Abstract
Context
The ability to predict survival accurately has implications in clinical decision making.
Objective
We determined the association of phase angle obtained from multi-frequency bioelectric impedance analysis (MF-BIA) with overall survival in patients with advanced cancer.
Methods
We included consecutive patients with advanced cancer who had an outpatient palliative care consultation. MF-BIA assessed phase angle at three different frequencies (5 kHz/50 kHz/250 kHz) on each hemibody (right/left). Survival analysis was conducted using the Kaplan Meier method, log rank test and multivariate Cox regression analysis.
Results
Among 366 patients, the median overall survival was 250 days (95% confidence interval 191–303 days). The mean phase angle for 5 kHz, 50 kHz and 250 kHz were 2.2°, 4.4°, 4.2° on the right, and 2.0°, 4.2° and 4.1° on the left, respectively. For all 6 phase angles, a lower value was significantly associated with a poorer overall survival (P<0.001). After adjusting for cancer type, performance status, weight loss and inflammatory markers, phase angle remained independently associated with overall survival (hazard ratio 0.85 per degree increase, 95% confidence interval 0.72–0.99; P=0.048).
Conclusion
Phase angle represents a novel objective prognostic factor in outpatient palliative cancer care setting, regardless of frequency and body sides.
Keywords: Electric impedance, forecasting, neoplasms, palliative care, prognosis, survival
Introduction
In the advanced cancer setting, a patient’s prognosis is an important determinant in clinical decision making. Recommendations regarding cancer treatments, palliative procedures, total parenteral nutrition and hospice admissions are dependent on a patient’s expected survival (1–3). Furthermore, an accurate understanding of prognosis allows patients and families to have a sense of control, prioritize their goals in life, and prepare for the end-of-life. Although many prognostic factors and prognostic models are available, they have not used routinely in the clinical setting because of some key limitations, such as subjectivity, low accuracy and difficulty in interpretation (4, 5).
Previous studies by our group and others have demonstrated that phase angle is a novel prognostic factor in patients with advanced cancer (6–8). Phase angle is a function of cellular membrane integrity and hydration level (9). It is typically assessed using single frequency bioelectric impedance analysis (SF-BIA) at 50 Hz over the right side of the body. The development of multi-frequency bioelectric impedance analysis (MF-BIA) allows assessment of body composition at different frequencies that range from 1 kHz to 1000 kHz typically. MF-BIAs have been found in several studies to have higher accuracy and greater precision compared to SF-BIAs in assessing body composition (10–14). MF-BIA devices can also assess phase angle at different frequencies over the right and left hemibody. However, the utility of phase angle at different frequencies has not been examined in the palliative care population. A better understanding of the prognostic utility of phase angle at different frequencies may assist clinicians to estimate survival more accurately. In this study, we determined the association of 6 different phase angles (3 frequencies and 2 sides of the body) with overall survival in patients with advanced cancer.
Methods
Study Setting and Criteria
This is a retrospective study of consecutive patients who had multi-frequency BIA completed between January 1, 2012 and March 31, 2014. Inclusion criteria included diagnosis of advanced cancer, defined as locally advanced, recurrent or metastatic disease for solid tumors or progressive/refractory/incurable disease for hematologic tumors, age 18 or greater, and seen at the Supportive Care outpatient clinic. The Institutional Review Board at The University of Texas MD Anderson Cancer Center approved this study, and waived the requirement for informed consent.
Data Collection
We collected baseline patient demographics on the day of multi-frequency BIA, including age, sex, race, cancer diagnosis (most active/serious cancer if multiple diagnoses), stage and Eastern Cooperative Oncology Group (ECOG) performance status.
We assessed phase angle using the InBody720 device (Inbody, Cerritos, CA). The test procedure was conducted according to the manufacturer’s instructions. Ambulatory patients stepped on the multi-frequency BIA device with bare feet and held onto the hand rails bilaterally, and remained on the device for 2 minutes. This analyzer uses an alternate current of 250mA and assesses phase angle at 5 kHz, 50 kHz, and 250 kHz. The MF-BIA device measures segmental impedances at the right arm (RA), left arm (LA), right leg (RL), left leg (LL) and trunk (TR) for all 3 frequencies. The phase angle for the each half of body at each frequency is then calculated using the following formula:
where Xc is reactance and R is resistance.
Symptom burden was assessed using the Edmonton Symptom Assessment Scale (ESAS), a validated 10-item symptom battery examining average intensity of pain, fatigue, nausea, depression, anxiety, drowsiness, and shortness of breath, appetite, feeling of well being and sleep over the past 24 hours using numeric rating scales ranging from 0 (none) to 10 (worst) (15, 16).
We also retrieved several objective laboratory-based prognostic variables that were collected within 2 weeks of phase angle, including leukocyte count, lymphocyte count, hemoglobin, serum albumin, calcium, and lactate dehydrogenase (8, 17–22).
Survival from time of multi-frequency BIA assessment was collected from institutional databases and electronic health records.
Statistical Analysis
We summarized the baseline demographics using descriptive statistics, including mean, standard deviation (SD), median, interquartile range (IQR), frequency and percentage. We examined the association between pairwise phase angle by frequency and body side using the Spearman rank correlation test.
We estimated overall survival using the Kaplan Meier method and compared among degrees of phase angle using the log rank test. We used the Contal and O’Quigley method to identify the optimal cutoff of a phase angle for overall survival.(23) We then applied this survival analysis to other laboratory variables based on pre-defined, established cutoffs from the literature, including leukocytosis (serum leukocyte >11,000/mm3), lymphopenia (lymphocyte <1%), anemia (hemoglobin <8.0 g/dL), and neutrophil-lymphocyte ratio >3, hypoalbuminemia (serum albumin <4.0 g/dL), hypercalcemia (corrected calcium >10.2 mg/dL), and elevated lactate dehydrogenase (>618 IU/L) (18, 22).
The Cox Proportional Hazard model was used to assess the effect of phase angle on overall survival, adjusting for patient characteristics and the objective laboratory-based prognostic factors. Stepwise selection was used to build the final multivariate model that included all covariates with P-value <0.05 in univariate analysis.
All computations were carried out in SAS 9.3 (SAS Institute, Cary, North Carolina), Splus 8.2 (TIBCO software Inc, Palo Alto, CA) and R 3.1.3 (University of Twente, Enschede, the Netherlands). A P-value of <0.05 is considered to be statistically significant.
Results
Patient characteristics
Table 1 shows the baseline characteristics of 366 patients at time of multi-frequency BIA assessment. The median age was 58 (range 21–90), 168 (46%) were female, and 242 (66%) were White. The most common cancer diagnoses were gastrointestinal (N=111, 30%), breast cancer (N=50, 14%) and head and neck cancer (N=48, 13%). The median overall survival this cohort was 250 days (95% confidence interval 191–303 days). Among the patients alive, the median followup was 924 days.
Table 1.
Baseline characteristics (N=366)
| Characteristics | N (%)* |
|---|---|
| Age, average (range) | 58 (21–90) |
| Female sex | 168 (46) |
| Race | |
| White | 242 (66) |
| Black | 59 (16) |
| Hispanic | 39 (11) |
| Other | 26 (7) |
| Cancer type | |
| Breast | 50 (14) |
| Gastrointestinal | 111 (30) |
| Genitourinary | 20 (6) |
| Gynecological | 34 (9) |
| Head and neck | 48 (13) |
| Hematologic | 8 (2) |
| Respiratory | 40 (11) |
| Other | 55 (15) |
| Edmonton Symptom Assessment Scale, mean (SD) | |
| Pain | 4.2 (3.0) |
| Fatigue | 5.2 (2.9) |
| Nausea | 2.0 (2.7) |
| Depression | 2.7 (2.9) |
| Anxiety | 3.1 (3.1) |
| Drowsiness | 3.5 (3.1) |
| Dyspnea | 3.1 (3.2) |
| Appetite | 4.7 (3.1) |
| Well being | 4.5 (2.9) |
| Sleep | 4.7 (2.9) |
| ECOG Performance status | |
| 0–1 | 81 (22.6) |
| 2 | 147 (41.1) |
| 3 | 130 (36.3) |
| 4 | 0 |
| Percentage weight loss over past 6 months, mean (SD) | 7.8 (14.6) |
| Serum albumin in g/dL, mean (SD) | 3.8 (0.7) |
| Hypercalcemia (corrected calcium >10.2 mg/dL) | 11 (4.1) |
| Lactate dehydrogenase in unit, mean (SD) | 778.5 (1226.2) |
| Leukocytosis (serum leukocyte >11,000/mm3) | 40 (12.0) |
| Lymphopenia (lymphocyte <1%) | 140 (42.2) |
| Anemia (hemoglobin <8.0 g/dL) | 13 (3.9) |
| Neutrophil-lymphocyte ratio, mean (SD) | 5.9 (7.0) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group
Unless otherwise specified
Phase angle values
Table 2 illustrates the average phase angle values by frequency and body side. The values for 50 kHz and 250 kHz were comparable, although the values for 5 kHz were significantly lower.
Table 2.
Distribution of Phase Angle
| Frequency/ hemibody |
<=2° N (%) |
>2–3° N (%) |
>3–4° N (%) |
>4–5° N (%) |
>5–6° N (%) |
>6° N (%) |
Mean (SD) |
|---|---|---|---|---|---|---|---|
| 5kHz right | 161 (44) | 167 (46) | 27 (7) | 7 (2) | 2 (0.6) | 2 (0.6) | 2.2 (0.8) |
| 50 kHz right | 4 (1) | 24 (7) | 105 (29) | 133 (36) | 83 (23) | 17 (5) | 4.4 (1.0) |
| 250 kHz right | 1 (0.3) | 7 (2) | 122 (33) | 211 (58) | 23 (6) | 2 (0.6) | 4.2 (0.6) |
| 5kHz left | 184 (50) | 163 (45) | 17 (5) | 2 (0.6) | 0 (0) | 0 (0) | 2.0 (0.6) |
| 50 kHz left | 5 (1) | 25 (7) | 125 (34) | 132 (36) | 68 (19) | 11 (3) | 4.2 (1.0) |
| 250 kHz left | 0 (0) | 9 (3) | 137 (37) | 201 (55) | 18 (5) | 1 (0.3) | 4.1 (0.6) |
Phase angle values between the right and left side of the body were also highly similar (Table 2). The Spearman correlation coefficient (ρ) between left and right side were 0.85, 0.91 and 0.87 for 5 kHz, 50 kHz and 250 kHz, respectively (Table 3). The lowest level of correlation was between the left 250 kHz measurement and right sided 5 kHz measurement (ρ=0.56, P<0.001). We found that phase angle was significantly associated with age, sex, body mass index at all frequencies and both sides, with the only exception at 250 kHz for sex (data not shown).
Table 3.
Correlation among Phase Angle at Different Frequencies*
| Frequency/ hemibody |
5kHz right |
50 kHz right |
250 kHz right |
5kHz left |
50 kHz left |
250 kHz left |
|---|---|---|---|---|---|---|
| 5kHz right | - | |||||
| 50 kHz right | 0.79 | - | ||||
| 250 kHz right | 0.63 | 0.85 | - | |||
| 5kHz left | 0.85 | 0.80 | 0.63 | - | ||
| 50 kHz left | 0.72 | 0.91 | 0.76 | 0.82 | - | |
| 250 kHz left | 0.56 | 0.78 | 0.87 | 0.63 | 0.84 | - |
All P-values <0.0001 from Spearman Rank Correlation test
Survival analysis
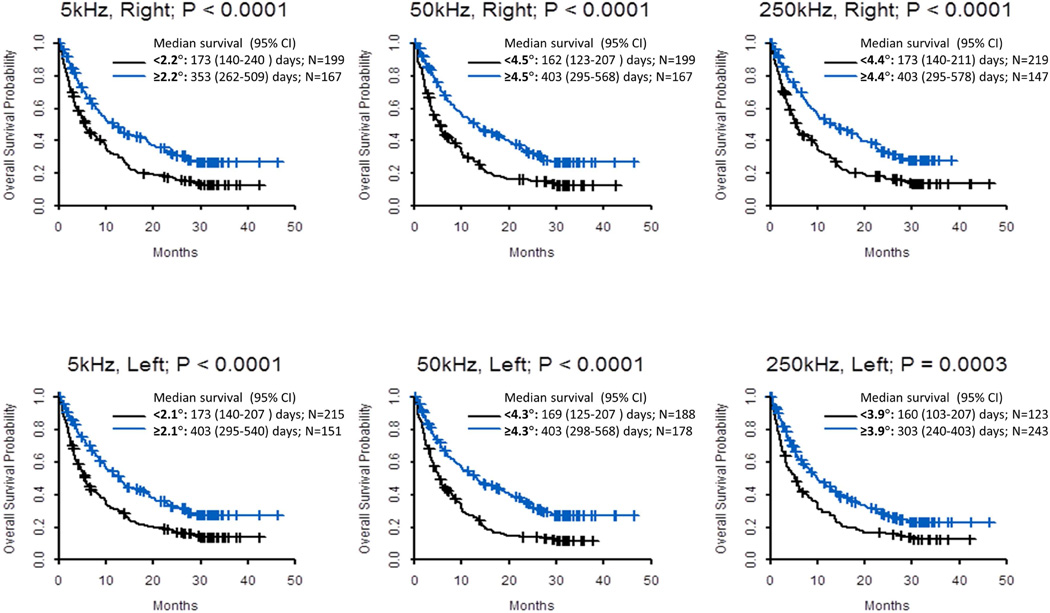
Univariate analysis was conducted with cutoff based on log rank test when phase angle was analyzed by degree (Table 4). A lower phase angle was associated with worse survival in all 6 measures (Figure 1). In multivariate analysis adjusting for patient characteristics and many known objective laboratory variables, phase angle remained significantly associated with overall survival (hazard ratio 0.85 per degree increase in phase angle, 95% CI 0.72 – 0.99; P=0.048; Table 4).
Table 4.
Univariate and Multivariate Cox Regression Analysis
| Univariate analysis | Multivariate analysis* | |||
|---|---|---|---|---|
| Hazard ratio (95% CI) |
P-value | Hazard ratio (95% CI) |
P-value | |
| Age (per year increase) | 1.004 (0.99 – 1.01) | 0.45 | ||
| Male sex (vs. Female) | 0.97 (0.76 – 1.24) | 0.82 | ||
| Race | ||||
| Asian vs. White | 1.16 (0.69 – 1.93) | 0.21 | ||
| Black vs. White | 1.19 (0.86 – 1.65) | |||
| Hispanic vs. White | 1.31 (0.88 – 1.96) | |||
| Other vs. White | 2.71 (1.001 – 7.36) | |||
| Cancer type | ||||
| Breast vs. Respiratory | 0.70 (0.43 – 1.15) | <0.0001 | ||
| Gastrointestinal vs. Respiratory | 1.36 (0.90 – 2.07) | |||
| Genitourinary vs. Respiratory | 0.73 (0.39 – 1.38) | |||
| Gynecological vs. Respiratory | 1.64 (0.98 – 2.74) | |||
| Head and neck vs. Respiratory | 0.39 (0.23 – 0.68) | |||
| Hematologic vs. Respiratory | 1.35 (0.56 – 3.26) | |||
| Other vs. Respiratory | 0.69 (0.42 – 1.12) | |||
| ECOG performance status | 1.77 (1.49 – 2.11) | <.0001 | 1.53 (1.25 – 1.89) | <0.0001 |
| Percentage weight loss over past 6 months | 0.98 (0.97 – 0.99) | 0.02 | ||
| Hypoalbuminemia (serum albumin <4.0 g/dL) | 2.42 (1.82 – 3.21) | <0.0001 | 1.71 (1.24 – 2.36) | 0.001 |
| Hypercalcemia (corrected calcium >10.2 mg/dL) | 1.82 (0.85 – 3.87) | 0.12 | ||
| Elevated lactate dehydrogenase (>618 unit) | 2.43 (1.81 – 3.26) | <0.0001 | 2.18 (1.59 – 2.98) | <0.0001 |
| Leukocytosis (serum leukocyte >11,000/mm3) | 1.55 (1.06 – 2.26) | 0.02 | ||
| Lymphopenia (lymphocyte % <1%) | 1.63 (1.26 – 2.10) | 0.0002 | ||
| Anemia (hemoglobin <8.0 g/dL) | 1.42 (0.77 – 2.60) | 0.26 | ||
| Neutrophil-lymphocyte ratio >3 | 2.02 (1.53 – 2.66) | <0.0001 | 1.65 (1.20 – 2.28) | 0.002 |
| Phase angle (per degree increase) | ||||
| 5kHz right | 0.76 (0.64 – 0.90) | 0.002 | ||
| 50 kHz right | 0.72 (0.64 – 0.82) | <0.0001 | 0.85 (0.72 – 0.99) | 0.048 |
| 250 kHz right | 0.65 (0.54 – 0.79) | <0.0001 | ||
| 5kHz left | 0.60 (0.49 – 0.74) | <0.0001 | ||
| 50 kHz left | 0.71 (0.62 – 0.80) | <0.0001 | ||
| 250 kHz left | 0.69 (0.56 – 0.84) | <0.0001 | ||
Abbreviations: ECOG, Eastern Cooperative Oncology Group
Covariates with P-value <0.05 were included in the multivariate Cox Regression Model, and included cancer type, percentage weight loss, ECOG performance status, hypoalbuminemia, elevated lactate dehydrogenase, leukocytosis, lymphopenia, neutrophil-lymphocyte ratio and phase angle 50 kHz right. We only entered one of 6 phase angles in the model because they were all highly correlated with each other. We selected 50 kHz right because it is most often used in single frequency assessments. Stepwise selection was used to build the final multivariate model.
Figure 1. Kaplan Meier Survival Curves by Phase Angle.
Overall survival was calculated from time of study assessment to last follow-up date or death. Cutoffs based on the Contal and O’Quigley method.
DISCUSSION
To our knowledge, this is the first study to examine phase angle at different frequencies measured using MF-BIA in the palliative care setting. We found that phase angle was strongly associated with survival in patients with advanced cancer. Survival prediction was highly similar among the different frequencies and between the two sides of the body. Upon further validation, this objective, non-invasive and relatively inexpensive prognostic tool may be useful to support clinical decision making.
Multiple studies have reported that low phase angle is associated with poorer survival in cancer and non-cancer patients (8, 24–27). However, only a handful of studies have specifically focused on the advanced cancer population, in which the survival was relatively homogeneous (6, 28, 29). In a recent study by our group, we enrolled 222 hospitalized patients with advanced cancer who were seen by palliative care team for consultation. The median survival was 106 days, and lower phase angle (assessed with SF-BIA) was associated with worse survival independent of other known prognostic variables such as the Palliative Prognostic Score, Palliative Prognostic Index, lean body mass and hypoalbumenia (8). Because survival can differ substantially between patients seen by inpatient and outpatient palliative care, our current study contributes to the literature by documenting phase angle values in ambulatory palliative care setting. With median survival of 250 days, the mean phase angle was 4.4 (SD 1.0).
In contrast to single frequency BIA which is most often conducted at 50 kHz, MF-BIAs assess impedance at different frequencies ranging between 1 and 1000 kHz. Lower frequency currents (<50 kHz) generally flows through the extracellular compartment, while higher frequency currents (>200 kHz) can penetrate cell membranes and pass through lean tissues.(30) This differential tissue penetration at various frequencies allows fat free mass, total body water, intracellular water and extracellular water to be delineated and measured accurately (31, 32). Several studies reported that MF-BIA was either comparable or more accurate than SF-BIA for assessment of body composition in healthy subjects (10, 11) and patients on hemodialysis (13, 14). A meta-analysis of 16 studies also reported the MF-BIA was more accurate than SF-BIA in estimating total body water in patients with CKD (12). More studies are needed to assess the utility of MF-BIA compared to SF-BIA in assessing body composition in the oncology setting.
To date, only a handful of studies have reported the use of MF-BIA in survival prediction. O’Lone included 529 patients on peritoneal dialysis, and reported that the overhydration to extracellular water ratio was an independent predictor of mortality when the BMI and lean tissue index were included in multivariate model (33). Caravaca et al. reported that phase angle at 50 kHz was associate with mortality (hazard ratio=0.49; P=0.026) in 175 patients with chronic kidney disease (34).
To date, only a few groups have studied phase angle at other frequencies. Malecka-Massalska et al. examined phase angle at 5 kHz, 50 kHz, 100 kHz and 200 kHz in 31 patients with head and neck cancer, and reported that phase angle was also significantly lower than normal control at all frequencies (35). Moreover, phase angle significantly decreased at followup after surgery at all frequencies except 200 kHz (36). Sarode et al. examined phase angle at 20 Hz, 50 kHz, 1.3 MHz, 2.5 MHz, 3.7 MHz and 5 MHz in 100 individuals, and reported that phase angle decreased with increasing frequency (37). Given the differential tissue penetration, phase angle assessed at 5 kHz, 50 kHz and 250 kHz could potentially provide different prognostic information. We found that the phase angle values at 50 kHz and 250 kHz were comparable to each other, while the phase angle values at 5 KHz was significantly lower. There was also a high level of correlation between the left and right side of the body, which was not surprising. Importantly, a lower phase angle value was consistently associated with shorter survival, and the phase angle at the 3 frequencies provided similar level of discrimination. The implications of our findings are as follows: (1) phase angle at 50 kHz alone is a reasonable measure because it is most frequently used and reference data are available (38); (2) phase angle at other frequencies may also be informative and strongly correlate with 50 kHz, and (3) further research is needed to examine if phase angle at other frequencies outside of our range (i.e. <5 kHz or >250 kHz) remain accurate for prognostication of survival.
We recently reported that objective prognostic factors have higher accuracy than clinician prediction of survival alone, supporting the use of objective measures for prognostication (39). Here, we found that phase angle was a predictor of survival independent of many established objective laboratory markers, such as hypoalbuminemia, leukocytosis, neutrophil to leukocyte ratio, and elevated lactate dehydrogenase. This may be because phase angle assesses a different physiologic aspect than these other variables. Further studies are needed to compare the accuracy of these measures, and to derive a prognostic score based solely on objective variables that can have high accuracy.
This study has several limitations. First, the retrospective nature of data gathering means that we were not able to include several key prognostic variables such as the Palliative Prognostic Score and C-reactive protein. Second, patients need to be able to stand on the scale for a few minutes to use the Inbody 720 device. Thus, a few individuals seen at the outpatient clinic were excluded due to muscle weakness. Thus, the Inbody 720 device may not be feasible in the inpatient setting, although other MF-BIAs are available that can be conducted with patient in a supine position.
In summary, phase angle represents a novel objective prognostic factor in outpatient palliative cancer care setting, regardless of frequency and body sides. Future studies should examine how phase angle values can be used to inform both patients with advanced cancer and clinicians in decision making on the many complex issues in the last months of life, such as palliative procedures, chemotherapy and nutrition.
Acknowledgments
Funding
This work was supported by the National Institutes of Health Cancer Center Support Grant (CA016672 to M.P. and D.L.). D.H. is supported in part by a National Institutes of Health grant (R21CA186000-01A1), an American Cancer Society Mentored Research Scholar Grant in Applied and Clinical Research (MRSG-14-1418-01-CCE), and an institutional startup grant (#18075582). The study device was provided by Inbody USA on loan. The company had no role in study design, data collection, analysis, interpretation, or writing of the report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None reported
REFERENCES
- 1.Weeks JC, Cook EF, O'Day SJ, et al. Relationship between cancer patients' predictions of prognosis and their treatment preferences. JAMA. 1998;279:1709–1714. doi: 10.1001/jama.279.21.1709. [DOI] [PubMed] [Google Scholar]
- 2.Lamont EB, Christakis NA. Physician factors in the timing of cancer patient referral to hospice palliative care. Cancer. 2002;94:2733–2737. doi: 10.1002/cncr.10530. [DOI] [PubMed] [Google Scholar]
- 3.Miner TJ. Palliative surgery for advanced cancer: lessons learned in patient selection and outcome assessment. Am J Clin Oncol. 2005;28:411–414. doi: 10.1097/01.coc.0000158489.82482.2b. [DOI] [PubMed] [Google Scholar]
- 4.Glare PA, Sinclair CT. Palliative Medicine review: prognostication. J Palliat Med. 2008;11:84–103. doi: 10.1089/jpm.2008.9992. [DOI] [PubMed] [Google Scholar]
- 5.Hui D. Prognostication of Survival in Patients With Advanced Cancer: Predicting the Unpredictable? Cancer Control. 2015;22:489–497. doi: 10.1177/107327481502200415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norman K, Stobaus N, Zocher D, et al. Cutoff percentiles of bioelectrical phase angle predict functionality, quality of life, and mortality in patients with cancer. Am J Clin Nutr. 2010;92:612–619. doi: 10.3945/ajcn.2010.29215. [DOI] [PubMed] [Google Scholar]
- 7.Santarpia L, Marra M, Montagnese C, Alfonsi L, Pasanisi F, Contaldo F. Prognostic significance of bioelectrical impedance phase angle in advanced cancer: preliminary observations. Nutrition. 2009;25:930–931. doi: 10.1016/j.nut.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Hui D, Bansal S, Morgado M, Dev R, Chisholm G, Bruera E. Phase angle for prognostication of survival in patients with advanced cancer: preliminary findings. Cancer. 2014;120:2207–2214. doi: 10.1002/cncr.28624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norman K, Stobaus N, Pirlich M, Bosy-Westphal A. Bioelectrical phase angle and impedance vector analysis--clinical relevance and applicability of impedance parameters. Clin Nutr. 2012;31:854–861. doi: 10.1016/j.clnu.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Pietrobelli A, Morini P, Battistini N, Chiumello G, Nunez C, Heymsfield SB. Appendicular skeletal muscle mass: prediction from multiple frequency segmental bioimpedance analysis. Eur J Clin Nutr. 1998;52:507–511. doi: 10.1038/sj.ejcn.1600592. [DOI] [PubMed] [Google Scholar]
- 11.Gaba A, Kapus O, Cuberek R, Botek M. Comparison of multi- and single-frequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for assessment of body composition in post-menopausal women: effects of body mass index and accelerometer-determined physical activity. J Hum Nutr Diet. 2015;28:390–400. doi: 10.1111/jhn.12257. [DOI] [PubMed] [Google Scholar]
- 12.Martinoli R, Mohamed EI, Maiolo C, et al. Total body water estimation using bioelectrical impedance: a meta-analysis of the data available in the literature. Acta Diabetol. 2003;40(Suppl 1):S203–S206. doi: 10.1007/s00592-003-0066-2. [DOI] [PubMed] [Google Scholar]
- 13.Raimann JG, Abbas SR, Liu L, et al. Agreement of single- and multi-frequency bioimpedance measurements in hemodialysis patients: an ancillary study of the Frequent Hemodialysis Network Daily Trial. Nephron Clin Pract. 2014;128:115–126. doi: 10.1159/000366447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teruel-Briones JL, Fernandez-Lucas M, Ruiz-Roso G, et al. Analysis of concordance between the bioelectrical impedance vector analysis and the bioelectrical impedance spectroscopy in haemodialysis patients. Nefrologia : publicacion oficial de la Sociedad Espanola Nefrologia. 2012;32:389–395. doi: 10.3265/Nefrologia.pre2012.Feb.11309. [DOI] [PubMed] [Google Scholar]
- 15.Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 16.Nekolaichuk C, Watanabe S, Beaumont C. The Edmonton Symptom Assessment System: a 15-year retrospective review of validation studies (1991–2006) Palliat Med. 2008;22:111–122. doi: 10.1177/0269216307087659. [DOI] [PubMed] [Google Scholar]
- 17.Maltoni M, Caraceni A, Brunelli C, et al. Prognostic factors in advanced cancer patients: evidence-based clinical recommendations--a study by the Steering Committee of the European Association for Palliative Care. J Clin Oncol. 2005;23:6240–6248. doi: 10.1200/JCO.2005.06.866. [DOI] [PubMed] [Google Scholar]
- 18.Li QQ, Lu ZH, Yang L, et al. Neutrophil count and the inflammation-based glasgow prognostic score predict survival in patients with advanced gastric cancer receiving first-line chemotherapy. Asian Pac J Cancer Prev. 2014;15:945–950. doi: 10.7314/apjcp.2014.15.2.945. [DOI] [PubMed] [Google Scholar]
- 19.Petrelli F, Cabiddu M, Coinu A, et al. Prognostic role of lactate dehydrogenase in solid tumors: a systematic review and meta-analysis of 76 studies. Acta Oncol. 2015;54:961–970. doi: 10.3109/0284186X.2015.1043026. [DOI] [PubMed] [Google Scholar]
- 20.Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Yao YH, Li BG, Yang Q, Zhang PY, Wang HT. Prognostic value of pretreatment serum lactate dehydrogenase level in patients with solid tumors: a systematic review and meta-analysis. Scientific reports. 2015;5:9800. doi: 10.1038/srep09800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donovan PJ, Achong N, Griffin K, Galligan J, Pretorius CJ, McLeod DS. PTHrP-mediated hypercalcemia: causes and survival in 138 patients. J Clin Endocrinol Metab. 2015:jc20144250. doi: 10.1210/jc.2014-4250. [DOI] [PubMed] [Google Scholar]
- 23.Contal C, O'Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Computational Statistics and Data Analysis. 1999;30:253–270. [Google Scholar]
- 24.Schwenk A, Beisenherz A, Romer K, Kremer G, Salzberger B, Elia M. Phase angle from bioelectrical impedance analysis remains an independent predictive marker in HIV-infected patients in the era of highly active antiretroviral treatment. The American Journal of Clinical Nutrition. 2000;72:496–501. doi: 10.1093/ajcn/72.2.496. [DOI] [PubMed] [Google Scholar]
- 25.Mushnick R, Fein PA, Mittman N, Goel N, Chattopadhyay J, Avram MM. Relationship of bioelectrical impedance parameters to nutrition and survival in peritoneal dialysis patients. Kidney Int. 2003;(87):S53–S56. doi: 10.1046/j.1523-1755.64.s87.22.x. [DOI] [PubMed] [Google Scholar]
- 26.Desport JC, Marin B, Funalot B, Preux PM, Couratier P. Phase angle is a prognostic factor for survival in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2008;9:273– 278. doi: 10.1080/17482960801925039. [DOI] [PubMed] [Google Scholar]
- 27.Abad S, Sotomayor G, Vega A, et al. The phase angle of the electrical impedance is a predictor of long-term survival in dialysis patients. Nefrologia : publicacion oficial de la Sociedad Espanola Nefrologia. 2011;31:670–676. doi: 10.3265/Nefrologia.pre2011.Sep.10999. [DOI] [PubMed] [Google Scholar]
- 28.Davis MP, Yavuzsen T, Khoshknabi D, et al. Bioelectrical impedance phase angle changes during hydration and prognosis in advanced cancer. The American Journal of Hospice & Palliative Care. 2009;26:180–187. doi: 10.1177/1049909108330028. [DOI] [PubMed] [Google Scholar]
- 29.Lee SY, Lee YJ, Yang JH, Kim CM, Choi WS. The Association between Phase Angle of Bioelectrical Impedance Analysis and Survival Time in Advanced Cancer Patients: Preliminary Study. Korean journal of family medicine. 2014;35:251–256. doi: 10.4082/kjfm.2014.35.5.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Utter AC, Nieman DC, Mulford GJ, et al. Evaluation of leg-to-leg BIA in assessing body composition of high-school wrestlers. Med Sci Sports Exerc. 2005;37:1395–1400. doi: 10.1249/01.mss.0000174901.05353.f2. [DOI] [PubMed] [Google Scholar]
- 31.Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis--part I: review of principles and methods. Clin Nutr. 2004;23:1226–1243. doi: 10.1016/j.clnu.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin Nutr. 2004;23:1430–1453. doi: 10.1016/j.clnu.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 33.O'Lone EL, Visser A, Finney H, Fan SL. Clinical significance of multi-frequency bioimpedance spectroscopy in peritoneal dialysis patients: independent predictor of patient survival. Nephrol Dial Transplant. 2014;29:1430–1437. doi: 10.1093/ndt/gfu049. [DOI] [PubMed] [Google Scholar]
- 34.Caravaca F, Martinez del Viejo C, Villa J, Martinez Gallardo R, Ferreira F. Hydration status assessment by multi-frequency bioimpedance in patients with advanced chronic kidney disease. Nefrologia : publicacion oficial de la Sociedad Espanola Nefrologia. 2011;31:537–544. doi: 10.3265/Nefrologia.pre2011.Apr.10936. [DOI] [PubMed] [Google Scholar]
- 35.Malecka-Massalska T, Smolen A, Morshed K. Altered tissue electrical properties in squamous cell carcinoma in head and neck tumors: Preliminary observations. Head Neck. 2013;35:1101–1105. doi: 10.1002/hed.23091. [DOI] [PubMed] [Google Scholar]
- 36.Malecka-Massalska T, Smolen A, Morshed K. Tissue electrical properties in head and neck tumors before and after surgery: Preliminary observations. Indian J Cancer. 2014;51:209–213. doi: 10.4103/0019-509X.146717. [DOI] [PubMed] [Google Scholar]
- 37.Sarode GS, Sarode SC, Kulkarni M, Karmarkar S, Patll S, Auciustine D. Bioimpedance Assessment of Oral Squamous Cell Carcinoma with Clinicopathological Correlation. J Contemp Dent Pract. 2015;16:715–722. doi: 10.5005/jp-journals-10024-1746. [DOI] [PubMed] [Google Scholar]
- 38.Barbosa-Silva MC, Barros AJ, Wang J, Heymsfield SB, Pierson RN., Jr Bioelectrical impedance analysis: population reference values for phase angle by age and sex. The American Journal of Clinical Nutrition. 2005;82:49–52. doi: 10.1093/ajcn.82.1.49. [DOI] [PubMed] [Google Scholar]
- 39.Hui D, Park M, Liu D, et al. Clinician prediction of survival versus the Palliative Prognostic Score: Which approach is more accurate? Eur J Cancer. 2016;64:89–95. doi: 10.1016/j.ejca.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]