Abstract
Background
Vitamin D has attracted considerable interest in recent years, and healthcare providers have reported large increases in vitamin D test requests. However, rates of diagnosis of vitamin D deficiency in clinical practice have not been investigated. We examined trends in the diagnosis of vitamin D deficiency in children in England over time, and by sociodemographic characteristics.
Methods
Cohort study using primary care electronic health records held in The Health Improvement Network database. 711 788 children aged 0-17 years were included. Incidence rates for diagnosis of vitamin D deficiency were calculated in each year between 2000-2014. Rate ratios exploring differences by age, sex, ethnicity, and social deprivation were estimated using multivariable Poisson regression.
Results
The crude rate of vitamin D deficiency diagnosis increased from 3.14 per 100 000 person-years in 2000 (95% confidence interval [CI]: 1.31-7.54) to 261 per 100 000 person-years in 2014 (95% CI: 241-281). After accounting for changes in demographic characteristics, a 15-fold (95% CI: 10-21 fold) increase in diagnosis was seen between 2008-2014. Older age (≥10 years), non-white ethnicity, and social deprivation were independently associated with higher rates of diagnosis. In children aged <5 years diagnosis rates were higher in boys than girls, whilst in children aged ≥10 they were higher in girls.
Conclusions
There has been a marked increase the diagnosis of vitamin D deficiency in children over the past decade. Future research should explore the drivers for this change in diagnostic behaviour and the reasons prompting investigation of vitamin D status in clinical practice.
Introduction
Vitamin D has attracted considerable clinical and academic interest over the last two decades. Regional studies and hospital case series in the United Kingdom (UK), United States (US), and Australia have suggested an increase in numbers of children presenting with symptomatic complications of vitamin D deficiency (rickets and hypocalcaemia).1–3 Furthermore, a large body of observational research has stimulated debate regarding the postulated role of vitamin D in modifying the risk of developing various diseases beyond its established function in bone metabolism and calcium homeostasis.4
As vitamin D has attracted increasing attention, hospitals in Australia and the UK have reported a surge in test requests.5,6 Primary care spending on vitamin D prescriptions in England increased from £28 million to £92 million between 2004-2014.7,8 Given the high prevalence of biochemical vitamin D deficiency in the general population, and uncertainty that treatment of asymptomatic individuals leads to improved health outcomes, some authors have questioned whether the large growth in testing may result in unnecessary health care costs and potential over-diagnosis.4,5,9 However, there has not been any empirical investigation of rates of diagnosis of vitamin D deficiency in clinical practice, and trends in testing and treatment have not been examined in children specifically. Using a large, population-based cohort of children in England, we determined longitudinal trends in rates of vitamin D deficiency diagnosis over the past 15 years, and explored differences by socio-demographic characteristics.
Methods
Data Source
We conducted a dynamic (open) cohort study using The Health Improvement Network (THIN) primary care database, which contains anonymised electronic health records of >11 million patients from 639 UK general practices. The THIN cohort is broadly representative of the UK population in terms of age, sex, prevalence of medical conditions, and mortality rates.10 THIN includes data regarding medical diagnoses, laboratory test results, medication prescriptions, and socio-demographic characteristics. Diagnoses are recorded using a hierarchical coding system called Read codes.11 Diagnoses made in secondary care may be coded from discharge summaries and outpatient letters. A subset of THIN practices in England (n=156) are linked to patient-level Hospital Episode Statistics (HES) data, available up to 31st March 2012. HES contains records of all hospital care episodes in England, although clinical diagnoses are only recorded for inpatient admissions. We used linked HES data to augment information regarding ethnicity.
Study Population
Children aged 0-17 years registered with a THIN practice linked to HES, at any point between 1st January 2000 and 31st December 2014, were included. Children with chronic renal disease, liver disease, or conditions associated with gastrointestinal malabsorption were excluded. The start of the observation period for each child was the latest of the date of practice registration (plus 3 months for children aged ≥1 year at registration), the date the practice met two pre-defined quality indicators for electronic data recording (acceptable mortality recording [AMR] and acceptable computer usage [ACU]),12,13 and 1st January 2000. Diagnoses recorded shortly after patient registration can represent historical information transferred from medical records rather than incident events.14 We observed greater recording of vitamin D deficiency diagnosis in the first 3 months after registration, therefore we excluded this period from observation for children aged ≥1 year at registration. The AMR and ACU criteria identify periods of incomplete use of computerised systems in primary care (e.g. following transition from paper records), and are described elsewhere.12,13 Exit from the observation period for each individual was the earliest of the date they transferred to a different practice, the date the practice stopped contributing data to THIN, the mid-point of their 18th year after birth, the date they died, 31st December 2014, or the date of the earliest record meeting the case definition for diagnosis of vitamin D deficiency.
Outcome
Diagnosis of vitamin D deficiency was defined as a record of any one of the following criteria in the THIN medical record: (1) a Read code related to vitamin D deficiency or rickets; (2) prescription of vitamin D (calciferol) at a ‘treatment dose’ (see below); or (3) a serum 25-hydroxyvitamin D (25-OH-D) test result <25 nmol/L (<10 ng/ml). Read code lists were developed using published guidelines.15 GPs do not always record diagnoses using Read codes, instead entering data as free text which is not routinely accessible.16 Using Read codes alone to identify cases can result in case under-ascertainment, therefore we also included prescription and test records in the case definition.
In order to capture prescriptions of colecalciferol or ergocalciferol issued for the treatment of established deficiency, as opposed to prophylactic supplementation or maintenance therapy, we used the following dose thresholds: (1) ≥1500 units/day if age <6 months; (2) ≥3000 units/day if age 6 months to 12 years; (3) ≥5000 units/day if age >12 years; (4) one-off (stoss) dose of ≥100 000 units at any age. These thresholds are higher than doses recommended for prophylaxis of between 400-1000 units/day,17 and represent half of the British National Formulary for Children treatment doses (≥3000 units/day if age 1-6 months, ≥6000 units/day if age 6 months to 12 years, and ≥10 000 units/day if age >12 years).18 A range of alternative dosage thresholds were explored using sensitivity analyses. The threshold of <25 nmol/L for 25-OH-D tests represents deficiency in UK guidance.17,19
Sensitivity analysis was performed additionally including ICD-10 codes for vitamin D deficiency and rickets from HES inpatient records in the case definition. This analysis was limited to follow-up to 31st December 2011.
Covariates
Socioeconomic position (SEP) was measured using the 2004 Index of Multiple Deprivation (IMD), an area-level indicator available in national quintiles.20 Recording of ethnicity in primary care databases is incomplete, but can be augmented by linkage with HES data.21 Ethnicity was grouped into the 2001 UK Census 5-category classification (white, mixed, Asian, black, or other). As consistency of ethnicity recording is greater in primary care data than HES, ethnicity was assigned from THIN where available, and supplemented with HES data.21 For individuals with multiple ethnicity categories recorded (0.3% of the cohort), the most frequently recorded category was used.
For children with missing ethnicity, maternal ethnicity was taken as a proxy measure for the child if available. Child-mother linkage was performed using similar methods to previous THIN studies.22,23 Children were linked to women sharing identical household identifiers with a pregnancy or delivery record where the expected or recorded date of delivery was in proximity to the child’s month of birth. Linked mothers were excluded if children matched to several women (0.3% of linked children), or >20 people shared the same household identifier (likely to represent a block of flats).
Statistical Analysis
Crude incidence rates were calculated for each year between 2000-2014. Differences in rates by sex, age group (<5, 5-9, 10-14, and 15-17 years), ethnicity, IMD, and calendar year were examined using multivariable Poisson regression. Multivariable analysis was limited to follow-up between 2008-2014, due to small numbers of cases per year before 2008. Interactions between explanatory variables were examined, and interaction terms retained in the final model if their inclusion resulted in both a qualitative change in parameter rate ratios and a significant likelihood ratio test (p<0.05). The multivariable model was run with and without inclusion of the general practice as a random effect to account for data clustering.
Missing data for ethnicity and IMD was handled using complete cases in the main analysis, and using multivariable multiple imputation for sensitivity analysis.24 The imputation model included all variables in the substantive model, plus auxiliary variables coding geographical region, and the ethnicity and IMD distributions of practice patients and of individuals sharing identical household identifiers. Analyses were performed using Stata 13.1 (StataCorp, College Station, TX).
The THIN data collection was approved by the NHS South-East Multicentre Research Ethics Committee in 2003. This study was approved by CSD Medical Research’s Scientific Review Committee.
Results
The study cohort contained 711 788 children from 156 practices, of whom 2918 were diagnosed with vitamin D deficiency between 2000-2014. Median observation time was 3.9 years (interquartile range 1.5-8.0). Descriptive characteristics are shown in Table 1.
Table 1.
Descriptive Characteristics of the Study Cohort (n=711 788)
| Characteristic | Value |
|---|---|
| Age at entry to follow-up, years, median (IQR) | 4.1 (0.40-10.5) |
| Sex, n (%) | |
| Male | 366 378 (51.5) |
| Female | 345 410 (48.5) |
| Ethnicity, n (%)a | |
| White | 491 962 (69.1) |
| Asian or Asian British | 34 521 (4.9) |
| Black or black British | 24 797 (3.5) |
| Mixed | 15 558 (2.2) |
| Chinese or other ethnic group | 13 443 (1.9) |
| Missing | 131 507 (18.5) |
| Index of multiple deprivation quintile, n (%): | |
| 1 (least deprived) | 158 866 (22.3) |
| 2 | 134 765 (18.9) |
| 3 | 138 264 (19.4) |
| 4 | 136 498 (19.2) |
| 5 (most deprived) | 95 656 (13.4) |
| Missing | 47 739 (6.7) |
IQR, interquartile range
Ethnicity data were available from the child’s THIN or HES record for 67.7% of the cohort, and maternal ethnicity was available as a proxy measure for 13.8%.
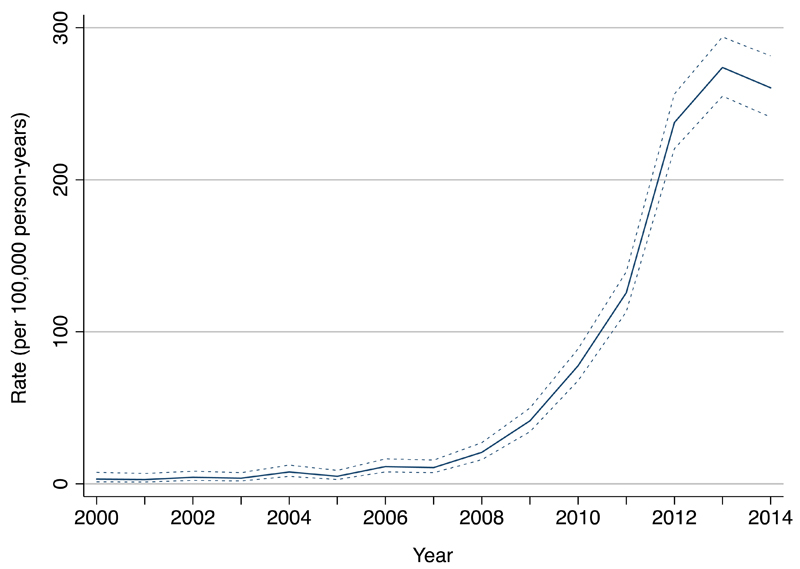
Analysis of time trends showed a marked increase in diagnosis of vitamin D deficiency after 2007 (Figure 1, and Supplemental Table 3). The crude incidence rate increased from 3.14 per 100 000 person-years at risk (PYAR) in 2000 (95% confidence interval [CI]: 1.31-7.54) to 261 per 100 000 PYAR in 2014 (95% CI: 241-281). After accounting for temporal changes in socio-demographic factors, a 15-fold increase in diagnosis (95% CI: 10-21 fold) was seen between 2008-2014 (Table 2).
Figure 1.
Time Trends in the Diagnosis of Vitamin D Deficiency in Children, 2000 to 2014. Crude incidence rates are shown, with 95% confidence limits represented by the dashed lines.
Table 2.
Associations Between Socio-Demographic Factors and Diagnosis of Vitamin D Deficiency (n=414 182)a
| Single-level Model |
Multilevel Model |
|||||
|---|---|---|---|---|---|---|
| Characteristic | Adjusted IRRb (95% CI) | P valuec | Adjusted IRRb (95% CI) | P valuec | ||
| Sex, stratified by age group | <0.001 | <0.001 | ||||
| 0-4 years: | Male | 1 | 1 | |||
| Female | 0.73 (0.57-0.93) | 0.72 (0.57-0.92) | ||||
| 5-9 years: | Male | 1 | 1 | |||
| Female | 1.06 (0.87-1.29) | 1.04 (0.86-1.27) | ||||
| 10-14 years: | Male | 1 | 1 | |||
| Female | 1.97 (1.71- 2.27) | 1.97 (1.71-2.27) | ||||
| 15-17 years: | Male | 1 | 1 | |||
| Female | 2.60 (2.18-3.11) | 2.65 (2.21-3.16) | ||||
| Age group, stratified by sex | <0.001 | <0.001 | ||||
| Males | 0-4 years | 1 | 1 | |||
| 5-9 years | 1.22 (0.99-1.50) | 1.20 (0.98-1.48) | ||||
| 10-14 years | 2.22 (1.83-2.70) | 2.19 (1.80-2.65) | ||||
| 15-17 years | 2.39 (1.93-2.96) | 2.36 (1.90-2.93) | ||||
| Females: | 0-4 years | 1 | 1 | |||
| 5-9 years | 1.77 (1.41-2.23) | 1.73 (1.37-2.18) | ||||
| 10-14 years | 6.00 (4.91-7.34) | 5.95 (4.86-7.27) | ||||
| 15-17 years | 8.52 (6.93-10.5) | 8.61 (7.00-10.6) | ||||
| Ethnicityd | <0.001 | <0.001 | ||||
| White | 1 | 1 | ||||
| Asian or Asian British | 22.4 (20.1-24.9) | 7.98 (6.98-9.13) | ||||
| Black or black British | 14.2 (12.5-16.2) | 5.47 (4.70-6.37) | ||||
| Mixed | 5.64 (4.52-7.03) | 2.99 (2.38-3.76) | ||||
| Chinese or other ethnic group | 8.91 (7.38-10.8) | 3.63 (2.96-4.45) | ||||
| IMD quintile | <0.001 | <0.001 | ||||
| 1 (least deprived) | 1 | 1 | ||||
| 2 | 1.98 (1.63-2.41) | 1.34 (1.07-1.67) | ||||
| 3 | 2.40 (2.00-2.88) | 1.41 (1.12-1.77) | ||||
| 4 | 2.67 (2.23-3.20) | 1.63 (1.29-2.05) | ||||
| 5 (most deprived) | 3.54 (2.96-4.24) | 1.96 (1.52-2.53) | ||||
| Calendar year | <0.001 | <0.001 | ||||
| 2008 | 1 | 1 | ||||
| 2009 | 2.20 (1.45-3.35) | 2.19 (1.44-3.34) | ||||
| 2010 | 3.87 (2.62-5.71) | 3.66 (2.48-5.40) | ||||
| 2011 | 6.61 (4.55-9.60) | 6.28 (4.32-9.12) | ||||
| 2012 | 12.7 (8.83-18.2) | 12.1 (8.43-17.4) | ||||
| 2013 | 14.7 (10.2-21.1) | 14.1 (9.85-20.3) | ||||
| 2014 | 14.7 (10.2-21.2) | 15.7 (10.9-22.6) | ||||
CI, confidence interval; IMD, Index of Multiple Deprivation; IRR, incidence rate ratio.
Results of multivariable Poisson regression models of rates of incident diagnosis of vitamin D deficiency. Missing data is handled using complete cases analysis.
Adjusted for all variables listed in the table, including an interaction term between age and sex (likelihood ratio test for interaction p value<0.001). The multilevel model additionally included the general practice as a random effect.
P values from likelihood ratio tests comparing nested models.
Ethnicity data was taken from the child’s THIN or HES record for 84.6% of children. Maternal ethnicity was used as a proxy measure for the remaining 15.4%.
Supplemental Figure 2 shows the overlap between cases identified from diagnosis codes, prescription records, and 25-OH-D test records. Results did not differ substantially in sensitivity analyses using alternative dosage thresholds for calciferol prescriptions (Supplemental Figure 3), or addition of ICD-10 diagnosis codes from HES inpatient records (Supplemental Figure 4), in the case definition.
In multivariable analysis older age, non-white ethnicity, and socioeconomic deprivation were associated with higher rates of vitamin D deficiency diagnosis (Table 2). There was an interaction between sex and age; among children aged ≥10 years diagnosis rates were higher in girls, whilst among children aged <5 years they were higher in boys (Supplemental Figure 5). No sex difference was seen in children aged 5-9 years. Although the magnitude of the effects of ethnicity and SEP were attenuated after accounting for clustering by practice, they remained strongly associated with the outcome (Table 2). There was a moderate proportion of missing data for ethnicity (12.7%) and IMD (8.3%). The results of analyses using multiple imputation were very similar to the main analyses using complete cases (Supplemental Table 4).
Discussion
In this large representative cohort of English children, there was a 15-fold increase in the diagnosis of vitamin D deficiency between 2008-2013, after which rates plateaued. Socio-demographic factors independently associated with higher rates of diagnosis included non-white ethnicity, socioeconomic deprivation, older age, female sex in children aged ≥10 years, and male sex in children aged <5 years.
Comparison with Other Studies
To the best of our knowledge, this is the first study to report national estimates for overall rates of diagnosis of vitamin D deficiency in clinical practice, in the UK or internationally. However, a number of studies have investigated the incidence of clinical complications of vitamin D deficiency in children. The annual incidence of symptomatic vitamin D deficiency presenting to paediatricians was reported to be 7.5 per 100 000 children aged 0-5 years in the West Midlands region of England, and between 2.2 to 2.9 per 100,000 children in New Zealand,25 Denmark,26 and Canada.27 The annual incidence of hypocalcaemic seizures secondary to vitamin D deficiency was 3.49 per million children age 0-15 years in the UK.28
Vitamin D deficiency was diagnosed considerably more frequently in Asian and black compared to white children, which was expected given that they have lower vitamin D levels and higher risk of symptomatic deficiency.28–30 Diagnosis was also more frequent in children from deprived backgrounds. Low SEP is associated with suboptimal vitamin D status in children independent of ethnicity, and with reduced use of vitamin D supplements.31–33 The magnitude of the effects of ethnicity and SEP were attenuated after accounting for clustering by practice. One explanation for this observation is that the socio-demographic characteristics of a practice population may have contextual effects on clinicians’ diagnostic behaviour, separate from the influence of individual patients’ characteristics. GPs working in practices with more deprived and ethnically diverse populations may be more likely to test for vitamin D deficiency even in low-risk patients, because of increased awareness of the condition. Possible explanations for greater diagnosis in older compared to younger children may include more frequent presentation to healthcare services with chronic pain or other medically unexplained symptoms,34,35 and higher thresholds for requesting vitamin D tests in younger children in primary care due to the practical challenges of phlebotomy. Among older children, factors contributing to higher diagnosis rates in girls compared to boys may include higher overall primary care consultation rates,36 and the influence of cultural dress in some communities.
Strengths and Limitations
Study strengths include the large sample size, and use of a prospectively collected database of healthcare records representative of real life clinical practice. As the THIN cohort has a similar age and sex distribution to the general population, our results should be broadly generalisable to England as a whole. However, it is somewhat over-representative of individuals from more affluent areas,10 therefore the observed diagnosis rates may underestimate true national rates to an extent. The inclusion of vitamin D prescriptions and tests in the case definition allowed identification of children where the diagnosis was not recorded using Read codes, helping to minimise case under-ascertainment. However, some cases may still have been missed, for example children who were diagnosed and received their full course of treatment in secondary care, if the diagnosis was not subsequently entered into the primary care record from hospital correspondence. Missing data for ethnicity was minimised by utilising linked HES data, and taking maternal ethnicity as a proxy measure where the child’s ethnicity was not available. However, the risk of misclassification will be greater where maternal ethnicity was used. Although there was a moderate proportion of missing data for ethnicity and IMD, complete case analysis and multiple imputation gave very similar results, suggesting that missing data did not substantially influence the findings under the missing at random assumption.24 Data was not available regarding other factors, such as body mass index, that are associated with vitamin D status and may influence testing in clinical practice.
Clinical Implications
Given the magnitude of the increase in diagnosis of vitamin D deficiency over a short period of time, it is very unlikely to be explained by changes in population vitamin D levels, incidence of clinical complications of vitamin D deficiency, or population demographics. It is likely that the rise in testing and treatment has been driven by increased awareness and consideration of vitamin D deficiency among clinicians’. There are several possible contributing factors for this: clinician education through the development of clinical guidelines and dissemination of Department of Health recommendations concerning vitamin D supplementation for high risk groups,37 and wide reporting in the lay media and medical literature of research suggesting a link between vitamin D status and numerous non-musculoskeletal health outcomes.38
The data available did not permit exploration of the clinical indications prompting investigation of vitamin D status. We do not know how much the increase in diagnosis is being driven by improved recognition of children with clinical features consistent with symptomatic vitamin D deficiency, or by testing in other clinical situations (for example screening of asymptomatic children, or testing prompted by the presence of non-musculoskeletal diseases that have been linked to vitamin D deficiency such as diabetes, atopic disorders, and infectious diseases). Sharp increases in vitamin D test requests in adults have been reported in Australia and the UK over the last decade.5,6 The introduction of a defined set of clinical criteria permitting 25-OH-D testing in Alberta, Canada, in 2015 resulted in a 92% reduction in the number of tests ordered, and annual cost savings of almost 4 million US dollars.39 This suggests that, prior to the intervention, the majority of vitamin D tests in adults were performed in individuals without specific clinical features or risk factors for deficiency. Further studies are required to explore the reasons for investigation of vitamin D status in children in clinical practice.
Biochemical vitamin D deficiency, as defined by current guidelines, has a high prevalence in the general population, and testing in any patient group is likely to identify a significant proportion of abnormal results.4 Whilst the benefits of treatment with pharmacological doses of vitamin D are clear in children with symptomatic deficiency, there is no evidence that testing and treating asymptomatic individuals results in improved health outcomes compared to prophylaxis with low-dose supplements.4,40 Whilst numerous observational studies have reported associations between low serum 25-OH-D levels and increased risk of various non-musculoskeletal diseases, their results are subject to reverse causality, confounding and bias, and findings from RCTs are generally null or inconsistent.5,40–42 The UK Scientific Advisory Committee on Nutrition (SACN), US Institute of Medicine, and European Society for Pediatric Gastroenterology, Hepatology, and Nutrition have concluded that there is insufficient evidence of a causative role for vitamin D deficiency in the aetiology of non-musculoskeletal health outcomes.40,43,44 Furthermore, there is limited evidence that optimising vitamin D status is beneficial for the management of these conditions once they have developed, for example in improving glycaemic control in diabetes or reducing disease severity in asthma.45,46
The UK National Institute for Health and Care Excellence, US Endocrine Society, and European Society for Paediatric Endocrinology recommend that vitamin D status should not be checked as a routine screening test,47–49 a position supported by the Choosing Wisely campaigns in North America and Australia.50–52 Shaw and Mughal proposed a set of clinical indications for the measurement of 25-OH-D in children, which relate to symptoms and signs directly attributable to vitamin D deficiency, biochemical or radiological evidence of metabolic bone disease, or the presence of disorders that can interfere with vitamin D metabolism.4 Testing outside of this context requires careful consideration of whether vitamin D deficiency is related to the child’s presentation or is a coincidental finding. The interpretation of 25-OH-D results is further complicated by the inconsistency of commonly used laboratory assays and the limited evidence base underpinning the threshold values used to define deficiency.40,43,44,53 At the population level, unnecessary testing can result in avoidable costs from the tests themselves and from prescription of pharmacological doses of vitamin D. From a public health perspective, resources may be better used if directed towards improving the currently low uptake of inexpensive vitamin D supplements, recommended by SACN and the American Academy of Pediatrics for the prevention of deficiency, by pregnant women and young children,54,55 particularly among high-risk ethnic groups.48,56
Conclusions
There has been a marked increase in the testing and diagnosis of vitamin D deficiency in children in England over the last decade. Future research should explore the drivers for this change in clinicians’ diagnostic behaviour, and the reasons prompting investigation of vitamin D status in clinical practice.
Supplementary Material
What’s Known on This Subject
Vitamin D has attracted considerable interest in recent years, and healthcare providers have reported large increases in vitamin D test requests. However, trends in the diagnosis of vitamin D deficiency in clinical practice have not been investigated.
What This Study Adds
There has been a marked increase in the testing and diagnosis of vitamin D deficiency in children in England over the last decade (15-fold between 2008-2014). Older age, non-white ethnicity, and social deprivation were associated with higher rates of diagnosis.
Acknowledgments
Funding source: This study was funded by a Doctoral Research Fellowship grant (DRF-2013-06-037) from the National Institute for Health Research (NIHR), and was supported by the NIHR Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London. This paper presents independent research funded by the NIHR. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Abbreviations
- 25-OH-D
25-hydroxyvitamin D
- ACU
acceptable computer usage
- AMR
acceptable mortality recording
- CI
confidence interval
- HES
Hospital Episode Statistics
- IMD
Index of multiple deprivation
- PYAR
person-years at risk
- SACN
Scientific Advisory Committee on Nutrition
- SEP
socioeconomic position
- THIN
The Health Improvement Network
- UK
United Kingdom
- US
United States
Footnotes
Potential Conflicts of Interest: The authors have indicated they have no potential conflicts of interest to disclose.
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
References
- 1.Ahmed SF, Franey C, McDevitt H, et al. Recent trends and clinical features of childhood vitamin D deficiency presenting to a children’s hospital in Glasgow. Arch Dis Child. 2011;96:694–6. doi: 10.1136/adc.2009.173195. [DOI] [PubMed] [Google Scholar]
- 2.Thacher TD, Fischer PR, Tebben PJ, et al. Increasing incidence of nutritional rickets: a population-based study in Olmsted County, Minnesota. Mayo Clin Proc. 2013;88(2):176–83. doi: 10.1016/j.mayocp.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson PD, Högler W, Craig ME, et al. The re-emerging burden of rickets: a decade of experience from Sydney. Arch Dis Child. 2006;91:564–68. doi: 10.1136/adc.2004.069575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw NJ, Mughal MZ. Vitamin D and child health: part 2 (extraskeletal and other aspects) Arch Dis Child. 2013;98:368–72. doi: 10.1136/archdischild-2012-302585. [DOI] [PubMed] [Google Scholar]
- 5.Sattar N, Welsh P, Panarelli M, Forouhi NG. Increasing requests for vitamin D measurement: costly, confusing, and without credibility. Lancet. 2012;379:95–6. doi: 10.1016/S0140-6736(11)61816-3. [DOI] [PubMed] [Google Scholar]
- 6.Bilinski K, Boyages S. Evidence of overtesting for vitamin D in Australia: an analysis of 4.5 years of Medicare Benefits Schedule (MBS) data. BMJ Open. 2013;3:e002955. doi: 10.1136/bmjopen-2013-002955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Health and Social Care Information Centre. Prescription Cost Analysis - England 2004. HSCIC; 2005. [Accessed May 26, 2016]. http://www.hscic.gov.uk/catalogue/PUB02249. [Google Scholar]
- 8.Health and Social Care Information Centre. Prescription Cost Analysis - England 2014. HSCIC; 2015. [Accessed May 26, 2016]. http://www.hscic.gov.uk/catalogue/PUB17274. [Google Scholar]
- 9.Bilinski K, Boyages S. The rise and rise of vitamin D testing. BMJ. 2012;345:e4743. doi: 10.1136/bmj.e4743. [DOI] [PubMed] [Google Scholar]
- 10.Blak BT, Thompson M, Dattani H, Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics , chronic disease prevalence and mortality rates. Inform Prim Care. 2011;19:251–5. doi: 10.14236/jhi.v19i4.820. [DOI] [PubMed] [Google Scholar]
- 11.Booth N. What are the Read Codes? Health Libr Rev. 1994;11:177–82. doi: 10.1046/j.1365-2532.1994.1130177.x. [DOI] [PubMed] [Google Scholar]
- 12.Maguire A, Blak BT, Thompson M. The importance of defining periods of complete mortality reporting for research using automated data from primary care. Pharmacoepidemiol Drug Saf. 2009;18:76–83. doi: 10.1002/pds.1688. [DOI] [PubMed] [Google Scholar]
- 13.Horsfall L, Walters K, Petersen I. Identifying periods of acceptable computer usage in primary care research databases. Pharmacoepidemiol Drug Saf. 2013;22:64–9. doi: 10.1002/pds.3368. [DOI] [PubMed] [Google Scholar]
- 14.Lewis JD, Bilker WB, Weinstein RB, Strom BL. The relationship between time since registration and measured incidence rates in the General Practice Research Database. Pharmacoepidemiol Drug Saf. 2005;14:443–51. doi: 10.1002/pds.1115. [DOI] [PubMed] [Google Scholar]
- 15.Davé S, Petersen I. Creating medical and drug code lists to identify cases in primary care databases. Pharmacoepidemiol Drug Saf. 2009;18:704–7. doi: 10.1002/pds.1770. [DOI] [PubMed] [Google Scholar]
- 16.Ford E, Nicholson A, Koeling R, et al. Optimising the use of electronic health records to estimate the incidence of rheumatoid arthritis in primary care: what information is hidden in free text? BMC Med Res Methodol. 2013;13:105. doi: 10.1186/1471-2288-13-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearce SHS, Cheetham TD. Diagnosis and management of vitamin D deficiency. BMJ. 2010;340:b5664. doi: 10.1136/bmj.b5664. [DOI] [PubMed] [Google Scholar]
- 18.Paediatric Formulary Committee. BNF for Children (online) London: BMJ Group, Pharmaceutical Press, and RCPCH Publications; May, 2016. [Accessed May 26, 2016]. http://www.medicinescomplete.com. [Google Scholar]
- 19.Arundel P, Ahmed SF, Allgrove J, et al. British Paediatric and Adolescent Bone Group’s position statement on vitamin D deficiency. BMJ. 2012;345:e8182. doi: 10.1136/bmj.e8182. [DOI] [PubMed] [Google Scholar]
- 20.Noble M, Wright G, Dibben C, et al. The English Indices of Deprivation 2004. London: Office of the Deputy Prime Minister; 2004. [Accessed May 26, 2016]. http://webarchive.nationalarchives.gov.uk/20100410180038/http://www.communities.gov.uk/archived/publications/communities/indicesdeprivation. [Google Scholar]
- 21.Mathur R, Bhaskaran K, Chaturvedi N, et al. Completeness and usability of ethnicity data in UK-based primary care and hospital databases. J Public Health (Oxf) 2014;36(4):684–92. doi: 10.1093/pubmed/fdt116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen I, McCrea RL, Sammon CJ, et al. Risks and benefits of psychotropic medication in pregnancy: cohort studies based on UK electronic primary care health records. Health Technol Assess. 2016;20:1–176. doi: 10.3310/hta20230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meeraus WH, Petersen I, Gilbert R. Association between antibiotic prescribing in pregnancy and cerebral palsy or epilepsy in children born at term: a cohort study using the health improvement network. PLoS One. 2015;10:e0122034. doi: 10.1371/journal.pone.0122034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30:377–99. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 25.Wheeler BJ, Dickson NP, Houghton LA, Ward LM, Taylor BJ. Incidence and characteristics of vitamin D deficiency rickets in New Zealand children: a New Zealand Paediatric Surveillance Unit study. Aust N Z J Public Health. 2015;39:380–3. doi: 10.1111/1753-6405.12390. [DOI] [PubMed] [Google Scholar]
- 26.Beck-Nielsen SS, Brock-Jacobsen B, Gram J, Brixen K, Jensen TK. Incidence and prevalence of nutritional and hereditary rickets in southern Denmark. Eur J Endocrinol. 2009;160:491–7. doi: 10.1530/EJE-08-0818. [DOI] [PubMed] [Google Scholar]
- 27.Ward LM, Gaboury I, Ladhani M, Zlotkin S. Vitamin D-deficiency rickets among children in Canada. CMAJ. 2007;177:161–6. doi: 10.1503/cmaj.061377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basatemur E, Sutcliffe A. Incidence of hypocalcemic seizures due to vitamin D deficiency in children in the United Kingdom and Ireland. J Clin Endocrinol Metab. 2015;100:E91–5. doi: 10.1210/jc.2014-2773. [DOI] [PubMed] [Google Scholar]
- 29.Absoud M, Cummins C, Lim MJ, Wassmer E, Shaw N. Prevalence and predictors of vitamin D insufficiency in children: a Great Britain population based study. PLoS One. 2011;6:e22179. doi: 10.1371/journal.pone.0022179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Callaghan AL, Moy RJD, Booth IW, Debelle G, Shaw NJ. Incidence of symptomatic vitamin D deficiency. Arch Dis Child. 2006;91:606–7. doi: 10.1136/adc.2006.095075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tolppanen AM, Fraser A, Fraser WD, Lawlor DA. Risk factors for variation in 25-hydroxyvitamin D3 and D2 concentrations and vitamin D deficiency in children. J Clin Endocrinol Metab. 2012;97:1202–10. doi: 10.1210/jc.2011-2516. [DOI] [PubMed] [Google Scholar]
- 32.Turer CB, Lin H, Flores G. Prevalence of vitamin D deficiency among overweight and obese US children. Pediatrics. 2013;131:e152–61. doi: 10.1542/peds.2012-1711. [DOI] [PubMed] [Google Scholar]
- 33.Millette M, Sharma A, Weiler H, Sheehy O, Bérard A, Rodd C. Programme to provide Quebec infants with free vitamin D supplements failed to encourage participation or adherence. Acta Paediatr. 2014;103:e444–9. doi: 10.1111/apa.12727. [DOI] [PubMed] [Google Scholar]
- 34.Roth-Isigkeit A, Thyen U, Stöven H, Schwarzenberger J, Schmucker P. Pain among children and adolescents: restrictions in daily living and triggering factors. Pediatrics. 2005;115:e152–62. doi: 10.1542/peds.2004-0682. [DOI] [PubMed] [Google Scholar]
- 35.Eminson DM. Medically unexplained symptoms in children and adolescents. Clin Psychol Rev. 2007;27:855–71. doi: 10.1016/j.cpr.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Hunt K, Nazareth I, Freemantle N, Petersen I. Do men consult less than women? An analysis of routinely collected UK general practice data. BMJ Open. 2013;3:e003320. doi: 10.1136/bmjopen-2013-003320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Department of Health. Vitamin D: an essential nutrient for all… but who is at risk of vitamin D deficiency? Important information for healthcare professionals. Central Office of Information; Dec, 2009. [Google Scholar]
- 38.Harvey NC, Cooper C. Vitamin D: some perspective please. BMJ. 2012;345:e4695. doi: 10.1136/bmj.e4695. [DOI] [PubMed] [Google Scholar]
- 39.Ferrari R, Prosser C. Testing Vitamin D Levels and Choosing Wisely. JAMA Intern Med. 2016;176(7):1019–1020. doi: 10.1001/jamainternmed.2016.1929. [DOI] [PubMed] [Google Scholar]
- 40.The Scientific Advisory Committee on Nutrition. Vitamin D and Health. Public Health England; 2016. [Accessed August 19, 2016]. https://www.gov.uk/government/publications/sacn-vitamin-d-and-health-report. [Google Scholar]
- 41.Reid IR. What diseases are causally linked to vitamin D deficiency? Arch Dis Child. 2016;101:185–189. doi: 10.1136/archdischild-2014-307961. [DOI] [PubMed] [Google Scholar]
- 42.Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035. doi: 10.1136/bmj.g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braegger C, Campoy C, Colomb V, et al. Vitamin D in the healthy European paediatric population. J Pediatr Gastroenterol Nutr. 2013;56:692–701. doi: 10.1097/MPG.0b013e31828f3c05. [DOI] [PubMed] [Google Scholar]
- 44.IOM (Institute of Medicine) Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 45.Riverin BD, Maguire JL, Li P. Vitamin D supplementation for childhood asthma: A systematic review and meta-analysis. PLoS One. 2015;10(8):e0136841. doi: 10.1371/journal.pone.0136841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seida JC, Mitri J, Colmers IN, et al. Effect of Vitamin D3 Supplementation on Improving Glucose Homeostasis and Preventing Diabetes: A Systematic Review and Meta-Analysis. J Clin Endocrinol Metab. 2014;99(10):3551–3560. doi: 10.1210/jc.2014-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munns CF, Shaw N, Kiely M, et al. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. J Clin Endocrinol Metab. 2016;101(2):394–415. doi: 10.1210/jc.2015-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.National Institute for Health and Care Excellence. Vitamin D: increasing supplement use among at-risk groups. NICE guideline (PH56). NICE; 2014. [Accessed May 26, 2016]. https://www.nice.org.uk/guidance/ph56. [DOI] [PubMed] [Google Scholar]
- 49.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 50. [Accessed May 26, 2016]; http://www.choosingwisely.org.
- 51. [Accessed May 26, 2016]; http://www.choosingwiselycanada.org.
- 52. [Accessed May 26, 2016]; http://www.choosingwisely.org.au.
- 53.Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 54.Perrine CG, Sharma AJ, Jefferds MED, Serdula MK, Scanlon KS. Adherence to vitamin D recommendations among US infants. Pediatrics. 2010;125(4):627–32. doi: 10.1542/peds.2009-2571. [DOI] [PubMed] [Google Scholar]
- 55.Moy RJ, McGee E, Debelle GD, Mather I, Shaw NJ. Successful public health action to reduce the incidence of symptomatic vitamin D deficiency. Arch Dis Child. 2012;97:952–4. doi: 10.1136/archdischild-2012-302287. [DOI] [PubMed] [Google Scholar]
- 56.Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–52. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.