Abstract
Objective:
Mitral annular plane systolic excursion (MAPSE) is a simple way to assess left ventricle (LV) function. MAPSE is also correlated to parameters, illustrating the close relation between systolic and diastolic function of LV. In this study, we evaluated whether MAPSE could help us in the determination the LV diastolic dysfunction (DD) in obese adults.
Methods:
Our study was a prospective cross-sectional study. Obese patients who were referred from the endocrinology clinic were enrolled into this study. The participants included 40 obese patients with early-stage DD (grade I and II) and 40 obese patients with normal diastolic function, with an equal number of males and females. The patients with DD were further divided into Obese DD+I, who had grade I DD, and Obese DD+II, who had grade II DD. Student t-test, Mann-Whitney U test, one-way analysis of variance, ROC curve analysis, and pairwise comparisons of the ROC curves were used for statistical analysis.
Results:
MAPSE was different in all groups, with the lowest value in the Obese DD+II group (p<0.001). E/Em ratio was also different among all groups and was highest in the Obese DD+II group (p<0.001). Furthermore, MAPSE was negatively correlated with E/Em ratio (r=-0.368, p=0.020). The optimal threshold point of MAPSE in the diagnosis of left ventricle diastolic dysfunction (LVDD) was ≥1.45 cm, with 92.5% sensitivity (95% CI 79.6-98.4) and 77.5% specificity (95% CI 61.5-89.2) in the ROC curve analysis. There was no difference in the pairwise comparisons of the ROC curves of MAPSE and E/Em ratio in the diagnosis of DD [area under the ROC curve 0.902 (0.033) vs. 0.927 (0.027); p=0.54].
Conclusion:
Consequently, we found significantly a close relationship between MAPSE with conventional echocardiographic parameters, especially with E/Em, in the detection of left ventricle diastolic dysfunction (LVDD) in obese adults with normal LV ejection fraction. We think that MAPSE is a simple, easily acquired and less time consuming measurement and may help us in the stratification of LVDD in obese adults.
Keywords: mitral annular plane systolic excursion, obesity, left ventricle diastolic dysfunction
Introduction
Obesity is a chronic disease resulting from the accumulation of body fat. It is one of the most important health problems in the world and is associated with a large spectrum of cardiovascular changes resulting from altered hemodynamics and inflammation (1, 2). Among these changes, left atrial (LA) enlargement and subclinical impairment of left ventricular (LV) function are precursors to more serious forms of cardiac dysfunction and heart failure (3). Moreover, the impact of obesity on LV structure results in systolic and diastolic dysfunction (DD) (4).
Tissue Doppler imaging (TDI) can be used to assess segmental and global ventricular function (5). Systolic excursion of the mitral annulus, which has a complex shape and motion, has been correlated with LV longitudinal function. Mitral annular plane systolic excursion (MAPSE) was recently reported to be correlated with diastolic parameters, which provides information about the relationship between systolic and diastolic functions (6). Clinical studies have reported impaired LV longitudinal contraction and relaxation in coronary artery disease, myocardial infarction, and hypertrophic cardiomyopathy (7).
However, to our knowledge, the usefulness of MAPSE in the assessment of the diastolic functions in obese adults has not been evaluated. In this study, we evaluated whether MAPSE could help us in the determination the left ventricular diastolic functions in obese adults with normal ejection fraction (EF).
Methods
Study design
This study was designed as a prospective cross-sectional study, which was performed in Adıyaman University Training and Research Hospital.
Obese patients who were referred from the endocrinology clinic at Adıyaman University Training and Research Hospital who provided informed consent were prospectively enrolled into this study. The participants included 40 obese patients [mean age 32.2±5.6 years; mean body mass index (BMI) 38.3±3.7 kg/m2] with early-stage left ventricle diastolic dysfunction (LVDD) and 40 obese patients with normal diastolic function (Obese DD-) (mean age 31.5±3.8 years; mean BMI 37.8±3.6 kg/m2), with an equal number of males and females. To detect an effect size of 0.10 at an alpha error of 0.05 and statistical power of 0.80, 34 participants was required for our study.
Patients and controls with a history or clinical evidence of LV wall motion abnormality, LVEF <55%, previous myocardial infarction, cardiomyopathy, diabetes mellitus, hypertension, valvular heart disease, myocarditis, thyroid dysfunction, anemia, chronic kidney disease, chronic obstructive pulmonary disease, systemic inflammatory disease, and usage of medications known to affect echocardiographic parameters (angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, β-blockers, calcium channel blockers, etc.) were excluded from this study. Thirteen patients were excluded from the study for having a bad acoustic window.
The study protocol was carried out according to the principles of the Declaration of Helsinki and approved by the İnönü University Faculty of Medicine Ethics Committee. Informed consent was obtained from all the participants.
Study protocol
Patients with LVDD were further divided into a grade I LVDD group as Obese DD+I and a grade 2 LVDD group as Obese DD+II. LVDD was defined as the presence of septal Em <8 cm/s, lateral Em <10 cm/s, and LA volume ≥34 mL/m2, according to the guidelines of the European Association of Echocardiography/American Society of Echocardiography for the evaluation of left ventricular diastolic function by echocardiography (8). Then, patients with the characteristics above were divided into two groups in this way: Grade I DD was defined as dT >200 ms, E/A ratio <0.8, and E/Em ratio ≤8, while grade II DD was defined as dT 160-200 ms, E/A ratio=0.8-1.5, and E/Em ratio 9-12.
Using standard laboratory methods, blood samples were taken after an overnight 12-hour fast to determine levels of blood glucose, blood urea nitrogen, creatinine, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglyceride.
For all participants, echocardiographic examinations (HD 11 XE, Philips Medical Systems, Bothell, WA) were performed by two experienced cardiologists who were blind to the clinical details and results of other investigations of each group. During conventional Doppler echocardiography, images were obtained in standard tomographic views of the LV (parasternal long-and short-axis and apical four-chamber, two-chamber views). LV end-diastolic and -systolic diameters, interventricular and posterior wall thicknesses, and LA diameter were measured from 2-dimensional targeted M-mode echocardiographic tracings in the parasternal long-axis, according to the criteria of the American Society of Echocardiography. LVEF was computed using a modified Simpson’s biplane method. Using pulsed-wave Doppler, mitral inflow velocities, such as peak early diastolic velocity (E), peak late diastolic velocity (A), and deceleration time (dT), were measured. LV myocardial velocities, such as systolic (Sm), early diastolic (Em), and late diastolic (Am) velocities; isovolumetric relaxation (IVRT) time; and isovolumetric contraction time (IVCT) were acquired from the septal and the lateral corners of the mitral annulus by TDI and then averaged. Three consecutive cycles were averaged for every parameter.
MAPSE was measured using the apical four-chamber view focused on the LV. An M-mode vector was placed through the mitral annulus close to the lateral, septal, anterior, and inferior walls, respectively. The vector was adjusted to be as parallel to the walls as possible using anatomical M-mode where necessary. MAPSE was measured in centimeters as previously described, shown in Figure 1 (9, 10). Values of the four walls were averaged. Resultant myocardial velocities were recorded, and at least five satisfactory cardiac cycles were obtained. The intraobserver variability of MAPSE was 3.7%, and the interobserver variability was 4.2%.
Figure 1.
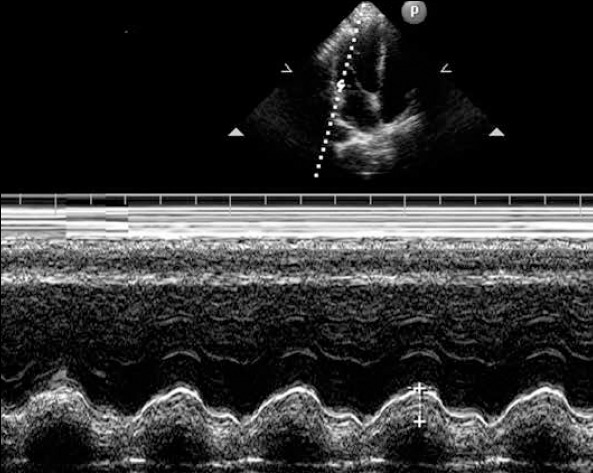
An illustration of the measurement of mitral annular plane systolic excursion from a mitral lateral annulus
Statistical analysis
Statistical analyses were performed using the SPSS package program, version 17.0 (SPSS Inc, Chicago, IL, USA). All continuous variables were expressed as mean±SD, and categorical variables were expressed as numbers and percentages. Kolmogorov-Smirnov test was used to determine the distribution of data. Continuous variables were compared between the two groups using student’s t-test or Mann-Whitney U test. One-way analysis of variance (ANOVA) or Kruskal-Wallis test, followed by Bonferroni posttest, was used to compare the three groups. Correlations were examined using Pearson correlation analysis. In order to predict the cut-off value of MAPSE for the diagnosis, LVDD in obese adults was analyzed using the receiver operating characteristics (ROC) curve analysis with Medcalc software (version 12.7.8, Mariakerke, Belgium). The ROC curve was also used to demonstrate the sensitivity and specificity of MAPSE and its cut-off value for predicting the DD. The area under curve (AUC) comparison of these scoring systems was performed using the Delong method (11). To compare the diagnostic performance of the MAPSE and E/Em ratio for LVDD in obese patients, pairwise comparisons of the ROC curves were performed. A p value less than 0.05 was considered to be significant.
Results
Baseline characteristics of the patient and control groups are presented in Table 1.
Table 1.
Baseline characteristics of the patients and control group
| Obese DD - (n=40) | Obese DD + (n=40) | P* | |
|---|---|---|---|
| Age, years | 31.5±3.8 | 32.2±5.6 | 0.502 |
| BMI, kg/m2 | 37.8±3.6 | 38.3±3.7 | 0.578 |
| BSA, m2 | 1.84±0.22 | 1.82±0.25 | 0.4431 |
| SBP, mm Hg | 117.2±7.1 | 115.1±8.6 | 0.233 |
| DBP, mm Hg | 75.5±4.6 | 73.8±7.2 | 0.231 |
| Heart rate, beat/min | 71.2±5.7 | 69.6±3.3 | 0.125 |
| Fasting glucose, mg/dL | 87.8±7.3 | 85.7±7.9 | 0.237 |
| BUN, mg/dL | 21.3±3.2 | 22.5±7.4 | 0.338 |
| Creatinine, mg/dL | 0.73±0.10 | 0.69±0.11 | 0.197 |
| Total cholesterol, mg/dL | 177.9±29.6 | 178.2±22.3 | 0.970 |
| HDL, mg/dL | 33.8±8.0 | 34.9±9.9 | 0.603 |
| LDL, mg/dL | 112.4±25.3 | 120.7±23.2 | 0.129 |
| Triglyceride, mg/dL | 124.8±28.1 | 132.4±29.9 | 0.244 |
BMI - body mass index; BSA - body surface area; BUN - blood urea nitrogen; DBP - diastolic blood pressure; HDL - high-density lipoprotein; LDL - low-density lipoprotein; NS - not significant; SBP - systolic blood pressure Values in mean±standard error.
Student’s t-test, Mann-Whitney U test
The echocardiographic and diastolic parameters of the study population are presented in Table 2. LV ejection fraction, LV end-diastolic and end-systolic diameters, interventricular septum, and LV posterior thickness were similar among all groups. LA volume and diameters were significantly higher in the Obese DD+ groups than in the Obese DD-group (p<0.001 for both) but were similar in the Obese DD+I and Obese DD+II groups.
Table 2.
Echocardiographic and diastolic parameters of the study population
| Obese DD - (n=40) | Obese DD+I (n=20) | Obese DD+II (n=20) | P1 | P2 | P3 | |
|---|---|---|---|---|---|---|
| LV EF, % | 62.4±2.9 | 61.6±2.3 | 62.6±3.4 | 0.987 | 0.998 | 0.827 |
| LVEDD, mm | 44.4±2.7 | 45.9±3.0 | 44.8±3.0 | 0.214 | 0.995 | 0.214 |
| LVESD, mm | 31.4±2.3 | 32.4±2.6 | 31.7±3.2 | 0.594 | 0.991 | 0.990 |
| IVST, mm | 10.8±0.8 | 11.2±0.8 | 10.9±1.2 | 0.337 | 0.966 | 0.952 |
| PWT, mm | 10.7±0.9 | 10.5±0.9 | 10.4±1.1 | 0.955 | 0.945 | 0.978 |
| LAD, mm | 32.4±3.1 | 35.9±3.0 | 36.6±2.2 | <0.001 | <0.001 | 0.955 |
| LA volume, mm3 | 27.5±3.2 | 40.1 ±3.8 | 42.2±3.2 | <0.001 | <0.001 | 0.157 |
| E, cm/s | 73.3±12.5 | 55.5±4.4 | 74.1±7.2 | <0.001 | 0.922 | <0.001 |
| A, cm/s | 51.1±8.5 | 82.3±7.1 | 67.6±10.9 | <0.001 | <0.001 | <0.001 |
| dT, ms | 182.3±15.1 | 219.8±12.9 | 178.8±8.0 | <0.001 | 0.978 | <0.001 |
| Em, cm/s | 12.2±1.2 | 7.3±0.3 | 7.1±0.4 | <0.001 | <0.001 | 0.912 |
| Am, cm/s | 8.6±2.2 | 8.9±1.9 | 8.8±1.4 | 0.922 | 0.857 | 0.869 |
| Sm, cm/s | 8.4±2.1 | 7.2±1.2 | 6.4±1.1 | 0.040 | <0.001 | 0.410 |
| IVRT, ms | 107.2±5.0 | 88.3±5.9 | 81.7±3.4 | <0.001 | <0.001 | <0.001 |
| IVCT, ms | 42.7±8.0 | 40.5±7.9 | 44.3±9.5 | 0.992 | 0.914 | 0.469 |
| E/A | 1.44±0.19 | 0.68±0.08 | 1.12±0.35 | <0.001 | <0.001 | <0.001 |
| E/Em | 6.08±1.28 | 7.64±0.60 | 10.39±0.85 | <0.001 | <0.001 | <0.001 |
| MAPSE, cm | 1.58±0.16 | 1.38±0.11 | 1. 18±0.16 | <0.001 | <0.001 | <0.001 |
A - mitral late diastolic velocity; Am - LV myocardial late diastolic velocity; dT - mitral E-wave deceleration time; E - mitral early diastolic velocity; Em - LV myocardial early diastolic velocity; IVCT - isovolumetric contraction time; IVRT - isovolumetric relaxation time; LV - left ventricular; LVEDD - LV end-diastolic dimension; LVESD - LV end-systolic dimension;
PW - posterior wall; Sm - LV systolic myocardial velocity
Values in mean±standard error. * One-way analysis of variance test
P value: Obese DD (-) vs. Obese DD (+I),
P value: Obese DD (-) vs. Obese DD (+II),
P value: Obese DD (+I) vs. Obese DD (+II).
Mitral E velocity was significantly lower in the Obese DD+I group than in the others (p<0.001). Mitral A velocity was significantly lower in the Obese DD-group than in the others (p<0.001). dT was higher in the Obese DD+I group than in the others (p<0.001) but was similar in the Obese DD- and Obese DD+II groups (p=0.586).
Em velocity was higher in the Obese DD- group (p<0.001) but was similar in the Obese DD+I and DD+II groups (p=0.897). Sm velocity was significantly different among all groups (p<0.001) and was lowest in the Obese DD+II group. IVRT was lower in the Obese DD+ group than in the Obese DD- group (p<0.001). Am velocity and IVCT were similar among all groups. The E/A ratio was different in all groups, with the lowest value in the Obese DD+I group (p<0.001). The E/Em ratio was also different among all groups and was highest in the Obese DD+II group (p<0.001).
MAPSE was different in all groups, with the lowest value in the Obese DD+II group (p<0.001). Furthermore, MAPSE was positively correlated with IVRT, A, dT, and Em and Sm velocity and negatively correlated with LA dimension, LA volume, E velocity, and E/A and E/Em ratios in the Obese DD+ group (Table 3).
Table 3.
Correlation of MAPSE with left ventricle echocardiographic parameters in obese patients with diastolic dysfunction
| Parameters | r | P* |
|---|---|---|
| LA diameter | -0.149 | 0.360 |
| LA volume | -0.294 | 0.066 |
| E | -0.322 | 0.043 |
| A | 0.646 | <0.001 |
| dT | 0.442 | 0.004 |
| Em | 0.156 | 0.336 |
| S | 0.658 | <0.001 |
| IVRT | 0.482 | 0.002 |
| E/A | -0.621 | <0.001 |
| E/Em | -0.368 | 0.020 |
A - mitral late diastolic velocity; dT - mitral E-wave deceleration time; E - mitral early diastolic velocity; Em - LV myocardial early diastolic velocity; IVRT - isovolumetric relaxation time; LA - left atrium; Sm - LV systolic myocardial velocity
Pearson correlation test
The ROC curve analysis to identify the optimal threshold point of MAPSE to detect LVDD in obese adults is shown in Figure 2. The cut-off value of MAPSE to detect LVDD was 1.45 cm, with a sensitivity of 92.5% and a specificity of 77.5% (AUC: 0.902, 95% CI: 0.814-0.957, p<0.001). We compared the differences in AUC of the MAPSE and E/Em ratio using the Delong method (11). The AUC comparisons of these values were performed based on the diagnosis of LVDD. Accordingly, there was no significant difference in the pairwise comparisons of the ROC curves of the MAPSE and E/Em ratio in the diagnosis of LVDD [area under the ROC curve 0.902 (0.033) vs. 0.927 (0.027); p=0.54] (Fig. 3).
Figure 2.
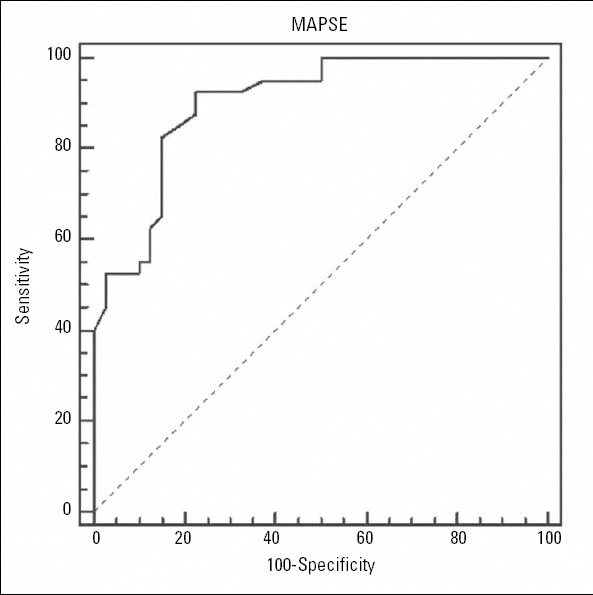
ROC curve analysis used to identify the optimal threshold point of MAPSE in the detection of LVDD in obese patients. The area under the ROC curve was 0.902, and the standard error was 0.033. The optimal threshold point of mitral annular motion was ≤1.45 cm, with 92.5% sensitivity (95% CI 79.6-98.4) and 77.5% specificity (95% CI 61.5-89.2)
Figure 3.
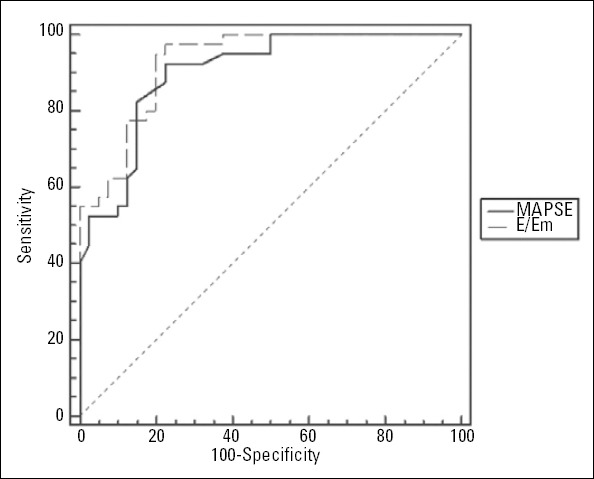
The pairwise comparisons of the ROC curves of MAPSE and the E/Em ratio in the diagnosis of DD [AUC 0.902 (0.033) vs. 0.927 (0.027); P=0.54]
Discussion
Our study is the first prospective study indicating that the MAPSE may be an easy and reliable parameter in the definition of LVDD in obese adults. In the current study, MAPSE was found to be different in all groups, with the lowest value in the Obese DD+II group. MAPSE was also positively correlated with IVRT, A, dT, and Em and Sm velocity and negatively correlated with LA dimension, LA volume, E velocity, and E/A and E/Em ratios in the Obese DD+group. Moreover, there was no significant difference in the pairwise comparisons of the ROC curves of the MAPSE and E/Em ratio in the diagnosis of the LVDD.
Cardiac disorders impair both longitudinal and radial contractile functions. However, it has been suggested that long-axis myocardial function is impaired first in some pathologic situations (12, 13). MAPSE, which reduces, even when LVEF remains normal, is a simple way to assess LV longitudinal function. Therefore, analysis of longitudinal myocardial function could help in the diagnosis of subclinical LV systolic dysfunction. MAPSE can also be used to assess both LV systolic and diastolic functions and to indicate left atrial function (14, 15). Matos et al. (10) reported that the average systolic excursion of the mitral annulus correlated excellently with LVEF. Previous studies also stated that chronic heart failure patients showed a significant reduction of MAPSE, and there was a good correlation between MAPSE and EF (16). Moreover, MAPSE significantly correlated with Doppler variables of LV diastolic filling, indicating that a reduction in MAPSE may be a result of impaired LV filling and/or systolic dysfunction (17). Brienza et al. (18) suggested that although global systolic function was comparable between obese children and controls, global longitudinal function was significantly reduced in obese subjects. In our study, we also found lower Sm values in obese DD+ adults, and MAPSE was positively correlated with Sm velocity. Although MAPSE may be a useful tool for assessing LV longitudinal functions of obese patients, our data can not support this statement by showing only a correlation in the MAPSE and Sm velocity. To clarify this issue, further studies using strain imaging or 3-dimentional echocar-diographic imaging are needed.
LVDD is common in the obese population and is associated with an adverse cardiovascular prognosis, related to changes in cardiac structure and function associated with hemodynamic volume overload (19, 20). Powell et al. (21) associated obesity with impaired LV relaxation and elevated LV diastolic filling pressures. Obesity may also change diastolic filling parameters due to altered load conditions (22). Atrial enlargement in obese subjects has been further related to expanded intravascular volume and altered LV filling properties (23). Since the MAPSE is a measurement that estimates LV systolic and diastolic functions, the pathological value of MAPSE can be associated to the presence of LVDD (24). In our study, in line with previous studies (24), MAPSE was found to be significantly lower in Obese DD+ patients with preserved systolic function, and the lowest value was observed in the Obese DD+II group. In concordance with our results, MAPSE has been shown to be correlated with diastolic parameters, which illustrates the close relationship between systolic and diastolic function, such as the E/Em ratio (8, 25). In our study, as expected, we found that diastolic parameters were significantly more impaired and that values indicating diastolic filling pressure, such as Em velocity and the E/Em ratio, were further increased in the Obese DD+II group. Moreover, MAPSE was negatively correlated with E/Em ratio. When we evaluated the pairwise comparisons of the ROC curves of MAPSE and the E/Em ratio, no significant difference was found. Based on these findings, MAPSE is demonstrated to be particularly correlated with the E/Em ratio in the diagnosis of LVDD in obese adults. In addition, obstructive sleep apnea syndrome (OSAS) is very common and is one of the major causes of LVDD in obese patients (26, 27). Although the study population was questioned on whether they had OSAS in the anamnesis, no specific test for the diagnosis of OSAS had been performed. So, more detailed and large-scale studies are needed to determine the effect of OSAS on MAPSE. Since poor echocardiographic imaging qualities, especially in obese patients, are often, we think that it is important to use MAPSE to rapidly and accurately achieve a diagnosis of LVDD in clinical practice. In spite of the routine use of newer echocardiographic technologies, we consider that MAPSE is still helpful in evaluating LVDD during daily routine echocardiography.
Study limitations
This study is limited by the use of a small patient population and cross-sectional design, which did not allow us to prospectively follow up on patients. Also, that significant differences could not be found in certain parameters between the two LVDD groups may be due to the insufficient number of the patients. Therefore, the power of MAPSE may have been poorer in the discrimination of the severity of LVDD. The other limitation is that the duration of obesity in our study population was unknown, and it is unclear whether the disease period affects MAPSE. Thus, a lengthier follow-up study is needed. The lack of invasive assessment of LV diastolic filling pressure is also a limitation. Our study also has a very refined group as young and normoten-sive and without metabolic syndrome, in light of the biochemical data. Because the vast majority of obese patients has many of the criteria mentioned and has therefore been using drugs in daily practice, we can not generalize our results to the general population.
Conclusion
In our study, we found a significantly close relationship between the MAPSE with conventional echocardiographic parameters, especially with E/Em, in the detection of LVDD in obese adults with normal LVEF. In the pairwise ROC comparison analysis, no statistically significant difference was found between MAPSE and the E/Em ratio for detection of LVDD. As a result, we think that MAPSE is a simple, easily acquired, and less time-consuming parameter, especially useful in patients with poor imaging qualities, and helps us in the stratification of LVDD in obese adults.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept - H.T., M.B., T.M., M.Ç., B.A.; Design - H.T., T.M., M.Ç.; Supervision - H.T., M.B., T.M., M.Ç., B.A., A.B., Y.Ö.O.; Data collection and/or processing - H.T., T.M., M.B., A.B., Y.Ö.O.; Analysis and/or Interpretation - H.T., M.Ç., A.B., Y.Ö.O.; Literature search - H.T., T.M., M.Ç., M.B., B.A., A.B., Y.Ö.O.; Writing - H.T., T.M., M.Ç., B.A., M.B., A.B., Y.Ö.O.; Critical review - H.T., M.Ç., B.A., M.B.
References
- 1.Çil H, Bulur S, Türker Y, Kaya A, Alemdar R, Karabacak A, et al. Impact of body mass index on left ventricular diastolic dysfunction. Echocardiography. 2012;29:647–51. doi: 10.1111/j.1540-8175.2012.01688.x. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 2.Gallego Pérez-Larraya J, Irimia P, Martinez-Vila E, Barba J, Guembe MJ, Varo N, et al. The influence of obesity on the assessment of carotid intima-media thickness. J Clin Ultrasound. 2012;40:479–85. doi: 10.1002/jcu.21916. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 3.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–6. doi: 10.1056/NEJM199005313222203. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 4.Khan MF, Movahed MR. Obesity cardiomyopathy and systolic function: obesity is not independently associated with dilated cardiomyopathy. Heart Fail Rev. 2013;18:207–17. doi: 10.1007/s10741-012-9320-4. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 5.Yurdakul S, Erdemir VA, Tayyareci Y, Yıldırımtürk O, Salih Gürel M, Aytekin S. Subclinical left and right ventricular systolic dysfunction in Behçet’s disease: A combined tissue Doppler and velocity vector imaging study. J Clin Ultrasound. 2013;41:347–53. doi: 10.1002/jcu.21985. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 6.Hu K, Liu D, Herrmann S, Niemann M, Gaudron PD, Voelker W, et al. Clinical implication of mitral annular plane systolic excursion for patients with cardiovascular disease. Eur Heart J Cardiovasc Imaging. 2013;14:205–12. doi: 10.1093/ehjci/jes240. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 7.Nikitin NP, Witte KK, Thackray SD, de Silva R, Clark AL, Cleland JG. Longitudinal ventricular function: normal values of atrioventricular annular and myocardial velocities measured with quantitative two-dimensional color Doppler tissue imaging. J Am Soc Echocardiogr. 2003;16:906–21. doi: 10.1016/S0894-7317(03)00279-7. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 8.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–93. doi: 10.1093/ejechocard/jep007. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 9.Bazaz R, Edelman K, Gulyasy B, Lôpez-Candales A. Evidence of robust coupling of atrioventricular mechanical function of the right side of the heart: insights from M-mode analysis of annular motion. Echocardiography. 2008;25:557–61. doi: 10.1111/j.1540-8175.2008.00676.x. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 10.Matos J, Kronzon I, Panagopoulos G, Perk G. Mitral annular plane systolic excursion as a surrogate for left ventricular ejection fraction. J Am Soc Echocardiogr. 2012;25:969–74. doi: 10.1016/j.echo.2012.06.011. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 11.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [CrossRef] [PubMed] [Google Scholar]
- 12.Yip G, Wang M, Zhang Y, Fung JW, Ho PY, Sanderson JE. Left ventricular long axis function in diastolic heart failure is reduced in both diastole and systole: time for a redefinition? Heart. 2002;87:121–5. doi: 10.1136/heart.87.2.121. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henein MY, Anagnostopoulos C, Das SK, O’Sullivan C, Underwood SR, Gibson DG. Left ventricular long-axis disturbances as predictors for thallium perfusion defects in patients with known peripheral vascular disease. Heart. 1998;79:295–300. doi: 10.1136/hrt.79.3.295. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wenzelburger FW, Tan YT, Choudhary FJ, Lee ES, Leyva F, Sanderson JE. Mitral annular plane systolic excursion on exercise: a simple diagnostic tool for heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:953–60. doi: 10.1093/eurjhf/hfr081. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 15.Elnoamany MF, Abdelhameed AK. Mitral annular motion as a surrogate for left ventricular function: correlation with brain natriuretic peptide levels. Eur J Echocardiogr. 2006;7:187–98. doi: 10.1016/j.euje.2005.05.005. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 16.Alam M, Hoglund C, Thorstrand C, Philip A. Atrioventricular plane displacement in severe congestive heart failure following dilated cardiomyopathy or myocardial infarction. J Intern Med. 1990;228:569–75. doi: 10.1111/j.1365-2796.1990.tb00281.x. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 17.Willenheimer R, Israelsson B, Cline C, Rydberg E, Broms K, Erhardt L. Left atrioventricular plane displacement is related to both systolic and diastolic left ventricular performance in patients with chronic heart failure. Eur Heart J. 1999;20:612–8. doi: 10.1053/euhj.1998.1399. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 18.Brienza C, Grandone A, Di Salvo G, Corona AM, Di Sessa A, Pascotto C, et al. Subclinical hypothyroidism and myocardial function in obese children. Nutr Metab Cardiovasc Dis. 2013;23:898902. doi: 10.1016/j.numecd.2012.04.006. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 19.Gülel O, Yüksel S, Soylu K, Kaplan O, Yılmaz O, Kahraman H, et al. Evaluation of left atrial functions by color tissue Doppler imaging in adults with body mass indexes >or=30 kg/m2 versus those <30 kg/m2. Int J Cardiovasc Imaging. 2009;25:371–7. doi: 10.1007/s10554-008-9403-4. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 20.Tigen MK, Karaahmet T, Gürel E, Dündar C, Pala S, Çevik C, et al. The role of isovolumic acceleration in predicting subclinical right and left ventricular systolic dysfunction in hypertensive obese patients. Turk Kardiyol Dern Ars. 2011;39:9–15. [PubMed] [Google Scholar]
- 21.Powell BD, Redfield MM, Bybee KA, Freeman WK, Rihal CS. Association of obesity with left ventricular remodeling and diastolic dysfunction in patients without coronary artery disease. Am J Cardiol. 2006;98:116–20. doi: 10.1016/j.amjcard.2006.01.063. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 22.Peterson LR, Waggoner AD, Schechtman KB, Meyer T, Gropler RJ, Barzilai B, et al. Alteration in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Card. 2004;43:1399–404. doi: 10.1016/j.jacc.2003.10.062. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 23.Iacobellis G, Ribaudo MC, Leto G, Zappaterreno A, Vecci E, Di Mario U, et al. Influence of excess fat on cardiac morphology and function: Study in uncomplicated obesity. Obes Res. 2002;10:76773. doi: 10.1038/oby.2002.104. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 24.Wierzbowska-Drabik K, Chrzanowski L, Kapusta A, Uznanska-Loch B, Plonska E, Krzeminska-Pakula M, et al. Severe obesity impairs systolic and diastolic heart function - the significance of pulsed tissue Doppler, strain, and strain rate parameters. Echocardiography. 2013;30:904–11. doi: 10.1111/echo.12164. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 25.Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;20:2539–50. doi: 10.1093/eurheartj/ehm037. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 26.Aslan K, Deniz A, Çaylı M, Bozdemir H, Sarıca Y, Şeydaoğlu G. Early left ventricular functional alterations in patients with obstructive sleep apnea syndrome. Cardiol J. 2013;20:519–25. doi: 10.5603/CJ.2013.0043. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 27.Kepez A, Niksarlıoğlu EY, Hazırolan T, Ranci O, Kabul HK, Demir AU, et al. Early myocardial functional alterations in patients with obstructive sleep apnea syndrome. Echocardiography. 2009;26:388–96. doi: 10.1111/j.1540-8175.2008.00809.x. [CrossRef] [DOI] [PubMed] [Google Scholar]


