Abstract
Although the gastrointestinal (GI) tract contains intrinsic neural plexuses that allow a significant degree of independent control over GI functions, the central nervous system provides extrinsic neural inputs that modulate, regulate and integrate these functions. In particular, the vagus nerve (VN) provides the parasympathetic innervation to the GI tract, co-ordinates the complex interactions between central and peripheral neural control mechanisms. This review will discuss the physiological roles of the afferent (sensory) and motor (efferent) vagus in regulation of appetite, mood and the immune system, as well as the pathophysiological outcomes of VN dysfunction resulting in obesity, mood disorders and inflammation. The therapeutic potential of VN modulation to attenuate or reverse these pathophysiological outcomes and restore autonomic homeostasis will also be discussed.
Introduction
The vagus nerve (VN), the longest cranial nerve in the body, not only regulates gut physiology, but is also involved in controlling the cardiovascular, respiratory, immune and endocrine systems. To date, its role in the regulation of appetite and obesity is increasingly recognized, and involves a complex interplay between central and peripheral mechanisms involving both afferent and efferent VN fibers. Similarly, it is increasingly recognized that the VN communicates with the immune system, i.e. inflammation in the periphery is detected by vagal afferents and integrated in the brainstem, affecting appetite, mood and sickness behavior generating, ultimately, an efferent vagal signal modulating the immune response. The “great wandering protector” plays a crucial role in the organism homeostasis, and is currently being explored as therapeutic target in a variety of disorders. Undoubtedly, the VN still hides many undiscovered mysteries relevant for better understanding of physiology and pathophysiology and the development of better treatments. In the present review, the current knowledge with respect to appetite regulation, mood and intestinal inflammation will be reviewed.
Vagal anatomy and neuroanatomy
Intrinsic neural networks within the gastrointestinal (GI) tract, including myenteric and submucosal plexuses as well as interstitial cells of Cajal (ICC’s), allow a substantial degree of autonomy over GI functions such as motility, secretion and absorption1. The central nervous system (CNS), however, provides extrinsic neural inputs which integrate, regulate, and modulate these responses. The sympathetic nervous system provides a principally inhibitory influence over GI muscle and mucosal secretion and, at the same time, regulates GI blood flow through neural-dependent vasoconstriction. The parasympathetic nervous system, in contrast, provides both excitatory and inhibitory control over gastric, intestinal and pancreatic functions, suggestive of a more complex homeostatic regulation (see 1 for review). The stomach and upper GI tract in particular receive an especially dense parasympathetic innervation, the density of which decreases as one progresses distally through the intestine 2,3 .
The parasympathetic innervation to the GI tract and pancreas are provided by the VN. As a mixed sensory-motor nerve, the vagus contains approximately 70–80% sensory fibers, depending on the species4. The cell bodies of these pseudounipolar sensory neurons are located in the paired nodose ganglia (inferior ganglion of the VN) located in the transverse process of the first cervical vertebra, although some cell bodies in the jugular (superior) ganglion may provide innervation to the GI tract. GI vagal afferents are principally unmyelinated C- or thinly myelinated Aδ-fibers and are classified based upon the location of their receptive field (mucosa, muscle or serosa-mesenteric), the region of GI tract innervated, primary stimulus modality (chemical, osmotic, mechanical), or their response to distention or pressure5. The majority of vagal afferents are sensitive to low pressure distention, although some vagal afferents can respond to high distention pressures; while the majority of GI vagal afferents traffic is interoceptive, therefore it is also likely that they play a role in nociception, or in the emotional-affective response to pain6.
The central terminals of vagal afferents enter the brainstem via the tractus solitarius (TS), and synapse onto neurons of the nucleus of the tractus solitarius (NTS) using glutamate as their principle neurotransmitter7. Some vagal afferents also make monosynaptic connections within the dorsal motor nucleus of the vagus (DMV)8, or with neurons of the area postrema (AP)9. Together, the NTS, DMV and AP, known as the dorsal vagal complex (DVC), function as a critical intersection in the integration of ascending interoceptive signals with descending visceromotor signals. The entire DVC area, which lies ventral to the 4th ventricle, is highly vascularized with fenestrated capillaries and is essentially a circumventricular organ10. An additional layer of subependymal cerebrospinal contacting neurons (CSF-cNs) are positioned between the CSF and DVC neurons and may integrate the detection of circulating signals with the modulation of autonomic, including GI, functions11.
The NTS appears organized in a viscerotopic manner according to the region of afferent input12. NTS neurons integrate the vast volume of sensory information together with inputs received from other brainstem and higher CNS nuclei involved in autonomic homeostatic regulation (see later). The integrated response is then relayed to the adjacent DMV which contains the preganglionic parasympathetic motor neurons that send the output response back to the viscera via the efferent VN. The NTS-DMV synapse uses glutamate, GABA or catecholamines as neurotransmitters, although evidence from several groups suggests that, under experimental conditions, GABA is the predominant neurotransmitter13–15. Vagal efferent outflow to the viscera, therefore, appears to be under a tonic inhibitory influence. Dendritic projections of NTS and DMV neurons intermingle within the various subnuclei, however, possibly providing a means by which autonomic reflexes may be integrated across organ system16.
Unlike the NTS, the DMV does not display a viscerotopic or organotopic organization. Instead, DMV neurons are organized in spindle-shaped ‘columns’ that extend throughout the rostro-caudal extent of the nucleus and innervate the GI tract via one of the five subdiaphragmatic vagal branches17. In humans, the DMV can be subdivided into nine distinct subnuclei with six distinct morphological and neurochemical neuronal phenotypes18. Most GI regions receive innervation from more than one subdiaphragmatic vagal branch, however; the stomach is innervated by the anterior and posterior gastric branches as well as the hepatic branch, the duodenum is innervated by all vagal branches, and the colon is innervated by both the celiac and accessory celiac branches3. In humans, the vagus innervates the right two-thirds of the transverse colon, with parasympathetic innervation to the left third of the transverse colon, the descending colon and rectum arising from the pelvic nerves19. Early neuronal tracing studies showed vagal efferent fibers were present within the celiac ganglion20 suggesting a role in the modulation of splenic nerves and a potential anatomical basis for the cholinergic anti-inflammatory pathway (CAIP). The use of the trans-synaptic, pseudorabies virus (PRV), to map the central neurocircuits innervating the spleen did not, however, find labeling in the DMV21. Vagal nerve stimulation clearly modulates splenic function (see later), but the anatomical and mechanistic basis of this action has yet to be clarified. For the purposes of this review, the principle CNS areas and inputs involved in the modulation of GI vagal reflexes by diet, inflammation and mood disorders are outlined in Table 1.
Table 1.
CNS nuclei with direct connections with vagal brainstem nuclei involved in the regulation of GI functions1
| Location | Neuroanatomical connections with brainstem vagal nuclei | GI functions | |
|---|---|---|---|
| Hindbrain | |||
| Spinal cord | GI afferents terminate within the thoracic (T1-T3) spinal cord | Spinal afferent inputs terminate within the NTS (spinosolitary tract) | Relay nociceptive information; spinal afferents also respond to low-pressure distention and innocuous chemostimulation |
| Area postrema | Dorsal to the NTS, adjacent to the 4th ventricle | AP neurons innervate the NTS;NTS and DMV neuronal dendrites reach the AP | Chemoreceptor trigger zone;Contain receptors for, and respond to, a variety of circulating factors including immune and inflammatory mediators |
| Midbrain | |||
| Paraventricular nucleus of the hypothalamus (PVN) | Hypothalamus; adjacent to 3rd ventricle within periventricular zone | Reciprocal connections with DVC; release neurotransmitters into neurohypophysis and hormones into hypophysial portal system; | Stress-induced GI responses, including arousal, anxiety and depression |
| Arcuate nucleus (Arc) | Hypothalamus; adjacent to the 3rd ventricle, in close apposition to median eminence | Reciprocal connections with DVC | Integration of endocrine and behavioral aspects of GI functions Modulation of food intake and satiety |
| Barrington’s nucleus | Dorsolateral pons | Receives inputs from NTS | Regulation of bladder and colon function;assimilation of emotional and cognitive behaviors with GI functions |
| Periaqueductal gray (PAG) | Surrounds cerebral aqueduct from dorsal tegmental nucleus to posterior commissure | Dense innervation from NTS | Integration of fear and anxiety; pain modulation |
| Locus coeruleus (LC) | A6 noradrenergic region;located at rostral pons at lateral floor of 4th ventricle | Reciprocal connections with DVC | Maintenance of arousal and attention;integration of emotional responses and cognitive inputs with GI functions; stress |
| Forebrain | |||
| Central nucleus of the amygdala (CeA) | Heterogeneous complex within anteromedial temporal lobe | Reciprocal connections with DVC | Learning and memory; integrative of emotional and aversive inputs with learning, memory and autonomic GI functions; prominent role in anxiety and stress-related GI disorders |
| Bed Nucleus of the Stria Terminalis (BNST) | Part of the extended amygdala | Reciprocal connections with DVC | Processing and consolidation of behavior and emotions; regulation of HPA axis and autonomic (GI) responses to stress |
| Cortex | Direct descending connections (infralimbic and prelimbic cortex) to DVC; indirect descending connections via hypothalamus and amygdala | Integration of affect, emotion and memory with autonomic (GI) functions |
Effects of diet and obesity on vago-vagal functions
The GI tract is one of several organs that contribute to the peripheral signaling of food intake and satiety and, increasingly, vagally-mediated reflexes are recognized as being critical to the neural control of energy homeostasis22 particularly the short-term (homeostatic), rather than longer-term (hedonic), control of appetite and food intake, although clearly the two pathways are inter-related and dependent23.
Gastric distention activates vagal afferent mechanoreceptors in a dose-dependent manner, suggesting that some mechanosensitive afferents control meal size via signaling of volume or load. In contrast, chemosensitive vagal afferents are activated in response to luminal pH, osmolality, and chemical stimulation, although they can also be activated by light stroking or compression of the mucosa5. Mucosal afferents are most abundant in the upper small intestine, where they innervate the mucosal villae or crypts24. Afferent terminals are found in close proximity to mucosal enteroendocrine cells24 and respond to the mediators they release. Over 30 GI neurohormones have been identified and many play critically important roles in digestion, absorption and satiety signaling25. The majority of these GI neurohormones activate their respective receptors present on vagal afferent nerve endings in a predominantly paracrine manner26–28, increasing vagal afferent firing and resulting, ultimately, in a range of visceral effects including gastric relaxation, reduced gastric emptying, altered motility and pancreatic secretion27–29. Ghrelin, in contrast, is released from the stomach and inhibits vagal afferent firing30 and is, to date, the only GI neurohormone that stimulates feeding directly, in addition to attenuating the anorexigenic actions of CCK and leptin31,32.
Despite being activated principally indirectly, some chemosensitive vagal afferents can respond directly to nutrients; discrete subpopulations of gastric vagal afferents are activated33 while hepatic vagal afferents34 are inhibited, by elevated glucose levels. Hepatic vagal afferents innervating the portal vein are in a unique anatomical position to respond rapidly to nutrients following intestinal absorption; their activity is also modulated by amino acids (increased or decreased, depending on the amino acid)35 suggesting a close correlation between hepatic vagal afferent activity and food ingestion.
Subsequent to their release, GI neurohormones enter the bloodstream from where they may also exert non-paracrine effects on vagal activity. In humans, circulating levels of platelet-free 5-HT increase 3 fold following meal ingestion36 while CCK levels increase 5–8 fold37. Originally thought to have a tight blood-ganglion barrier38, circulating substances39 readily access, hence potentially alter the activity of, vagal afferent somata directly. GI vagal afferent somata display a variety of receptors for neurotransmitters/modulators40 and in virtually every instance studied to date, the actions of GI neurohormones on vagal afferent neurons mimic those at their peripheral terminals (see 32,33,41,42).
Several of these GI neurohormones also act centrally to modulate the activity of brainstem vagal neurons. CCK, GLP-1 and 5-HT, for example, also all act as neurotransmitters/modulators within the brainstem to affect vagal afferent and efferent responses either directly or indirectly43–46. We have demonstrated previously that CCK can activate NTS neurons even after selective surgical section of vagal afferent rootlets, implying a direct central action44. While the contribution of paracrine vs hormonal modulation of vagal neurocircuits by GI neurohormones is not clear, GI neurohormones appear capable of exerting coordinated, but temporally distinct, actions to regulate vagally dependent functions.
High fat diet and obesity compromise vagal plasticity
Studies from several laboratories have demonstrated that, in both humans and animal models, the ability of vagal afferents to respond to GI neuropeptides is compromised by obesity or exposure to a high fat diet. The actions of CCK, for example, to increase vagal afferent activity is reduced in obese rodents as well as humans47–49. Similarly, subpopulations of vagal afferent neurons from rats fed a high fat diet become leptin resistant at the onset of hyperphagia50. It should also be noted though that, rather than the actions of leptin being attenuated uniformly following conditions of excess energy intake, an action of leptin to inhibit gastric tension receptors in mice only becomes apparent after exposure to a high fat diet. Such changes appear to act in concert, however, to promote increased food intake51. Several groups have demonstrated that vagal neurocircuits exhibit a significant degree of plasticity and their ‘response phenotype’, is modulated by ongoing physiological, and pathophysiological, conditions52–54. Under fasting conditions, when circulating CCK levels are low, the density of cannabinoid (CB1), and melanin concentrating hormone (MCH1) receptors on vagal afferent neurons is increased; this ‘orexigenic phenotype’ is associated with an increase in food intake. Conversely, following re-feeding, or an increase in circulating CCK levels, Y2 receptor expression increases while CB1 receptor expression decreases; this ‘anorexigenic phenotype’ is associated with a decrease in food intake52. In diet-induced obesity, however, vagal afferent neurons appear fixed in an orexigenic phenotype, regardless of feeding status, supportive of an increased ‘drive’ for food intake or a decreased satiety response52. Furthermore, in diet-induced obese mice, the mechanosensitivity of vagal afferents is reduced55 and one recent study suggested that the ghrelin’s inhibitory actions on vagal afferent firing are lost56, suggestive of a more wide-spread dysregulation of vagal afferents, not merely a loss of satiety signaling. In contrast, other studies demonstrated that, rather than losing efficacy, in obese mice ghrelin is able to inhibit the activation of both gastric mucosal and tension sensitive vagal afferents, which would be expected to increase its orexigenic actions55.
While technical differences between studies may explain some of these differences, clearly the effects of diet and obesity on vagal afferent responsiveness is multifactorial and is likely to be affected by vagal afferent type, sex, time of day and feeding status. In general, both vagal afferent and efferent neurons appear less excitable in diet-induced obese rodent models48–50,52–57; a generalized decrease in neuronal excitability would compromise the ability of vagal afferents and efferents to respond to any signal, mechanical or chemical47,50,55,57.
A decrease in neuronal excitability is unlikely to be the sole mechanism behind loss of vagal afferent responsiveness, however. Gene expression levels of the growth hormone secretagogue (ghrelin) receptor shows a diurnal rhythm58 and the mechanosensitivity of gastric vagal afferents shows a striking circadian rhythm, decreasing three-fold during the dark cycle when feeding in mice is prevalent59 implying that vagal afferent responsiveness to food ingestion is dictated by feeding cycles and the time of day. This circadian dependency is lost in obesity, however, in concert with a loss in ability of leptin to increase mechanosensitivity, increasing daytime feeding60.
It remains to be determined whether these changes in vagal afferent properties with obesity are correlative or causative, however. One recent study suggests that, in rats, the ability of glucose to modulate serotonin signaling in GI vagal afferents is lost after only 3–7 days of exposure to a high fat diet, implying that reduced vagal sensory signaling is affected prior to obesity. Even one day of exposure to a high fat diet increases the recruitment of classically activated macrophages (M1) to the nodose ganglion of mice, suggesting that even short term exposure can induce inflammation in the vagal afferent system61.
Effects of gut microbiota on vagal afferents
A significant, and growing, body of literature supports the actions of gastrointestinal luminal microorganisms to modulate gut-brain signaling via vagal afferents, the so-called microbiota-gut-brain axis (MGB axis; see reviews62–64). While gut microbiota might normally be expected to activate vagal afferents directly only under conditions where intestinal permeability is compromised (e.g. following inflammation or stress), luminal bacteria may activate vagal afferents indirectly, subsequent to stimulation and release of neuroactive mediators from enteroendocrine cells or gut associated lymphoid tissue (GALT). In addition to their potential role in the regulation of gut functions including motility and secretion, and activation of immune responses, the gut microbiota have well-described roles in ‘higher’ CNS functions, including mood, stress-related psychiatric conditions and memory63,64. Administration of Citrobacter rodentium to mice, for example, increases anxiety-like behaviors in a vagally-dependent manner65 while Bifidobacterium longum NC3001 normalized anxiety-like behavior following nematode-induced GI inflammation, again in a manner involving the vagus nerve66. Clinical studies have also suggested that ingestion of probiotics may decrease anxiety and depression67. Large clinical trials evaluating the efficacy of antibiotic as well as probiotic (“psychobiotic”) therapies, as well as in-depth sequencing of the microbiome, is required as a matter of urgency to further elaborate on the role of the MGB axis in several pathophysiological states.”
Inflammation and modulation of vagal afferents
Vagal afferents and neurons respond directly to cytokines and endotoxin, not only contributing to the induction of fever and sickness behavior, but also affecting GI function. Indeed, interleukin-1 (IL-1) receptors are expressed on vagal afferents and glomus cells adjacent to the VN68,69 while intestinal inflammation, triggered by Campylobacter jejuni infection70 or intestinal manipulation71 activates NTS neurons. Inflammation, including intestinal inflammation, and an increase in circulating levels of cytokines are well recognized sequelae of obesity72. Indeed, chronic exposure to low doses of the bacterial endotoxin, lipopolysaccharide (LPS), mimics several obesity-associated outcomes including attenuated vagal afferent leptin signaling, hyperphagia, and decreased CCK-induced satiation73 suggesting that inflammation plays a causal role in obesity development. Vagal afferents can not only respond to cytokines and inflammatory mediators74, but inflammation induces neuroplasticity, including the upregulation of transient receptor potential (TRP) channels TRPV1 and TRPA175, upregulation of P2X receptors and augmentation of ATP signaling76, modulation of sodium ion channel density and function53, reactive gliosis of satellite glial cells76, and increased mechanosensitivity77 suggesting a means by which vagal afferent signaling and sensitivity may be modulated by inflammation.
Increases in circulating levels of cytokines, including tumor necrosis factor alpha (TNF-a) decreases gastric motility and emptying associated with nausea and vomiting via actions at central vagal neurocircuits. Calcium imaging studies suggest that TNF-a potentiates vagal afferent terminal responses, thus activates NTS neurons78, increases DVC microglial activation and induces DMV apoptosis via PAR1 and PAR2 receptor activation79,80. Vagal motoneurons are also inhibited by IL-1b, partly via local synthesis of prostaglandins81, suggesting that inflammation-associated GI disorders may result, at least in part, from dysregulation of both afferent and efferent VN. Finally, inflammation activates DMV neurons as part of the “inflammatory reflex”, which will be discussed in more detail below.
The inflammatory reflex
In 2000, the concept of the cholinergic anti-inflammatory pathway (CAIP) was introduced by Kevin Tracey’s group. In a model of sepsis, VN stimulation (VNS) increased survival by reducing TNF production in the liver, and especially the spleen82. This anti-inflammatory effect could be reproduced in vitro using isolated human macrophage cultures; the release of TNF, IL-1b, IL-6 and IL-18 in response to endotoxin was significantly reduced by acetylcholine and nicotine. While searching for a pharmacological target of VNS, Wang et al.83 identified the alpha7 subtype of the nicotinic acetylcholine receptor (α7nAChR) as the main receptor by which splenic macrophages are modulated. Based on these findings, the “cholinergic anti-inflammatory pathway” was introduced whereby the VN exerts its anti-inflammatory effect by direct modulation of macrophages and cytokine production, via α7nAChR, mainly in the spleen. By restraining the magnitude of a potentially fatal peripheral immune response, this mechanism is believed to provide an additional protective mechanism against the lethal effects of cytokines84. In comparison with the HPA axis or the local production of anti-inflammatory cytokines, this cholinergic control seems to have several properties favoring a central role in immune homeostasis. Considering the speed of neural conductance, it is capable of providing an instantaneous modulatory input to the inflamed region. Moreover, as the nervous system can adapt its output based on information obtained from different host regions, the modulatory influence of the CAIP is not only fast, but integrated with respect to the general host well-being. This reasoning eventually has led to the introduction of the “inflammatory reflex”84.
The Cholinergic Anti-Inflammatory Pathway – controversies and unresolved issues
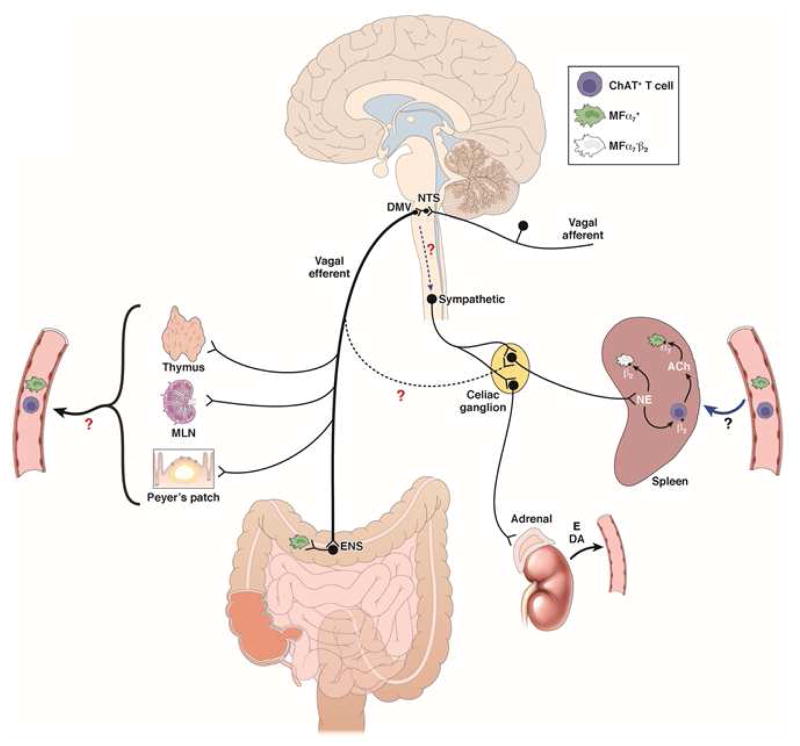
Although the anti-inflammatory effect of VNS is well accepted and demonstrated in a variety of disease models, the main problem accepting the concept as proposed initially is the absent, or very limited, vagal innervation of the spleen85. The adrenergic innervation of the spleen is more abundant, however, arising from prevertebral sympathetic ganglia, particularly from the celiac ganglion20,86. Vagal efferents have been proposed to activate these adrenergic neurons through interaction with α7nAChR86 affecting cytokine production in the spleen via sympathetic fibers running in the splenic nerve. These fibers are indeed in close proximity to ChAT expressing splenic CD4+ T cells, further characterized as CD44highCD62low memory T cells87. These T cells are rather rare in the spleen of naïve mice, however85. Nevertheless, VNS in nude mice (deficient in T cells) fails to reduce endotoxin-induced TNF production, while adoptive transfer of memory T cells restored the anti-inflammatory potential of VNS87. Based on these findings, the hypothesis was put forward that adrenergic, rather than cholinergic, nerve fibers activate acetylcholine producing memory T cells, via β-adrenoceptor activation, subsequently dampening splenic macrophages and cytokine production via interaction with α 7nAChRs (Figure 1).
Figure 1. Schematic representation of the cholinergic anti-inflammatory pathway.
VNS of the intact vagus nerve stimulates both afferent and efferent fibers. Electrical stimulation of afferent nerve fibers activates neurons in the NTS leading to activation of not only both ipsi- and contralateral efferent vagus nerves, but also of an adrenergic pathway resulting in release of norepinephrine (NE) in the spleen and the production of dopamine (DA) in the adrenal gland. In the spleen, NE reduces TNF production by splenic macrophages both directly, via actions on β2 adrenoceptor activation, and indirectly, via activation of CHAT+ T cells releasing ACh. Activation of presynaptic α7nAChR on adrenergic nerve fibers156 by choline or other α7nAChR agonists may increase NE release contributing to their anti-inflammatory properties. Stimulation of the efferent vagus nerve dampens α7nAChR+ resident muscular macrophages in the gastrointestinal tract via activation of cholinergic enteric neurons. The immune cell(s) modulated in the lamia propria, however, still need to be identified. The extent to which efferent vagus fibers exert an anti-inflammatory effect in the spleen by synapsing with adrenergic postganglionic (α7nAChR+) neurons in the celiac ganglion is a matter of debate since no anatomical or electrophysiological evidence supporting this connection is available. As the vagus nerve innervates the thymus, Peyers’ patches and other myeloid organs, one may hypothesize that cholinergic modulation of immune cells (α7nAChR+ macrophages, CHAT+, T cells) occurs in these organs. Under conditions of systemic inflammation, these cells subsequently migrate, or get trapped in the spleen, via the circulation.
Still, several important discrepancies and issues remain. First, using antegrade and/or retrograde tracing, no synaptic connection can be detected between vagal efferents and adrenergic prevertebral neurons innervating the spleen88,89. Second, although splenic denervation and reserpine-induced depletion of adrenergic nerves abolish the anti-inflammatory effect of VNS, splenic nerve activity cannot be detected in response to vagal efferent nerve stimulation89,90. Third, splenic nerve activation reduces splenic cytokine production in a α7nAChR independent manner86. This suggests that α7nAChR+ target cells may be located outside the spleen that migrate to, and are sequestered in, the spleen, especially in conditions of systemic inflammation. In view of the dense innervation of almost all lymphoid structures (lymph nodes, Peyer’s patches, thymus), neuromodulation may primarily occur in these structures, after which immune cells travel to the spleen. Clearly, more research is warranted to unravel the exact neuroanatomy of the vagal anti-inflammatory pathway to the spleen.
Another, thus far largely neglected, issue is the fact that electrical stimulation of the entire VN activates not only efferent, but also afferent, nerve fibers leading to stimulation of the contralateral vagus via a central pathway86,90. Indeed, activation of the central end of the cut VN seemingly has the same anti-inflammatory properties as stimulation of the intact or efferent VN86,90. Of note, activation of the intact (hence also afferent) VN recruits additional, distinct neural pathways. For example, stimulation of the intact, but not efferent, VN evokes action potentials in the renal sympathetic nerve90 and recruits an α7nACh-independent anti-inflammatory pathway86. Furthermore, electrical stimulation of the central end of the cut VN has a protective effect in a model of renal ischemia, even when the contralateral VN is blocked90 suggesting the recruitment of a vago-sympathetic reflex or activation of the hypothalamic-pituitary-adrenal axis. Finally, VNS reduces cytokine production in sepsis via vagal activation of adrenal dopamine production91. Clearly, many issues remain to be resolved with respect to the neural pathways activated by VNS, including whether, and where exactly, the vagal nerve endings interact with the immune system, at least with respect to the spleen (Figure 1).
The Cholinergic Anti-Inflammatory Pathway in the gut
As the spleen has been repeatedly shown to be the major source of cytokine production in conditions of systemic inflammation (like sepsis), it is certainly a critical site for neural control of distant organ inflammation. In conditions of more subtle or local inflammation, such as in postoperative ileus or mild colitis, however, the spleen is not itself the site of the inflammatory insult, and other anti-inflammatory pathways may be involved. Indeed, we demonstrated recently that VNS prevents inflammation of the muscular externa evoked by intestinal manipulation and improves postoperative ileus92, an effect that is independent of T cells and does not involve the spleen93. This inflammatory response was associated with activation of the NTS and DMV neurons innervating the inflamed area, compatible with the existence of a hardwired inflammatory reflex in the gut, independent of the spleen88. This observation suggests that the CAIP may comprise two levels of activation; the first involving local modulation of inflammation that is independent of the spleen, whereas vagal modulation of the splenic immune response is recruited when the inflammatory process becomes more generalized.
The GI tract receives the majority of the vagal efferent nerve fibers and harbors the most immune cells in the body. It is to be expected, therefore, that the GI tract may be an important site of vagal neuroimmune modulation. It is unlikely, however, that vagal efferents interact directly with immune cells; using an anterograde tracing technique, we confirmed that the VN synapses mainly with the myenteric and submucosal plexuses, but maintains no direct contact with the mucosal/submucosal or myenteric/muscular network of immune cells88,93. ChAT+ fibers originating from enteric neurons are, however, present abundantly in the submucosa and lamina propria along the axis of each individual villus. Notably, varicose fibers approach individual immune cells closely (less than 1uM)85 while resident macrophages in the muscularis externa and the myenteric plexus are in close proximity to cholinergic nerve fibers. These data indicate that vagal neuromodulation in the intestinal wall is indirect, and occurs via the enteric nervous system. At the level of Peyer’s patches, ChAT+ fibers are absent from follicle and dome areas, but are consistently present within the interfollicular area where high-endothelial vessels and many lymphocytes are contained85.
Before discussing the importance of the CAIP in the GI tract, it is fundamental to underscore that the immune cell population residing in the submucosal/mucosal compartment largely differs from that in the muscularis externa. The latter population is less well studied and consists mainly of resident macrophages located between the longitudinal and circular muscle layer at the level of the myenteric plexus. These resident macrophages have a rather tolerogenic phenotype94, play a role in diabetic-induced gastroparesis95, postoperative ileus96 and LPS-induced septic ileus97 and seem to represent the gatekeepers of the enteric nervous system or the “little brain of the gut”. Of note, they are in close contact with cholinergic nerve fibers and express α7nAChRs88,93. As with splenocytes, the α7nAChR agonists nicotine and choline dampen the activation of this macrophage population, indicating that these immune cells are sensitive to cholinergic modulation (see below).
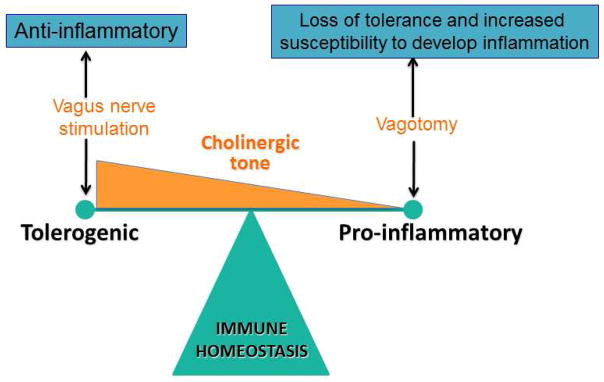
The mucosal immune system is however more complex, mainly as the constant challenge by the intestinal microbiota requires a perfectly balanced equilibrium between tolerance and defense against foreign antigens. One of the key mechanisms for discrimination of innocent and harmful antigens is oral tolerance, a mechanism orchestrated by the mucosal immune system and dependent on FoxP3+ regulatory T cells (Tregs). We showed recently that vagotomy reduced the ability to develop oral tolerance, a finding that was associated with a reduction in Tregs in the lamina propria and mesenteric lymph nodes98. Of note, vagotomized mice were more susceptible to developing colitis following exposure to the mucosal irritant, dextran sulfate sodium (DSS), suggesting that, in addition to TGF-β, retinoic acid and TSLP99, vagal input, or cholinergic tone, may be a new player in determining the degree of lamina propria tolerance (Figure 2). This may be relevant for several intestinal, as well as extra-intestinal, immune-mediated diseases; decreased tolerance to microbiota, for example, is proposed as a main pathogenic mechanism in inflammatory bowel disease (Crohn’s disease and ulcerative colitis). Food allergy is another well-known example of loss of tolerance, in which the immune system reacts to food antigens leading potentially to severe anaphylactic shock and even death100,101. In both cases, the immune system overreacts to innocent antigens leading to severe tissue damage, morbidity and mortality.
Figure 2.
The cholinergic tone determines immune homeostasis either shifting the balance towards tolerance (normal to enhanced tone) or inflammation (decreased tone).
The following paragraphs will discuss the current evidence supporting the relevance of the CAIP in the GI tract. For more information on the CAIP and its physiological importance, the reader is referred to several excellent reviews102–104.
The Cholinergic Anti-Inflammatory Pathway in intestinal inflammatory disorders Preclinical evidence
Colitis
Inflammatory bowel disease (IBD) is a debilitating and chronic inflammatory GI disease. Based on clinical presentation, endoscopic appearance, and histology, two major subtypes of the disease have been identified, i.e. Crohn’s disease with transmural inflammation versus ulcerative colitis which involves mostly superficial inflammation confined to the mucosa. Preclinical models provide convincing evidence that the cholinergic innervation of the gut has a major impact on the intestinal immune system. As indicated above, vagotomized animals fail to develop oral tolerance98 and develop more severe colitis with increased levels of NF-kB and cytokines in DSS and hapten-induced (dinitrobenzene sulfonic acid or DNBS) colitis98,105,106. Similarly, mice with depressive-like behavior following chronic reserpine treatment, which is associated with reduced intestinal levels of acetylcholine, developed more severe DSS and DNBS-induced colitis107. Of interest, treatment with the anti-depressant desimipramine reversed these effects in a vagus-dependent manner. In contrast, vagotomy does not affect T cell transfer-induced colitis98,108, indicating that T cells are less likely to be under direct vagal/cholinergic control. Macrophages or dendritic cells may be more likely to be involved; vagotomy had no effect on colitis in macrophage deficient mice105, while adoptive transfer of macrophages isolated from vagotomized mice or mice treated with reserpine to macrophage-deficient M-CSF-/- mice produced a modest, but significant, increase in severity of colitis109. These data may at least partly explain how psychological factors such as depressive mood associated with anxiety predict the onset in IBD patients109,110. Taken together, the above would suggest that the intestinal cholinergic tone, through vagal input, has a significant modulatory effect on the gut immune system, determining the balance between tolerance and inflammation, and affecting the susceptibility to develop colitis. (Figure 2)
Data on VNS in colitis models is rather limited and restricted to rats, principally because the electrodes for chronic VNS available need to be reduced in size to be suitable for implantation in mice. A single episode of VNS significantly improves oxazolone-induced111 and DSS-induced (unpublished data) colitis in mice, an effect that is independent of the spleen. In a rat model of TNBS colitis, chronic VNS slightly reduced disease activity index, histological scores, MPO activity, iNOS, TNF-α and IL-6, an effect mediated by disruption of the NF-κB pathway112,113. A more pronounced reduction in DSS and DNBS-induced colitis was noted using nicotine, an effect resistant to vagotomy106, and following activation of brainstem vagal neurocircuits using centrally acting drugs like the M1 muscarinic agonist McN-A-343, the M2 muscarinic antagonist methoctramine, or the acetylcholine esterase inhibitor galantamine114.The beneficial effect of central pharmacological activation of the VN was associated with decreased MHC class II expression and reduced cytokine production of splenic CD11c+ splenocytes, most likely resulting in decreased priming of CD4+CD25− T cells in the spleen. How these findings relate to the reduction in colonic inflammation, however, remains to be explored. As splenic denervation abolished the beneficial effect of central cholinergic activation, the authors proposed the involvement of a vagal-splenic nerve circuit114. Of note, however, splenic denervation increased colitis to a similar extent as vagotomy. Given that splenic macrophages are mainly modulated by adrenergic input115, with increased splenic macrophage activation and TNF-α production following adrenergic denervation of the spleen, splenic denervation will undoubtedly obscure the beneficial effect of central VN activation. It is impossible, therefore, to dissect out the extent to which central vagus activation affects splenic function via the splenic nerve. Moreover, as pointed out earlier, no anatomic or electrophysiological evidence is available to support such neurocircuitry. Direct modulation of the intestinal immune system, especially in view of the dense cholinergic innervation of the intestine, may be an alternative explanation for the beneficial effect of central acting compounds, with entrapment of intestine- or secondary lymphoid tissue-modulated immune cells in the spleen. Clearly, this hypothesis, and the exact role of the spleen in colitis, require further study. Nevertheless, the concept of central activation of the vagal anti-inflammatory pathway is very appealing and may be a promising new approach to treat IBD.
In models of sepsis, α7nAChRs have been repeatedly shown to mediate the beneficial effect of VNS4,83. Their role in colitis, however, is somewhat controversial. We showed recently the intact development of oral tolerance and lack of increased susceptibility for development of DSS-induced or T cell transfer colitis in α7nAChR−/− mice98, while DSS colitis was worsened by treatment with selective α7nAChRs agonists (AR-R17779, GSK1345038A)116. Conversely, others reported more severe DSS-induced colitis and increased IL-1b and IL-6 levels in colonic tissue of α7nAChR−/− mice110 while cytokine production of splenocytes and splenic CD11c+ cells isolated from colitis mice is reduced by the α7nAChR agonist GTS-21. Such controversies are not restricted to preclinical models since, in IBD patients, it remains to be determined why nicotine and smoking is associated with improvement of disease activity in ulcerative colitis but has an opposite effect in Crohn’s colitis. Differences in expression of α7nAChR, depending on the state of maturation and the inflammatory microenvironment, may partly explain these seemingly contradictory observations. For example, oxazolone-induced colitis was associated with an IL-4 mediated upregulation of α7nAChR on colonic CD4 T cells which resulted in a beneficial response to nicotine. Conversely, α7nAChR expression was down-regulated in an IL-12 dependent manner in TNBS-induced colitis and was associated with a worsening of colitis by nicotine117. To what extent this also applies to IBD patients remains however to be confirmed.
Ischemia –reperfusion intestinal damage
Ischemia due to severe blood loss, cardiac arrest or arterial occlusion results in severe cell damage and organ injury. Tissue damage, in addition to endotoxemia resulting from disruption of the intestinal barrier due to ischemia, is a potent trigger for the innate immune system resulting in a systemic inflammatory response. Indeed, hemorrhagic shock increases intestinal mucosal permeability with bacterial translocation and detectable endotoxin levels as well as systemic release of pro-inflammatory cytokines such as TNF-α and IL-6 . Of note, high-fat enteral nutrition prevents these changes, an effect mediated via cholecystokinin-induced activation of vagal afferents triggering the CAIP119. In addition to its known effect on macrophages, a recent study showed vagal-mediated expansion of enteric neural stem cells with an increase in enteric glia and recovery of barrier function120. In agreement with these studies, VNS protected against burn-induced intestinal injury and restored barrier function through activation of enteric glia cells, independently from the spleen121. Of note, and similar to colitis, administration of nicotinic agonists and central activation of the vagal CAIP by ghrelin and melanocortins reduces the inflammatory response and restores organ function122,123.
Postoperative and endotoxin-induced ileus
Every abdominal surgical intervention leads to impaired motility of the entire GI tract lasting several days with symptoms like nausea, vomiting, intolerance to food and absence of defecation, referred to as postoperative ileus (POI). Although some would argue that this represents a physiological response to the surgical insult and should not be regarded as a clinical problem, this iatrogenic condition is a major source of patient morbidity and a significant economic burden to health care96,124. For more than a decade, a subtle microscopic inflammation of the intestinal muscularis, triggered by activation of resident macrophages residing between the longitudinal and circular muscle layer of the intestinal wall, has been identified as key process in the pathophysiology of POI and endotoxin-induced ileus. Activation of these phagocytes results in cytokine and chemokine release, followed by influx of mainly leukocytes and monocytes starting approximately 3–4 hours after surgery. As this inflammatory response has a major impact on neuromuscular function, treatments or interventions hampering this process may be instrumental in reducing POI96.
Several lines of preclinical evidence indicate that activation of the CAIP may be an efficient strategy to prevent POI. Stimulation of the vagus, either electrically92, via enteral feeding125 or central application of semapimod116, all reduced the influx of immune cells into the muscularis, dampened cytokine production, and improved GI transit. The effect of VNS is mediated by vagal activation of cholinergic myenteric neurons, resulting in reduced activation of resident muscular macrophages. The latter express α7nAChR, explaining the lack of a beneficial effect of VNS in α7nAChR−/− mice93. Of note, pharmacological stimulation of these neurons with the 5-hydroxytryptamine 4 receptor agonists mosapride or prucalopride mimics the effect of VNS126,127. Finally, the selective α7nAChR agonist, AR-R17779, improved intestinal transit and reduced intestinal inflammation116. Of note, and in contrast to the neurocircuitry proposed in sepsis models, vagal modulation of intestinal resident macrophages is independent of T cells and results from a direct input to the gut; selective denervation of the spleen leaves the modulatory effect of the VN untouched, arguing against the spleen as site of neuromodulation, at least in this model of subtle intestinal inflammation93.
Human evidence
Accepting that the VN has a major impact on the immune system, one would anticipate increased incidence of intestinal inflammation in patients who undergo vagotomy. To date, however, solid data supporting this assumption are not available. One study reports increased ulcer disease, septicemia and mortality in patients that underwent vagotomy following traumatic injury128 although this increased incidence of inflammatory-mediated adverse outcomes may be a consequence of an increased need for gastrectomy and vagotomy in the more severe cases128. One possible explanation for the lack of increased intestinal inflammation may be that cholinergic tone is restored by compensatory increase within the enteric nervous system. In mice, the increased susceptibility to DSS colitis following vagotomy indeed normalizes after several weeks129. To what extent similar compensatory mechanisms are activated in other regions of the body remains to be studied.
There is however increasing indirect evidence, based on heart rate variability monitoring, to support an immune-modulatory role of the CAIP in humans (reviewed in 130,131). In healthy subjects, reduced heart rate variability indices (= low vagal tone) are independently associated with increased serum CRP and IL-6 levels and increased TNF and IL-6 production in endotoxin-stimulated whole blood. Also, in immune-mediated diseases like rheumatoid arthritis, VN activity was reduced compared to healthy controls and associated with increased levels of serum HMGB1, while parasympathetic tone is inversely related to inflammatory markers (IL-6 and CRP) in patients with cardiovascular disease. Finally, increased morbidity and mortality following cardiac surgery, myocardial infarction, sepsis, RA, IBD, lupus erythematosus and sarcoidosis has been reported to be associated with decreased VN activity. These data suggest that reduced vagal tone increases the setpoint of the immune response with a subtle rise in pro-inflammatory cytokines and increased disease risk.
The final evidence that activation of the vagal anti-inflammatory pathway is, indeed, a breakthrough for the clinical management of immune-mediated inflammatory diseases will ultimately have to come from clinical trials evaluating specific nicotinic agonists or electrical, pharmacological or nutritional activation of the CAIP. The effect of nicotine (rectal enemas or transdermal application) has been extensively evaluated in patients with ulcerative colitis albeit with controversial results132. Side effects due to interaction with several nAChR subtypes and toxicity issues most likely prevent effective dosing with nicotine. Activation of the CAIP by early enteral nutrition improved clinical recovery in patients undergoing major rectal surgery and was associated with less anastomic leakage133. In contrast, despite being supposed to activate the VN, gum chewing fails to improve postoperative outcome after colorectal surgery134. Finally, electrical stimulation of the VN, either in the neck, the abdominal cavity, or the ear, may be considered as treatment for chronic inflammatory diseases such as IBD and rheumatoid arthritis (RA), and will be discussed in more detail in the following paragraph.
Treatment options – VNS in humans
VNS is currently routinely and safely used in patients with intractable epilepsy, depression and migraine135. To this end, a coiled electrode is surgically positioned around the cervical VN and connected to a pacemaker. Transcutaneous auricular VNS, contrast, stimulates the auricular branch of the VN using a dedicated intra-auricular electrode. Finally, the cervical VN can be stimulated transcutaneously by low voltage electrical signals delivered by a handheld device (reviewed in136).
VNS refers, commonly, to electrical stimulation of the left cervical vagus, the right vagus being thought to be more closely associated with cardiovascular functions. While not effective acutely, long-term (>9–12 months) VNS has been shown to have clinically relevant outcomes, in both unipolar and bipolar depression, in patients that fail to respond to multiple antidepressant drugs137. The mechanism of VNS action to modulate mood is not understood fully, but since the NTS (the principle termination site of vagal afferents) projects to multiple brainstem, midbrain and forebrain nuclei, VNS has the potential to modulate several CNS-dependent functions. Both animal and human studies have suggested that VNS alters neurotransmitter levels centrally; increased serotonin levels138, increased locus ceruleus norepinephrine signaling139, altered NTS GABA and glutamate signaling140, and enhanced cortical inhibition141 have all been proposed to be responsible for the efficacy of VNS in treating epilepsy.
The anti-inflammatory effects of VNS were evaluated initially in epilepsy patients; VNS reduced IL-6 and increased IL-10 serum levels142,143 and diminished IL-8, TNF, IL-1B and IL-6 production by isolated peripheral blood mononuclear cells144,145. Recently, a small open pilot study in patients with RA provided the first evidence in humans that VNS, indeed, has the potential to improve immune-mediated diseases145. Cervical VNS improved RA disease activity, reduced TNF production and dampened cytokine production of peripheral blood. Similarly, 5 out of 7 patients with Crohn’s disease improved following cervical VNS and remained in remission during the 6 months follow-up146. Finally, abdominal VNS during abdominal surgery reduced IL-6 and IL-8 production of LPS-stimulated peripheral blood147. Randomized and sham-controlled trials are ongoing in Crohn’s disease (NCT02311660) and postoperative ileus (NCT02524626, NCT02425774) hopefully further confirming the therapeutic potential of VNS.
The use of VNS in the treatment of obesity has also gained some initial attention148, despite evidence of only a rather modest weight loss; studies in obese minipig models have shown that long-term bilateral VNS prevents further weight gain and decreases food consumption149 rather than reversing obesity per se. Again, the mechanism responsible for these effects is unclear, although activation of the olfactory bulb and its projection pathways, as well as activation of the hippocampus and pallium (involved in assigning hedonic value to ingestive signals) have been reported149. Shorter periods of VNS have been shown to activate the mesolimbic dopaminergic system suggesting some of the effects on food intake and weight gain may be related to activation of central reward pathways150.
In contrast to the modest effects of VNS to reduce or reverse obesity, vagal nerve blockade (vBloc), designed as an alternative to standard bariatric surgery, has proven much more efficient in inducing significant weight loss, early and more prolonged satiation and improved glycemic regulation151,152. While this appears initially to directly contradict the actions of VNS to induce weight loss, it is perhaps not that surprising since it has been known for some time that truncal vagotomy improves obesity, including exogenous (hypothalamic) obesity in rodents83 and humans153,154. Vbloc uses a cuff electrode that encircles the VN and employs kilohertz frequency alternating current, with zero net charge delivery, to produce a very localized, true nerve conduction (rather than a neurotransmitter) block, that is rapidly reversible and does not induce any apparent nerve degeneration or damage (reviewed in155). Biophysical modeling studies suggest that a depolarization induced inactivation of sodium channels is responsible for the nerve block in mammalian nerves155 although the contribution of afferent versus efferent VN block still remains to be elucidated.
Conclusion
While a few decades ago, cutting the vagus nerve represented a well accepted treatment for peptic ulcer disease, it is now clear that this nerve is extremely precious not only for the homeostasis of a variety of organ systems, but also for the regulation of appetite, mood and inflammation. The therapeutic potential of stimulating or blocking the VN, either electrically or pharmacologically, is being explored gradually, and the exact pathways and mechanisms of action are becoming understood. Non-invasive devices to stimulate the VN have been developed, although the optimal stimulation parameters and the type of nerve fibers to be stimulated remain to be determined. Clinical studies are ongoing and will hopefully soon confirm that the VN should be handled with great care rather than being cut.
Acknowledgments
Funding: GEB is supported by a European Research Council (ERC) Advanced Grant (ERC-2013-Adg): 340101 Cholstim. SV is supported by a FWO postdoctoral research fellowship. KNB is supported by a National Institutes of Health grant NIH NIDDK 078364 and National Science Foundation grant NSF IOS1148978.
Footnotes
Financial arrangements – conflicts of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference list
- 1.Browning KN, Travagli RA. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr Physiol. 2014;4:1339–1368. doi: 10.1002/cphy.c130055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschuler SM, Escardo J, Lynn RB, Miselis RR. The Central Organization of the vagus nerve innervating the colon of the rat. Gastroenterol. 1993;104:502–509. doi: 10.1016/0016-5085(93)90419-d. [DOI] [PubMed] [Google Scholar]
- 3.Berthoud HR, Carlson NR, Powley TL. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol. 1991;260:R200–R207. doi: 10.1152/ajpregu.1991.260.1.R200. [DOI] [PubMed] [Google Scholar]
- 4.Beyak M, Grundy D. In: Advances in Vagal Afferent Neurobiology. Undem BJ, Weinreich D, editors. CRC Press; 2005. pp. 315–350. [Google Scholar]
- 5.Brookes SJ, Spencer NJ, Costa M, Zagorodnyuk VP. Extrinsic primary afferent signalling in the gut. Nat Rev Gastroenterol Hepatol. 2013;10:286–296. doi: 10.1038/nrgastro.2013.29. [DOI] [PubMed] [Google Scholar]
- 6.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 7.Andresen MC, Yang M. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol. 1990;259:H1307–H1311. doi: 10.1152/ajpheart.1990.259.4.H1307. [DOI] [PubMed] [Google Scholar]
- 8.Rinaman L, Card JP, Schwaber JS, Miselis RR. Ultrastructural Demonstration of a Gastric Monsynaptic Vagal Cirucit in the Nucleus of the Solitary Tract in Rat. J Neurosci. 1989;9:1985–1996. doi: 10.1523/JNEUROSCI.09-06-01985.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leslie RA, Gwyn DG. Neuronal connections of the area postrema. Fed Proc. 1984;43:2941–2943. [PubMed] [Google Scholar]
- 10.Cottrell GT, Ferguson AV. Sensory circumventricular organs: central roles in integrated autonomic regulation. Regul Pept. 2004;117:11–23. doi: 10.1016/j.regpep.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Orts-Del’immagine A, et al. Properties of subependymal cerebrospinal fluid contacting neurones in the dorsal vagal complex of the mouse brainstem. J Physiol. 2012;590:3719–3741. doi: 10.1113/jphysiol.2012.227959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altschuler SM, Bao X, Bieger D, Hopkins DA, Miselis RR. Viscerotopic Representation of the Upper Alimentary Tract in the Rat: Sensory Ganglia and Nuclei of the Solitary and Spinal Trigeminal Tracts. J Comp Neurol. 1989;283:248–268. doi: 10.1002/cne.902830207. [DOI] [PubMed] [Google Scholar]
- 13.Sivarao DV, Krowicki ZK, Hornby PJ. Role of GABAA receptors in rat hindbrain nuclei controlling gastric motor function. Neurogastroenterol Motil. 1998;10:305–313. doi: 10.1046/j.1365-2982.1998.00110.x. [DOI] [PubMed] [Google Scholar]
- 14.Travagli RA, Gillis RA, Rossiter CD, Vicini S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol. 1991;260:G531–G536. doi: 10.1152/ajpgi.1991.260.3.G531. [DOI] [PubMed] [Google Scholar]
- 15.Davis SF, Derbenev AV, Williams KW, Glatzer NR, Smith BN. Excitatory and inhibitory local circuit input to the rat dorsal motor nucleus of the vagus originating from the nucleus tractus solitarius. Brain Res. 2004;1017:208–217. doi: 10.1016/j.brainres.2004.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shapiro RE, Miselis RR. The Central Organization of the Vagus Nerve Innervating the Stomach of the Rat. J Comp Neurol. 1985;238:473–488. doi: 10.1002/cne.902380411. [DOI] [PubMed] [Google Scholar]
- 17.Fox EA, Powley TL. Longitudinal columnar organization within the dorsal motor nucleus represents separate branches of the abdominal vagus. Brain Res. 1985;341:269–282. doi: 10.1016/0006-8993(85)91066-2. [DOI] [PubMed] [Google Scholar]
- 18.Huang XF, Tork I, Paxinos G. Dorsal motor nucleus of the vagus nerve: a cyto- and chemoarchitectonic study in the human. J Comp Neurol. 1993;330:158–182. doi: 10.1002/cne.903300203. [DOI] [PubMed] [Google Scholar]
- 19.NRB, Standring S. Gray’s Anatomy. The anatomical basis of clinical practice. Elsevier; 2008. [Google Scholar]
- 20.Berthoud HR, Powley TL. Characterization of vagal innervation to the rat celiac, suprarenal and mesenteric ganglia. J Auton Nerv Syst. 1993;42:153–69. doi: 10.1016/0165-1838(93)90046-w. [DOI] [PubMed] [Google Scholar]
- 21.Cano G, Sved AF, Rinaman L, Rabin BS, Card JP. Characterization of the central nervous system innervation of the rat spleen using viral transneuronal tracing. J Comp Neurol. 2001;439:1–18. doi: 10.1002/cne.1331. [DOI] [PubMed] [Google Scholar]
- 22.Berthoud HR. The vagus nerve, food intake and obesity. Regul Pept. 2008;149:15–25. doi: 10.1016/j.regpep.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 24.Powley TL, Spaulding RA, Haglof SA. Vagal afferent innervation of the proximal gastrointestinal tract mucosa: chemoreceptor and mechanoreceptor architecture. J Comp Neurol. 2011;519:644–660. doi: 10.1002/cne.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinert RE, Beglinger C. Nutrient sensing in the gut: interactions between chemosensory cells, visceral afferents and the secretion of satiation peptides. Physiol Behav. 2011;105:62–70. doi: 10.1016/j.physbeh.2011.02.039. [DOI] [PubMed] [Google Scholar]
- 26.Raybould HE. Mechanisms of CCK signaling from gut to brain. Curr Opin Pharmacol. 2007;7:570–574. doi: 10.1016/j.coph.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owyang C. Physiological mechanisms of cholecystokinin action on pancreatic secretion. Am J Physiol. 1996;271:G1–G7. doi: 10.1152/ajpgi.1996.271.1.G1. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi T, Owyang C. Mechanism of cholecystokinin-induced relaxation of the rat stomach. J Auton Nerv Syst. 1999;75:123–130. doi: 10.1016/s0165-1838(98)00181-7. [DOI] [PubMed] [Google Scholar]
- 29.Zhu JX, Wu XY, Owyang C, Li Y. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J Physiol. 2001;530:431–442. doi: 10.1111/j.1469-7793.2001.0431k.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Date Y. Ghrelin and the vagus nerve. Methods Enzym. 2012;514:261–269. doi: 10.1016/B978-0-12-381272-8.00016-7. [DOI] [PubMed] [Google Scholar]
- 31.Date Y, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 32.Grabauskas G, et al. KATP channels in the nodose ganglia mediate the orexigenic actions of ghrelin. J Physiol. 2015;593:3973–3989. doi: 10.1113/JP270788. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Grabauskas G, Song I, Zhou SY, Owyang C. Electrophysiological identifications of glucose-sensing neurons in the rat nodose ganglia. J Physiol. 2010;588:617–632. doi: 10.1113/jphysiol.2009.182147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niijima A. Glucose-sensitive afferent nerve fibres in the hepatic branch of the vagus nerve in the guinea-pig. J Physiol. 1982;332:315–323. doi: 10.1113/jphysiol.1982.sp014415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niijima A, Meguid MM. An electrophysiological study on amino acid sensors in the hepato-portal system in the rat. Obes Res. 1995;3(Suppl 5):741S–745S. doi: 10.1002/j.1550-8528.1995.tb00494.x. [DOI] [PubMed] [Google Scholar]
- 36.Houghton LA, Atkinson W, Whitaker RP, Whorwell PJ, Rimmer MJ. Increased platelet depleted plasma 5-hydroxytryptamine concentration following meal ingestion in symptomatic female subjects with diarrhoea predominant irritable bowel syndrome. Gut. 2003;52:663–670. doi: 10.1136/gut.52.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest. 1985;75:1144–1152. doi: 10.1172/JCI111809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.ten Tusscher MP, Klooster J, Vrensen GF. Satellite cells as blood-ganglion cell barrier in autonomic ganglia. Brain Res. 1989;490:95–102. doi: 10.1016/0006-8993(89)90434-4. [DOI] [PubMed] [Google Scholar]
- 39.Lacolley P, et al. Occipital artery injections of 5-HT may directly activate the cell bodies of vagal and glossopharyngeal afferent cell bodies in the rat. Neuroscience. 2006;143:289–308. doi: 10.1016/j.neuroscience.2006.08.047. [DOI] [PubMed] [Google Scholar]
- 40.Zhuo H, Ichikawa H, Helke CJ. Neurochemistry of the nodose ganglion. Prog Neurobiol. 1997;52:79–107. doi: 10.1016/s0301-0082(97)00003-8. [DOI] [PubMed] [Google Scholar]
- 41.Gaisano GG, Park SJ, Daly DM, Beyak MJ. Glucagon-like peptide-1 inhibits voltage-gated potassium currents in mouse nodose ganglion neurons. Neurogastroenterol Motil. 2010;22:470–9. e111. doi: 10.1111/j.1365-2982.2009.01430.x. [DOI] [PubMed] [Google Scholar]
- 42.Troy AE, Browning KN. High fat diet decreases glucose-dependent modulation of 5-HT responses in gastrointestinal vagal afferent neurons. J Physiol. 2016;594:99–114. doi: 10.1113/JP271558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Browning KN, Travagli RA. Characterisation of the in vitro effects of 5-Hydroxytryptamine (5-HT) on identified neurones of the rat dorsal motor nucleus of the vagus (DMV) Br J Pharmacol. 1999;128:1307–1315. doi: 10.1038/sj.bjp.0702908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baptista V, Browning KN, Travagli Ra. Effects of cholecystokinin-8s in the nucleus tractus solitarius of vagally deafferented rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1092–100. doi: 10.1152/ajpregu.00517.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Browning KN, Wan S, Baptista V, Travagli RA. Vanilloid, purinergic, and CCK receptors activate glutamate release on single neurons of the nucleus tractus solitarius centralis. Am J Physiol Regul Integr Comp Physiol. 2011;301:R394–R401. doi: 10.1152/ajpregu.00054.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Viard E, Zheng Z, Wan S, Travagli RA. Vagally mediated, nonparacrine effects of cholecystokinin-8s on rat pancreatic exocrine secretion. Am J Physiol Gastrointest Liver Physiol. 2007;293:G493–G500. doi: 10.1152/ajpgi.00118.2007. [DOI] [PubMed] [Google Scholar]
- 47.Covasa M, Grahn J, Ritter RC. High fat maintenance diet attenuates hindbrain neuronal response to CCK. Regul Pept. 2000;86:83–88. doi: 10.1016/s0167-0115(99)00084-1. [DOI] [PubMed] [Google Scholar]
- 48.Daly DM, Park SJ, Valinsky WC, Beyak MJ. Impaired intestinal afferent nerve satiety signalling and vagal afferent excitability in diet induced obesity in the mouse. J Physiol. 2011;589:2857–2870. doi: 10.1113/jphysiol.2010.204594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Little TJ, Horowitz M, Feinle-Bisset C. Modulation by high-fat diets of gastrointestinal function and hormones associated with the regulation of energy intake: implications for the pathophysiology of obesity. Am J Clin Nutr. 2007;86:531–541. doi: 10.1093/ajcn/86.3.531. [DOI] [PubMed] [Google Scholar]
- 50.de LG, Barbier de la S, Espero E, Lee J, Raybould HE. Diet-induced obesity leads to the development of leptin resistance in vagal afferent neurons. Am J Physiol Endocrinol Metab. 2011;301:E187–E195. doi: 10.1152/ajpendo.00056.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kentish SJ, et al. Gastric vagal afferent modulation by leptin is influenced by food intake status. J Physiol. 2013;591:1921–34. doi: 10.1113/jphysiol.2012.247577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dockray GJ. Gastrointestinal hormones and the dialogue between gut and brain. J Physiol. 2014;592:2927–2941. doi: 10.1113/jphysiol.2014.270850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bielefeldt K, Ozaki N, Gebhart GF. Experimental ulcers alter voltage-sensitive sodium currents in rat gastric sensory neurons. Gastroenterology. 2002;122:394–405. doi: 10.1053/gast.2002.31026. [DOI] [PubMed] [Google Scholar]
- 54.Kang YM, Bielefeldt K, Gebhart GF. Sensitization of mechanosensitive gastric vagal afferent fibers in the rat by thermal and chemical stimuli and gastric ulcers. J Neurophysiol. 2004;91:1981–1989. doi: 10.1152/jn.01097.2003. [DOI] [PubMed] [Google Scholar]
- 55.Kentish S, et al. Diet-induced adaptation of vagal afferent function. J Physiol. 2012;590:209– 221. doi: 10.1113/jphysiol.2011.222158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Naznin F, et al. Diet-induced obesity causes peripheral and central ghrelin resistance by promoting inflammation. J Endocrinol. 2015;226:81–92. doi: 10.1530/JOE-15-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Browning KN, Fortna SR, Hajnal A. Roux-en-Y gastric bypass reverses the effects of diet-induced obesity to inhibit the responsiveness of central vagal motoneurones. J Physiol. 2013;591:2357–2372. doi: 10.1113/jphysiol.2012.249268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sato M, Nakahara K, Miyazato M, Kangawa K, Murakami N. Regulation of GH secretagogue receptor gene expression in the rat nodose ganglion4591. J Endocrinol. 2007;194:41–46. doi: 10.1677/JOE-06-0078. [DOI] [PubMed] [Google Scholar]
- 59.Kentish SJ, Frisby CL, Kennaway DJ, Wittert Ga, Page AJ. Circadian variation in gastric vagal afferent mechanosensitivity. J Neurosci. 2013;33:19238–42. doi: 10.1523/JNEUROSCI.3846-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kentish SJ, Vincent AD, Kennaway DJ, Wittert GA, Page AJ. High-Fat Diet-Induced Obesity Ablates Gastric Vagal Afferent Circadian Rhythms. J Neurosci. 2016;36:3199–3207. doi: 10.1523/JNEUROSCI.2710-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Waise TM, et al. One-day high-fat diet induces inflammation in the nodose ganglion and hypothalamus of mice4592. Biochem Biophys Res Commun. 2015;464:1157–1162. doi: 10.1016/j.bbrc.2015.07.097. [DOI] [PubMed] [Google Scholar]
- 62.Bravo JA, et al. Communication between gastrointestinal bacteria and the nervous system. Curr Opin Pharmacol. 2012;12:667–72. doi: 10.1016/j.coph.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 63.Bercik P, Collins SM, Verdu EF. Microbes and the gut-brain axis. Neurogastroenterol Motil. 2012;24:405–13. doi: 10.1111/j.1365-2982.2012.01906.x. [DOI] [PubMed] [Google Scholar]
- 64.Borre YE, Moloney RD, Clarke G, Dinan TG, Cryan JF. The impact of microbiota on brain and behavior: mechanisms & therapeutic potential. Adv Exp Med Biol. 2014;817:373–403. doi: 10.1007/978-1-4939-0897-4_17. [DOI] [PubMed] [Google Scholar]
- 65.Bravo JA, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050–5. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bercik P, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. 2011;23:1132–9. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dinan TG, Cryan JF. Melancholic microbes: a link between gut microbiota and depression? Neurogastroenterol Motil. 2013;25:713–719. doi: 10.1111/nmo.12198. [DOI] [PubMed] [Google Scholar]
- 68.Hansen MK, O’Connor Ka, Goehler LE, Watkins LR, Maier SF. The contribution of the vagus nerve in interleukin-1beta-induced fever is dependent on dose. Am J Physiol Regul Integr Comp Physiol. 2001;280:R929–34. doi: 10.1152/ajpregu.2001.280.4.R929. [DOI] [PubMed] [Google Scholar]
- 69.Marquette C, et al. IL-1beta, TNFalpha and IL-6 induction in the rat brain after partial- body irradiation: role of vagal afferents. Int J Radiat Biol. 2003;79:777–85. doi: 10.1080/09553000310001610998. [DOI] [PubMed] [Google Scholar]
- 70.Goehler LE, et al. Activation in vagal afferents and central autonomic pathways: Early responses to intestinal infection with Campylobacter jejuni. Brain Behav Immun. 2005;19:334–344. doi: 10.1016/j.bbi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 71.Cailotto C, et al. Neuroanatomical evidence demonstrating the existence of the vagal anti-inflammatory reflex in the intestine. Neurogastroenterol Motil. 2012;24 doi: 10.1111/j.1365-2982.2011.01824.x. [DOI] [PubMed] [Google Scholar]
- 72.Karagiannides I, Pothoulakis C. Obesity, innate immunity and gut inflammation. Curr Opin Gastroenterol. 2007;23:661–666. doi: 10.1097/MOG.0b013e3282c8c8d3. [DOI] [PubMed] [Google Scholar]
- 73.de La Serre CB, de LG, Raybould HE. Chronic exposure to low dose bacterial lipopolysaccharide inhibits leptin signaling in vagal afferent neurons. Physiol Behav. 2015;139:188–194. doi: 10.1016/j.physbeh.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goehler LE, et al. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton Neurosci. 2000;85:49–59. doi: 10.1016/S1566-0702(00)00219-8. [DOI] [PubMed] [Google Scholar]
- 75.Schwartz ES, et al. Synergistic role of TRPV1 and TRPA1 in pancreatic pain and inflammation4599. Gastroenterology. 2011;140:1283–1291. doi: 10.1053/j.gastro.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hanani M. Role of satellite glial cells in gastrointestinal pain. Front Cell Neurosci. 2015;9:412. doi: 10.3389/fncel.2015.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu CY, Mueller MH, Grundy D, Kreis ME. Vagal modulation of intestinal afferent sensitivity to systemic LPS in the rat. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1213–G1220. doi: 10.1152/ajpgi.00267.2006. [DOI] [PubMed] [Google Scholar]
- 78.Rogers RC, Van Meter MJ, Hermann GE. Tumor necrosis factor potentiates central vagal afferent signaling by modulating ryanodine channels. J Neurosci. 2006;26:12642–12646. doi: 10.1523/JNEUROSCI.3530-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang H, et al. Functional protease-activated receptors in the dorsal motor nucleus of the vagus3544. Neurogastroenterol Motil. 2009 doi: 10.1111/j.1365-2982.2009.01391.x. at < http://www.ncbi.nlm.nih.gov/pubmed/19719510>. [DOI] [PMC free article] [PubMed]
- 80.Fritze D, Zhang W, Li JY, Chai B, Mulholland MW. TNFalpha causes thrombin-dependent vagal neuron apoptosis in inflammatory bowel disease4601. J Gastrointest Surg. 2014;18:1632–1641. doi: 10.1007/s11605-014-2573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mo ZL, Katafuchi T, Hori T. Effects of IL-1 beta on neuronal activities in the dorsal motor nucleus of the vagus in rat brain slices4602. Brain Res Bull. 1996;41:249–255. doi: 10.1016/s0361-9230(96)00196-7. [DOI] [PubMed] [Google Scholar]
- 82.Borovikova LV, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 83.Wang H, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–8. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 84.Tracey KJ. The inflammatory reflex. Nature. 420:853–9. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 85.Gautron L, et al. Neuronal and nonneuronal cholinergic structures in the mouse gastrointestinal tract and spleen. J Comp Neurol. 2013;521:3741–67. doi: 10.1002/cne.23376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vida G, Peña G, Deitch EA, Ulloa L. α7-cholinergic receptor mediates vagal induction of splenic norepinephrine. J Immunol. 2011;186:4340–6. doi: 10.4049/jimmunol.1003722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rosas-Ballina M, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cailotto C, et al. Neuro-anatomical evidence indicating indirect modulation of macrophages by vagal efferents in the intestine but not in the spleen. PLoS One. 2014;9:e87785. doi: 10.1371/journal.pone.0087785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bratton BO, et al. Neural regulation of inflammation: no neural connection from the vagus to splenic sympathetic neurons. Exp Physiol. 2012;97:1180–5. doi: 10.1113/expphysiol.2011.061531. [DOI] [PubMed] [Google Scholar]
- 90.Inoue T, et al. Vagus nerve stimulation mediates protection from kidney ischemia-reperfusion injury through α7nAChR+ splenocytes. J Clin Invest. 2016;126:1939–52. doi: 10.1172/JCI83658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Torres-Rosas R, et al. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med. 2014;20:291–5. doi: 10.1038/nm.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Jonge WJ, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–51. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 93.Matteoli G, et al. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut. 2013:1–11. doi: 10.1136/gutjnl-2013-304676. [DOI] [PubMed]
- 94.Gabanyi I, et al. Neuro-immune Interactions Drive Tissue Programming in Intestinal Macrophages. Cell. 2016;164:378–391. doi: 10.1016/j.cell.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Choi KM, et al. CD206-Positive M2 Macrophages That Express Heme Oxygenase-1 Protect Against Diabetic Gastroparesis in Mice. Gastroenterology. 2010;138 doi: 10.1053/j.gastro.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boeckxstaens GE, de Jonge WJ. Neuroimmune mechanisms in postoperative ileus. Gut. 2009;58:1300–11. doi: 10.1136/gut.2008.169250. [DOI] [PubMed] [Google Scholar]
- 97.de Winter BY, et al. Role of oxidative stress in the pathogenesis of septic ileus in mice. Neurogastroenterol Motil. 2005;17:251–61. doi: 10.1111/j.1365-2982.2004.00618.x. [DOI] [PubMed] [Google Scholar]
- 98.Di Giovangiulio M, et al. Vagotomy affects the development of oral tolerance and increases susceptibility to develop colitis independently of the alpha-7 nicotinic receptor. Mol Med. 2016;22 doi: 10.2119/molmed.2016.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rescigno M. Intestinal Dendritic Cells. Adv Immunol. 2010;107:109–138. doi: 10.1016/B978-0-12-381300-8.00004-6. [DOI] [PubMed] [Google Scholar]
- 100.Bischoff SC. Food allergy and eosinophilic gastroenteritis and colitis. Curr Opin Allergy Clin Immunol. 2010;10:238–45. doi: 10.1097/ACI.0b013e32833982c3. [DOI] [PubMed] [Google Scholar]
- 101.Bischoff SC. Food allergies. Curr Treat Options Gastroenterol. 2007;10:34–43. doi: 10.1007/s11938-007-0055-6. [DOI] [PubMed] [Google Scholar]
- 102.Pavlov VA, Tracey KJ. Neural circuitry and immunity. Immunol Res. 2015;63:38–57. doi: 10.1007/s12026-015-8718-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Olofsson PS, Rosas-Ballina M, Levine YA, Tracey KJ. Rethinking inflammation: neural circuits in the regulation of immunity. Immunol Rev. 2012;248:188–204. doi: 10.1111/j.1600-065X.2012.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Andersson U, Tracey KJ. Neural reflexes in inflammation and immunity. J Exp Med. 2012;209:1057–68. doi: 10.1084/jem.20120571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF, Collins SM. The Vagus Nerve: A Tonic Inhibitory Influence Associated With Inflammatory Bowel Disease in a Murine Model. Gastroenterology. 2006;131:1122–1130. doi: 10.1053/j.gastro.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 106.O’Mahony C, van der Kleij H, Bienenstock J, Shanahan F, O’Mahony L. Loss of vagal anti-inflammatory effect: in vivo visualization and adoptive transfer. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1118–26. doi: 10.1152/ajpregu.90904.2008. [DOI] [PubMed] [Google Scholar]
- 107.Ghia JE, Blennerhassett P, Collins SM. Impaired parasympathetic function increases susceptibility to inflammatory bowel disease in a mouse model of depression. J Clin Invest. 2008;118:2209–18. doi: 10.1172/JCI32849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van der Kleij H, O’Mahony C, Shanahan F, O’Mahony L, Bienenstock J. Protective effects of Lactobacillus rhamnosus [corrected] and Bifidobacterium infantis in murine models for colitis do not involve the vagus nerve. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1131–7. doi: 10.1152/ajpregu.90434.2008. [DOI] [PubMed] [Google Scholar]
- 109.Ghia JE, Park AJ, Blennerhassett P, Khan WI, Collins SM. Adoptive transfer of macrophage from mice with depression-like behavior enhances susceptibility to colitis. Inflamm Bowel Dis. 2011;17:1474–89. doi: 10.1002/ibd.21531. [DOI] [PubMed] [Google Scholar]
- 110.Ghia J-E, et al. Reactivation of inflammatory bowel disease in a mouse model of depression. Gastroenterology. 2009;136:2280–2288. e1–4. doi: 10.1053/j.gastro.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 111.Meroni E, et al. Su1564 Improvement of Oxazolone-Induced Colitis by Vagus Nerve Stimulation. Gastroenterology. 2016;150:S527. [Google Scholar]
- 112.Meregnani J, et al. Anti-inflammatory effect of vagus nerve stimulation in a rat model of inflammatory bowel disease. Auton Neurosci. 2011;160:82–89. doi: 10.1016/j.autneu.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 113.Sun P, et al. Involvement of MAPK/NF-κB signaling in the activation of the cholinergic anti-inflammatory pathway in experimental colitis by chronic vagus nerve stimulation. PLoS One. 2013;8:e69424. doi: 10.1371/journal.pone.0069424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ji H, et al. Central cholinergic activation of a vagus nerve-to-spleen circuit alleviates experimental colitis. Mucosal Immunol. 2014;7:335–47. doi: 10.1038/mi.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain, Behavior, and Immunity. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.The F, et al. Central activation of the cholinergic anti-inflammatory pathway reduces surgical inflammation in experimental post-operative ileus. Br J Pharmacol. 2011;163:1007–16. doi: 10.1111/j.1476-5381.2011.01296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Galitovskiy V, et al. Cytokine-induced alterations of α7 nicotinic receptor in colonic CD4 T cells mediate dichotomous response to nicotine in murine models of Th1/Th17- versus Th2-mediated colitis. J Immunol. 2011;187:2677–87. doi: 10.4049/jimmunol.1002711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Luyer MD, et al. Strain-specific effects of probiotics on gut barrier integrity following hemorrhagic shock. Infect Immun. 2005;73:3686–92. doi: 10.1128/IAI.73.6.3686-3692.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Luyer MD, et al. Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J Exp Med. 2005;202:1023–9. doi: 10.1084/jem.20042397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Langness S, Coimbra R, Eliceiri BP, Costantini TW. Vagus nerve mediates the neural stem cell response to intestinal injury. J Am Coll Surg. 2015;221:871–879. doi: 10.1016/j.jamcollsurg.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 121.Costantini TW, et al. Vagal nerve stimulation protects against burn-induced intestinal injury through activation of enteric glia cells. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1308–18. doi: 10.1152/ajpgi.00156.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu R, et al. Orexigenic hormone ghrelin attenuates local and remote organ injury after intestinal ischemia-reperfusion. PLoS One. 2008;3:e2026. doi: 10.1371/journal.pone.0002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Giuliani D, et al. Melanocortins and the cholinergic anti-inflammatory pathway. Adv Exp Med Biol. 2010;681:71–87. doi: 10.1007/978-1-4419-6354-3_6. [DOI] [PubMed] [Google Scholar]
- 124.Bauer AJ, Boeckxstaens GE. Mechanisms of postoperative ileus. Neurogastroenterol Motil. 2004;16(Suppl 2):54–60. doi: 10.1111/j.1743-3150.2004.00558.x. [DOI] [PubMed] [Google Scholar]
- 125.Lubbers T, et al. Lipid-rich enteral nutrition reduces postoperative ileus in rats via activation of cholecystokinin-receptors. Ann Surg. 2009;249:481–7. doi: 10.1097/SLA.0b013e318194d187. [DOI] [PubMed] [Google Scholar]
- 126.Tsuchida Y, et al. Neuronal stimulation with 5-hydroxytryptamine 4 receptor induces anti-inflammatory actions via α7nACh receptors on muscularis macrophages associated with postoperative ileus. Gut. 2011;60:638–47. doi: 10.1136/gut.2010.227546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gomez-Pinilla PJ, et al. 416 Prucalopride Activates the Intestinal Cholinergic Anti-Inflammatory Pathway and Prevents Postoperative Ileus. Gastroenterology. 2014;146:S-89. [Google Scholar]
- 128.Peterson CY, Krzyzaniak M, Coimbra R, Chang DC. Vagus nerve and postinjury inflammatory response. Arch Surg. 2012;147:76–80. doi: 10.1001/archsurg.2011.237. [DOI] [PubMed] [Google Scholar]
- 129.Ghia JE, Blennerhassett P, Collins SM. Vagus nerve integrity and experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G560–7. doi: 10.1152/ajpgi.00098.2007. [DOI] [PubMed] [Google Scholar]
- 130.Huston JM, Tracey KJ. The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. J Intern Med. 2011;269:45–53. doi: 10.1111/j.1365-2796.2010.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bonaz B, Sinniger V, Pellissier S. Vagal tone: effects on sensitivity, motility, and inflammation. Neurogastroenterol Motil. 2016;28:455–62. doi: 10.1111/nmo.12817. [DOI] [PubMed] [Google Scholar]
- 132.Nikfar S, Ehteshami-Ashar S, Rahimi R, Abdollahi M. Systematic Review and Meta-Analysis of the Efficacy and Tolerability of Nicotine Preparations in Active Ulcerative Colitis. Clinical Therapeutics. 2010;32:2304–2315. doi: 10.1016/j.clinthera.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 133.Boelens PG, et al. Reduction of postoperative ileus by early enteral nutrition in patients undergoing major rectal surgery: prospective, randomized, controlled trial. Ann Surg. 2014;259:649–55. doi: 10.1097/SLA.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 134.Atkinson C, et al. Randomized clinical trial of postoperative chewing gum versus standard care after colorectal resection. Br J Surg. 2016;103:962–70. doi: 10.1002/bjs.10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Beekwilder JP, Beems T. Overview of the clinical applications of vagus nerve stimulation. J Clin Neurophysiol. 2010;27:130–8. doi: 10.1097/WNP.0b013e3181d64d8a. [DOI] [PubMed] [Google Scholar]
- 136.Yuan H, Silberstein SD. Vagus Nerve and Vagus Nerve Stimulation, a Comprehensive Review: Part II. Headache. 2016;56:259–66. doi: 10.1111/head.12650. [DOI] [PubMed] [Google Scholar]
- 137.Kosel M, Schlaepfer TE. Mechanisms and state of the art of vagus nerve stimulation4604. J ECT. 2002;18:189–192. doi: 10.1097/00124509-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 138.Ben-Menachem E, et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures4605. Epilepsy Res. 1995;20:221–227. doi: 10.1016/0920-1211(94)00083-9. [DOI] [PubMed] [Google Scholar]
- 139.Krahl SE, Clark KB, Smith DC, Browning RA. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation4608. Epilepsia. 1998;39:709–714. doi: 10.1111/j.1528-1157.1998.tb01155.x. [DOI] [PubMed] [Google Scholar]
- 140.Walker BR, Easton A, Gale K. Regulation of limbic motor seizures by GABA and glutamate transmission in nucleus tractus solitarius4607. Epilepsia. 1999;40:1051–1057. doi: 10.1111/j.1528-1157.1999.tb00818.x. [DOI] [PubMed] [Google Scholar]
- 141.Marrosu F, et al. Correlation between GABA(A) receptor density and vagus nerve stimulation in individuals with drug-resistant partial epilepsy4606. Epilepsy Res. 2003;55:59–70. doi: 10.1016/s0920-1211(03)00107-4. [DOI] [PubMed] [Google Scholar]
- 142.Aalbers MW, et al. The effects of vagus nerve stimulation on pro- and anti-inflammatory cytokines in children with refractory epilepsy: an exploratory study. Neuroimmunomodulation. 2012;19:352–8. doi: 10.1159/000341402. [DOI] [PubMed] [Google Scholar]
- 143.Majoie HJM, et al. Vagus nerve stimulation in refractory epilepsy: effects on pro- and anti-inflammatory cytokines in peripheral blood. Neuroimmunomodulation. 2011;18:52–6. doi: 10.1159/000315530. [DOI] [PubMed] [Google Scholar]
- 144.De Herdt V, et al. Effects of vagus nerve stimulation on pro- and anti-inflammatory cytokine induction in patients with refractory epilepsy. J Neuroimmunol. 2009;214:104–108. doi: 10.1016/j.jneuroim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 145.Koopman FA, et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2016;113:8284–9. doi: 10.1073/pnas.1605635113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bonaz B, et al. Chronic vagus nerve stimulation in Crohn’s disease: a 6-month follow-up pilot study. Neurogastroenterol Motil. 2016;28:948–53. doi: 10.1111/nmo.12792. [DOI] [PubMed] [Google Scholar]
- 147.Stakenborg N, et al. Su1563 Electrical Stimulation of the Abdominal Vagus Nerve Is as Effective as Cervical Nerve Stimulation in Reducing Postoperative Ileus. Gastroenterology. 2016;150:S527. [Google Scholar]
- 148.Guiraud D, et al. Vagus nerve stimulation: state of the art of stimulation and recording strategies to address autonomic function neuromodulation4611. J Neural Eng. 2016;13:41002. doi: 10.1088/1741-2560/13/4/041002. [DOI] [PubMed] [Google Scholar]
- 149.Val-Laillet D, Biraben A, Randuineau G, Malbert CH. Chronic vagus nerve stimulation decreased weight gain, food consumption and sweet craving in adult obese minipigs4609. Appetite. 2010;55:245–252. doi: 10.1016/j.appet.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 150.Malbert CH. [The brain-gut axis: insights from the obese pig model]4610. Bull Acad Natl Med. 2013;197:1683–1694. [PubMed] [Google Scholar]
- 151.Sarr MG, et al. The EMPOWER study: randomized, prospective, double-blind, multicenter trial of vagal blockade to induce weight loss in morbid obesity4617. Obes Surg. 2012;22:1771–1782. doi: 10.1007/s11695-012-0751-8. [DOI] [PubMed] [Google Scholar]
- 152.Morton JM, et al. Effect of Vagal Nerve Blockade on Moderate Obesity with an Obesity-Related Comorbid Condition: the ReCharge Study4618. Obes Surg. 2016;26:983–989. doi: 10.1007/s11695-016-2143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kral JG. Vagotomy for treatment of severe obesity4612. Lancet. 1978;1:307–308. doi: 10.1016/s0140-6736(78)90074-0. [DOI] [PubMed] [Google Scholar]
- 154.Kral JG, Gortz L. Truncal vagotomy in morbid obesity4613. Int J Obes. 1981;5:431–435. [PubMed] [Google Scholar]
- 155.Kilgore KL, Bhadra N. Reversible nerve conduction block using kilohertz frequency alternating current4619. Neuromodulation. 2014;17:242–254. doi: 10.1111/ner.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Downs AM, Bond CE, Hoover DB. Localization of alpha7 nicotinic acetylcholine receptor mRNA and protein within the cholinergic anti-inflammatory pathway. Neuroscience. 2014;266:178–185. doi: 10.1016/j.neuroscience.2014.02.011. [DOI] [PubMed] [Google Scholar]