Abstract
Objective:
Morning blood pressure surge (MBPS) is an independent predictor of atherothrombotic cardiovascular events in hypertensive patients. There is evidence from studies supporting the validity of mean platelet volume (MPV) as a marker of vascular risk and predictor of thrombotic complications. The aim of this study is to investigate the relationship between MPV and MBPS in hypertensive patients.
Methods:
Measurements were obtained from 298 patients with newly diagnosed essential hypertension (Mean age 51.9±11.7 years). The patients were divided into two groups (MPVlow group; <10.8 fL, MPVhigh group; ≥10.8 fL). The MBPS was calculated as mean systolic BP during the 2 hours after awaking minus the mean systolic BP during the 1 hour that included the lowest sleep BP.
Results:
MPV was independently associated with MBPS (β=0.554, p<0.001) and hs-CRP level (β=0.286, p<0.001).
Conclusion:
Finally, higher MPV values related to enhanced MBPS which are associated with atherothrombotic cardiovascular events.
Keywords: mean platelet volume, morning blood pressure surge, CRP
Introduction
Essential hypertension (HT) is an established major independent risk factor for cardiovascular diseases. HT causes target organ damage by the direct physical effect of increased blood pressure (BP) as well as the active promotion of atherosclerosis and thrombogenesis (1, 2). Evidence for the prothrombotic or hypercoagulable state in HT has been shown. The main complications of HT are generally thrombotic in nature rather than hemorrhagic (3). Platelet dysfunction in hypertensive patients is a potential cause of increased cardiovascular morbidity and mortality (4).
Mean platelet volume (MPV) is an indicator of platelet activation and size and it has been reported to increase in HT (5, 6). There is evidence from both retrospective and prospective studies supporting the validity of MPV as a marker of vascular risk and predictor of thrombotic complications at hypertensive patients (7, 8). On the other hand, occurrence of major cardiovascular complications, including myocardial infarction (MI), stroke, and sudden cardiac death, peaks in the early morning hours (8-10).
Blood pressure (BP) also exhibits a similar diurnal variation, with a decrease during sleep and a surge in the morning (11). It has been shown that enhanced morning BP surge (MBPS) is an independent predictor of cardiovascular events including composite of cardiovascular death, nonfatal MI, nonfatal stroke, and heart failure requiring hospitalization in hypertensive patients (12-16).
In the present study, we hypothesized that ambulatory BP measurements (ABPM) including MBPS will be associated with platelet size. Therefore, we aimed to investigate the relationship between MPV and ABPM values in newly diagnosed hypertensive patients.
Methods
Study populations
In Adana Numune Training and Research Hospital between January 2013 and June 2013, 344 patients with newly diagnosed essential HT according to office BP measurements enrolled to this prospective cross-sectional study. Exclusion criteria were secondary or malignant HT, heart failure, positive history or clinical signs of ischemic heart disease, cerebrovascular disease, valve disease, atrial fibrillation, receiving any drugs, renal insufficiency, hepatic dysfunction, major non-cardiovascular diseases such as autoimmune disease, hematological disease, cancer, thrombocytopenia and systemic inflammatory conditions, and known diabetes or fasting glycemia 126≥ mg/dL. Of 344 patients having office BP measurement ≥140/90 mm Hg, 46 patients were excluded because of their BP was normal according to ABPM (White coat HT). Measurements were obtained from 298 patients with newly diagnosed essential HT (mean age; 51.9±11.7 years, male/female; 111/187). Institutional Ethics Committee approved the study and written informed consent for participation in the study was obtained from all individuals.
Body mass index (BMI) was computed as weight divided by height squared (kg/m2). Body surface area of all subjects was computed (m2).
Blood pressure and ambulatory blood pressure measurements
BP was measured using a mercury sphygmomanometer in an office setting. Systolic BP (SBP) and diastolic BP (DBP) were taken. Office BP measurements were done by the same person, following the guidance of the seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High BP, on at least two separate occasions on different days (17). Noninvasive 24 hours ABPM was performed with a portable, compact digital recorder (Tracker NIBP2, Delmar Reynolds Ltd., Hertford, UK) and analyzed using customized analytical software (Delmar Reynolds Medical Inc., Model 2169, Hertford, UK). All subjects wore an ABPM device for a single 24 h period. The device was programmed to inflate and record BP at pre-specified intervals (every 15 min during daytime hours and every 30 min during nighttime hours), which provided approximately 80 BP recordings during the 24 h period. The display of the ABPM was inactivated so that viewing each BP reading did not distract the subjects. For the analysis of the data reports, reports generated from a session of ABPM contained BP recordings for the entire 24 h, heart rate, mean arterial pressure and BP load, as well as summary statistics for the overall 24 h, daytime and nighttime periods. When the readings exceeded at least 80% of the total readings programmed for the testing period, the recording was considered valid and satisfactory.
Diagnosis of hypertension
In each subject, BP was measured on at least three separate days after 15 min of sitting comfortably and was then averaged. Each subject then underwent 24 h ABPM. Individuals who had systolic BP ≥140 mm Hg and/or a diastolic BP ≥90 mm Hg in the office setting, and in ABPM, an average 24 h systolic BP>130 mm Hg and/or diastolic BP >80 mm Hg, an average daytime systolic BP >135 mm Hg and/or diastolic BP >85 mm Hg or an average nighttime systolic BP >125 mm Hg and/or diastolic BP >75 mm Hg were diagnosed as hypertensive (18). In addition, the subjects who had a <10% reduction in BP from the daytime to the nighttime period were defined as NDH, and the subjects who had a BP reduction ≥10% from the daytime to the nighttime period were considered DH, and the subjects who had a BP reduction ≥20% from the daytime to the nighttime period were considered extreme DH (10).
Morning blood pressure surge
To calculate the MBPS, we determined the awake and asleep intervals from the subjects’ diary. The MBPS was determined as the difference between the average BP during the 2 hours after awakening and the lowest nighttime BP (ie, the average of the lowest BP and the 2 readings immediately preceding and after the lowest value) (11, 12).
Blood samples
Blood samples were drawn in the morning following a fasting period of 12 h. Glucose, creatinine and lipid profiles for blood samples were analyzed for each patient. MPV was measured from tripotassium EDTA (0.05 mL K3)-based anticoagulated blood samples drawn in the morning after a 20-minute rest, stored at +4°C and assessed by a Sysmex K-1000 autoanalyzer which uses optical light scatter (Block Scientific, Bohemia, New York) within 30 minutes of sampling. The patients were divided in to the median MPV values (MPVlow group; <10.8 fL, MPVhigh group; ≥10.8 fL). HsCRP was measured with an autoanalyzer [Aeroset by using a commercial spectrophotometric kit (Scil Diagnostics GmbH, Viernheim, Germany)].
Echocardiography
Standard 2-dimensional and Doppler echocardiography were performed using a commercially available echocardiographic machine (Vivid 7R GE Medical System, Horten, Norway). Left ventricule (LV) end-diastolic diameters (LVDd), end-diastolic interventricular septal thickness (IVSth) and end-diastolic left ventricular posterior wall thickness (PWth) were measured at end-diastole according to established standards of the American Society of Echocardiography (19). LV ejection fraction (EF) was determined by the biplane Simpson’s method (20).
Left ventricular mass (LVM) was calculated using the Devereux formula (21): LVM=1:04[(LVDd+IVSth+PWth)3 -(LVDd)3]-13.6.
Statistical analysis
All analyses were conducted using SPSS 17.0 (SPSS for Windows 17.0, Chicago, IL, USA). Comparison of categorical variables between the groups was performed using the chi-square (χ2) test. Analysis of normality was performed with the Kolmogorov– Smirnov test. Independent samples t-test was used in the analysis of continuous variables. The correlations between MPV and laboratory, hemodynamic, ABPM and echocardiographic parameters were assessed by the Pearson correlation test. A multiple linear regression analysis was performed to identify the independent associations of MPV. All significant (p<0.05) parameters in the univariate analysis were selected in the multivariate model. A two-tailed p<0.05 was considered as statistically significant.
Results
The patients were divided into two groups according to their median MPV values: MPVlow group 149 patients; <10.8 fL (mean age; 52.8±11.9, male/female: 54/95) and MPVhighgroup 149 patients; ≥10.8 fL (mean age: 50.9±11.4, male/female: 57/92). Comparison of baseline, laboratory, echocardiographic and clinical characteristics between the groups were showed in Table 1. Age, gender, BMI and frequencies of diabetes, smoking and hyperlipidemia were not differ between the groups (p>0.05, for all). hs-CRP levels were higher and platelet count were lower in MPVhigh group compared with MPVlow group (p<0.05, for all). LVM, EF and the other parameters were not different between the groups (p>0.05, for all).
Table 1.
Comparison of baseline, echocardiographic and laboratory findings of groups
| Variables | MPVlow Group (149 patients) | MPVhigh Group (149 patients) | P |
|---|---|---|---|
| Baseline characteristics | |||
| Age, years | 52.8±11.9 | 50.9±11.4 | 0.152 |
| Gender, male | 54 (36.2%) | 57 (38.3%) | 0.405 |
| BMI, kg/m2 | 30.7±5.3 | 30.8±5.7 | 0.973 |
| Heart rate, beat/minute | 78.7±11.8 | 80.2±11.7 | 0.287 |
| Smoking, n (%) | 33 (22.1%) | 43 (28.9%) | 0.116 |
| Laboratory findings | |||
| Glucose, mg/dL | 94.6±8.8 | 94.7±7.5 | 0.899 |
| Total Chol, mg/dL | 217.1±45.8 | 210.6±41.1 | 0.203 |
| Triglyceride, mg/dL | 179.2±91.8 | 179.2±84.6 | 0.997 |
| HDL Chol, mg/dL | 47.8±10.8 | 46.4±11.6 | 0.300 |
| LDL Chol, mg/dL | 145.5±35.1 | 145.7±34.2 | 0.969 |
| Creatinin, mg/dL | 0.82±0.43 | 0.82±0.22 | 0.850 |
| hs-CRP, mg/dL | 0.64±0.20 | 0.77±0.18 | <0.001 |
| Platelet count, x109/L | 285.2±79.7 | 261.3±58.6 | 0.003 |
| Echocardiographic findings | |||
| LAD, mm | 36.4±3.9 | 36.8±4.3 | 0.442 |
| LVID, mm | 45.2±4.2 | 45.7±4.4 | 0.391 |
| LVM, g | 191.5±46.1 | 200.2±49.5 | 0.117 |
| Ejection fraction, % | 62.1±4.9 | 61.1±4.4 | 0.054 |
BMI - body mass index; Chol - cholesterol; HDL - high density lipoprotein; hs-CRP - high sensitive C reactive protein; LAD - left atrial diameter; LDL - low density lipoprotein; LVID - left ventricle internal diameter; LVM - left ventricle mass; MPV - mean platelet volume “chi-square”
Ambulatory blood pressure measurements (Table 2)
Table 2.
Office and ambulatory blood pressure measurements
| Variables | MPVlow Group (149 patients) | MPVhigh Group (149 patients) | P | ||
|---|---|---|---|---|---|
| Blood pressure measurements (mm Hg) | |||||
| Office SBP | 160.4±17.9 | 159.1±18.6 | 0.530 | ||
| Office DBP | 98.7±10.0 | 97.1±9.6 | 0.162 | ||
| Average 24-hours SBP | 136.8±8.0 | 137.8±10.0 | 0.367 | ||
| Average 24-hours DBP | 83.8±7.8 | 84.5±8.0 | 0.434 | ||
| Average daytime SBP | 144.5±8.0 | 144.2±9.4 | 0.731 | ||
| Average daytime DBP | 91.0±6.7 | 90.7±6.9 | 0.715 | ||
| Average nighttime SBP | 129.0±10.2 | 131.3±12.7 | 0.093 | ||
| Average nighttime DBP | 76.6±10.4 | 78.3±10.4 | 0.157 | ||
| Average morning SBP | 145.3±7.8 | 144.8±9.2 | 0.560 | ||
| Average morning DBP | 91.6±11.6 | 91.7±9.6 | 0.760 | ||
| Morning BP Surge | 24.2±16.9 | 44.3±15.3 | <0.001 | ||
| Dipper hypertension, n (%) | 72 (48.3%) | 63 (42.3%) | 0.176 | ||
| Non-dipper hypertension, n (%) | 70 (47.0%) | 85 (57.0%) | 0.049 | ||
| Extreme dipper hypertension, n (%) | 6 (4.0%) | 1 (0.7%) | 0.060 | ||
BP - blood pressure; DBP - diastolic blood pressure; MPV - mean platelet volume; SBP - systolic blood pressure
The frequency of non-dipper hypertension was higher in MPVhigh group compared with MPVlow group (p<0.05). Average nighttime systolic BP value of MPVhigh group was higher than MPVlow group, but there was no statistically significance (p>0.05). MBPS values were higher in MPVhigh group compared with MPVlow group (p<0.05, for all).
Bivariate and multiple relationships of mean plateletvolume (Table 3)
Table 3.
Bivariate and multiple associations of mean platelet volume
| Variables | Pearson correlation coefficient | P | Standardized β regression coefficients | P |
|---|---|---|---|---|
| hs-CRP, mg/dL | 0.447 | <0.001 | 0.286 | <0.001 |
| Platelet count, x109/L | -0.157 | 0.006 | -0.073 | 0.093 |
| Average nighttime SBP, mm Hg | 0.158 | 0.006 | -0.011 | 0.865 |
| Non-dipper hypertension | 0.221 | <0.001 | 0.198 | 0.173 |
| Dipper hypertension | -0.213 | <0.001 | 0.111 | 0.289 |
| Morning BP Surge, mm Hg | 0.626 | <0.001 | 0.554 | <0.001 |
BP - blood pressure; hs-CRP - high sensitive C reactive protein
We did bivariate and multiple analysis for whole groups. MPV was associated with hs-CRP (r=0.447, p<0.001), platelet count (r=-0.157, p=0.006), average nighttime systolic BP (r=0.158, p=0.006), non-dipper hypertension (r=0.221, p<0.001), dipper hypertension (r=-0.213, p<0.001) and MBPS (r=-0.626, p<0.001) in bivariate analysis.
Multiple linear regression analysis showed that MPV was independently associated with hs-CRP (β=0.286, p<0.001) and MBPS (β=0.554, p<0.001) in hypertensive patients.
Relationships between MPV level with MBPS and hs-CRP were shown in Figure 1 and Figure 2, respectively.
Figure 1.
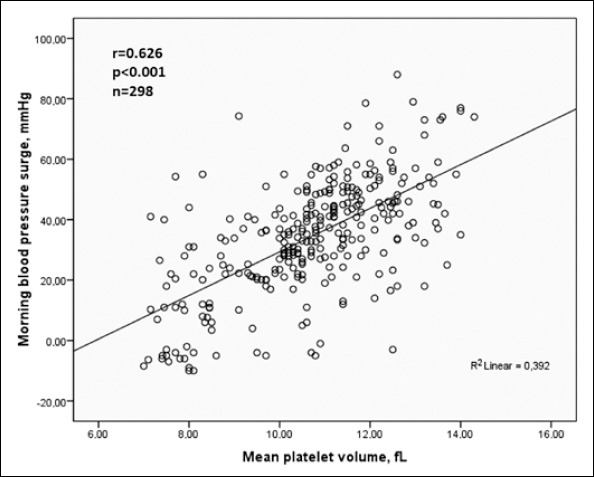
Relationship between morning blood pressure surge and mean platelet volume
Figure 2.
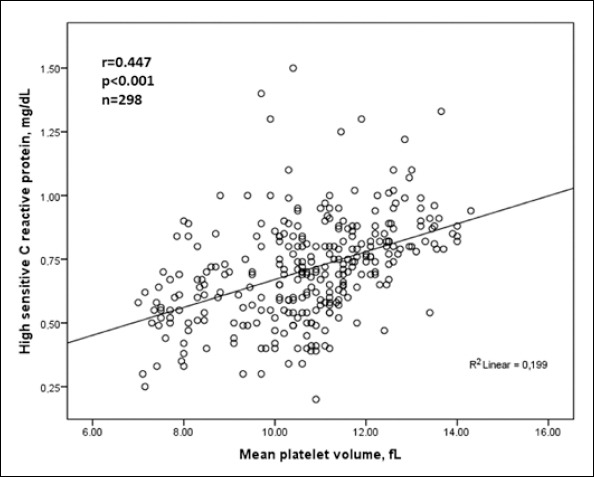
Relationship between high sensitive C reactive protein and mean platelet volume
Discussion
In the present study, for the first time in literature, enhanced MBPS, but not 24-h BP, was found to be significantly associated with an increased platelet activity is reflected by MPV in newly diagnosed hypertensive patients.
Blood pressure (BP) is characterized with alteration of rhythm along 24 h in hypertensive patients. Several prospective studies have established that ABPM gives a better prediction of the risk of cardiovascular morbidity than office BP measurements (22). The rate of the morning surge in 24-hour ABPM is greater in hypertensive patients than in normotensive subjects. It has been proposed that the MBPS may be particularly harmful, since many of the cardiovascular morbid events show an increased frequency during the morning hours (12-16). Indeed, several large epidemiological studies have reported a circadian pattern of adverse cardiac events as well as cerebrovascular accidents, with a peak incidence of MI, sudden cardiac death, ischaemic and haemorrhagic stroke occurring in the morning (06.00-12.00 h), after a nadir at night (9, 23). On the other hand, the studies investigating the association between cardiovascular events and MBPS have conflicting results. The most of study groups who have investigated the MBPS have suggested that an enhanced MBPS is highly correlated with cardiovascular events (12-14). In contrary, Verdecchia et al. (13). reported that blunted MBPS was an independent predictor of cardiovascular events. The mechanisms involved in the morning increase in cardiovascular diseases have been unclear. However, it has been suggested that excessive MBPS upon awakening from sleep is thought to be one of the important contributors to this phenomenon (12).
Present study showed that MPV, which reflects platelet activity was independently associated with enhanced MBPS as well as hs-CRP. Recent prospective data raise the possibility that a hypercoagulable state is not merely a marker or consequence of target organ damage but may contribute to the pathogenesis of cardiovascular events in hypertensive patients (24). In recent years, it has become evident that the prothrombotic state is present in hypertensive patients; particular attention has been directed toward the role of platelets in the pathogenesis of the complications of hypertension (7, 25). Kario et al. (12) reported that both excess MBPS and impaired coagulant or fibrinolytic activity were independently and additively associated with an increased risk of stroke in older hypertensive patients. However, in that study, authors reported that there was no association between the extent of MBPS and procoagulant or hypofibrinolytic activity (12). On the other hand, in another study, Kario et al. (26) showed that extent of MBPS was associated with increased activity of morning platelet aggregation in hypertensive patients. In hypertensive patients, the precise pathophysiological mechanisms between increased platelet activity and higher MBPS are still unknown. Several mechanisms may be responsible for this relationship. Altered platelet function in hypertensive patients, and a positive association between platelet aggregation and BP level have been reported (25). Platelets can be activated by excess BP increase itself or high shear stress at the site of atherosclerotic stenotic lesion (27). Moreover, neurohumoral factors such as sympathetic activity and the renin-angiotensinaldosterone system potentiated in the morning can affect not only MBPS but also platelet activation (12, 25). In this regard, increased platelet activity assessed with MPV may mediate the association between MBPS and cardiovascular events.
The present study also showed that MPV was independently associated with hs-CRP as well as MBPS. Previous studies showed that MPV is an inflammatory indicator in different diseases (28, 29). Circulating markers or mediators of inflammation, such as C-reactive protein (CRP), are associated with the risk of atherothrombotic events (30). CRP contribute to platelet activation and thus increase the risk of coronary heart disease (30, 31). The correlation between MPV and hs-CRP indicates molecular interaction between activated platelets and inflammatory cells (31). Therefore, high MPV values may be part of low-grade chronic inflammation in hypertensive patients (5, 28).
Finally, the relationships between MPV with platelet count, systolic blood pressure and non-dipper pattern were reported in previous studies (32, 33). In present study, MPV was correlated with non-dipper hypertension, nighttime BP and platelet count in bivariate analysis. However, similar relationships were not observed in multivariate regression analysis.
Study limitations
Smoking may have an effect on MPV and EMBPS. However, frequencies of smoking in groups were not different. Also, coronary artery disease may affect MPV levels in this patient group. Although coronary angiography was not performed in our patients, patients with coronary artery disease has been excluded according to clinical characteristics and patient history, electrocardiography, and treadmill exercise test.
Conclusion
In conclusion, high MPV was independently associated with enhanced MBPS values as well as higher hs-CRP levels. Higher MPV values related to enhanced MBPS which are associated with atherothrombotic cardiovascular events.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept - H.U., M.G.; Design - M.Y.G., A.K.; Supervision - Z.K., S.A.; Resource - O.K., Z.E.;Materials- D.Y.Ş.; Data collection &/or processing - H.U., C.T.; Analysis &/or interpretation - T.Ş., S.A.; Literature search - H.U., M.G.; Writing - H.U., M.Ç.; Critical review - M.Ç., A.K.
References
- 1.Lip GY. Target organ damage and the prothrombotic state in hypertension. Hypertension. 2000;36:975–7. doi: 10.1161/01.hyp.36.6.975. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 2.Yıldız G, Hür E, Özçiçek A, Candan F, Kayataş M. The mean platelet volume and atherogenic index of plasma in nondipper normotensive individuals compared to dippers. Clin Exp Hypertens. 2013;35:35–9. doi: 10.3109/10641963.2012.689043. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 3.Varughese GI, Lip GY. Is hypertension a prothrombotic state? Curr Hypertens Rep. 2005;7:168–73. doi: 10.1007/s11906-005-0005-4. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 4.Karan A, Güray Y, Güray U, Demirkan B, Astan R, Baysal E, et al. Mean platelet volume and the extent of coronary atherosclerosis in patients with stable coronary artery disease. Turk Kardiyol Dern Ars. 2013;41:45–50. doi: 10.5543/tkda.2013.26235. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 5.Elbasan Z, Gür M, Şahin DY, Tanboğa IH, Çaylı M. Mean platelet volume and abnormal left ventricle geometric patterns in patients with untreated essential hypertension. Platelets. 2013;24:521–7. doi: 10.3109/09537104.2012.738839. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 6.Lande K, Os I, Kjeldsen SE, Westheim A, Hjermann I, Eide I, et al. Increased platelet size and release reaction in essential hypertension. J Hypertens. 1987;5:401–6. [CrossRef] [PubMed] [Google Scholar]
- 7.Nadar SK, Blann AD, Kamath S, Beevers DG, Lip GY. Platelet indexes in relation to target organ damage in high-risk hypertensive patients:a substudy of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) J Am Coll Cardiol. 2004;44:415–22. doi: 10.1016/j.jacc.2004.03.067. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 8.Bath P, Algert C, Chapman N, Neal B Collaborative Group PROGRESS. Association of mean platelet volume with risk of stroke among 3134 individuals with history of cerebrovascular disease. Stroke. 2004;35:622–6. doi: 10.1161/01.STR.0000116105.26237.EC. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 9.Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313:1315–22. doi: 10.1056/NEJM198511213132103. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 10.Muller JE, Ludmer PL, Willich SN, Tofler GH, Aylmer G, Klangos I, et al. Circadian variation in the frequency of sudden cardiac death. Circulation. 1987;75:131–8. doi: 10.1161/01.cir.75.1.131. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 11.Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med. 2006;354:2368–74. doi: 10.1056/NEJMra060433. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 12.Kario K, Pickering TG, Umeda Y, Hoshide S, Hoshide Y, Morinari M, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives:a prospective study. Circulation. 2003;107:1401–6. doi: 10.1161/01.cir.0000056521.67546.aa. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 13.Verdecchia P, Angeli F, Mazzotta G, Garofoli M, Ramundo E, Gentile G, et al. Day-night dip and early-morning surge in blood pressure in hypertension:prognostic implications. Hypertension. 2012;60:34–42. doi: 10.1161/HYPERTENSIONAHA.112.191858. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 14.White WB. Relevance of blood pressure variation in the circadian onset of cardiovascular events. J Hypertens Suppl. 2003;21:9–15. doi: 10.1097/00004872-200307006-00003. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 15.Kuzeytemiz M, Karaağaç K, Vatansever F, Özlük OA, Yılmaz M, Arslan B, et al. The Effect of non-dipper and dipper blood pressure patterns on aortic elasticity in patients with metabolic syndrome. Clin Exp Hypertens. 2013;35:632–6. doi: 10.3109/10641963.2013.776572. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 16.Kario K. Morning surge in blood pressure and cardiovascular risk:evidence and perspectives. Hypertension. 2010;56:765–73. doi: 10.1161/HYPERTENSIONAHA.110.157149. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 17.Stergiou GS, Salgami EV. World Health Organization-International Society of Hypertension (WHO-ISH);USA Joint National Committee on prevention, detection, evalutiaon, and treatment of high blood pressure (JNC-7);European Soceity of Hypertension-European Society of Cardiology (ESH-ESC). New European, American and International guidelines for hypertension management:agreement and disagreement. Expert Rev Cardiovasc Ther. 2004;2:359–68. doi: 10.1586/14779072.2.3.359. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 18.Verdecchia P, Schillaci G, Porcellati C. Dippers versus non-dippers. J Hypertens Suppl. 1991;9:42–8. [PubMed] [Google Scholar]
- 19.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Chamber Quantification Writing Group. Recommendations for chamber quantification:A report from the American Society of Echocardiography’s Guidelines and Standards Committee and The Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 20.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–67. doi: 10.1016/s0894-7317(89)80014-8. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 21.Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–8. doi: 10.1161/01.cir.55.4.613. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 22.Eguchi K, Pickering TG, Hoshide S, Ishikawa J, Ishikawa S, Schwartz JE, et al. Ambulatory blood pressure is a better marker than clinic blood pressure in predicting cardiovascular events in patients with/without type 2 diabetes. Am J Hypertens. 2008;21:443–50. doi: 10.1038/ajh.2008.4. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willich SN, Levy D, Rocco MB, Tofler GH, Stone PH, Muller JE. Circadian variation in the incidence of sudden cardiac death in the Framingham Heart Study population. Am J Cardiol. 1987;60:801–6. doi: 10.1016/0002-9149(87)91027-7. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 24.Kario K, Yano Y, Matsuo T, Hoshide S, Eguchi K, Shimada K. Additional impact of morning haemostatic risk factors and morning blood pressure surge on stroke risk in older Japanese hypertensive patients. Eur Heart J. 2011;32:574–80. doi: 10.1093/eurheartj/ehq444. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 25.Gkaliagkousi E, Passacquale G, Douma S, Zamboulis C, Ferro A. Platelet activation in essential hypertension:implications for anti-platelet treatment. Am J Hypertens. 2010;23:229–36. doi: 10.1038/ajh.2009.247. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 26.Kario K, Yano Y, Matsuo T, Hoshide S, Asada Y, Shimada K. Morning blood pressure surge, morning platelet aggregation, and silent cerebral infarction in older Japanese hypertensive patients. J Hypertens. 2011;29:2433–9. doi: 10.1097/HJH.0b013e32834cf1c0. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 27.Kawano K, Yoshino H, Aoki N, Udagawa H, Watanuki A, Hioki Y, et al. Shear-induced platelet aggregation increases in patients with proximal and severe coronary artery stenosis. Clin Cardiol. 2002;25:154–60. doi: 10.1002/clc.4960250405. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume:A link between thrombosis and inflammation. Curr Pharm Des. 2011;17:47–58. doi: 10.2174/138161211795049804. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 29.Sharma G, Berger JS. Platelet activity and cardiovascular risk in apparently healthy individuals:a review of the data. J Thromb Thrombolysis. 2011;32:201–8. doi: 10.1007/s11239-011-0590-9. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 30.Danesh J, Wheeler JG, Hirshfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–97. doi: 10.1056/NEJMoa032804. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 31.Eisen A, Bental T, Assali A, Kornowski R, Lev EI. Mean platelet volume as a predictor for long-term outcome after percutaneous coronary intervention. J Thromb Thrombolysis. 2013;36:469–74. doi: 10.1007/s11239-013-0876-1. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 32.Uçar H, Gür M, Koyunsever NY, Şeker T, Türkoğlu C, Kaypaklı O, et al. Mean platelet volume is independently associated with renal dysfunction in stable coronary artery disease. Platelets. 2013 Jun 17; doi: 10.3109/09537104.2013.805406. [Epup ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.İnanç T, Kaya MG, Yarlioglues M, Ardıç I, Özdoğru I, Doğan A, et al. The mean platelet volume in patients with non-dipper hypertension compared to dippers and normotensives. Blood Press. 2010;19:81–5. doi: 10.3109/08037050903516284. [CrossRef] [DOI] [PubMed] [Google Scholar]


