Abstract
Objective:
To evaluate the left venticular myocardial deformation parameters in normotensive obese children and adolescents by using 2-D speckle tracking echocardiography.
Methods:
This observational cross-sectional study included 69 children and adolescents (aged between 10-18), 38 were normotensive obese and 31 were normal weighted. All children underwent detailed two- dimentional, Doppler and two-dimentional speckle tracking echocardiography. Student t-test, Mann-Whitney U test, chi-square test and Pearson’s correlation were used in statistical analysis. Multiple linear regression analysis was used the determine independent variables on global longitudinal strain (GLS).
Results:
While in normal limits, diastolic blood pressure was significantly higher in obese group. Left ventricular end-diastolic diameter (LVDd) and end-systolic diameter (LVDs), interventricular septal thickness (IVSd), left ventricular posterior wall thickness (LPWD) and left ventricular mass index (LVM)/height2.7 were significantly higher in obese group compared to healthy peers (p=0.004, p=0.011, p<0.001, p=0.001, p<0.001) respectively. Obese subjects had reduced global longitudinal strain (GLS) values (p=0.001). Multiple linear regression analysis using the step-wise method were performed to assess the independent variables (age, body mass index, insulin resistance, systolic blood pressure, diastolic blood pressure, left ventricular diameters and LVM index (g/m2.7) affecting the dependent variable GLS. GLS was found significantly correlated with body mass index (BMI) (β:0.440, p:0.001; 95% CI: 0.104-0.311).
Conclusion:
Left ventricular strain parameters obtained by two dimentional speckle tracking echocardiography were diminished in obese children compared to normal subjects indicating that obesity in childhood is linked to decreased myocardial deformation even in the absence of comorbidities in early stages.
Keywords: echocardiography, obesity, regression analysis, strain
Introduction
Obesity has become a major health problem both in developed and developing countries (1-3). Nearly 30% of children and adolescents in United States are either overweight or obese (4). The prevalance of the comorbidities associated with obesity has increased proportionally. Obese children are more likely to become obese adults. In United States about 70% of adolescents with severe obesity remained severely obese in adulthood (5).
Childhood and adolescence obesity has various effects on cardiovascular system related to increased cardiovascular risk in adulthood. The process of cardiac involvement begins early in life which suggests that morbidity and mortality may occur at younger ages (6, 7). Dilatation of left ventricular structures, left ventricular (LV) hypertrophy, heart failure, systolic and diastolic dysfunctions are the major obesity-induced changes (8). The causes of these changes are controversial. On the other hand, these changes may also reflect the role of comorbidities that contribute to LV dysfunction such as hypertension, diabetes mellitus, coronary artery disease and obstructive sleep apnoea. Detection of these cardiovascular abnormalities in early stages is important because appropriate treatment may reverse the process.
2-dimentional speckle tracking echocardiography (2DSTE) is a newly developed method that evaluates ventricular deformation parameters (strain, strain rate) early in stages of cardiac involvement even in the absence of clinical deterioration.
There are few studies evaluating the LV myocardial deformation parameters in normotensive obese children and adolescents. In this study we aimed to evaluate the LV functions by 2DSTE in obese childen and adolescents to show the preclinical effects of obesity on cardiovascular system.
Methods
Study design
This study was designed as a cross-sectional observational study.
Study population
This study included 69 children and adolescents (aged between 10-18), who admitted to pediatric cardiology department and general outpatient clinics at Çanakkale Onsekiz Mart University Hospital from October 2012 to July 2013. Thirty-eight of them were obese and 31 were normal weighted. Only 2 obese child (2.8%) were excluded because of poor echocardiographic images. The parents and children were informed of the aim of the study and provided written and verbal consent, respectively and the local Ethics Committee of Çanakkale Onsekiz Mart University approved the study.
Exclusion criteria
Children with history of functional and structural cardiovascular diseases (acquired and congenital), chronic systemic diseases, hypertension, sleep apnea, endocrinological disorders were excluded. Patients with poor image quality were also excluded.
Study protocol
Baseline variables
All subjects underwent detailed physical examination. Height, weight, blood pressures and body mass index (BMI) were calculated for each patient.
Body mass index (BMI)
Body mass index was calculated by the formula; BMI- weight (kg)/height (m)2. Obesity was considered if BMI exceeded the 95th percentile for sex and age based reference values.
Blood pressure (BP)
Blood pressure was measured with an appropriate cuff after children had rested for 10 minutes in the supine position in a silent room. To avoid white coat hypertension, measurements were repeated at the end of echoacardiographic examination. Participants who had systolic and diastolic BP (both at least 3 different occasions) measurements below the 90th percentile for sex and height of the Turkish pediatric population were included in the study. Patients with borderline values were underwent 24- hour ambulatory monitoring to confirm the absence of hypertension.
Laboratory investigations
Only obese children underwent detailed hematologic and biochemical laboratory evaluation. Fasting blood glucose and insulin level, homeostatic model assessment of insulin resistance (HOMA-IR), total cholesterol and triglycerids were performed only in obese children and adolescents. (HOMA-IR) was calculated using the following formula: HOMA-IR=fasting insulin (mIU/mL) x fasting blood glucose (mmol/L)/22.5. Insulin resistance was defined as HOMA-IR exceeded 2.5 (9).
Echocardigraphic evaluation
Transthoracic echocardiography was performed by using a Vivid 7 GE Vingmed, Horten, Norway© echocardiograph. B-Mode grayscale images were obtained from apical 4-chamber, and parasternal short-axis views at the level of the papillary muscle. M-modes of two-dimensional images were obtained from the parasternal long-axis views. Interventricular septal wall thickness, left ventricular posterior wall thickness, and left ventricular internal diameters were measured in all children. Cardiac chamber sizes, and left ventricular systolic and diastolic functions were assessed in accordance with the guidelines of the American Society of Echocardiography (10). Systolic functions of the left ventricle (LV) were evaluated using shortening fraction, ejection fraction. Left ventricular mass was calculated using the Devereux formula (11). Left ventricular hypertrophy was considered if the left ventricular mass index (LVMI) was above the 95th percentile according to age and sex (12). Relative wall thickness (RWT) was calculated by using the formula: RWT=2× (posterior wall thickness/left ventricular end-diastolic volume).
Pulsed Doppler measurements were obtained with the transducer in the apical 4-chamber view, with the Doppler beam aligned perpendicular to the plane of the mitral annulus. Measurements included peak early left ventricular filling wave (E), peak late left ventricular filling wave (A), isovolumic relaxation time, and deceleration time (DT). Tissue Doppler imaging (TDI) was used to determine peak systolic (S’), early diastolic (E’), and late diastolic (A’) myocardial velocities at the lateral mitral annulus.
Grayscale images were obtained from the apical 4-chamber and short-axis views (at the level of the papillary muscle) of the LV using tissue harmonic imaging with frame rates between 60 and 90 per second. These images were then processed and stored in cineloop format for subsequent offline analysis. 2D strain values were measured by using a dedicated, customized software package (EchoPAC, Vingmed, General Electric, Horten, Norway) accordance with the guidelines of the American Society of Echocardiography (10). The software calculated longitudinal and transversal strains for the respective segments (Peak Systolic Strain, PSS), and the global longitudinal strain (GLS) for the entire U-shaped length of LV myocardium, including the basal, mid, and apical segments of 2 opposite walls in each view (Fig. 1).
Figure 1.
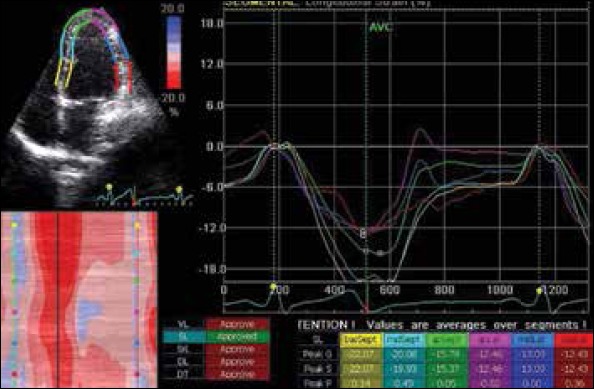
Diagram showing the calculation of longitudinal strain from apical 4-chamber view
Statistical analysis
All results were expressed as mean±SD. The Kolmogorov-Smirnov tests were used to detect the normality distribution of the data. The student’s t-test was used to compare normally distributed variables and Mann-Whitney U test was used for abnormally distributed variables among groups. Chi-square test was used for comparison of categorical variables. Correlations between variables were evaluated by Pearson’s rank correlation test. Multiple linear regression analyses using the stepwise method were performed to assess the independent variables (age, body mass index, insulin resistance, systolic blood pressure, diastolic blood pressure, left ventricular diameters and LVMI (g/m2.7) affecting the dependent variable GLS. SPSS v 13.0 (SPSS Inc., Chicago, IL, USA) was used for analyses, with p values <0.05 considered significant.
Results
Clinical features
Thirty eight obese children and adolescents (20 male, 18 female) and 31 (21 male, 10 female) normal weighted children were recruited. Table 1 summarizes the sample characteristics of the children enrolled in the study.
Table 1.
Clinical features of study groups
| Parameter | Obese group (n:38) | Control group (n:31) | P* |
|---|---|---|---|
| Age, year | 12.2 (10-17) | 15 (11-16) | 0.003 |
| Sex, M/F | 20/18 | 21/10 | 0.118 |
| Weight, kg | 73.20±14.44 | 50.80±13.26 | <0.001 |
| Height, cm | 155.06±11.40 | 157.29±15.21 | 0.490 |
| BSA, m2 | 1.77±0,22 | 1.48±0.24 | <0.001 |
| BMI, kg/m2 | 30.32±4.65 | 20.58±5.80 | <0.001 |
| SBP, mm Hg | 110 (90-130) | 110 (80-129) | 0.075 |
| DBP, mm Hg | 70 (50-80) | 60 (48-80) | <0.001 |
Data are presented as the mean value±SD, median (interquartile range) or number or percentage of patients.
Student t-test, Mann -Whitney U test, chi-square test p<0.05 considered statistically significant.
BMI - body mass index; BSA - body surface area; DBP - diastolic blood pressure; SBP - systolic blood pressure
Although statistically insignificant, obese children had increased systolic blood pressures compared with control group (p=0.0759) but diastolic blood pressure was significantly higher in obese group (p<0.001).
Conventional and tissue Doppler echocardiographic parameters
Indicators of left ventricular systolic functions (ejection fraction and fractional shortening) were not different between groups. Left ventricular end-diastolic diameter (LVDd), left ventricular end-systolic diameter (LVDs), interventricular septal thickness (IVSD), left ventricular posterior wall thickness (LPWD) and left ventricular mass (LVM) corrected for height2.7 were significantly higher in obese group. RWT was similar in both groups. M-mode measurements, conventional Doppler and tissue Doppler-derived parameters in obese and control group are shown in Table 2. Left ventricular diastolic functions were not different between groups. Only DT was significantly higher in obese patients (<0.001).
Table 2.
M-mode, conventional and tissue Doppler echocardiographic parameters of study groups
| Parameter | Obese group (n:38) | Control group (n:31) | P* |
|---|---|---|---|
| LVDd, mm | 45.13±5.04 | 42.19±2.48 | 0.004 |
| LVDs, mm | 28.57±3.90 | 26.14±3.74 | 0.011 |
| IVSd, mm | 8.87±1.41 | 7.34±1.11 | <0.001 |
| LPWD, mm | 8.50±1.57 | 7.25±1.36 | 0.001 |
| LVMI, g | 129.13±34.88 | 101.22±30.14 | 0.001 |
| LVMI, g/m2.7 | 39.51±9.71 | 29.95±8.18 | <0.001 |
| LVMI, g/m2 | 72.50±15.23 | 67.77±12.99 | 0.176 |
| RWT, mm | 0.38±0.08 | 0.34±0.06 | 0.055 |
| EF, % | 66.39±5.66 | 65.67±4.48 | 0.568 |
| FS, % | 36.28±4.34 | 35.90±3.54 | 0.692 |
| E, m/sec | 1.02±0.18 | 1.01±0.19 | 0.908 |
| A, m/sec | 0.6±0.17 | 0.58±0.15 | 0.622 |
| E/A | 1.72±0.41 | 1.79±0.41 | 0.490 |
| E’, m/sec | 0.19±0.3 | 0.19±0.03 | 0.992 |
| A’, m/sec | 0.07±0.02 | 0.08±0.09 | 0.670 |
| E’/A’ | 2.59±0.79 | 3.01±0.99 | 0.052 |
| DT, msec | 158.52±28.95 | 116.77±30.87 | <0.001 |
Data are presented as the mean value±SD.
Student t-test, p<0.05 considered statistically significant.
DT - deceleration time; EF - ejection fraction; FS - fractional shortening; IVSd -interventricular septum end-diastolic thickness; LVDd - left ventricular end-diastolic diameter; LVDs - left venticular end-systolic diameter; LVM - left ventricular mass; LVMI - left ventricular mass index; LPWD - left ventricular posterior wall thickness; RWT - relative wall thickness
2-D speckle tracking echocardiography
Longitudinal strain values of all lateral segments were significantly lower in obese group. Mid and apical septal strain values were also lower in obese group. Global longitudinal strain values were lower in obese group (p=0.001) (Table 3).
Table 3.
Longitudinal 2D speckle tracking-strain values
| Apical 4 chamber strain | Septal | Segment | Obese (n:38) | Control (n:31) | P |
| Basal | -20.02±3.09 | -19.99±3.21 | 0.968 | ||
| Mid | -19.63±3.78 | -21.31±2.56 | 0.038 | ||
| Apical | -16.86±5.47 | -19.95±3.58 | 0.009 | ||
| Lateral | Basal | -14.26±6.46 | -18.24±4.39 | 0.005 | |
| Mid | -13.75±5.69 | -18.10±3.68 | <0.001 | ||
| Apical | -13.83±5.22 | -17.05±4.97 | 0.012 | ||
| Longitudinal strain | -16.39±3.64 | -19.11±2.22 | 0.001 | ||
Data are presented as the mean value±SD
*Student t-test, p<0.05 considered statistically significant
Radial strain values of anteroseptal, anterior and lateral segments were lower in obese children but they were statistically insignificant (Table 4).
Table 4.
Radial 2D speckle tracking-strain values
| Segment | Obese (n:38) | Control (n:31) | P | |
|---|---|---|---|---|
| Radial Strain | Anteroseptal | 36.09±13.90 | 38.72±17.96 | 0.495 |
| Anterior | 37.40±18.15 | 43.05±20.76 | 0.232 | |
| Lateral | 41.46±19.92 | 46.24±21.30 | 0.340 | |
| Posterior | 44.54±21.44 | 48.47±19.50 | 0.433 | |
| Inferior | 47.73±18.57 | 45.52±16.72 | 0.609 | |
| Septal | 44.30±16.32 | 40.21±16.15 | 0.302 | |
| Global radial strain | 41.92±16.11 | 43.70±16.03 | 0.646 | |
Data are presented as the mean value±SD
*Student t-test, p<0.05 considered statistically significant
Circumferential strain values of anteroseptal, anterior, posterior, inferior and septal segments were lower in obese children but compared with control group only posterior and inferior values were significantly lower (Table 5).
Table 5.
Circumferential 2D speckle tracking-strain values
| Segment | Obese (n:38) | Control (n:31) | P | |
|---|---|---|---|---|
| Circumferential Strain | Anteroseptal | -22.09±6.23 | -22.87±3.64 | 0.537 |
| Anterior | -18.97±6.53 | -21.04±8.36 | 0.252 | |
| Lateral | -11.34±9.9 | 11.32±5.17 | 0.999 | |
| Posterior | -6.26±10.86 | -11.16±4.61 | 0.022 | |
| Inferior | -11.44±6.71 | -15.26±4.53 | 0.009 | |
| Septal | -19.73±6.47 | -21.05±4.57 | 0.343 | |
| Global circumferential strain | -14.97±5.60 | -16.96±2.05 | 0.065 | |
Data are presented as the mean value±SD.
*Student t-test, p<0.05 considered statistically significant.
Patients with higher LVM and higher BMI had reduced GLS values (p=0.026, r=0.269/ p<0.001, r=0.440) (Fig. 2, 3). There was a significant positive correlation between HOMA-IR and LV mass (g) (p=0.046, r=0.326). There was not a significant correlation between GLS and HOMA-IR, SBP (p=0.967, p=0.170). But we found positive correlation between GLS and LVDd, GLS and DBP (p=0.033 and p=0.004). Final stepwise multiple linear regression analysis didn’t determine any correlation between GLS and DBP as well as LVDd.
Figure 2.
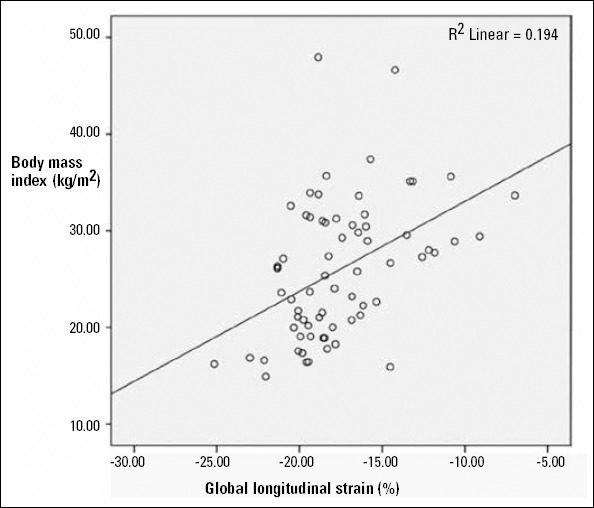
Correlation of GLS with BMI
Figure 3.
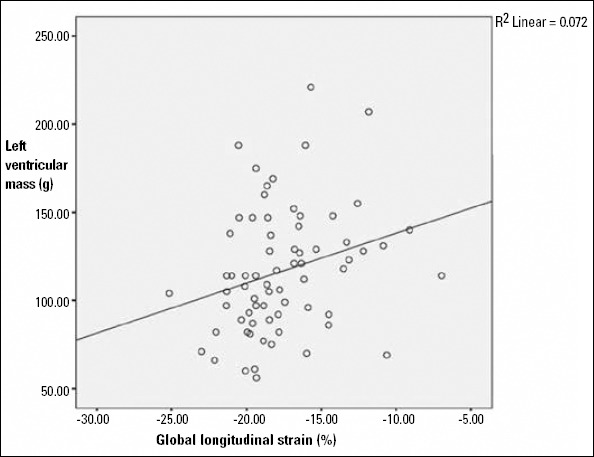
Correlation of GLS with LV mass
In multivariable analysis global left ventricular longitudinal strain (GLS) was significantly correlated with BMI (β: 0.440, p:0.001; 95% CI: 0.104-0.311) (Table 6), whereas age, HOMA-IR, SBP, DBP, left ventricular diameters and LVMI (g/m2.7) were not significantly correlated.
Table 6.
Results of multiple regression analysis for dependent variable BMI
| β | P | 95% CI | |
|---|---|---|---|
| Model 1 | |||
| BMI | 0.440 | 0.001 | 0.104-0.311 |
Dependent variable: Global longitudinal strain Independent variables: age, body mass index, insulin resistance, systolic blood pressure, diastolic blood pressure, left ventricular diameters and LVMI (g/m2.7)
Discussion
Obese children and adolescents had reduced LV strain values obtained by 2DSTE in the absence of hypertension when compared with normal weighted peers.
Obesity can cause functional and structural abnormalities in cardiovascular system such as increased LV diameters and mass, eccentric hypertrophy, systolic and diastolic dysfunctions and heart failure (13-16). Elevation of peripheral vascular resistance due to excessive fatty tissue, elevated peripheral stiffness, increased pre- and afterload are possible mechanisms (17).
There are many studies reporting systolic dysfuntion in obesity. But in contrast, few studies found systolic functions in normal limits (18). Because EF can easily be effected from pre-and afterload changes, this parameter should not be used to evaluate systolic functions in obesity. Diastolic dysfunction has also been described in children and adolescents (19-21) and many studies suggested that LV diastolic dysfunction may be an early finding in obesity. The reason of diastolic dysfunction in obesity is the reduced myocardial relaxation due to obesity induced-LV hypertrophy.
Studies using newer and more sensitive parameters such as strain and strain rate, had showed incipient systolic dysfunction even in the presence of normal LV ejection fraction (22, 23). 2DSTE provides several advantages, including better reproducibility, evaluation of radial and circumferential strains, and quantitative evaluation of twist, rotation and torsion movements. In comparison to tissue Doppler echocardiography, 2DSTE is angle-independent and it is not affected from pre-and afterload variations and does not require as high frame rates, and allows straightforward measurement of radial and circumferential strain in addition to longitudinal strain (24). In our study, conventional M-mode echocardiography determined higher LVDd, IVSD, LVM in obese as expected and conventional indices of systolic function, such as EF did not differ between groups, whereas LV strain values were found to be lower in the obese patients. This result suggests incipient systolic dysfunction.
Insulin resistance, hypertension and type 2 diabetes mellitus are comorbide problems in obesity. Myocardial dysfunction worsens due to the contribution of insulin resistance with hypertension (25). So it is of clinical importance to determine the early effects of comorbidities in normotensive obese children. Because evaluating the effect of obesity in children without hypertension, and without other appreciable cause of heart disease, might have offered the unique clinical opportunity to exclude the effects of possible comorbidities on LV functions. Di Salvo et al. (26) showed that obesity, in the absence of hypertension, is associated with significant reduction in systolic myocardial deformation parameters. Our results of 2DSTE showed signs of early myocardial deformation in the presence of normal standart systolic indices. Insulin resistance measured by HOMA-IR, is another comorbide problem in obesity. It is especially a main risk factor for cardiovascular diseases. Our results showed significant positive correlations of HOMA-IR with LV mass in the obese children. Therefore, these data suggest that increased insulin resistance is significantly associated with increased LV mass, indicative of LV myocardial dysfunction in obese children. But we could not find any correlation between HOMA-IR and GLS.
All obese patients irrespective of their GLS values, were scheduled for one year later to undergo 2DSTE for the evaluation of recovery of myocardial deformation parameters (especially for GLS) after the treatment for insulin resistance and after weight loosing. Because duration of morbid obesity is an important determinant of LV mass, systolic function, and diastolic filling (27).
Study limitations
Our study group is a relatively small group. Additionally the study group didn’t undergo echocardiographic examination after the treatment for insulin resistance. But in future, we planned to evaluate cardiac changes after treatment for insulin resistance and after losing weight. The other limitation is that twist, rotation and torsion were not evaluated in our study which are other methods to detect subclinical dysfunction. The last limitation is that we did not perform intra and interobserver variability because the echocardiologist has enough experience about strain and strain rate echocardiography.
Conclusion
In conclusion, in normotensive obese children and adolescents, systolic parameters which are evaluated by standart echocardiographic methods may be in normal limits. However, LV strain parameters obtained by 2DSTE were diminished compared to normal subjects indicating that obesity in childhood is linked to decreased myocardial deformation even in the absence of comorbidities in early stages. This result should provide the pediatricians to struggle against this public health problem more strongly.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept - F.K.B., Ş.Y., N.T.; Design - M.T., H.A.; Supervision - F.K.B., N.K.; Resource - F.K.B., Ş.Y., N.T.; Materials - H.A., N.K.; Data collection &/or processing - N.K., M.T.; Analysis &/or interpretation - F.K.B., M.T.; Literature search - H.K., N.T.; Writing - F.K.B., Ş.Y.; Critical review - H.K., N.K.; Other -H.K.
References
- 1.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999-2000. JAMA. 2002;288:1728–32. doi: 10.1001/jama.288.14.1728. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 2.Strauss RS, Pollack HA. Epidemic increase in childhood overweight 1986-1998. JAMA. 2001;286:2845–8. doi: 10.1001/jama.286.22.2845. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 3.Wang Y. Cross-national comparison of childhood obesity:the epidemic and the relationship between obesity and socioeconomic status. Int J Epidemiol. 2001;30:1129–36. doi: 10.1093/ije/30.5.1129. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents 1999-2010. JAMA. 2012;307:483–90. doi: 10.1001/jama.2012.40. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The NS, Suchindran C, North KE, Popkin BM, Gordon-Larsen P. Association of adolescent obesity with risk of severe obesity in adulthood. JAMA. 2010;304:2042–7. doi: 10.1001/jama.2010.1635. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorof J, Daniels S. Obesity hypertension in children:a problem of epidemic proportions. Hypertension. 2002;40:441–7. doi: 10.1161/01.hyp.0000032940.33466.12. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 7.Alpert MA. Obesity cardiomyopathy:pathophysiology and evolution of the clinical syndrome. Am J Med Sci. 2001;321:225–36. doi: 10.1097/00000441-200104000-00003. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 8.Pascual M, Pascual DA, Soria F, Vicente T, Hernandez AM, Tebar FJ, et al. Effects of isolated obesity on systolic and diastolic left ventricular function. Heart. 2003;89:1152–6. doi: 10.1136/heart.89.10.1152. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment:insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 10.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. American Society of Echocardiography’s Guidelines and Standards Committee;European Association of Echocardiography Recommendations for chamber quantification:a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 11.Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–8. doi: 10.1161/01.cir.55.4.613. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 12.Khoury PR, Mitsnefes M, Daniels SR, Kimball TR. Age-specific reference intervals for indexed left ventricular mass in children. J Am Soc Echocardiogr. 2009;22:709–14. doi: 10.1016/j.echo.2009.03.003. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 13.Alam M, Wardell J, Andersson E, Samad BA, Nordlander R. Characteristics of mitral and tricuspid annular velocities determined by pulsed wave Doppler tissue imaging in healthy subjects. J Am Soc Echocardiogr. 1999;12:618–28. doi: 10.1053/je.1999.v12.a99246. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 14.Alpert MA, Lambert CR, Terry BE, Cohen MV, Mukerji V, Massey CV, et al. Influence of left ventricular mass on left ventricular diastolic filling in normotensive morbid obesity. Am Heart J. 1995;130:1068–73. doi: 10.1016/0002-8703(95)90210-4. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez L, Garcia M, Ares M, Griffin BP, Nakatani S, Thomas JD. Assessment of mitral annular dynamics during diastole by Doppler tissue imaging:comparison with mitral Doppler inflow in subjects without heart disease and in patients with left ventricular hypertrophy. Am Heart J. 1996;31:982–7. doi: 10.1016/s0002-8703(96)90183-0. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 16.Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH, et al. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol. 1997;30:474–80. doi: 10.1016/s0735-1097(97)88335-0. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 17.Di Bello V, Santini F, Di Cori A, Pucci A, Palagi C, Delle Donne MG, et al. Obesity cardiomyopathy:is it a reality? An ultrasonic tissue characterization study. J Am Soc Echocardiogr. 2006;19:1063–71. doi: 10.1016/j.echo.2006.03.033. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 18.Pirat B, Zoghbi WA. Echocardiographic assessment of left ventricular diastolic function. Anadolu Kardiyol Derg. 2007;7:310–5. [PubMed] [Google Scholar]
- 19.Kibar AE, Paç FA, Ballı S, Oflaz MB, Ece İ, Bas VN, et al. Early subclinical left-ventricular dysfunction in obese nonhypertensive children:a tissue Doppler imaging study. Pediatr Cardiol. 2013;34:1482–90. doi: 10.1007/s00246-013-0674-8. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 20.Alpert MA, Lambert CR, Terry BE, Cohen MV, Mukerji V, Massey CV, et al. Interrelationship of left ventricular mass, systolic function and diastolic filling in normotensive morbidly obese patients. Int J Obes Relat Metab Disord. 1995;19:550–7. [PubMed] [Google Scholar]
- 21.Movahed MR, Saito Y. Lack of association between obesity and left ventricular systolic dysfunction. Echocardiography. 2009;26:128–32. doi: 10.1111/j.1540-8175.2008.00764.x. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 22.Binnetoğlu FK, Babaoğlu K, Altun G, Kayabey O. Effects that different types of sports have on the hearts of children and adolescents and the value of two-dimensional strain-strain-rate echocardiography. Pediatr Cardiol. 2014;35:126–39. doi: 10.1007/s00246-013-0751-z. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 23.Orhan AL, Uslu N, Dayı SU, Nurkalem Z, Uzun F, Erer HB, et al. Effects of isolated obesity on left and right ventricular function:a tissue Doppler and strain rate imaging study. Echocardiography. 2010;27:236–43. doi: 10.1111/j.1540-8175.2009.01024.x. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 24.Blessberger H, Binder T. Two dimensional speckle tracking echo-cardiography:clinical applications. Heart. 2010;96:2032–40. doi: 10.1136/hrt.2010.199885. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 25.Levent E, Gökşen D, Özyürek AR, Darcan S, Coker M. Usefulness of the myocardial performance index (MPI) for assessing ventricular function in obese pediatric patients. Turk J Pediatr. 2005;47:34–8. [PubMed] [Google Scholar]
- 26.Di Salvo G, Pacileo G, Del Giudice EM, Natale F, Limongelli G, Verrengia M, et al. Abnormal myocardial deformation properties in obese, non-hypertensive children:an ambulatory blood pressure monitoring, standard echocardiographic, and strain rate imaging study. Eur Heart J. 2006;27:2689–95. doi: 10.1093/eurheartj/ehl163. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 27.Alpert MA, Lambert CR, Panayiotou H, Terry BE, Cohen MV, Massey CV, et al. Relation of duration of morbid obesity to left ventricular mass, systolic function, and diastolic filling, and effect of weight loss. Am J Cardiol. 1995;76:1194–7. doi: 10.1016/s0002-9149(99)80338-5. [CrossRef] [DOI] [PubMed] [Google Scholar]


