Abstract
Objective:
In this study we assess the value of left atrial dyssynchrony time measured by tissue Doppler imaging (TDI) to predict recurrences after pulmonary vein isolation (PVI) in patients with paroxysmal and persistent atrial fibrillation (AF).
Methods:
One hundred sixty patients (57±7.5 years, 122 males) with symptomatic drug-refractory paroxysmal and persistent AF, undergoing PVI were enrolled in our study. PA peak time by tissue Doppler imaging (PApeak-TDI) is defined as the time measured from the start of P wave in lead II to the peak of A wave on the tissue Doppler tracing. Left atrial dyssynchrony was measured by subtracting the PApeak-TDI time measured at the mid-inter atrial septum from the PA peak-TDI time measured at the left atrial midlateral free wall, (LA dyssynchrony=PApeak TDI lateral-PApeak TDI septal).
Results:
During a mean follow-up of 12±3 months, recurrences occurred in 50 out of 160 patients. Patients with recurrence of atrial fibrillation had larger left atrial dyssynchrony time (26.5±2.4 ms vs. 23.5±2.3 ms, p<0.001). Left atrial dyssynchrony time of 25 ms has the best combined sensitivity and specificity (74% and 63% respectively) along with positive predictive value 53% and negative predictive value 85.5%. LA dyssynchrony time ≥25 ms was found to discriminate patients prone to AF recurrences over time. Multivariate regression analysis showed that left atrial dyssynchrony time (HR per ms: 1.69, p<0.001) was identified as independent predictor of AF recurrence.
Conclusion:
Left atrial dyssynchrony time is good clinical predictor of recurrence of AF after PVI in patients with paroxysmal and persistent AF.
Keywords: atrial remodeling, left atrial dyssynchrony, atrial fibrillation, pulmonary vein isolation
Introduction
Atrial fibrillation (AF) is one of the most common cardiac arrhythmia which leads to circulatory instability and stroke. The main goals in management of AF are rate or rhythm control and anticoagulation. If rhythm control is desired and cannot be maintained by medication or cardioversion then catheter ablation may be attempted.
Radiofrequency catheter ablation (RFCA) of AF is effective in 70-80% of the cases but recurrences are frequent (1-4). The natural history of AF is characterized by self-perpetuating mechanisms, rate-induced electrophysiogical changes and structural remodeling that involve the atrial myocardium (5).
Atrial remodeling which is the main substrate of AF divides mainly into structural and electrical remodeling and is represented clinically by enlargement of the atria and slow atrial conduction velocity. Remodeling of the left atrium (LA) can be evaluated by several methods by using; electrocardiograph (ECG), tissue Doppler imaging (TDI), or invasively by measuring cardiac intervals through using intracardiac electrodes.
TDI allows the measurement of peak systolic velocity of different regions of the myocardium. Moreover, precise timing of peak systolic velocity is possible when the TDI tracings are related to the electrical activity (P wave and QRS complex).
Integration of this information allows accurate assessment of electromechanical coupling, and evaluation of inter- and intramyocardial walls dyssynchrony (6).
The loss of coordinated myocardial contraction that occurs as a result of conduction delay is referred to as dyssynchrony and may be evaluated in a number of ways, usually involving echocardiography. Assessment of dyssynchrony by two segmental models was previously evaluated by Garrigue et al. (7) using pulsed wave TDI obtained in two segments. Non-invasive prediction of paroxysmal atrial fibrillation (PAF) was also previously evaluated by Karapınar et al. (8) through investigating the relationship between the atrial electromechanical coupling time and PAF.
Using LA dyssynchrony time measured by TDI is cheap, feasible and can give us more accurate assessment of the presence and extent of LA remodeling than conventional echocardiographic parameters. In our study we evaluate the usage of this parameter to predict AF recurrences after pulmonary vein isolation (PVI) for patients with paroxysmal and persistent AF.
Methods
Population
From our ongoing clinical registry 298 patient with persistent and paroxysmal AF were enrolled from September 2010 till August 2012. Patients having structure heart disease, patients who were not compliant to the antiarrhythmic drugs, patients who needed ablation in non-pulmonary vein sites at the first session and patients who were not sinus during echocardiographic examination were excluded from the study. Finally 160 consecutive patients (57±7.6 years, 122 males, and 38 females) with symptomatic drug-refractory paroxysmal and persistent AF, undergoing RFCA for the first time were enrolled. AF was classified as paroxysmal when episodes were generally self-terminating and lasted no longer than 7 days and was classified as persistent when AF episodes lasted longer than 7 days or requiring termination by cardioversion according to the European Society of Cardiology guidelines definitions (9). Informed consents from all patients were taken and our study was approved by the Ethical Committee of our institute and the procedures we followed complied with the Declaration of Helsinki. All antiarrhythmic medications were stopped for about four to five half-lives (amiodarone stopped at least one month before ablation), patients who did not comply for stoppage of the antiarrhythmic drugs were excluded from the study.
Transthoracic echocardiography was done to a control group of 40 normal subjects (31 males; 9 females; mean age 57.1±8.2 years) without any history of AF, structural heart disease, hypertension and diabetes to define normal values for total atrial conduction time and LA dyssynchrony time measured by TDI.
Prior to the procedure
Standard 12 lead ECG
Standard 12 lead ECGs were performed for all patients to make sure that they were sinus during the echocardiographic assessment, ECGs were done by a commercially available machine (MAC 1200 ST General Electric medical information technology), the 12-lead ECGs were recorded for 10 seconds at a sweep speed of 25 mm/s and calibrated to 1 mV/cm in the standard leads, Scanning and digitizing ECG signals from paper records using an optical scanner were performed for all ECG recordings, the onset and offset of the P wave were defined as the start of the upward deflection of the P wave pattern and its return to the isoelectric baseline in lead II (10). P wave duration in lead II was measured and assessed for correlation with other study variables.
Standard 2-D, Doppler and transesophageal echocardiography
All included patients were subjected to a standard 2-D and Doppler echocardiogram 24 hours prior to ablation using (Vivid 7, General Electric Vingmed, Milwaukee, WI, USA), equipped with a 3.5 MHz transducer at a depth of 16 cm. ECG-triggered images of standard parasternal short- and long-axis views as well as apical 2-and 4-chamber views were digitized during three consecutive cardiac cycles in cine-loop format for off-line analyses using (EchoPac 108.1.5, General Electric Medical Systems, Horten, Norway). Doppler measurements were obtained at end-expiratory apnea. Average values of three sequential beats were used for analysis. All patients underwent transesophageal echo-cardiography before the ablation procedure to exclude left atrial thrombi.
Left ventricular (LV) ejection fraction was calculated by modified Simpson method. Measurement of LA volumes from the apical 4-chamber and apical 2-chamber views using biplane area length method indexed to body surface area at two phases of the cardiac cycle: LA maximum volume at the end-systolic phase (just before mitral valve opening) and LA minimum volume at the end-diastolic phase (just before mitral valve closure) (11), LA ejection fractions were measured according to the formula: (maximal volume-minimal volume)/maximal volume x 100.
LV diastolic function was evaluated using pulsed-wave Doppler recordings of the mitral valve inflow pattern (E-wave, A-wave), the diastolic function grade was classified as either normal, Grade 1 (impaired relaxation), Grade 2 (pseudonormalization), or Grade 3 (restrictive filling pattern) (12).
Tissue Doppler imaging
In our study we used the two segmental models to assess LA dyssynchrony, PA peak time by TDI (PA peak-TDI) is defined as the time measured from the start of P wave in lead II to the peak of A wave on the tissue Doppler tracing. The sample volume was put on the middle septal and middle lateral LA walls. Attempts were made to align the atrial wall parallel to the Doppler beam and the following measurements were done; PA peak-TDI lateral which is the time measured from the start of P wave in lead II to the peak of A-wave measured from lateral LA wall, known also as the total atrial conduction time (13) and LA dyssynchrony was measured by subtracting the PA peak-TDI time measured at the mid interatrial septum (PA peak-TDI septal) from the PA peak-TDI lateral measured at the LA mid-lateral free wall (LA dyssynchrony=PA peak TDI lateral-PA peak TDI septal). Doppler measurements were obtained at end-expiratory apnea, Doppler beams are well and carefully aligned with the tested areas. Average values of three sequential beats were used for analysis.
For further verification of our measurements, intra-procedural measurement of the total atrial conduction time using the intracardiac electrodes was used by measuring the time interval from the right atrial activation to activation of distal coronary sinus and assessment of correlation with the total atrial conduction measured by TDI was done (14).
Radiofrequency catheter ablation procedure
The ablation procedure was performed in a fasting state. The right femoral vein was used for the insertion of catheters, a single trans-septal puncture was performed using (FAST-CATH trans-septal guiding introducer SL1 8.5 F St. Jude medical). Two catheters were inserted: one duodecapolar Lasso catheter for PV recording (introduced through the SL0 long sheath and positioned at the ostium of each PV sequentially), other 4 mm irrigated-tip ablation catheter for circumferential isolation. The right internal jugular vein was used for the insertion of one decapolar (2 mm spacing) steerable catheter in the distal coronary sinus. After transseptal puncture, a bolus of 5000 U of intravenous heparin was administered and activated clotting time was maintained above 250 s throughout the procedure.
Three-dimensional electro-anatomical mapping was performed in all cases using the CARTO 3 system (Biosence Webster, Jhonson&Jhonson, San Francisco, CA-USA). Circumferential isolation of pulmonary veins was performed using the ablation catheter [maximum power 30-35 W; maximal temperature 40 Celsius; duration of the radiofrequency (RF) application 60 s]. The endpoint of RF application was complete PVI, demonstrated by the absence of PV potentials in the PV during sinus rhythm or coronary sinus pacing and by the absence of PV-LA conduction during PV pacing. Reconfirmation of PV isolation was performed 30 min after ablation for each PV. Patients were followed with continuous ECG monitoring for 24 h and were discharged from the hospital two days after the procedure.
All measurements were evaluated by two independent blinded investigators and Bland-Altman analyses (15) were performed to assess the inter- and intra-observer reproducibility of total atrial conduction time measured by TDI (PA peak-TDI lateral), total atrial conduction time measured by electrophysiological study and left atrial dyssynchrony time measured by TDI, Measurements show minimal biases (1.2±6 and 1.3±5 ms, respectively for PA peak-TDI lateral), (1.4±5 and 1.3±5 ms, respectively for total atrial conduction time measured by electrophysiological study), (1.2±5 and 1.3±4 ms, respectively for left atrial dyssynchrony time measured by TDI).
Follow up
All patients were evaluated at the outpatient clinic during a mean follow up of 12±3 months. Electrocardiogram (ECG) recordings were acquired on each visit and 24 h Holter recordings were scheduled to be done every 3 months of follow-up. Importantly, all patients were encouraged to immediately obtain an ECG recording when experiencing palpitations. Recurrence of AF was defined as any recording of AF on ECG or an episode longer than 30 s on 24 h Holter recording. Recurrent patients had another trial of medical management mainly class IC and class III antiarrhythmic drugs, recurrent patients who did not respond to the medical treatment were offered another trial of ablation and assessment of the presence of non-pulmonary vein triggers of AF was done. Moreover measurement of the left atrial dyssynchrony time by TDI was repeated for further evaluation to all patients after one year from doing the first session of RFCA.
Statistical analysis
Data are expressed as mean±standard deviation for continuous variables and frequencies for categorical variables. Differences between groups were assessed using chi-square statistics for categorical variables and group means for continuous variables with normal and non-normal distributions were compared using student’s t-tests and Mann-Whitney U tests, respectively. A p value <0.05 was considered significant. Pearson’s correlation coefficient analysis using significant variables was performed to assess correlation between continuous variables. Univariate and multiple Cox regression analysis were performed to investigate predictors of AF recurrence after RFCA. All variables mentioned in Table 1 were included in the univariate analyses. Variables with a p value <0.05 in the univariate analysis were included into the multiple Cox regression analysis. Multiple Cox regression analysis was performed using a backward stepwise conditional approach. Variables with a p>0.05 were excluded from the model. A receiver operating characteristic (ROC) curve was generated to evaluate left atrial dyssynchrony as a predictor of AF recurrences after PVI and different cut-off values for LA dyssynchrony time were chosen to evaluate probability of AF recurrences. The AF-free rates according to LA dyssynchrony time of <25 ms and ≥25 ms were calculated using Kaplan–Meier analysis with the log-rank test. Statistical analyses were performed using SPSS version 16.0 statistical software (SPSS Inc., Chicago, IL, USA).
Table 1.
Clinical characteristics of the study population along with comparison between recurrence and no recurrence group
| Clinical characteristics | Total | No recurrence | Recurrence | P |
|---|---|---|---|---|
| Number of patients | 160 | 110 | 50 | |
| Age, years | 57±7.5 | 53.9±5.9 | 64.6±5.1 | <0.001 |
| Gender, male/female | 122/38 | 82/28 | 40/10 | 0.54 |
| Hypertension, n/t | 87/160 | 52/110 | 35/50 | 0.01 |
| Diabetes, n/t | 12/160 | 6/110 | 6/50 | 0.19 |
| Stroke, n/t | 1/160 | 0/110 | 1/50 | 0.31 |
| Body mass index, kg/m2 | 27.5±1.1 | 27.4±1.2 | 27.78±1 | 0.06 |
| Duration of AF, months | 11.3±6.7 | 8.9±3.8 | 16.6±8.5 | <0.001 |
| AF type, persistent/paroxysmal | 54/106 | 25/85 | 29/21 | <0.001 |
| (Arrhythmic drugs) Class IC/Class III, n | 74/86 | 52/58 | 22/28 | 0.7 |
| Left atrium maximum volume, mL/m2 | 38.7±3.9 | 37.2±2.9 | 42.2±3.7 | 0.001 |
| Left atrium minimum volume, mL/m2 | 19.4±2 | 18.6±1.4 | 21.1±1.9 | 0.002 |
| Left atrium ejection fraction, % | 49.9±1.29 | 49.9±1.24 | 49.8±1.3 | 0.08 |
| Left ventricle ejection fraction, % | 59.9±1.2 | 59.9±1.2 | 59.8±1.3 | 0.15 |
| Pulsed wave Doppler E wave, cm/s | 62.1±2.6 | 61.9±2.9 | 62.4±1.9 | 0.25 |
| Pulsed wave Doppler A wave, cm/s | 53.9±5.6 | 55.5±5.5 | 50.4±3.9 | 0.005 |
| Diastolic function grade, n | ||||
| Normal | 72 | 52 | 20 | 0.356 |
| Grade 1 | 78 | 53 | 25 | |
| Grade 2 | 10 | 5 | 5 | |
| Grade 3 | 0 | 0 | 0 | |
| P wave duration, ms | 109.4±15.6 | 103.2±13.7 | 123.1±9.7 | 0.001 |
| Total atrial conduction time, ms | 130.8±11.8 | 128.8±12.1 | 135.2±10.1 | 0.001 |
| Left atrial dyssynchrony time, ms | 24.4±2.7 | 23.5±2.3 | 26.5±2.4 | <0.001 |
ms - millisecond; n - number; t - total
Results
Comparison between control group and AF patients showed that control cases had shorter total atrial conduction time (110.7±8.2 ms vs. 130.8±11.8 ms, p<0.001), lower LA dyssynchrony time (14.7±4.6 ms vs. 24.4±2.7 ms, p<0.001).
All patients had successfully isolated pulmonary veins according to the end points mentioned before, only 35 out of the 160 patients needed direct current (DC) cardioversion at the end of the procedure to achieve sinus rhythm while the rest of the patients turned sinus without the need of DC shock. No recurrence of AF was observed during the first 24 h after the ablation procedure. During a mean follow-up of 12±3 months, recurrences had occurred in 31.2% (50/160). Patients with recurrence of AF had larger LA dyssynchrony time in comparison with patients with no recurrence (26.5±2.4 ms vs. 23.5±2.3 ms, p<0.001), the clinical characteristics of the study population along with comparison between recurrence and no recurrence group are summarized in Table 1. Persistent AF patients had larger LA dyssynchrony time in comparison with paroxysmal AF patients (26.3±2.3 ms vs. 23.5±2.4 ms, p<0.001), the clinical characteristics of the study population along with comparison between recurrence and no recurrence group are summarized in Table 1. Persistent AF patients had larger LA dyssynchrony time in comparison with paroxysmal AF patients (26.3±2.3 ms vs. 23.5±2.4 ms, p<0.001). Comparison between paroxysmal AF and persistent AF patients is summarized in (Table 2). Assessment of correlation between total atrial activation time measured by intracardiac electrodes and that measured by TDI showed strong correlation (r=0.7, p<0.001).
Table 2.
Comparison between patients with paroxysmal AF and persistent AF
| Clinical characteristics | Persistent AF | Paroxysmal AF | P |
|---|---|---|---|
| Number of patients | 54 | 106 | |
| Age, years | 60.1±7.5 | 55.7±7.2 | 0.001 |
| Gender, male/female, n | 42/12 | 80/26 | 0.8 |
| Hypertension, n/t | 37/54 | 50/106 | 0.012 |
| Diabetes, n/t | 5/54 | 7/106 | 0.54 |
| Stroke, n/t | 1/54 | 0/106 | 0.99 |
| Body mass index, kg/m2 | 27.7±1.1 | 27.4±1.2 | 0.22 |
| Left atrium maximum volume, mL/m2 | 40±4 | 38.2±3.9 | 0.03 |
| Left ventricle ejection fraction, % | 59.8±1.3 | 59.9±1.2 | 0.26 |
| Pulsed wave Doppler A wave, cm/s | 52.3±5.4 | 54.7±5.5 | 0.01 |
| P wave duration, ms | 113.5±15.4 | 107±15.3 | 0.01 |
| Total atrial conduction time, ms | 133.8±10.1 | 129.3±12.3 | 0.02 |
| Left atrial dyssynchrony time, ms | 26.3±2.3 | 23.5±2.4 | <0.001 |
| AF recurrence post PVI, n/t | 29/54 | 21/106 | <0.001 |
n - number; t – total
Receiver operating characteristic (ROC) of both total atrial conduction time and left atrial dyssynchrony time ROC curve analysis were done for different total atrial conduction time and LA dyssynchrony time cut-off points, the area under the curve for total atrial conduction time was 0.69 (95% confidence interval 0.6-0.78) (p<0.001), the area under the curve for left atrial dyssynchrony time was 0.8 (95% confidence interval 0.73-0.88) (p<0.001). As shown from the ROC curves left atrial dyssynchrony time could have better predictive power for predicting recurrence of AF after PVI. This can be illustrated by presenting one of our recurrent patients who had large LA dyssynchrony time while his total atrial conduction time was normal (Fig. 1). By observing different cut-off values, left atrial dyssynchrony time of 25 ms has the best combined sensitivity and specificity (74% and 63% respectively) along with positive predictive value 53% and negative predictive value 85.5%. LA dys synchrony time ≥25ms was found to discriminate patients prone to AF recurrences over time according to log-rank test (Fig. 2).
Figure 1.
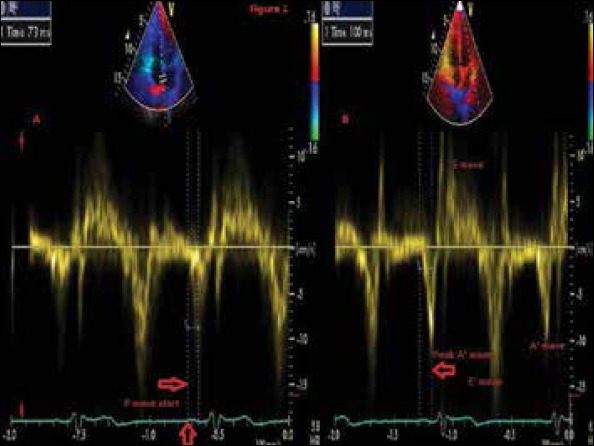
Tissue Doppler imaging for a patient that suffer recurrence of AF post PVI with normal total atrial conduction time-100ms along with large left atrial dyssynchrony time-27 ms
Figure 2.
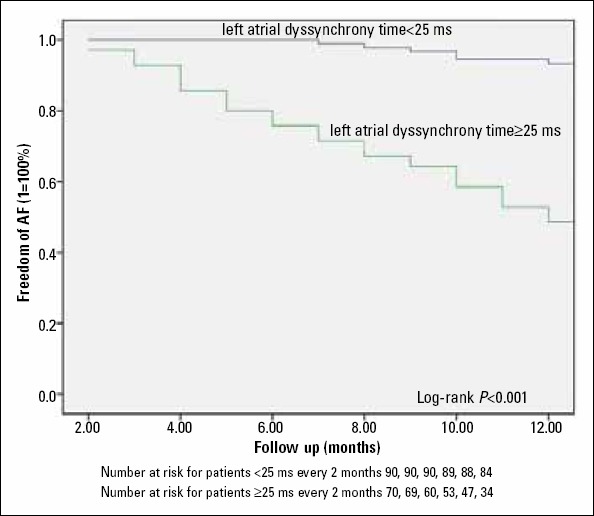
Kaplan-Meier event-free analysis for patients with left atrial dyssynchrony time ≥25 ms compared with patients with left atrial dyssynchrony time <25 ms
Clinical predictors of AF recurrence
Univariate and multiple cox regression analysis were performed to find out the predictors of AF recurrence after RFCA. In univariate analysis all the variables in (Table 1) were tested and the results were shown in (Table 3). In multiple cox regression analysis hazard ratio (HR) of significant independent predictors of AF recurrence were defined as follows; LA maximum volume index (HR per mL/m2: 1.16, p<0.001), age (HR per year 1.18, p=0.001) and LA dyssynchrony time (HR per ms: 1.69, p<0.001), LA dyssynchrony time ≥25 ms clinical cut-off value was tested in univariate analysis [HR- 5.2, confidence interval (CI): 2.7-9.8, p<0.001] and it was an independent predictor of AF recurrence in multiple analysis (HR- 3.4, CI: 1.13-5.3, p<0.001) (Table 4). Importantly, multiple analyses demonstrated that there was no interaction between LA maximum volume index or LA dyssynchrony time and the type of AF. This illustrates that both LA maximum volume index and LA dyssynchrony time have a similar prognostic value in patients with paroxysmal or persistent AF.
Table 3.
Univariate analysis for clinical predictors of AF recurrence
| Variables | Hazard ratio | Confidence interval | P |
|---|---|---|---|
| Age, years | 1.19 | 1.14-1.25 | <0.001 |
| Body mass index | 1.25 | 0.99-1.58 | 0.06 |
| Hypertension | 1.16 | 1.1-1.2 | 0.02 |
| Diabetes mellitus | 0.55 | 0.23-1.29 | 0.17 |
| Stroke | 0.16 | 0.02-1.19 | 0.07 |
| AF duration | 1.07 | 1.05-1.09 | <0.001 |
| P wave duration | 1.1 | 1.07-1.14 | <0.001 |
| LV ejection fraction | 0.48 | 0.22-1.02 | 0.08 |
| Peak A wave velocity | 0.87 | 0.83-0.91 | <0.001 |
| LA maximum volume | 1.28 | 1.2-1.36 | <0.001 |
| LA minimum volume | 1.65 | 1.45-1.88 | <0.001 |
| LA ejection fraction | 0.48 | 0.22-1.02 | 0.05 |
| TACT | 1.06 | 1.02-1.1 | <0.001 |
| Type of AF | 3.7 | 2.1-6.5 | <0.001 |
| LA dyssynchrony time | 1.97 | 1.65-2.34 | <0.001 |
| LA dyssynchrony time ≥25 ms | 5.2 | 2.7-9.8 | <0.001 |
LA - left atrium; LV - left ventricle; TACT - total atrial conduction time
Table 4.
Multivariate analysis for clinical predictors of AF recurrence
| Variables | Hazard ratio | Confidence interval | P |
|---|---|---|---|
| Age, year | 1.18 | 1.11-1.25 | <0.001 |
| LA dyssynchrony time | 1.69 | 1.91-2.04 | <0.001 |
| LA maximum volume | 1.16 | 1.13-1.2 | <0.001 |
| LA dyssynchrony time ≥25 ms | 3.4 | 1.13-5.3 | <0.001 |
LA - left atrium
Improvement of dyssynchrony time after one year
After one year from performing ablation procedure, measuring LA dyssynchrony time for all patients showed total improvement of the LA dyssynchrony time (22.4±3.6 ms vs. 24.4±2.7 ms, p<0.001). By analyzing for LA dyssynchrony time measured one year after ablation, it showed that patients with recurrence of AF had larger dyssynchrony time (25.8±2.6 ms vs. 20.9±2.9 ms, p<0.001) in comparison with non-recurrent patients further more patients who were initially classified as persistent AF had larger dyssynchrony time than patients who were initially classified as paroxysmal AF (24.7±3.2 ms vs. 21.3±3.2 ms, p<0.001).
Follow up of recurrent patients
All the recurrent patients had another trial of medical management mainly class IC and class III antiarrhythmic drugs, 13 patients respond well with no recurrence for at least 6 months follow up while other 37 patients decided to repeat ablation. Data collected from patients who repeated ablation showed presence of non pulmonary vein triggers in 27 patients with large LA dyssynchrony time 26.7±2.7 ms. The 27 patient had an additional ablation sites as follows; two in the superior vena cava, five in the ligament of Marshall, six in coronary sinus, fourteen in the LA posterior wall of whom five were having also pulmonary veins reconnection. By analyzing the initial classification of the patients who showed non pulmonary vein triggers on follow up 17 out of the 27 were originally having persistent AF while 10 were originally having paroxysmal AF. The remaining 10 out of the 37 patients had pulmonary veins reconnection only.
Discussion
In our study we propose a non-invasive easy applicable predictor for AF recurrence after ablation. From our results we found that there is significant difference between recurrent patients and patients without recurrence regarding age, type of AF, duration of AF, hypertension, LA maximum volume, LA minimum volume, A wave measured by pulsed wave Doppler, P wave duration, total atrial conduction time and LA dyssynchrony time, while there were no difference between the two groups regarding gender, diabetes, LV systolic function, LV diastolic function, body mass index, left atrial EF, stroke and the history of antiarrhythmic drugs usage. LA dyssynchrony time in our study were prolonged in patients with AF recurrence (26.5±2.4 ms vs. 23.5±2.3 ms, p<0.001) along with patients who were originally having persistent AF had larger LA dyssynchrony time than paroxysmal AF (26.3±2.3 ms vs. 23.5±2.4 ms, p<0.001).
AF ablation now is very important as it is believed that it can cure the arrhythmia however the recurrence of AF after ablation is still high. A large number of parameters have been related to a high risk of AF recurrence after RFCA, such as age, type of AF, LA size, and impaired LV systolic function. Interestingly, these parameters except LA size seem to either cause or reflect the presence and extent of atrial remodeling. A high extent of atrial remodeling is thought to limit the efficacy of RFCA for AF.
In addition to atrial dilatation, slow atrial conduction velocity may be another consequence of atrial remodeling (16). Atrial enlargement and slow atrial conduction velocity can result in a larger number of re-entrant wavelets inside the atria. This situation favors the development and perpetuation of AF (17). Karapınar et al. (8) also demonstrated that atrial electromechanical delays could be showed non-invasively by tissue Doppler echocardiography and delayed right atrial lateral electromechanical coupling relative to the septal one can be associated with paroxysmal atrial fibrillation.
Recently, Allessie et al. (18) underlined the importance of electrical remodeling in the development of substrate for AF by demonstrating a substantially higher degree of functional reentry during longstanding AF as compared with acute AF. Furthermore, Choi et al. (19) demonstrated that by decreasing the total atrial conduction time with linear triple-site pacing, burst-induction of AF could be prevented in some patients with persistent AF. Areas of slow conduction and conduction block are important prerequisites for re-entry known to underlie AF. Experimental and clinical data show that AF is associated with global lowering of atrial propagation velocity and the presence of fibrotic changes in the atrial musculature (20).
Previous studies had proved that usage of atrial dyssynchrony is of clinical importance to predict the occurrence of AF; Sakabe et al. (21) proved that interatrial dyssynchrony on TDI predicts progression to chronic atrial fibrillation in patients with non-valvular paroxysmal atrial fibrillation. Cho et al. (22) evaluated the usage of left atrial dyssynchrony assessed by strain imaging in predicting future development of atrial fibrillation in patients with heart failure, although this study had demonstrated that atrial dyssynchrony based on strain is the strongest univariate and multivariate predictor for new onset AF in hospitalized patients with congestive heart failure, however it concluded that atrial dyssynchrony based on tissue Doppler during the atrial contraction period failed to predict the development of new onset AF, we have some considerations regarding this point, the type of patients selected were congestive heart failure patients with no history of AF which differs from our study population, also one study by Van Beeumen et al. (23) demonstrated that interatrial dyssynchrony, not intra-atrial dyssynchrony, was documented in patients with heart failure.
Evranos et al. (24) had used the intra left atrial electromechanical delay with cut-off value 29.5 ms as a predictor of AF recurrence after AF ablation. The main differences between our study and their study can be summarized as follows; first our study population is much larger (160 patients vs. 61 patients), second point is the method used for measuring the PA time interval by TDI, we measure it from the start of P wave on the surface ECG to the peak of A wave on the TDI tracing while in their study the measure it from the start of P wave on the surface ECG to the beginning of A wave on the TDI tracing, third point is the follow up, we repeat the measurement of the LA dyssynchrony time one year post ablation, at last we also observe in our study the presence of association between large LA dyssynchrony time and non-pulmonary vein triggers of AF.
Improvement of left atrial dyssynchrony after one year post ablation
Improvement of LA dyssynchrony time reinforces the concept of atrial reverse remodeling after PVI, and stresses on the idea that AF is characterized by a self-perpetuating mechanisms. Treating AF patients with RFCA improves the atrial electrical conduction velocity protecting the atria from further remodeling.
Kuppahally et al. (25) had demonstrated that after catheter ablation of AF there is reverse remodeling of the LA structure and function which is significantly better when ablation is performed in early stage of the arrhythmia with mild LA structural remodeling. The long term success of RFCA seems to be dependent on the stage of LA structure reverse remodeling.
Improvement of atrial function post PVI is recently proved by Küçükdurmaz et al. (26). They demonstrated how AF ablation contributes to cardiac function in patients with persistent AF, improvement for LA dimensions and LAEF was seen during the first 3 months and continued up to 2 years.
Non-pulmonary vein triggers of AF in recurrent patients
The present study showed that recurrent patients associated with non-pulmonary vein triggers of AF had large LA dyssynchrony time. LA dyssynchrony time could be a marker of advanced atrial remodeling and slow atrial conduction velocity and represent a risk of increased automaticity and increased trigger activity in human diseased atrial fibers. Thus, it is possible that sites other than pulmonary veins could be the source of spontaneous ectopy in patients with advanced atrial remodeling and based on the theory of multiple re-entrant wavelets, AF could be more easily induced and maintained in larger atrial sizes and slower conduction velocities.
Study limitations
AF duration data were derived from the patient’s history and ECG recordings; thus, the existence of asymptomatic AF cannot be excluded and the duration of AF might not be accurate. The asymptomatic episodes of AF may also confound the results of AF recurrence during the follow-up. LA dyssynchrony time measured in our study cannot be used in patients presenting with AF at the time of the echo assessment. The area under the curve although in favor for LA dyssynchrony time it is not too large along with the sample size is relatively small and results of the present study should be confirmed by larger prospective trials.
Conclusion
LA dyssynchrony time is a good clinical predictor of recurrence of AF after PVI. Moreover LA dyssynchrony time of 25 ms can distinguish patients with paroxysmal or persistent AF who will gain benefit from pulmonary vein isolation. Patients who have intra LA dyssynchrony at baseline, attempts at finding extra-pulmonary vein foci during the procedure might be necessary before the decision to terminate the procedure and additional lines or fractionated potentials should be targeted.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept - A.S., S.Z.; Design - A.S., X.L., Q.L.; Supervision - A.S., L.T., X.L., S.Z.; Resource - A.S., H.Y., Q.L.; Materials - A.S., H.Y., S.Z.; Data collection &/or processing - A.S., H.Y., L.T., Q.L.; Literature search - A.S., L.T., Q.L.; Writing - A.S., H.Y., X.L., S.Z.; Critical review - A.S., L.T., Q.L.; Other - A.S., S.Z.
References
- 1.Tzou WS, Marchlinski FE, Zado ES, Lin D, Dixit S, Callans DJ, et al. Long-term outcome after successful catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:237–42. doi: 10.1161/CIRCEP.109.923771. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 2.Letsas KP, Weber R, Bürkle G, Mihas CC, Minners J, Kalusche D, et al. Pre-ablative predictors of atrial fibrillation recurrence following pulmonary vein isolation:the potential role of inflammation. Europace. 2009;11:158–63. doi: 10.1093/europace/eun309. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 3.Shah AN, Mittal S, Sichrovsky TC, Cotiga D, Arshad A, Maleki K, et al. Long-term outcome following successful pulmonary vein isolation:pattern and prediction of very late recurrence. J Cardiovasc Electrophysiol. 2008;19:661–7. doi: 10.1111/j.1540-8167.2008.01101.x. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 4.Oakes RS, Badger TJ, Kholmovski EG, Akoum N, Burgon NS, Fish EN, et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119:1758–67. doi: 10.1161/CIRCULATIONAHA.108.811877. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carnes CA, Chung MK, Nakayama T, Nakayama H, Baliga RS, Piao S, et al. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res. 2001;89:E32–8. doi: 10.1161/hh1801.097644. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 6.Yu CM, Bax JJ, Monaghan M, Nihoyannopoulos P. Echocardiographic evaluation of cardiac dyssynchrony for predicting a favourable response to cardiac resynchronisation therapy. Heart. 2004;90:17–22. doi: 10.1136/hrt.2004.048322. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garrigue S, Reuter S, Labeque JN, Jais P, Hocini M, Shah DC, et al. Usefulness of biventricular pacing in patients with congestive heart failure and right bundle branch block. Am J Cardiol. 2001;88:1436–41. doi: 10.1016/s0002-9149(01)02131-2. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 8.Karapınar H, Acar G, Kırma C, Kaya Z, Karavelioğlu Y, Küçükdurmaz Z, et al. Delayed right atrial lateral electromechanical coupling relative to the septal one can be associated with paroxysmal atrial fibrillation. Eur Rev Med Pharmacol Sci. 2013;17:2172–8. [PubMed] [Google Scholar]
- 9.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. European Heart Rhythm Association;European Association for Cardio-Thoracic Surgery. Guidelines for the management of atrial fibrillation:the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Europace. 2010;12:1360–420. doi: 10.1093/europace/euq350. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 10.Dilaveris P, Batchvarov V, Gialafos J, Malik M. Comparison of different methods for manual P wave duration measurement in 12-lead electrocardiograms. Pacing Clin Electrophysiol. 1999;22:1532–8. doi: 10.1111/j.1540-8159.1999.tb00358.x. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 11.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification:a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 12.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–33. doi: 10.1016/j.echo.2008.11.023. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 13.den Uijl DW, Gawrysiak M, Tops LF, Trines SA, Zeppenfeld K, Schalij MJ, et al. Prognostic value of total atrial conduction time estimated with tissue Doppler imaging to predict the recurrence of atrial fibrillation after radiofrequency catheter ablation. Europace. 2011;13:1533–40. doi: 10.1093/europace/eur186. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 14.Deniz A, Şahiner L, Aytemir K, Kaya B, Kabakçı G, Tokgözoğlu L, et al. Tissue Doppler echocardiography can be a useful technique to evaluate atrial conduction time. Cardiol J. 2012;19:487–93. doi: 10.5603/cj.2012.0089. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 15.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [CrossRef] [PubMed] [Google Scholar]
- 16.Gaspo R, Bosch RF, Talajic M, Nattel S. Functional mechanisms underlying tachycardia-induced sustained atrial fibrillation in a chronic dog model. Circulation. 1997;96:4027–35. doi: 10.1161/01.cir.96.11.4027. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 17.Moe GK, Rheinboldt WC, Abildskov JA. A computer model of atrial fibrillation. Am Heart J. 1964;67:200–20. doi: 10.1016/0002-8703(64)90371-0. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 18.Allessie MA, de Groot NM, Houben RP, Schotten U, Boersma E, Smeets JL, et al. Electropathological substrate of long-standing persistent atrial fibrillation in patients with structural heart disease:longitudinal dissociation. Circ Arrhythm Electrophysiol. 2010;3:606–15. doi: 10.1161/CIRCEP.109.910125. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 19.Choi JI, Ryu K, Park E, Benser ME, Jang JK, Lee HS, et al. Atrial activation time and pattern of linear triple-site vs. single-site atrial pacing after cardioversion in patients with atrial fibrillation. Europace. 2010;12:508–16. doi: 10.1093/europace/eup407. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 20.Platonov PG. Interatrial conduction in the mechanisms of atrial fibrillation:from anatomy to cardiac signals and new treatment modalities. Europace. 2007;9(Suppl 6):10–6. doi: 10.1093/europace/eum201. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 21.Sakabe K, Fukuda N, Fukuda Y, Morishita S, Shinohara H, Tamura Y. Interatrial dyssynchrony on tissue Doppler imaging predicts progression to chronic atrial fibrillation in patients with non-valvular paroxysmal atrial fibrillation. Heart. 2009;95:988–93. doi: 10.1136/hrt.2008.152561. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 22.Cho GY, Jo SH, Kim MK, Kim HS, Park WJ, Choi YJ, et al. Left atrial dyssynchrony assessed by strain imaging in predicting future development of atrial fibrillation in patients with heart failure. Int J Cardiol. 2009;134:336–41. doi: 10.1016/j.ijcard.2008.08.019. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 23.Van Beeumen K, Duytschaever M, Tavernier R, Van de Veire N, De Sutter J. Intra- and interatrial asynchrony in patients with heart failure. Am J Cardiol. 2007;99:79–83. doi: 10.1016/j.amjcard.2006.07.066. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 24.Evranos B, Aytemir K, Oto A, Okutucu S, Karakulak U, Şahiner L, et al. Predictors of atrial fibrillation recurrence after atrial fibrillation ablation with cryoballoon. Cardiol J. 2013;20:294–303. doi: 10.5603/CJ.2013.0075. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 25.Kuppahally SS, Akoum N, Badger TJ, Burgon NS, Haslam T, Kholmovski E, et al. Echocardiographic left atrial reverse remodeling after catheter ablation of atrial fibrillation is predicted by preablation delayed enhancement of left atrium by magnetic resonance imaging. Am Heart J. 2010;160:877–84. doi: 10.1016/j.ahj.2010.07.003. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Küçükdurmaz Z, Kato R, Erdem A, Gölcük E, Tobiume T, Nagase T, et al. Catheter ablation for atrial fibrillation results in greater improvement in cardiac function in patients with low versus normal left ventricular ejection fraction. J Interv Card Electrophysiol. 2013;37:179–87. doi: 10.1007/s10840-013-9794-6. [CrossRef] [DOI] [PubMed] [Google Scholar]


