Abstract
Objective:
The relationship between severity of coronary artery disease (CAD) and left ventricler (LV) hypertrophy in hypertensive patients is well known. However, the association between the extent and complexity of CAD assessed with SYNTAX score (SS) and different LV geometric patterns has not been investigated. We aimed to investigate the association between SYNTAX score and different LV geometric patterns in hypertensive patients.
Methods:
The study had been made in our clinic between January 2013 and August 2013. We studied 251 CAD patients who had hypertension and who underwent coronary angiography (147 males, 104 females; mean age 61.61±9.9 years). Coronary angiography was performed based on clinical indications. SS was determined in all patients. Echocardiographic examination was performed in all subjects. Four different geometric patterns were determined in patients according to LV mass index (LVMI) and relative wall thickness (RWT) (Groups: NG-normal geometry, CR-concentric remodeling, EH-eccentric hypertrophy, and CH-concentric hypertrophy). Biochemical markers were measured in all participants.
Results:
The highest SS values were observed in the CH group compared with the NG, CR, and EH groups (p<0.05 for all). Also, the SS values of the EH group were higher than in the NG and CR groups (p<0.05 for all). Multivariate linear regression analysis showed that SS was independently associated with LV geometry (β=0.316, p=0.001), as well as age (β=0.163, p=0.007) and diabetes (β=-0.134, p=0.022).
Conclusion:
SYNTAX score is independently related with LV geometry in hypertensive patients. This result shows that LV remodeling is parallel to the increase in the extent and complexity of CAD in our study patients.
Keywords: SYNTAX, geometry, hypertrophy, hypertension
Introduction
The Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery (SYNTAX) score quantifies the extent and complexity of angiographic disease (1). SYNTAX score (SS) has been used to make revascularization decisions and predict long-term mortality and morbidity in patients with coronary artery disease (CAD) (2, 3). Moreover, SS has the power to predict adverse cardiac events, such as contrast-induced nephropathy and no-reflow in patients with ST elevation myocardial infarction (MI) treated with primary percutaneous intervention (4). On the other hand, SS is strongly associated with left ventricle hypertrophy (LVH) in hypertensive patients and different patient groups (5-9).
There are four different geometric patterns of the left ventricle (LV) in hypertension (HT), and these geometric patterns are different for prognosis and LV function (6-8). LV geometric patterns incorporate normal LV structure (NG) and concentric remodeling (CR), in addition to LVH [eccentric hypertrophy (EH) and concentric hypertrophy (CH)] (7). Previously, it has been shown that higher systolic blood pressures are associated with CH (7). Demographic factors, such as age and gender, diabetes mellitus, neurohormonal activation, or coronary artery disease can also modulate the development of different LV geometric patterns (8).
Although SS is associated with LVH in hypertensive patients (7, 9, 10), it has not been investigated in different LV geometric patterns. Therefore, we aimed to investigate the association between the extent and complexity of CAD, assessed by SYNTAX score, and different LV geometric patterns in patients with CAD who have hypertension.
Methods
Study populations
The study had been made in our clinic between January 2013 and August 2013, we evaluated 251 CAD patients who had hypertension and underwent coronary angiography (147 males, 104 females; mean age 61.61±9. years). Coronary angiography was performed for the investigation of ischemic heart disease based on clinical indications (typical chest discomfort and/or abnormal stress test results, such as positive treadmill test, dobutamine stress echo, and myocardial perfusion scintigraphy). Patients with coronary lesions with a diameter stenosis of ≥50% in ≥1.5-mm vessels were included in the study. All patients were clinically stable and had a history of hypertension. In each subject, blood pressure (BP) was measured on at least three separate days after 15 min of sitting comfortably and was then averaged. Individuals who had systolic BP ≥140 mm Hg and/or diastolic BP ≥90 mm Hg in the office setting were diagnosed as hypertensive (11). Only 70% of hypertensive individuals selected for the present analysis had regularly used anti-hypertensive therapy with one or more medications.
The exclusion criteria were the presence of neoplastic disease, heart failure, recent major surgical procedure, or chronic liver or kidney disease. Patients with previous MI and angina episodes 48 hours before hospitalization, those who had previous coronary angioplasty or bypass surgery, and those with valvular, myocardial, or pericardial disease were also excluded. The study was conducted according to the recommendations set forth by the Declaration of Helsinki on biomedical research involving human subjects. The institutional Ethics Committee approved the study protocol, and each participant provided written informed consent.
After a detailed medical history and complete physical examination, each participant was questioned for major cardiovascular risk factors, such as age, sex, diabetes mellitus, smoking status, previous medications, and hypertension. In addition, body mass index was calculated, and systolic BP (SBP) and diastolic BP (DBP) were recorded.
Also, fasting venous blood samples were obtained from all patients to determine their plasma levels of fasting blood glucose, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglyceride, creatinine, and hemoglobin.
Coronary angiography and SYNTAX score
All patients underwent coronary angiography with the Judkins technique. Coronary angiography was performed with a standard femoral approach with a 6-F diagnostic catheter. Coronary lesions leading to ≥50% diameter stenosis in ≥1.5-mm vessels were scored separately and added together to provide the cumulative SYNTAX score, which was prospectively calculated using the SYNTAX score algorithm on the baseline diagnostic angiogram (1). Two experienced interventional cardiologists analyzed the SYNTAX score; the opinion of a third analyst was obtained, and the final judgment was made by consensus in cases of a disagreement. The final score was calculated from the individual lesion scores by analysts who were blinded to the procedural data and clinical outcome.
Echocardiography
Standard two-dimensional and Doppler echocardiographies were performed using a commercially available echocardiography machine (Vivid 7R GE Medical System, Horten, Norway) with a 2.0-3.5-MHz transducer. Measurements were made during normal breathing at end-expiration. LV end-systolic (LVESD) and end-diastolic diameters (LVEDD), end-diastolic interventricular septal thickness (IVSth), and end-diastolic LV posterior wall thickness (PWth) were measured at end-diastole according to established standards of the American Society of Echocardiography. LV ejection fraction (EF) was determined by the biplane Simpson’s method (12). LV mass (LVM) was calculated using the Devereux formula (13).
LVM=(1.04 [(LVEDD+ IVSth+PWth)3-(LVEDD)3]-13.6).
Then, the LV mass index (LVMI, g/m2) was obtained with the following formula: LVM/body surface area. LVH was defined according to more stringent criteria as LVMI values exceeding 125 g/m2 in men and 110 g/m2 in women (14). Relative wall thickness (RWth, cm) was measured at end-diastole as the ratio of (2xPWth)/LVEDD. Increased RWth was defined as ≥0.45.
All echocardiographic measurements were repeated by a second observer (MG) who was blinded to the values obtained by the first observer (HU). Interobserver variability was assessed by calculating the coefficient of variation. The coefficient of variation was <8% for all measurements. Any discrepancy was resolved by consensus. All echocardiographic measurements were repeated 1 week later by an observer (HU) who was blinded to the results of the previous measurements, and the intraobserver variability was <5% for all measurements.
Patterns of left ventricular geometry
Geometric patterns were based on the upper normal limits for LVMI and RWth: (i) normal geometry (NG; normal LVMI and normal RWth); (ii) concentric remodeling (CR; normal LVMI and increased RWth); (iii) concentric hypertrophy (CH; increased LVMI and increased RWth); and (iv) eccentric hypertrophy (EH; increased LVMI and normal RWth) (8).
Reproducibility
All echocardiographic measurements [except coronary flow reserve (CFR)] were repeated 1 week later by the same sonographer (H.U.), and the intraobserver variability was calculated as the difference in the 2 measurements of the same patient by the observer divided by the mean value. Intra-observer variabilities were less than 5% for all measurements.
Statistical analysis
All analyses were conducted using SPSS 17.0 (SPSS for Windows 17.0, Chicago, IL, USA). The distribution of continuous variables was assessed with the one-sample Kolmogorov-Smirnov test. Comparison of categorical variables between the groups was performed using the chi-square test. Analysis of variance (ANOVA) was used in the analysis of continuous variables. Spearman’s test was performed to identify bivariate correlation between continuous and categorical variables. A stratified post hoc analysis of echocardiographic, clinical, and laboratory variables was performed according to the LV geometric patterns. The associations of SYNTAX score were assessed by the Pearson’s correlation test. Multiple linear regression analysis was performed to identify the independent associations of the SYNTAX score. A two-tailed p<0.05 was considered statistically significant.
Results
Baseline characteristics
Baseline clinical, laboratory, echocardiographic, and angiographic characteristics of the groups are shown in Table 1. In the present study, four different geometric patterns were determined according to LVMI and RWth: (1) 79 patients with NG, (2) 64 patients with CR, (3) 39 patients with EH, and (4) 69 patients with CH. Age, body surface area (BSA), and SBP and DBP values were significantly different among the groups (p<0.05 for all).
Table 1.
Comparison of baseline, laboratory, echocardiographic, and angiographic characteristics among the groups
| Variables | NG Group | CR Group | EH Group | CH group | ANOVA P |
|---|---|---|---|---|---|
| Age, years | 60.1±9.04 | 58.46±9.6 | 61.7±9.8 | 66.10±9.8a | <0.001 |
| Gender× male | 38 (46.0%) | 31 (45.8%) | 21 (37.5%) | 37(46.2%) | 0.852 |
| BMI, kg/m2 | 29.01±4.4 | 29.6±5.3b | 29.3±5.2 | 28.8±5.3 | 0.911 |
| BSA, m2 | 1.87±0.17 | 1.93±0.21 | 1.83±0.17 | 1.83±0.17 | 0.020 |
| SBP, mm Hg | 145.2±15.8 | 146.7±18.5 | 149.4±18.1 | 157.0±17.1c | <0.001 |
| DBP, mm Hg | 92.2±11.3 | 94.2±11.2 | 95.6±11.3 | 104.6±13.2d | <0.001 |
| Heart rate, beats/minute | 76.5±12.3 | 75.2±8.5 | 76.1±13.0 | 75.4±9.7 | 0.332 |
| Diabetes×, n (%) | 36 (45.6%) | 23 (31.9%) | 21 (43.8%) | 31 (44.9%) | 0.345 |
| Hyperlipidemia×, n (%) | 36 (45.6%) | 35 (54.7%) | 17 (33.6%) | 35 (50.7%) | 0.628 |
| Smoking×, n (%) | 24 (27.6%) | 23 (35.9%) | 22 (46.3%) | 31 (44.9%) | 0.152 |
| Family history×, n (%) | 26 (32.9%) | 21 (31.8%) | 13 (23.3%) | 16 (23.2%) | 0.518 |
| Laboratory findings | |||||
| Glucose, mg/dL | 140.8±8.2 | 148.3±9.3 | 149.3±13.6 | 147.4±10.3 | 0.740 |
| LDL-C, mg/dL | 117.7±23.1 | 111.2±24.8 | 109.4±20.9 | 110.7±33.1 | 0.170 |
| HDL-C, mg/dL | 44.4±6.7 | 42.4±6 | 43.7±8.5 | 44.2±7.2 | 0.463 |
| Triglyceride, mg/dL | 162.12±53 | 158.64±65 | 151.41±37 | 145.52±42 | 0.237 |
| Hemoglobin, mg/dL | 15.5±1.4 | 13.8±1.3 | 14.1.0±1.4 | 15.2±1.3 | 0.767 |
| Creatinine, mg/dL | 0.92±0.4 | 0.88±0.27 | 0.92±0.31 | 0.97±0.49 | 0.636 |
| Echocardiographic findings | |||||
| LVEDD, cm | 5.1±4.6 | 4.3.2±3.6 | 5.1±3.9 | 4.7.2±3.6 | 0.238 |
| IVSth, cm | 0.98±.0.1e | 1.12±0.11f | 1.15±0.12g | 1.23±0.1 | <0.001 |
| PWth, cm | 0.9±0.08h | 1.08±0.1k | 1.02±0.1l | 1.21 ±0.09 | <0.001 |
| EF, % | 54.8±8.4aa | 55.8±7.6bb | 50.6±8.7 | 53.7±6.8 | 0.011 |
| RWth, cm | 0.39±0.03cc | 0.50±0.04dd | 0.40±0.04ee | 0.51±0.04 | <0.001 |
| LVMI, g/m2 | 96.3±17.6ff | 102.3±17.8gg | 146.1 ±32.3 | 147.6±23.9 | <0.001 |
| Angiographic findings | |||||
| SYNTAX score | 9.2±7.02hh | 10.1 ±6.9kk | 12.6±7.1 | 15.2±6.8 | <0.001 |
| Previous medications | |||||
| ACE-I use, n (%) | 24 (27.6%) | 21 (31.8%) | 21 (53.8%) | 31 (44.3%) | 0.153 |
| ARB use, n (%) | 26 (32.9%) | 23 (35.9%) | 17 (43.6%) | 35 (50.7%) | 0,164 |
| Beta-blocker use, n (%) | 24 (27.6%) | 23 (35.9%) | 22 (46.3%) | 31 (44.3%) | 0.347 |
| Statin use, n (%) | 36 (45.6%) | 35 (54.7%) | 17 (33.6%) | 35 (50.7%) | 0.628 |
| OAD use, n (%) | 36 (45.6%) | 23 (31.9%) | 21 (43.8%) | 31 (44.9%) | 0.345 |
ACE-I - angiotensin-converting enzyme inhibitor; ARB - angiotensin receptor blocker; BMI - body mass index; BSA - body surface area; CH - concentric hypertrophy; CR – concentric remodeling; DBP - diastolic blood pressure; EF - ejection fraction; EH - eccentric hypertrophy; HDL-C - high-density lipoprotein cholesterol; IVSth - interventricular septal thickness; LDL-C - low-density lipoprotein cholesterol; LVEDD - left ventricle end-diastolic diameter; LVMI - left ventricle mass index; NG - normal geometry; OAD - oral antidiabetic; PWth -posterior wall thickness; RWth - relative wall thickness; SBP - systolic blood pressure
Chi-square
P<0.001 vs. NG, CR, and EH groups;
P=0.014 vs. EH group, P=0.005 vs. CH group;
P<0.001 vs. NG, CR, and EH groups;
P<0.001 vs. NG, CR, and EH groups:
P<0.001 vs. CR, EH, and CH groups;
P<0.001 vs. CH group;
P<0.001 vs. CH group;
P<0.001 vs. CR, EH, and CH groups;
P<0.001 vs. EH and CH groups;
P<0.001 vs. CH group;
P=0.007 vs. EH group;
P=0.001 vs. EH group;
P<0.001 vs. CR and CH groups;
P<0.001 vs. EH group;
P<0.001 vs. CH group;
P<0.001 vs. EH and CH groups;
P<0.001 vs. EH and CH groups;
P=0.012 vs. EH group; P<0.001 vs. CH group;
P<0.001 vs. CH group
The comparison of SS values among the groups is shown in Table 1 and Figure 1. The highest SYNTAX score values were observed in the CH group compared with the other groups (p<0.05 for all). Also, SS values of the EH group were higher than in the NG and CR groups (p<0.05 for all). However, SS values of the NG and CR groups were similar (p>0.05).
Figure 1.
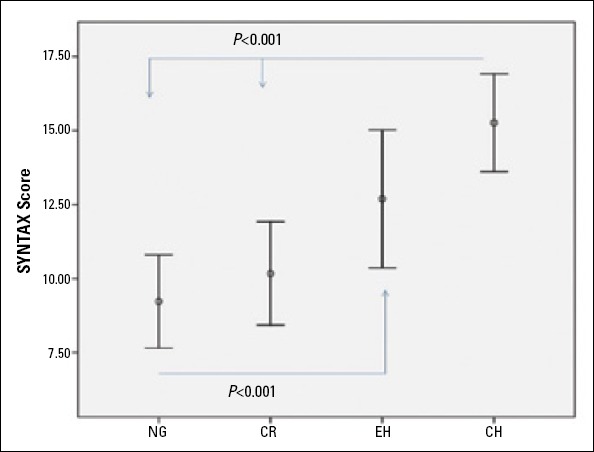
SYNTAX score in different left ventricle geometry patterns
CH - concentric hypertrophy; CR - concentric remodeling; EH - eccentric hypertrophy; NG - normal geometry
IVSth, PWth, RWth LVMI, and EF values were different among the groups. The highest LVMI and RWth values were detected in the CH group compared with the other groups (p<0.05 for all).
Bivariate and multivariate relationships of SYNTAX score
The bivariate and multivariate relationships of the SYNTAX score are demonstrated in Table 2. SS was associated with BMI (r=-0.129, p=0.041), age (r=0.150, p=0.017), diabetes (r=0.144, p=0.023), HDL (r=0.132, p=0.036), creatinine (r=0.138, p=0.028), EF (r=-0.180, p=0.004), LVMI (r=0.252, p<0.001), RWth (r=0.152, p=0.016), and LV geometry (r=0.331, p<0.001) in the bivariate analysis.
Table 2.
Bivariate and multivariate relationships of SYNTAX score
| Variables | Pearson correlation coefficient | P | Standardized β-regression coefficients | P |
|---|---|---|---|---|
| BMI, kg/m2 | -0.129 | 0.041 | -0.004 | 0.82 |
| Age, years | 0.150 | 0.017 | 0.163 | 0.007 |
| Diabetes, n (%) | 0.144 | 0.023 | 0.134 | 0.022 |
| HDL, mg/dL | 0.132 | 0.036 | 0.107 | 0.065 |
| Creatinine, mg/dL | 0.138 | 0.028 | 0.111 | 0.060 |
| EF, % | -0.180 | 0.004 | -0.132 | 0.034 |
| LVMI g/m2 | 0.252 | <0.001 | -0.032 | 0.710 |
| RWth, cm | 0.152 | 0.016 | 0.025 | 0.721 |
| LV Geometry | 0.331 | <0.001 | 0.316 | 0.001 |
BMI - body mass index; EF - ejection fraction; HDL - high-density lipoprotein; LV - left ventricle; LVMI - left ventricular mass index; RWth - relative wall thickness
Multivariate regression analysis showed that SS was independently associated with age (β=0.163, p=0.007), diabetes mellitus (DM) (β=0.134, p=0.022), and LV geometry (β=0.316, p=0.001).
Discussion
This is the first study that has investigated the relationship between SYNTAX score and different LV geometric patterns in hypertensive CAD. The present study shows that SS is independently associated with left ventricle geometry, as well as age and diabetes. In the present study, the CH group had the highest SS values among all study groups. Also, the SS values of the EH group were higher than in NG and CR groups.
An association between SS and LVH in hypertensive patients has been showed in previous studies (6, 7). Several mechanisms have been proposed for this relationship (15). There is evidence that several factors may induce parallel changes in LV mass and atherosclerotic lesions (16). Hypertension stimulates both LVH and atherosclerosis (16, 17). The renin-angiotensin-aldosterone system can be the most important mechanism. Angiotensin II and angiotensin II type 1 receptor activation promotes intracellular reactions that may lead to both cardiac hypertrophy and the progression of complex atherosclerotic lesions through the proliferation of vascular smooth muscle cells and the production of extracellular matrix protein (18, 19). LVH in hypertensive patients leads to shifts toward glycolytic metabolism, disorganization of the sarcomere, alterations in calcium handling, changes in contractility, loss of myocytes with fibrotic replacement, systolic and diastolic dysfunction, and electrical remodeling, resulting in alterations in myocardial metabolism, structure, and function with increasing severity of LVH (20). These structural, metabolic, and functional alterations possibly increase the extent of atherosclerosis.
Although the association between LVH and SS is well known in hypertensive patients (6, 7, 10), the relationship between LV geometry and SS has not been investigated previously. The present study shows that SS is associated with LV geometry independently of LVH. Also, in our study, SS values were associated with LV geometry but not with LVMI or RWth alone in the multivariate analysis. Previous studies have reported that abnormal LV geometric patterns are associated with a greater risk of hypertensive complications (6, 7). A subgroup study of the Framingham heart study (21) demonstrated that patients with concentric LVH had the worst prognosis, followed by those with EH, CR, and normal geometry. In the LIFE study (10), the prevalence of CAD was almost twice as high in patients with CH as in the other subgroups. The lowest cardiovascular risk was observed in the group with NG (7). Therefore, the highest SS in the CH group may contribute to a poorer prognosis compared with the other geometric patterns. A CH geometric pattern is the endpoint of hypertension, and both LVMI and RWth increase in this geometric pattern. Our study revealed that the SS values were significantly higher among hypertensive subjects with CH and EH compared to those in the CR and NG groups. This result suggests that LVMI is a more important parameter than RWth. Increased left ventricle mass, volume and pressure overloads, or a combination of both cause different LV geometric adaptations, including NG, CR, EH, and CH. Geometric patterns identify distinctive pathophysiologic patterns and may be added to LV mass for risk stratification (6, 7, 21, 22).
The pathophysiological mechanisms underlying the association between different LV geometry with the extent and complexity of CAD are still unclear. Traditionally, it was thought that the activation of neuro-humoral mechanisms triggers a progressive increase in LVMI and the progression of atherosclerosis in hypertensive patients. The increase in LV mass needs to compensate for increased cardiac load when the LV geometry is concentric or eccentric (23, 24). Also, it is known that increased oxidative stress has been regarded as one of the most important contributors to the progression of atherosclerosis in hypertensive patients with different LV geometries (25, 26). Oxidative stress alters normal endothelial function, supporting proinflammatory, prothrombotic, proliferative, and vasoconstrictor mechanisms that support the atherogenic process (27, 28). In addition, patients with EH and CH were older, which may also have affected the severity of CAD.
Also, in our study, SS was independently associated with DM. A relationship between diabetes and SYNTAX score was shown in previous studies (29, 30). DM appears to be involved in each step of the atherosclerotic process. It triggers endothelial dysfunction in humans in vivo (31) and leads to adverse modifications in lipid (32-35) and coagulation factors (33, 36). Chronic hyperglycemia can damage the kidneys, leading to vascular damage and secondary hypertension (32, 33, 37). It may also exert direct toxic effects on the vasculature, potentiating the development of atherosclerosis (37, 38).
Study limitations
Two-dimensional echocardiography is limited in its accuracy for measuring LV mass, because all methods assume a uniform LV thickness. However, the M-mode methods that are based on the simple cube-function formula have repeatedly been shown to give reasonably accurate LV mass measurements in necropsy validation studies. In addition, the simplicity and ease of this technique have made it possible to apply it to large-scale clinical and epidemiological studies and to relate LV mass and its change over time to clinical outcomes (39).
Conclusion
In hypertensive patients with coronary artery disease, the severity and complexity of coronary artery disease progressively increase from normal geometry to concentric hypertrophic geometry. So, it can be thought that ventricular remodeling in hypertensive patients may be parallel to the severity of coronary artery disease.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept - H.U., M.G.; Design - A.B., M.G.; Supervision - T.Ş., A.B.; Materials - G.Y.K., T.Ş.; Data collection &/or processing - M.Ç., A.Y., Ö.Ş., M.K., A.O.B.; Analysis &/or interpretation - M.Ç., H.U., M.G.; Literature search - A.O.B., M.Ç., Ö.Ş.; Writing - H.U.; Critical review - M.Ç., A.Y.
References
- 1.Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, et al. The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. Euro Intervention. 2005;1:219–27. [PubMed] [Google Scholar]
- 2.Kaya A, Tanboğa İH, Kurt M, Işık T, Kaya Y, Günaydın ZY, et al. Relation of ABO blood groups to coronary lesion complexity in patients with stable coronary artery disease. Anadolu Kardiyol Derg. 2014;14:55–60. doi: 10.5152/akd.2013.4728. [DOI] [PubMed] [Google Scholar]
- 3.Capodanno D, Capranzano IP, Di Salvo ME, Caggegi A, Tomasello D, Cincotta G, et al. Usefulness of SYNTAX score to select patients with left main coronary artery disease to be treated with coronary artery bypass graft. JACC Cardiovasc Interv. 2009;2:731–8. doi: 10.1016/j.jcin.2009.06.003. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 4.Şahin DY, Gür M, Elbasan Z, Kuloğlu O, Şeker T, Kıvrak A, et al. SYNTAX score is a predictor of angiographic no-reflow in patients with ST-elevation myocardial infarction treated with a primary percutaneous coronary intervention. Coron Artery Dis. 2013;24:148–53. doi: 10.1097/MCA.0b013e32835c4719. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 5.Verma A, Meris A, Skali H, Ghali JK, Arnold JM, Bourgoun M, et al. Prognostic implications of left ventricular mass and geometry following myocardial infarction: the VALIANT (VALsartan In Acute myocardial iNfarcTion) Echocardiographic Study. JACC Cardiovasc Imaging. 2008;1:582–91. doi: 10.1016/j.jcmg.2008.05.012. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 6.Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–52. doi: 10.7326/0003-4819-114-5-345. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 7.Ganau A, Devereux RB, Roman MJ, de Simone G, Pickering TG, Saba PS, et al. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19:1550–8. doi: 10.1016/0735-1097(92)90617-v. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 8.Negri F, Sala C, Re A, Mancia G, Cuspidi C. Left ventricular geometry and diastolic function in the hypertensive heart: impact of age. Blood Press. 2013;22:1–8. doi: 10.3109/08037051.2012.707307. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 9.Elbasan Z, Gür M, Şahin DY, Kırım S, Akyol S, Kuloğlu O, et al. N-Terminal pro-brain natriuretic peptide levels and abnormal geometric patterns of left ventricle in untreated hypertensive patients. Clin Exp Hypertens. 2014;36:153–8. doi: 10.3109/10641963.2013.804538. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 10.Dahlöf B, Devereux R, de Faire U, Fyhrquist F, Hedner T, Ibsen H, et al. The Losartan Intervention For Endpoint reduction (LIFE) in Hypertension study: rationale, design, and methods. The LIFE Study Group. Am J Hypertens. 1997;10:705–13. [CrossRef] [PubMed] [Google Scholar]
- 11.White WB. Relevance of blood pressure variation in the circadian onset of cardiovascular events. J Hypertens Suppl. 2003;21:S9–S15. doi: 10.1097/00004872-200307006-00003. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 12.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Chamber Quantification Writing Group. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 13.Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–8. doi: 10.1161/01.cir.55.4.613. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 14.Friehs I, del Nido PJ. Increased susceptibility of hypertrophied hearts to ischemic injury. Ann Thorac Surg. 2003;75:S678–84. doi: 10.1016/s0003-4975(02)04692-1. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 15.Verdecchia P, Angeli F, Pittavini L, Gattobigio R, Benemio G, Porcellati C. Regression of left ventricular hypertrophy and cardiovascular risk changes in hypertensive patients. Ital Heart J. 2004;5:505–10. [PubMed] [Google Scholar]
- 16.Roman MJ, Pickering TG, Schwartz JE, Pini R, Devereux RB. Association of carotid atherosclerosis and left ventricular hypertrophy. J Am Coll Cardiol. 1995;25:83–90. doi: 10.1016/0735-1097(94)00316-i. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 17.Young W, Gofman JW, Tandy R, Malamud N, Waters ES. The quantitation of atherosclerosis: II. quantitative aspects of the relationship of blood pressure and atherosclerosis. Am J Cardiol. 1960;6:294–9. doi: 10.1016/0002-9149(60)90318-0. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 18.Dzau VJ. Tissue renin-angiotensin system in myocardial hypertrophy and failure. Arch Intern Med. 1993;153:937–42. [CrossRef] [PubMed] [Google Scholar]
- 19.Sadoshima J, Xu Y, Slayter HS, Izumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac muscle in vitro. Cell. 1993;75:977–84. doi: 10.1016/0092-8674(93)90541-w. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 20.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–80. doi: 10.1056/NEJMra072139. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 21.Krumholz HM, Larson M, Levy D. Prognosis of left ventricular geometric patterns in the Framingham Heart Study. J Am Coll Cardiol. 1995;25:879–84. doi: 10.1016/0735-1097(94)00473-4. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 22.Muiesan ML, Salvetti M, Monteduro C, Bonzi B, Paini A, Viola S, et al. Left ventricular concentric geometry during treatment adversely affects cardiovascular prognosis in hypertensive patients. Hypertension. 2004;43:731–8. doi: 10.1161/01.HYP.0000121223.44837.de. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 23.Mureddu GF, Pasanisi F, Palmieri V, Celentano A, Contaldo F, de Simone G. Appropriate or inappropriate left ventricular mass in the presence or absence of prognostically adverse left ventricular hypertrophy. J Hypertens. 2001;19:1113–9. doi: 10.1097/00004872-200106000-00017. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 24.de Simone G, Verdecchia P, Pede S, Gorini M, Maggioni AR. Prognosis of inappropriate left ventricular mass in hypertension: the MAVI Study. Hypertension. 2002;40:470–6. doi: 10.1161/01.hyp.0000034740.99323.8a. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 25.Kaysen GA, Eiserich JR. The role of oxidative stress-altered lipo-protein structure and function and microinflammation on cardiovascular risk in patients with minor renal dysfunction. J Am Soc Nephrol. 2004;15:538–48. doi: 10.1097/01.asn.0000111744.00916.e6. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 26.Rizzi E, Ceron CS, Guimaraes DA, Prado CM, Rossi MA, Gerlach RF, et al. Temporal changes in cardiac matrix metalloproteinase activity, oxidative stress, and TGF-βin renovascular hypertension-induced cardiac hypertrophy. Exp Mol Pathol. 2013;94:1–9. doi: 10.1016/j.yexmp.2012.10.010. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 27.Emdin M, Pompella A, Paolicchi A. Gamma-glutamyltransferase, atherosclerosis, and cardiovascular disease: triggering oxidative stress within the plaque. Circulation. 2005;112:2078–80. doi: 10.1161/CIRCULATIONAHA.105.571919. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 28.Nedeljkovic ZS, Gokce N, Loscalzo J. Mechanisms of oxidative stress and vascular dysfunction. Postgrad Med J. 2003;79:195–9. doi: 10.1136/pmj.79.930.195. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ndrepepa G, Braun S, Mehilli J, Birkmeier KA, Byrne RA, Ott I, et al. Prognostic value of sensitive troponin T in patients with stable and unstable angina and undetectable conventional troponin. Am Heart J. 2011;161:68–75. doi: 10.1016/j.ahj.2010.09.018. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 30.Korosoglou G, Lehrke S, Mueller D, Hosch W, Kauczor HU, Humpert PM, et al. Determinants of troponin release in patients with stable coronary artery disease: insights from CT angiography characteristics of atherosclerotic plaque. Heart. 2011;97:823–31. doi: 10.1136/hrt.2010.193201. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 31.Williams SB, Goldfine AB, Timimi FK, Ting HH, Roddy MA, Simonson DC, et al. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation. 1998;97:1695–701. doi: 10.1161/01.cir.97.17.1695. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 32.Aronson D, Bloomgarden Z, Rayfield EJ. Potential mechanisms promoting restenosis in diabetic patients. J Am Coll Cardiol. 1996;27:528–35. doi: 10.1016/0735-1097(95)00496-3. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 33.Bierman EL. George Lyman Duff Memorial Lecture. Atherogenesis in diabetes. Atheroscler Thromb. 1992;12:647–56. doi: 10.1161/01.atv.12.6.647. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 34.Kreisberg RA. Diabetic dyslipidemia. Am J Cardiol. 1998;82:67U–73U. doi: 10.1016/s0002-9149(98)00848-0. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 35.Stern MP, Mitchell BD, Haffner SM, Hazuda HR. Does glycemic control of type 2 diabetes suffice to control diabetic dyslipidemia? A community perspective. Diabetes Care. 1992;15:638–44. doi: 10.2337/diacare.15.5.638. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 36.Ceriello A. Coagulation activation in diabetes mellitus: a role of hyperglycemia and therapeutic prospects. Diabetologia. 1993;36:1119–25. doi: 10.1007/BF00401055. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 37.Stout RW. Insulin and atheroma: 20-year perspective. Diabetes Care. 1990;13:631–54. doi: 10.2337/diacare.13.6.631. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 38.Lorenzi M, Cagliero E, Toledo S. Glucose toxicity for human endothelial cells in culture. Delayed replication, disturbed cell cycle, and accelerated death. Diabetes. 1985;34:621–7. doi: 10.2337/diab.34.7.621. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 39.Devereux RB, Pini R, Aurigemma GP, Roman MJ. Measurement of left ventricular mass: methodology and expertise. J Hypertens. 1997;15:801–9. doi: 10.1097/00004872-199715080-00002. [CrossRef] [DOI] [PubMed] [Google Scholar]


