term prognosis in patients with acute symptomatic pulmonary embolism. J Thromb Haemost. 2010;8:651–8. doi: 10.1111/j.1538-7836.2010.03762.x. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 31.Kjaergaard J, Schaadt BK, Lund JO, Hassager C. Prognostic importance of quantitative echocardiographic evaluation in patients suspected of first non-massive pulmonary embolism. Eur J Echocardiogr. 2009;10:89–95. doi: 10.1093/ejechocard/jen169. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 32.Kurzyna M, Torbicki A, Pruszczyk P, Burakowska B, Fijalkowska A, Kober J, et al. Disturbed right ventricular ejection pattern as a new Doppler echocardiographic sign of acute pulmonary embolism. Am J Cardiol. 2002;90:507–11. doi: 10.1016/s0002-9149(02)02523-7. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 33.Torbicki A, Kurzyna M, Ciurzynski M, Pruszczyk P, Pacho R, Kuch-Wocial A, et al. Proximal pulmonary emboli modify right ventricular ejection pattern. Eur Respir J. 1999;13:616–21. doi: 10.1183/09031936.99.13361699. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 34.Cirillo M. Rationale, pros and cons of GFR estimation: the Cockcroft-Gault and MDRD equations. G Ital Nefrol. 2009;26:310–7. [PubMed] [Google Scholar]
- 35.Ray JG. Dyslipidemia, statins, and venous thromboembolism: a potential risk factor and a potential treatment. Curr Opin Pulm Med. 2003;9:378–84. doi: 10.1097/00063198-200309000-00007. [CrossRef] [DOI] [PubMed] [Google Scholar]


