Abstract
Vocal communication is required for successful social interactions in numerous species. During the breeding season, songbirds produce songs that are reinforced by behavioral consequences (e.g., copulation). However, some songbirds also produce songs not obviously directed at other individuals. The consequences maintaining or reinforcing these songs are less obvious and the neural mechanisms associated with undirected communication are not well-understood. Previous studies indicate that undirected singing is intrinsically rewarding and mediated by opioid or dopaminergic systems; however, endocannabinoids are also involved in regulating reward and singing behavior. We used a conditioned place preference paradigm to examine song-associated reward in European starlings and quantitative real-time PCR to measure expression of endocannabinoid-related neural markers (CB1, FABP7, FABP5, FAAH, DAGLα), in brain regions involved in social behavior, reward and motivation (ventral tegmental area [VTA], periaqueductal gray [PAG], and medial preoptic nucleus [POM]), and a song control region (Area X). Our results indicate that starlings producing high rates of song developed a conditioned place preference, suggesting that undirected song is associated with a positive affective state. We found a significant positive relationship between song-associated reward and CB1 receptors in VTA and a significant negative relationship between song-associated reward and CB1 in PAG. There was a significant positive relationship between reward and the cannabinoid transporter FABP7 in POM and a significant negative relationship between reward and FABP7 in PAG. In Area X, FABP5 and DAGLα correlated positively with singing. These results suggest a role for endocannabinoid signaling in vocal production and reward associated with undirected communication.
Keywords: conditioned place preference, endocannabinoid, reward, songbird, vocal communication
Animals produce vocalizations in a variety of contexts. Often there are obvious behavioral consequences that occur after a vocalization is produced, resulting in reinforcement of the vocal behavior. Examples of behavioral outcomes that can follow a vocalization include: attracting a mate, deterring a predator, or scaring off a rival. In each of these examples, the vocal behavior is reinforced (i.e., a mate is obtained; a predator or competitor stays away) and the vocalization is more likely to be produced again in the future. In other contexts, the function of vocalizing, or the reinforcer maintaining the vocal behavior, is less clear. For example, some songbirds produce songs that are not obviously directed at other individuals and appear to be ignored by potential recipients (Dunn and Zann, 1996). This type of song was first termed ‘undirected song’ in zebra finches (Taeniopygia guttata) (Sossinka and Böhner, 1980; Dunn and Zann 1996), with similar forms of communication observed in other species (e.g., Bengalese finches, Lonchura striata domestica, Dunning et al., 2014; European starlings, Sturnus vulgaris, Riters et al., 2000). Although the precise functions of undirected song may vary across species, this type of communication is facilitated by the presence of conspecifics (Jesse and Riebel, 2012); for example, starlings produce high rates of song during the nonbreeding season while in large affiliative flocks (Feare, 1984). This communication is proposed to maintain social groups (Hausberger et al., 1995; Eens, 1997) and is important for song learning and maintenance (Adret-Hausberger et al., 1990; Böhner et al., 1990; Chaiken et al., 1994; Kao et al., 2005). Females may use male undirected song to assess potential mates (Holveck and Riebel, 2007), but, unlike female-directed song, undirected songs are not accompanied by courtship displays or followed by mating (Morris, 1954; Sossinka and Böhner, 1980). In other words, there is not an obvious external reinforcer that follows this form of undirected song (in contrast to female-directed song, which may be reinforced by copulation). It has been proposed that because there is no immediate, obvious external reinforcer for undirected song, producing this type of song may be facilitated or maintained by intrinsic reward mechanisms (Riters, 2010, 2011, 2012).
Consistent with the idea that the reward mechanisms associated with female-directed and undirected song vary, there is evidence in male starlings and zebra finches that producing undirected song may be intrinsically rewarding and associated with a positive affective state, while song directed at conspecifics (e.g., female-directed song) is more tightly linked to the reinforcement provided by the receiver's behavior (Riters and Stevenson, 2012). Using a conditioned place preference paradigm, male zebra finches and male starlings developed a conditioned place preference associated with producing song that was not directed at other individuals (i.e., undirected song), while males did not develop a conditioned place preference associated with producing directed song.
Across vertebrates, opioid neuropeptides are strongly implicated in reward and several studies demonstrate opioid neuropeptides to be tightly coupled to undirected singing behavior (Riters et al., 2005; Kelm-Nelson et al., 2012) and to reward associated with undirected singing behavior (Riters et al. 2014). There is also evidence that dopamine may be involved in singing in this context (Heimovics et al., 2009; Merullo et al., 2016), and it is likely that other neurochemical systems also play a role. The endocannabinoid system is a likely candidate for potentiating reward processes, as endocannabinoids interact with other neurochemical systems that modulate motivation and reward, including the opioid and dopaminergic systems (for review see Solinas et al., 2007; 2008).
Endocannabinoids, their receptors and enzymes are distributed across the brains of vertebrates (Elphick, 2012) and are involved in a variety of rewarding behaviors (for review see Fattore et al., 2010). The best characterized endogenous ligands of the endocannabinoid system are anandamide and 2-arachidonylglycerol (2-AG). Both anandamide and 2-AG are hydrophobic, synthesized “on demand,” and transported via intracellular carriers, such as fatty acid-binding proteins (FABP; Sanson et al., 2013). Anandamide and 2-AG, which primarily signal in a retrograde manner, are released from the postsynaptic membrane and interact with cannabinoid receptors on presynaptic neurons, including the cannabinoid receptor CB1. CB1 receptors are G-protein coupled receptors abundant in the CNS (for review see Wilson and Nicoll, 2002; Castillo et al. 2012) and activation of CB1 receptors suppresses neurotransmitter release (Kano et al., 2009). Given the role of endocannabinoids in reward and the relationship with other reward- and undirected song-related neurotransmitters (i.e., dopamine, opioids), the endocannabinoid system may also have a role in undirected singing.
The objective of this study was to examine the extent to which mRNA expression for endocannabinoid-related markers is associated with singing behavior and reward. In order to examine song-associated reward, we observed male European starlings singing undirected song while in nonbreeding flocks and used a conditioned place preference (CPP) task to measure reward state associated with song production (as in Riters and Stevenson 2012; Riters et al. 2014). Following the CPP procedure, we collected neural tissue and used quantitative real-time PCR (qPCR) to measure expression levels of mRNA encoding CB1 receptors, two endocannabinoid transporters (FABP7 and FABP5; Kaczocha et al., 2009), fatty acid amide hydrolase (FAAH; the degradative enzyme for anandamide), and diacylglycerol lipase-ɑ (DAGLɑ; the synthesizing enzyme for 2-AG). We examined mRNA expression of these neural markers in brain regions involved in undirected song and reward, including medial preoptic area (POM) and two brain regions that are interconnected with POM: ventral tegmental area (VTA) and periaqueductal gray (PAG; Riters and Alger, 2004). We also examined Area X, a song control nucleus that is involved in song learning and receives direct projections from VTA (Lewis et al., 1981).
Experimental procedures
Subjects
Twenty-four male European starlings were captured on a farm in Madison, WI using baited fly-in traps. Following capture, starlings were brought to the facilities at the University of Wisconsin-Madison and were housed in colony rooms in stainless steel cages (91 cm × 47 cm × 47 cm) in same-sex groups with no more than five individuals per cage. Colored leg bands allowed identification of individual birds. Birds were housed indoors and placed on 18L:6D light cycle for at least six weeks. Starlings maintained under this photoperiod become photorefractory, during which birds have regressed gonads and are not in a reproductive state, which mimics fall nonbreeding conditions (Dawson et al., 2001).
During the experiment, males were housed in groups of four in indoor aviaries (3.5 m × 2.25 m × 2 m) containing four perches, a nest box, and a water bath. Birds were provided with ad libitum access to food and water. Birds were housed in the aviaries for at least two weeks before the start of behavioral testing. All procedures were conducted in accordance with the guidelines in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and a protocol approved by the Institutional Animal Care and Use Committee at the University of Wisconsin-Madison.
Behavioral observations
Behavior was observed for 20 min prior to CPP conditioning (described below) by a single experimenter hidden behind a one-way mirror. We measured the number of songs produced and several non-vocal behaviors including: feeding bouts, drinking bouts, and preening bouts. A bout was defined as a behavior that was separated from a previous behavior by at least 2 s. Behavior was also observed for 20 min prior to sacrifice (at least one day following the final day of the CPP procedure).
Behavioral testing: Conditioned place preference
The CPP procedure consisted of three phases, each occurring on a separate day: habituation day, conditioning day, and test day. The CPP apparatus was a cage (118 cm × 59 cm × 59 cm) divided into two distinct compartments. Each compartment contained distinct visual cues (i.e., red or blue construction paper) to visually distinguish between the two sides of the cage.
On habituation day, a single bird was removed from its aviary (caught using a net) and placed into the CPP apparatus for 30 minutes in order to habituate the bird to the apparatus. The bird was allowed to freely explore both sides of the apparatus and the amount of time spent (in seconds) on each side of the apparatus was recorded. Following this habituation period, each bird was returned to its home aviary.
Conditioning was conducted on the following day. On conditioning day, once a bird was singing, it was observed for 20 min while in its home aviary and we measured the number of songs produced and other non-vocal behaviors (feeding bouts, drinking bouts, preening bouts; see Behavioral observations, above). Following the behavioral observations, each bird was quickly captured and placed singly into one side of the CPP apparatus for 30 min. An opaque barrier separated the two sides of the CPP apparatus, so a bird was restricted to one distinct side (i.e., the conditioned side) of the apparatus. The conditioned side of the apparatus was pseudo-randomly assigned and counterbalanced across birds, so that half of the birds were conditioned to the red side and half to the blue side. Following the conditioning period, each bird was returned to its home aviary.
The extent to which an individual developed a conditioned preference was tested the next day (i.e., CPP test day). On test day, a single bird was removed from its aviary and placed into the CPP apparatus. On this day, the opaque barrier in the apparatus was removed and the bird was allowed to freely move between the two distinct sides of the CPP apparatus for 30 min. Similar to habituation day, the total amount of time that a bird spent on each side of the apparatus on test day was recorded.
The rationale for this CPP procedure is that singing (or other behavior) is associated with a particular affective state before the birds are placed into the CPP apparatus. This affective state is the unconditioned stimulus. This affective state is then paired with one side of the CPP apparatus. That side of the apparatus becomes the conditioned stimulus and is linked with the affective state associated with producing song. If song is associated with a positive (or rewarding) affective state, males should develop a CPP. We employ this CPP procedure because birds rarely produce song while in the CPP apparatus. This method has produced consistent results in previous studies examining song-associated reward in songbirds (Riters and Stevenson, 2012; Riters et al., 2014).
We removed four birds from the analyses that did not explore (i.e., spend at least 1min on each side) the CPP apparatus on the habituation day (these subjects were not habituated to both sides of the apparatus before conditioning day) and one bird that did not explore on test day. All other subjects visited each side of the apparatus on the habituation and test days. Three additional birds were removed due to external confounds during testing that interfered with the bird's exploration of the apparatus. We lost one brain during extraction (see below), resulting in n = 15. Final samples sizes for each statistical test are given in the results.
Tissue preparation for qPCR
Following the last behavioral observation, birds were rapidly decapitated. Brains were extracted, frozen using isopentane (Catalog No. 277258, Sigma, St. Louis, MO) cooled with dry ice, and stored at -80° C until further processing. One brain was lost during extraction. Coronal sections (200μm) were collected using a cryostat between -14° and -16° C and placed onto slides. Fine Science Tools Sample Corers (Item Nos. 18035-01 and 18035-02; Foster City, CA) were used to collect samples from target brain regions. A 1 mm diameter punch was taken from VTA (2 bilateral punches on 1 section), 2 mm diameter punches were taken from Area X (2 bilateral punches on 2 sections), POM (1 medial punch from 3 sections), and PAG (1 medial punch from 1 section). See Fig. 1 for approximate size and location of tissue punches. Tissue from each individual was placed in separate centrifuge tubes (one tube for each brain region) and stored at -80° C.
Figure 1.
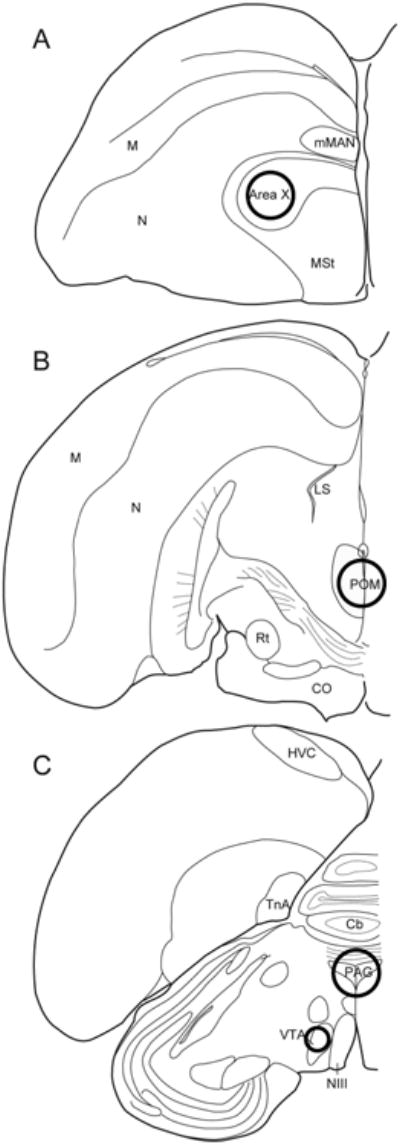
Coronal sections showing approximate sizes and locations of tissue punches in (A) Area X (2 mm diameter), (B) POM (2 mm diameter), and (C) VTA (1 mm diameter) and PAG (2 mm diameter). Bilateral tissue punches were collected, except for POM and PAG (for each of these regions, a single central punch was taken). Abbreviations: Cb: cerebellum; CO: optic chiasm; HVC: letter-based proper name; LS: lateral septum; M: mesopallium; mMAN: medial magnocellular nucleus; MSt: medial striatum; N: nidopallium; NIII: third cranial nerve; Rt: nucleus rotundus; TnA: nucleus taenia of the amygdala
For RNA extraction, tissue was homogenized in a 1.7 mL cone-tipped tube with a cone-tipped Dremel tool and RNA was extracted with the Bio-Rad Aurum Total RNA Fatty and Fibrous Tissue Kit (Catalog No. 732-6830; Bio-Rad, Hercules, CA). PureZol was used to isolate RNA and RNA was treated with DNAse. Using 30 μL of nuclease free water, RNA was eluted and a NanoDrop system (Thermo Scientific, Wilmington, DE) was used to measure RNA concentration. 100 ng of RNA was converted into single-stranded cDNA using the Invitrogen SuperScript III First-Strand Synthesis System (Catalog No. 18080-051; Life Technologies, Carlsbad, CA). cDNA from surrounding sections were pooled and used as standard tissue for qPCR. Relative gene expression for CB1, FABP7, FABP5, FAAH, and DAGLα was quantified using qPCR.
qPCR analysis
We used NCBI Primer Blast to design primers using zebra finch, European starling, or chicken (Gallus gallus) genome. Primers were prepared by the University of Wisconsin Biotechnology Center. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and hydroxymethylbilane synthase (HMBS) were used as reference genes for each brain region. For some genes, we used forward and reverse primers that have been reported previously (GAPDH: Riters et al., 2014; HMBS: Merullo et al., 2015; CB1: DeVries et al., 2016; FAAH: Dickens et al., 2015). See Table 1 for primer information. Sanger sequencing using the forward and reversed primers was conducted to sequence the qPCR reaction product at the University of Wisconsin Biotechnology Center and matched the intended targets using NCBI BLAST (Table 2).
Table 1.
Primers used for endocannabinoid-related genes (CB1, FABP7, FABP5, FAAH, DAGLα) and reference genes (GAPDH, HMBS).
| Gene | Accession No. | Direction | Sequence | Ta (°C) | Product (bp) |
|---|---|---|---|---|---|
| CB1 | NM_001038652.1 | Forward | GGTCTTCTGTGGACTTAGGG | 58.5 | 113 |
| Reverse | CTCCTCTATTCCTTTGTTGCTC | ||||
| FABP7 | XM_002188827.3 | Forward | TGGTAGACAGCCACAACTTC | 61.7 | 237 |
| Reverse | CTCCATCCAGGGTCACAAC | ||||
| FABP5 | NM_001245600.1 | Forward | TTTGGGTAAATGGTGCCTC | 58.3 | 143 |
| Reverse | GACGGTGATTGTGTCCC | ||||
| FAAH | XM_014872201.1 | Forward | AGGTCTGCTCTATGGAGTGC | 59.5 | 109 |
| Reverse | GTCCTCTGCTGCAGGTTTAT | ||||
| DAGLα | XM_014878708.1 | Forward | GTGATTGAGTTCGTGTACGCC | 61.5 | 118 |
| Reverse | CCACCCAGTTACACACCACC | ||||
| GAPDH | NM_204305.1 | Forward | AGCAATGCTTCCTGCACTAC | 57.5 | 121 |
| Reverse | CTGTCTTCTGTGTGGCTGTG | ||||
| HMBS | XM_002187761.1 | Forward | ACCTGCCAACTTCTCTTCCT | 58 | 140 |
| Reverse | GGTTCCAATCACACTCTTTTCA |
Table 2.
Sanger sequencing of the qPCR reaction product from primers for each gene
| Gene | qPCR reaction product sequence |
|---|---|
| CB1 | TAATTTTTCCCCCTCCTTTTCTTTCACATGCATATGAGACTAACAGCAACAAAGG AATAGAGGAGA |
| FABP7 | GGGGTCGCACACTGTAGNNNGGNATGTGACTAAANNCNCTGTGCATTATCANG NTNGCAGGGGGACANGNGTANTGATCNGAANCCNNNGCACTTTTCNAAAACAT AGAAATCNGCCTTTAAACTCGAGATAGGAATTTGANGAAACTACTCCCGATGAT AGAAANTGCANNTCANTTGTGACCCTGGATGGAG |
| FABP5 | GGACTNGGTGTGGGTATGGCTATGAGAAAAATGGGAAGCATGGCCAAACCANC ACGTTTTACNTAATTAAGGATGGGGACACAATCACCGTTCA |
| FAAH | CATNGNCGCCAGGAGTNAGTACTTCCACGNTTGGGGCTTCATAAAACACCGTCC ATAAACCTGTCTGGCAGNCNGACA |
| DAGLα | CAGTACTCACCTCCTGCACGACATCACAGCCAAGAGTGTCACCCCTGGGAATGG TGGTGTGTAACTGGGGTGG |
| GAPDH | AGGGCTGGCAGGTATCCATGACACTTTGGCATCGTGGAGGGTCTCATGACCACT GGTCCATGCCATCACAGCCACACAGAAGACAGAT |
| HMBS | TCGCAAAGGGAAACCCACCTTGNATGCTGTTGTCTTTCATCCCAAAAACTGTGG AAAAACACTGAGCCTCCTTCCTGAAAAGAGTGTGATTGGAACNAA |
For qPCR, we used the BioRad CFX96 Touch Real-Time PCR Detection System (Catalog No. 185-5195; Bio-Rad, Hercules, CA). To amplify cDNA, samples were mixed with Sso Fast Evagreen Supermix (Catalog No. 172-5204; Bio-Rad, Hercules, CA), nuclease-free H2O, and forward and reverse primers. Along with each plate of samples, a run included five standards (1:10 serial dilution with a starting concentration of 500ng/μl; an exception was that the plate for FABP7 in Area X used standards with a 1:12 serial dilution) and a negative control consisting of nuclease free H2O in place of cDNA. On each plate, samples and standards were run in triplicate. A run consisted of an initiation step at 95° C for 30 s, followed by 40 cycles of 95° C for 5 s, an annealing phase for 30 s at the annealing temperature (Ta) specific to the primer (see Table 1), followed by an elongation phase at 72° C for 30 s. Plates went through a melt curve from 65° C to 88° C, 0.5° C for each 5 s step. All runs followed the MIQE guidelines (Bustin et al., 2009) and had an efficiency between 90-110%, an R2 of at least 0.990, and a melt curve with a single peak, which verified primer specificity.
We used the Pfaffl method to determine the relative levels of gene expression (for a detailed description see Cordes et al., 2015; Pfaffl, 2001). In brief, the amplification threshold was set at 200 RFU and the average number of cycles that crossed this threshold (Ct) was transformed for each sample. For each brain region, the geometric mean of the Ct values for the two reference genes was calculated and used to calculate the Ct values for each gene (i.e., CB1, FABP7, FABP5, FAAH, or DAGLα) as a normalized ratio.
Statistical analysis
Our prediction was that song production would be associated with individual reward state and endocannabinoid-related expression. We were interested in the relationship between singing and constitutive mRNA expression in individuals. Consistent with past studies (e.g., Riters et al. 2014), a correlation analyses revealed that the song rate of a given individual positively correlated between observation days (r = 0.57, r2 = 0.33, p = 0.026), demonstrating that an individual's propensity to sing is relatively constant. This suggests that mRNA expression on the day that tissue was collected is indicative of an individual's consistent tendency to produce song.
In order to examine the relationship between the absolute amount of time that the subject spent on the conditioned side of the apparatus on test and our behavioral measures we conducted correlation analyses. We conducted one correlation analyses with the number of songs the bird produced on conditioning day and we conducted a separate correlation analyses with bouts of non-vocal behaviors on conditioning day. We combined bouts of feeding, drinking and preening for our measure of non-vocal behavior, because overall birds produced few bouts of each behavior (range: 0-6 bouts). To account for multiple comparisons we used a Bonferroni correction for our alpha value (α = 0.05/2 comparisons = 0.025).
We conducted multiple regression analyses with the absolute amount of time that the subject spent on the conditioned side of the apparatus on test day as the dependent variable. We included two behavioral measures from conditioning day as predictor variables: (1) number of songs and (2) bouts of non-vocal behaviors. We also included mRNA expression in VTA, PAG, and POM as predictor variables. We conducted a separate regression analysis for each neural marker (i.e., CB1, FABP7, FABP5, FAAH, DAGLα). The neural markers were intercorrelated with one another in some brain regions, so inclusion in one regression analyses was not appropriate. See Table 3 for the predictor variables included in each regression analysis. In order to examine endocannabinoid-related expression in the song control nucleus, Area X, we first conducted a regression analysis with the absolute amount of time that the subject spent on the conditioned side of the apparatus on test day as the dependent variable. For the predictor variables, we included our two behavioral measures (number of songs and bouts of non-vocal behaviors), and expression in Area X for each neural marker (CB1, FABP7, FABP5, FAAH, DAGLα). While, Area X may play a role in reinforcement related to vocal learning (e.g., Hoffman et al., 2016), it does not have a definitive role in reward processes comparable to other brain regions (e.g., PAG, POM, VTA; thus we would not predict a relationship between CPP and Area X mRNA expression, a prediction we confirmed below). However, Area X is critical for song learning (Sohrabji et al., 1990; Scharff and Nottebohm, 1991) and variability in song production (Leblois et al., 2010; Leblois and Perkel, 2012); therefore, we also conducted separate correlation analyses for each neural marker to examine the relationship between the number of songs produced and mRNA expression in Area X. We examined Area X because it is the nucleus involved in song learning, compared to other nuclei in the song control system (HVC and the robust nucleus of the arcopallium, RA), which are proposed to regulate the temporal structure of song production (Fee et al., 2004). In some instances, data were missing for a sample because it did not meet our inclusion criteria (e.g., a sample ran after 35 cycles), reducing our sample size. Below we note when sample size was reduced or when we excluded a brain region from the analysis because of low sample size. All statistics were conducted with Statistica (StatSoft, Inc., Tuslsa, OK).
Table 3.
Results of regression models demonstrating behaviors and brain regions in which endocannabinoid-related expression explain variance in song-associated reward (measured via CPP).
| Endocannabinoid-related gene | Regression model | Variables | Beta | p |
|---|---|---|---|---|
|
| ||||
| CB1 receptor n = 14 |
Adjusted R2 = 0.770 F5,8 = 9.69 p = 0.0030 |
VTA | 0.374 | 0.0466* |
| PAG | -0.468 | 0.0131* | ||
| POM | -0.028 | 0.8724 | ||
| Number of songs | 0.398 | 0.0495* | ||
| Non-vocal behaviors | 0.007 | 0.9653 | ||
|
| ||||
| FABP7 n = 13 |
Adjusted R2 = 0.687 F5,7 = 6.27 p = 0.0160 |
VTA | -0.058 | 0.7932 |
| PAG | -0.460 | 0.0476* | ||
| POM | 0.771 | 0.0133* | ||
| Number of songs | -0.020 | 0.9475 | ||
| Non-vocal behaviors | -0.091 | 0.6432 | ||
|
| ||||
| FABP5 n = 13 |
Adjusted R2 = 0.409 F5,7 = 2.66 p = 0.1172 |
VTA | -0.047 | 0.8541 |
| PAG | -0.132 | 0.7388 | ||
| POM | 0.751 | 0.1366 | ||
| Number of songs | 0.298 | 0.3319 | ||
| Non-vocal behaviors | -0.078 | 0.7933 | ||
|
| ||||
| FAAH† n = 12 |
Adjusted R2 = 0.565 F4,7 = 4.57 p = 0.0394 |
PAG | -0.186 | 0.3908 |
| POM | -0.281 | 0.2485 | ||
| Number of songs | 0.369 | 0.1787 | ||
| Non-vocal behaviors | 0.452 | 0.0867 | ||
|
| ||||
| DAGLᆆ n = 15 |
Adjusted R2 = 0.385 F3,11 = 3.92 p = 0.0398 |
POM | 0.027 | 0.9033 |
| Number of songs | 0.611 | 0.0340* | ||
| Non-vocal behaviors | 0.177 | 0.4934 | ||
Indicates a significant predictor variable (p ≤ 0.05).
Indicates VTA was not included in regression model (see text for details).
Indicates VTA and PAG were not included in regression model (see text for details).
Results
Relationship between CPP and behavioral measures
A correlation analysis revealed that the song rate of a given individual positively correlated with the amount of time on the conditioned side of the apparatus on test day (r = 0.703; r2 = 0.494; p = 0.0035; n = 15; see Fig. 2), a result consistent with previous studies (Riters and Stevenson, 2012; Riters et al., 2014). A second correlation analysis revealed that bouts of non-vocal behaviors (feeding, drinking, preening) did not significantly correlate with the amount of time on the conditioned side of the apparatus on test day (r = 0.506; r2 = 0.256; p = 0.0542; n = 15). To account for multiple comparisons we used a Bonferroni correction (α = 0.025).
Figure 2.
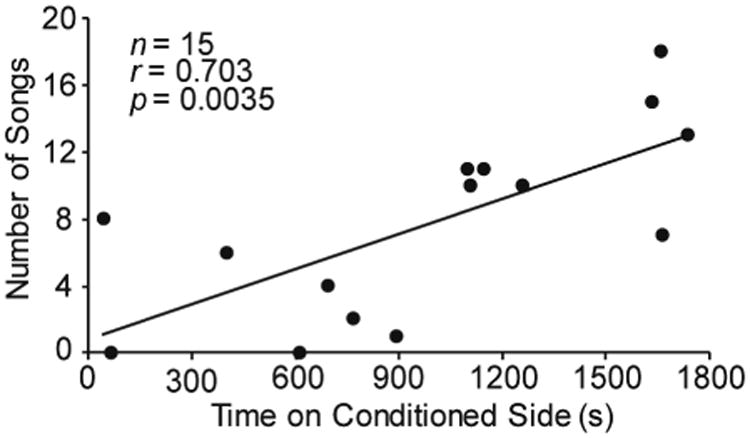
Correlation between the number of songs produced and CPP (measured as the amount of time (s) on test day spent on the conditioned side of the apparatus; n = 15). Line indicates a significant correlation (p ≤ 0.025).
Song-associated preference and CB1
We conducted a multiple regression analysis with the amount of time on the conditioned side of the apparatus on test day as the dependent variable and number of songs, bouts of non-vocal behaviors, mRNA expression for the cannabinoid receptor CB1 in VTA, CB1 mRNA expression in PAG, and CB1 mRNA expression in POM as predictor variables. We were missing mRNA expression in VTA for 1 bird, reducing the sample size to 14. The model explained a significant amount of the variance (adjusted R2 = 0.770, N = 14, p = 0.0030). Number of songs produced (Beta = 0.398, SE = 0.172, t(8) = 2.312, p = 0.0495), CB1 mRNA expression in VTA (Beta = 0.374, SE = 0.159, t(8) = 2.351, p = 0.0466), and CB1 mRNA expression in PAG (Beta = -0.468, SE = 0.148, t(8) = -3.176, p = 0.0131) statistically explained variance in our measure of CPP. No other variables were significant. See Table 3; Fig. 3.
Figure 3.
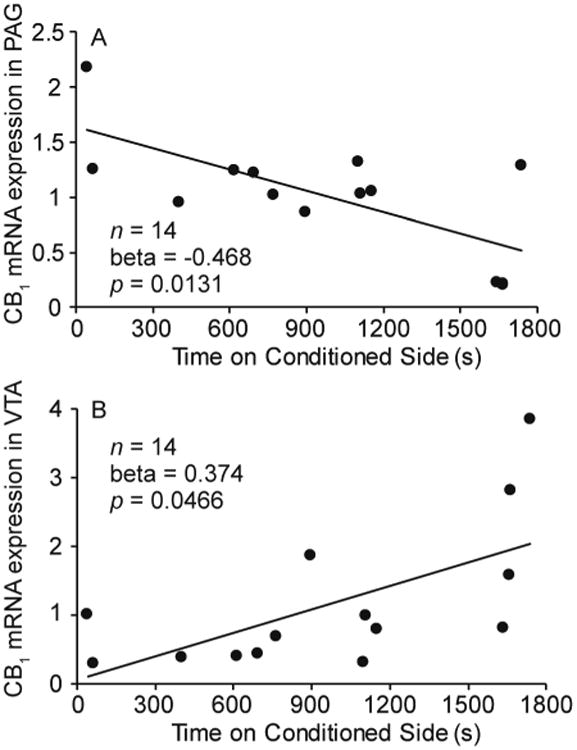
Relationship between CPP (measured as the amount of time (s) on test day spent on the conditioned side of the apparatus) and CB1 mRNA expression in (A) PAG and (B) VTA. Beta and p values were determined with a multiple regression model (n = 14). Linear regression lines indicate a significant relationship (p ≤ 0.05).
Song-associated preference and FABP7
We conducted a multiple regression analysis with the amount of time on the conditioned side of the apparatus on test day as the dependent variable and number of songs, bouts of non-vocal behaviors, mRNA expression for the endocannabinoid transporter FABP7 in VTA, FABP7 mRNA expression in PAG, and FABP7 mRNA expression in POM as predictor variables. We were missing mRNA expression in VTA for 2 birds, reducing the sample size to 13. The model explained a significant amount of the variance (adjusted R2 = 0.687, N = 13, p = 0.0160). FABP7 mRNA expression in POM (Beta = 0.771, SE = 0.234, t(7) = 3.289, p = 0.0133), and FABP7 mRNA expression in PAG (Beta = -0.460, SE = 0.192, t(7) = -2.399, p = 0.0476) statistically explained variance in our measure of CPP. No other variables were significant. See Table 3; Fig. 4.
Figure 4.
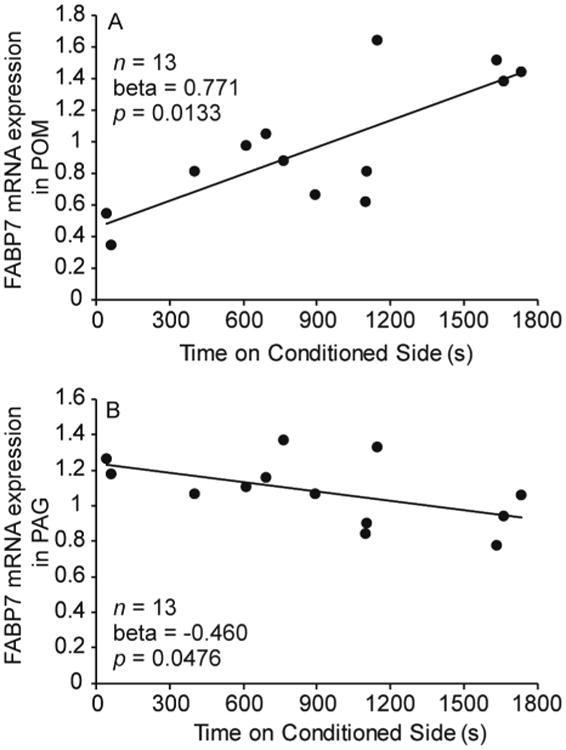
Relationship between CPP (measured as the amount of time (s) on test day spent on the conditioned side of the apparatus) and FABP7 mRNA expression in (A) POM and (B) PAG. Beta and p values were determined with a multiple regression model (n = 13). Linear regression lines indicate a significant relationship (p ≤ 0.05).
Song-associated preference and FABP5
We conducted a multiple regression analysis with the amount of time on the conditioned side of the apparatus on test day as the dependent variable and number of songs, bouts of non-vocal behaviors, mRNA expression for the endocannabinoid transporter FABP5 in VTA, FABP5 mRNA expression in PAG, and FABP5 mRNA expression in POM as predictor variables. We were missing mRNA expression in VTA for 2 birds, reducing the sample size to 13. The multiple regression analysis was not significant (adjusted R2 = 0.409, N = 13, p = 0.1172) and there were no significant predictor variables (all ps ≥ 0.1366; see Table 3) for any of the brain regions measured.
Song-associated preference and FAAH
We conducted a multiple regression analysis with the amount of time on the conditioned side of the apparatus on test day as the dependent variable and number of songs, bouts of non-vocal behaviors, mRNA expression for the degradative enzyme FAAH in PAG, and FAAH mRNA expression in POM as predictor variables. FAAH mRNA expression in VTA was missing for 5 birds, likely because expression for this neural marker is low in this brain region, so we excluded VTA from the analysis. We were missing mRNA expression in POM for 1 bird and PAG for 2 birds, reducing the sample size to 12. The model explained a significant amount of the variance (adjusted R2 = 0.565, N = 12, p = 0.0394); however, none of the individual predictor variables were significant (all ps ≥ 0.0867; see Table 3).
Song-associated preference and DAGLɑ
We conducted a multiple regression analysis with the amount of time on the conditioned side of the apparatus on test day as the dependent variable and number of songs, bouts of non-vocal behaviors, mRNA expression for the synthesizing enzyme DAGLα in POM as predictor variables. DAGLα mRNA expression in VTA was missing for 11 birds and DAGLα mRNA expression in PAG was missing for 6 birds, so we excluded these brain regions from the analysis. The model explained a significant amount of the variance (adjusted R2 = 0.385, N = 15, p = 0.0398). The number of songs produced (Beta = 0.611, SE = 0.252, t(11) = 2.420, p = 0.0340) statistically explained variance in our measure of CPP. No other variables were significant. See Table 3.
Song production and endocannabinoid-related expression in Area X
We conducted a multiple regression analysis with the amount of time on the conditioned side of the apparatus on test day as the dependent variable and number of songs, bouts of non-vocal behaviors, CB1, FABP7, FABP5, FAAH, and DAGLα mRNA expression in Area X as predictor variables. We were missing FABP7 mRNA expression for 1 bird, reducing the sample size to 14. As predicted, mRNA expression in Area X did not significantly predict our measure of reward (amount of time on the conditioned side of the apparatus on test day; all ps ≥ 0.2103). The number of songs produced (Beta = 0.98, SE = 0.36, t(6) = 2.69, p = 0.0360) statistically explained variance in our measure of reward; however, the overall regression model was not significant (adjusted R2 = 0.54, N = 14, p = 0.0877).
In order to examine the relationship between song production and endocannabinoid-related markers in Area X, we conducted correlation analyses. We used a Bonferroni correction for our alpha value (α = 0.05/5 comparisons = 0.01) to account for multiple comparisons. We were missing FABP7 mRNA expression for 1 bird, reducing the sample size to 14 for the analysis of FABP7. The number of songs produced positively correlated with DAGLα mRNA expression in Area X (r = 0.67; r2 = 0.45; p = 0.006) and FABP5 expression in Area X (r = 0.64; r2 = 0.41; p = 0.01; Fig. 5).
Figure 5.
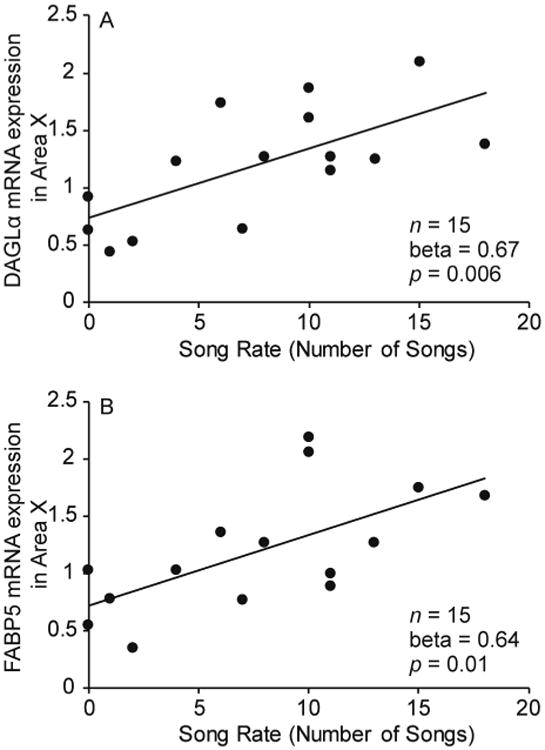
Correlations between the number of songs and (A) DAGLɑ mRNA expression in Area X and (B) FABP5 mRNA expression in Area X (n = 15). Lines indicate a significant correlation (p ≤ 0.01).
Discussion
Our results show that the affective state associated with the production of undirected song in male starlings is associated with cannabinoid-related gene expression in brain regions implicated in motivation and reward. We identified strong, linear relationships between song-associated reward (as measured via CPP) and genes involved in cannabinoid activity in brain regions implicated in motivation and reward (VTA, PAG, POM). It is possible that endocannabinoids in these regions induce a positive affective state that then facilitates singing behavior. Alternatively, but not mutually exclusively, singing behavior could influence endocannabinoid activity which then induces a positive affective state. Our data are correlational and it is possible that a third variable explains these relationships; however, given the role of endocannabinoids in reward we suggest that endocannabinoids may directly link the act of song production with a positive affective state. Relationships between endocannabinoid markers and song were also identified for the song control region, Area X, which we interpret to suggest a role for cannabinoids in Area X in song learning as suggested previously in studies of zebra finches (Soderstrom and Johnson, 2003).
Undirected song is related to reward state
This is the fourth study to provide evidence for a positive linear relationship between undirected singing and reward state (measured via CPP; Riters and Stevenson, 2012; Riters et al., 2014). Our hypothesis regarding reward associated with undirected singing is that neurochemicals associated with reward are released prior to or during the act of singing. Given the nature of singing behavior, song production and the lack of song production cannot be equally paired with one side of the apparatus during the conditioning phase (discussed in detail in Riters and Stevenson 2012), so one side of the apparatus (i.e., the conditioned side) may be more familiar to the individual. However, we do not have compelling reasoning to explain why the familiar side of the apparatus would be more appealing to birds that sing higher rates of songs (compared to birds that sing fewer songs); therefore, we suggest that the more parsimonious explanation is that singing high rates of song is associated with a positive affective state and birds that sing high rates of song spend more time on the previously-conditioned side of the apparatus because it is associated with this positive affective state. In addition, we only included birds that explored both sides of the apparatus to ensure that neither side was completely novel to the individual. See Riters and Stevenson (2012) for a full discussion of the interpretational limitations of our CPP methodology.
Consistent with the notion that individual propensity to sing is constant, and the related neural expression is constitutive, we found evidence that individual song rate was consistent across days; similar findings of consistency in song production across days have been reported by previous studies (e.g., Naguib et al., 2010; Riters et al., 2014). The current results support the hypothesis that undirected singing is stimulated and/or reinforced by intrinsic reward processes, as birds that developed a song-associated preference sang more songs compared to birds that did not demonstrate a conditioned preference.
CB1 expression in VTA and PAG relate to song-associated reward
There was a significant positive relationship between CPP (measured as the amount of time on test day spent on the conditioned side of the apparatus), the number of songs a bird produced, and relative CB1 expression in VTA. VTA is a brain region strongly implicated in reward and motivation (Fields et al., 2007; O'Connell and Hofmann, 2011; Riters, 2012). In mammals, there is evidence that endocannabinoids in VTA regulate reward and motivational processes by influencing dopamine release (Oleson et al., 2012; Sagheddu et al., 2015). Specifically, in VTA, dopamine neurons release endocannabinoids, which then interact with CB1 receptors on GABAergic and glutamatergic axon terminals and inhibit neurotransmitter release (Melis et al., 2004; Riegel & Lupica, 2004). In VTA, endocannabinoids reduce the GABAergic inhibition of dopaminergic neurons (Szabo et al., 2002) and this is a possible mechanism for dopaminergic neurons in VTA to fine-tune their own activity (Riegel and Lupica, 2004; Wang and Lupica, 2014) and mediate reward-related behavior (Oleson et al., 2012). In addition, VTA is well-positioned to influence the motivation to produce vocalizations, as VTA projects directly to song control nuclei including Area X (Lewis et al., 1981; Appeltants et al., 2000). Consistent with the idea that CB1 receptors in VTA play a role in the motivation associated with social communication, a previous study with male European starlings in breeding condition found that CB1 receptors in VTA positively correlated with singing and males with nest sites produced more song, suggesting that singing was related to a bird's motivational state (i.e., males with nest sites are more sexually-motivated compared to males without nest sites; DeVries et al., 2016). Our results also indicate a positive relationship between CB1 receptors in VTA and reward or motivation associated with singing.
We also found a significant negative relationship between CPP and relative CB1 expression in PAG. This is consistent with prior studies suggesting a role for PAG in undirected song (Lynch et al., 2008; Kelm-Nelson and Riters, 2013). PAG has a role in reward (e.g., Olmstead and Franklin, 1997) and vocal communication in mammals (Jürgens, 1994). In songbirds there are projections from PAG to brain regions critical for song production (Appeltants et al., 2000) and PAG receives projections from other brain regions involved in motivation (such as POM; Riters and Alger, 2004). PAG is thus anatomically well-positioned to integrate information about motivational state and then send this information to brain regions critical for vocal production, resulting in an individual producing vocalizations indicative of its affective state, as proposed in studies in mammals (Jürgens and Pratt, 1979; Dubbeldam and den Boer-Visser, 2002; Dujardin & Jürgens, 2005).
In addition to reward, cannabinoids (Finn et al., 2003) and opioids (Jensen and Yaksh, 1986) in PAG induce analgesia in mammals and analgesia can thus serve as an indirect measure of opioid or cannabinoid release. Consistent with this, previous studies with male starlings found that undirected song was tightly coupled to analgesia (Kelm-Nelson et al., 2012) and mu opioid receptor (MOR) labeling in PAG related to undirected song, with high and low singers exhibiting lower receptor labeling (compared to intermediate singers), resulting in a curvilinear relationship between undirected song and MOR (Kelm-Nelson and Riters, 2013). The results of the current study indicate a negative linear relationship between CB1 and song-associated reward. MOR and CB1 receptors co-localize in PAG (Wilson-Poe et al., 2012), so it is possible that our results are highlighting the negative portion of the curve found by Kelm-Nelson and Riters (2013). However, double-labeling studies examining MOR and CB1 receptors in PAG in males producing undirected song are needed.
FABP7 expression in PAG and POM relate to song-associated reward
We found significant relationships between CPP and FABP7 in PAG and FABP7 in POM. While our correlation analysis revealed a significant positive relationship between song rate and CPP, song rate was not a significant predictor variable in this regression analysis with FABP7, so it is possible that the relationship between FABP7 and CPP is not mediated by singing. FABPs are intracellular transporters of endocannabinoids (Kaczocha et al., 2009; Sanson et al., 2013); however, FABPs may also store endocannabinoid ligands, (Howlett et al., 2011), suggesting that endocannabinoids can accumulate within a cell. Our results indicate that individuals that are in a positive affective state may have fewer endocannabinoid ligands in PAG compared to birds not in a positive affective state, which is consistent with the negative relationship we found between CPP and CB1 expression in PAG.
Birds that developed a song-associated preference (compared to those that did not) had more FABP7 expression in POM. There are direct connections between POM and other brain regions in which endocannabinoids are involved in motivation and reward (e.g., VTA: Oleson et al., 2012; Sagheddu et al., 2015), including regions that the current study found significantly related to song-associated reward (i.e., VTA, PAG). POM also projects both directly and indirectly to song control regions (Riters and Alger, 2004). Opioids in POM relate to reward (Ågmo and Gómez, 1991; Ågmo and Gómez, 1993) and in POM are tightly coupled to song-associated CPP (Riters et al., 2014). The current results suggest that birds in a positive affective state may have more endocannabinoids in POM; however, more research is needed to examine the relationship between endocannabinoids in POM and reward.
DAGLɑ and FABP5 expression in Area X relate to undirected song
Area X, a song control nucleus in songbirds that is involved in song learning (Sohrabji et al., 1990; Scharff and Nottebohm, 1991) and song variability (Leblois et al., 2010; Leblois and Perkel, 2012) does not have a definitive role in reward processes comparable to other brain regions. Consistent with this, endocannabinoid-related genes in Area X did not relate to reward. We examined the relationship between undirected song production and endocannabinoid-related genes in Area X, because CB1 is expressed in Area X (Soderstrom and Tian, 2006) and cannabinoid exposure during development alters songbird vocal production (Soderstrom and Johnson, 2003), leading to long-term impairment of the endocannabinoid system (Soderstrom et al., 2011). It has also been proposed that DAGLɑ and CB1 in Area X have a role in vocal learning (Soderstrom and Tian, 2006; Soderstrom and Wilson, 2013). Our results are consistent with this previous research suggesting that the endocannabinoid system has a role in songbird vocal development and production; we found significant positive correlations between how much song a bird produced and FABP5 (an intracellular transporter) and DAGLα (the synthesizing enzyme for 2-AG) expression in Area X. As adults, European starlings continue to learn and add elements into their songs. Singing outside of the breeding season (such as the undirected singing examined in the current study) may be important for this song modification and practice.
We did not find a significant relationship between singing and CB1 expression in Area X, a result that is consistent with a previous study examining CB1 receptor expression in male starlings producing breeding song (DeVries et al., 2016). Taken together, these results suggest that in adult starlings there are similar levels of relative CB1 receptor expression in Area X regardless of how much a bird sings. However, other endocannabinoid-related genes responsible for the production and transport of endocannabinoids have higher levels in birds that sing more undirected song, suggesting that high singers may have more endocannabinoid ligands in Area X. Area X indirectly projects to VTA via the ventral pallidum and dopaminergic neurons in VTA project back to Area X, forming an anatomical loop (Gale et al., 2008). This could be a mechanism for endocannabinoids produced in Area X to mediate reward-related behaviors by interacting with CB1 receptors in VTA, thus motivating song production. Consistent with this idea, not only do the current results indicate that an endocannabinoid synthesizing enzyme (DAGLα) in Area X positively correlates with singing, but the results also indicate that CB1 receptor expression in VTA positively correlates with song-associated reward.
Conclusions
Endocannabinoids have been implicated in the reward processes associated with numerous behaviors including eating, social interactions, and social play (reviewed in Fattore et al., 2010), and the current results indicate a role for endocannabinoids in undirected communication associated with a positive affective state. Previous studies have provided evidence linking dopamine (Heimovics et al., 2009; Merullo et al., 2016) and opioids (Khurshid et al., 2010; Riters et al., 2014) with undirected communication. Given that the endocannabinoid system interacts with these and other neurochemical systems to modulate motivation and reward (Solinas et al., 2007; 2008), future work should examine how these neural systems interact in the reward processes associated with vocal communication. It should be noted that in the current study we quantified mRNA expression and not the final protein product. Therefore the final site of action for the translated protein may be different from the regions in which we quantified mRNA expression. Additionally, the current results are correlational, but a critical first step in examining the extent to which endocannabinoid-related genes relate to song-associated reward. Additional studies are now needed to determine the causal role of endocannabinoids in song production and song-associated reward.
Male starlings producing a high rate of undirected song developed a song-associated conditioned place preference
CB1 mRNA expression in VTA and FABP7 mRNA expression in POM positively correlated with song-associated reward
CB1 and FABP7 mRNA expression in PAG negatively correlated with song-associated reward
DAGLα and FAPB5 mRNA expression in Area X positively correlated with singing
Endocannabinoid signaling may play a role in the reward associated with undirected communication
Acknowledgments
This work was supported by National Institute of Mental Health grant R01MH080225 to LVR. AHH was supported by a Michael Guyer Postdoctoral Fellowship from the UW-Madison Department of Zoology. We thank Dr. Melissa A. Cordes for technical assistance and Chris Elliott, Kate Skogen, and Jeffrey Alexander for animal care.
Abbreviations
- 2-AG
2-arachidonylglycerol
- CPP
conditioned place preference
- DAGLɑ
diacylglycerol lipase-ɑ
- FAAH
fatty acid amide hydrolase
- FABP
fatty acid-binding protein
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HMBS
hydroxymethylbilane synthase
- PAG
periaqueductal gray
- POM
medial preoptic area
- qPCR
quantitative real-time polymerase chain reaction
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ågmo A, Gómez M. Conditioned place preference produced by infusion of metenkephalin into the medial preoptic area. Brain Res. 1991;550:343–346. doi: 10.1016/0006-8993(91)91339-3. [DOI] [PubMed] [Google Scholar]
- Ågmo A, Gómez M. Sexual reinforcement is blocked by infusion of naloxone into the medial preoptic area. Behav Neurosci. 1993;107:812–818. doi: 10.1037//0735-7044.107.5.812. [DOI] [PubMed] [Google Scholar]
- Adret-Hausberger M, Güttinger HR, Merkel FW. Individual life history and song repertoire changes in a colony of starlings (Sturnus vulgaris) Ethology. 1990;84:265–280. [Google Scholar]
- Appeltants D, Absil P, Balthazart J, Ball GF. Identification of the origin of catecholaminergic inputs to HVc in canaries by retrograde tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Chem Neuroanat. 2000;18:117–133. doi: 10.1016/s0891-0618(99)00054-x. [DOI] [PubMed] [Google Scholar]
- Böhner J, Chaiken M, Ball GF, Marler P. Song acquisition in photosensitive and photorefractory male European starlings. Horm Behav. 1990;24:582–594. doi: 10.1016/0018-506x(90)90043-w. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Younts TJ, Chávez AE, Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron. 2012;76:70–81. doi: 10.1016/j.neuron.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiken M, Böhner J, Marler P. Repertoire turnover and the timing of song acquisition in European starlings. Behaviour. 1994;128:25–39. [Google Scholar]
- Cordes MA, Stevenson SA, Driessen TM, Eisinger BE, Riters LV. Sexually-motivated song is predicted by androgen- and opioid-related gene expression in the medial preoptic nucleus of male European starlings (Sturnus vulgaris) Behav Brain Res. 2015;278:12–20. doi: 10.1016/j.bbr.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic Control of Seasonality in Birds. J Biol Rhythm. 2001;16:365–380. doi: 10.1177/074873001129002079. [DOI] [PubMed] [Google Scholar]
- DeVries MS, Cordes MA, Rodriguez JD, Stevenson SA, Riters LV. Neural endocannabinoid CB1 receptor expression, social status, and behavior in male European starlings. Brain Res. 2016;1644:240–24. doi: 10.1016/j.brainres.2016.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens MJ, Vecchiarelli HA, Hill MN, Bentley GE. Endocannabinoid signaling in the stress response of male and female songbirds. Endocrinology. 2015;156:4649–4659. doi: 10.1210/en.2015-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubbeldam JL, den Boer-Visser AM. The central mesencephalic grey in birds: nucleus intercollicularis and substantia grisea centralis. Brain Research Bulletin. 2002;57:349–352. doi: 10.1016/s0361-9230(01)00689-x. [DOI] [PubMed] [Google Scholar]
- Dujardin E, Jürgens U. Afferents of vocalization-controlling periaqueductal regions in the squirrel monkey. Brain Res. 2005;1034:114–131. doi: 10.1016/j.brainres.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Dunn AM, Zann RA. Undirected song in wild zebra finch flocks: Contexts and effects of mate removal. Ethology. 1996;102:529–539. [Google Scholar]
- Dunning JL, Pant S, Bass A, Coburn Z, Prather JF. Mate Choice in Adult Female Bengalese Finches: Females Express Consistent Preferences for Individual Males and Prefer Female-Directed Song Performances. PLoS ONE. 2014;9(2):e89438. doi: 10.1371/journal.pone.0089438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eens M. Understanding the complex song of the European starling: An integrated ethological approach. Adv Stud Behav. 1997;26:355–434. [Google Scholar]
- Elphick MR. The evolution and comparative neurobiology of endocannabinoid signalling. Philos T R Soc B. 2012;367:3201–3215. doi: 10.1098/rstb.2011.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Melis M, Fadda P, Pistis M, Fratta W. The endocannabinoid system and nondrug rewarding behaviours. Exp Neurol. 2010;224:23–36. doi: 10.1016/j.expneurol.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Feare CJ. The Starling. Oxford: Oxford University Press; 1984. [Google Scholar]
- Fee MS, Kozhevnikov AA, Hahnloser RHR. Neural mechanisms of vocal sequence generation in the songbird. Ann NY Acad Sci. 2004;1016:153–170. doi: 10.1196/annals.1298.022. [DOI] [PubMed] [Google Scholar]
- Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- Finn DP, Jhaveri MD, Beckrett SRG, Roe CH, Kendall DA, Marsden CA, Chapman V. Effects of direct periaqueductal gray administration of a cannabinoid receptor agonist on nociceptive and aversive responses in rats. Neuropharmacology. 2003;45:594–604. doi: 10.1016/s0028-3908(03)00235-1. [DOI] [PubMed] [Google Scholar]
- Gale SD, Person AL, Perkel DJ. A novel basal ganglia pathway forms a loop linking a vocal learning circuit with its dopaminergic input. J Comp Neurol. 2008;508:824–839. doi: 10.1002/cne.21700. [DOI] [PubMed] [Google Scholar]
- Hausberger M, Richard-Yris MA, Henry L, Lepage L, Schmidt I. Song sharing reflects the social organization in a captive group of European starlings (Sturnus vulgaris) J Comp Psychol. 1995;109:222–241. [Google Scholar]
- Heimovics SA, Cornil CA, Ball GF, Riters LV. D1-like dopamine receptor density in nuclei involved in social behavior correlates with song in a context-dependent fashion in male European starlings. Neuroscience. 2009;159:962–973. doi: 10.1016/j.neuroscience.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman LA, Saravanan V, Wood AN, He L, Sober SJ. Dopaminergic contributions to vocal learning. J Neurosci. 2016;36:2176–2189. doi: 10.1523/JNEUROSCI.3883-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holveck MJ, Riebel K. Preferred songs predict preferred males: consistency and repeatability of zebra finch females across three test contexts. Anim Behav. 2007;74:297–309. [Google Scholar]
- Howlett AC, Reggio PH, Childers SR, Hampson RE, Ulloa NM, Deutsch DG. Endocannabinoid tone versus constitutive activity of cannabinoid receptors. Brit J of Pharmacol. 2011;163:1329–1343. doi: 10.1111/j.1476-5381.2011.01364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TS, Yaksh TL. Comparison of antinociceptive action of morphine in the periaqueductal gray, medial and paramedical medulla in rat. Brain Res. 1986;1:99–113. doi: 10.1016/0006-8993(86)90662-1. [DOI] [PubMed] [Google Scholar]
- Jesse F, Riebel K. Social facilitation of male song by male and female conspecifics in the zebra finch, Taeniopygia guttata. Behav Process. 2012;91:262–266. doi: 10.1016/j.beproc.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Jürgens U. The role of the periaqueductal grey in vocal behaviour. Behav Brain Res. 1994;62:107–117. doi: 10.1016/0166-4328(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Jürgens U, Pratt R. Role of the periaqueductal grey in vocal expression of emotion. Brain Res. 1979;167:367–378. doi: 10.1016/0006-8993(79)90830-8. [DOI] [PubMed] [Google Scholar]
- Kaczocha M, Glaser ST, Deutsch DG. Identification of intracellular carriers for the endocannabinoid anandamide. P Natl Acad Sci USA. 2009;106:6375–6380. doi: 10.1073/pnas.0901515106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Kao MH, Doupe AJ, Brainard MS. Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature. 2005;433:638–643. doi: 10.1038/nature03127. [DOI] [PubMed] [Google Scholar]
- Kelm-Nelson CA, Riters LV. Curvilinear relationships between mu-opioid receptor labeling and undirected song in male European starlings (Sturnus vulgaris) Brain Res. 2013;1527:29–39. doi: 10.1016/j.brainres.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm-Nelson CA, Stevenson SA, Riters LV. Context-dependent links between song production and opioid-mediated analgesia in male European starlings (Sturnus vulgaris) PLoS ONE. 2012;7:e46721. doi: 10.1371/journal.pone.0046721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurshid N, Jayaprakash N, Hameed LS, Mohanasundaram S, Iyengar S. Opioid modulation of song in male zebra finches (Taenopygia guttata) Behav Brain Res. 2010;208:359–370. doi: 10.1016/j.bbr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Leblois A, Perkel DJ. Striatal dopamine modulates song spectral but not temporal features through D1 receptors. Eur J Neurosci. 2012;35:1771–1781. doi: 10.1111/j.1460-9568.2012.08095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblois A, Wendel BJ, Perkel DJ. Striatal Dopamine Modulates Basal Ganglia Output and Regulates Social Context-Dependent Behavioral Variability through D1 Receptors. J Neurosci. 2010;30:5730–5743. doi: 10.1523/JNEUROSCI.5974-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JW, Ryan SM, Arnold AP, Butcher LL. Evidence for a catecholaminergic projection to area X in the zebra finch. J Comp Neurol. 1981;196:347–354. doi: 10.1002/cne.901960212. [DOI] [PubMed] [Google Scholar]
- Lynch KS, Diekamp B, Ball GF. Catecholaminergic cell groups and vocal communication in male songbirds. Phy Behav. 2008;93:870–876. doi: 10.1016/j.physbeh.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Pistis M, Perra S, Muntoni AL, Pillolla G, Gessa GL. Endocannabinoids mediate presynaptic inhibition of glutamatergic transmission in rat ventral tegmental area dopamine neurons through activation of CB1 receptors. J Neurosci. 2004;24:53–62. doi: 10.1523/JNEUROSCI.4503-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merullo DP, Angyal CS, Stevenson SA, Riters LV. Song in an affiliative context relates to the neural expression of dopamine- and neurotensin-related genes in male European starlings. Brain Behav Evolut. 2016 doi: 10.1159/000448191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merullo DP, Cordes MA, DeVries MS, Stevenson SA, Riters LV. Neurotensin neural mRNA expression correlates with vocal communication and other highly-motivated social behaviors in male European starlings. Physiol Behav. 2015;151:155–161. doi: 10.1016/j.physbeh.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. The reproductive behavior of the zebra finch (Poephila guttata), with special reference to pseudofemale behavior and displacement activities. Behaviour. 1954;6:271–322. [Google Scholar]
- Naguib M, Kazek A, Schaper SV, van Oers K, Visser ME. Singing activity reveals personality traits in great tits. Ethology. 2010;116:763–769. [Google Scholar]
- O'Connell LA, Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: A comparative synthesis. J Comp Neurol. 2011;519:3599–3639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Beckert MV, Morra JT, Lansink CS, Cachope R, Abdullah RA, Loriaux AL, Schetters D, Pattij T, Roitman MF, Lichtman AH, Cheer JF. Endocannabinoids shape accumbal encoding of cue-motivated behavior via CB1 receptor activation in the ventral tegmentum. Neuron. 2012;73:360–373. doi: 10.1016/j.neuron.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead MC, Franklin KBJ. The development of a conditioned place preference to morphine: Effects of microinjections into various CNS sites. Behav Neurosci. 1997;111:1324–1334. doi: 10.1037//0735-7044.111.6.1324. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegel AC, Lupica CR. Independent presynaptic and postsynaptic mechanisms regulate endocannabinoid signaling at multiple synapses in the ventral tegmental area. J Neurosci. 2004;24:11070–11078. doi: 10.1523/JNEUROSCI.3695-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV. Evidence for opioid involvement in the motivation to sing. J Chem Neuroanat. 2010;39:141–150. doi: 10.1016/j.jchemneu.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV. Pleasure seeking and birdsong. Neurosci Biobehavi R. 2011;35:1837–1845. doi: 10.1016/j.neubiorev.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV. The role of motivation and reward neural systems in vocal communication in songbirds. Front Neuroendocrin. 2012;33:194–209. doi: 10.1016/j.yfrne.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV, Alger SJ. Neuroanatomical evidence for indirect connections between the medial preoptic nucleus and the song control system: possible neural substrates for sexually motivated song. Cell Tissue Res. 2004;316:35–44. doi: 10.1007/s00441-003-0838-6. [DOI] [PubMed] [Google Scholar]
- Riters LV, Eens M, Pinxten R, Duffy DL, Balthazart J, Ball GF. Seasonal changes in courtship song and the medial preoptic area in male European starlings (Sturnus vulgaris) Horm Behav. 2000;38:250–261. doi: 10.1006/hbeh.2000.1623. [DOI] [PubMed] [Google Scholar]
- Riters LV, Schroeder MB, Auger CJ, Eens M, Pinxten R, Ball GF. Evidence for opioid involvement in the regulation of song production in male European starlings (Sturnus vulgaris) Behav Neurosci. 2005;119:245–255. doi: 10.1037/0735-7044.119.1.245. [DOI] [PubMed] [Google Scholar]
- Riters LV, Stevenson SA. Reward and vocal production: Song-associated place preference in songbirds. Physiol Behav. 2012;106:87–94. doi: 10.1016/j.physbeh.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV, Stevenson SA, DeVries MS, Cordes MA. Reward associated with singing behavior correlates with opioid-related gene expression in the medial preoptic nucleus in male European starlings. PLoS ONE. 2014;9:e115285. doi: 10.1371/journal.pone.0115285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagheddu C, Muntoni AL, Pistis M, Melis M. Endocannabinoid signalling in motivation, reward, and addiction: influences on mesocorticolimbic dopamine function. Int Rev Neurobiol. 2015;125:257–302. doi: 10.1016/bs.irn.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Sanson B, Wang T, Sun J, Wang L, Kaczocha M, Ojima I, Deutsch D, Li H. Crystallographic study of FABP5 as an intracellular endocannabinoid transporter. Acta Crystallogr D. 2013;70:290–298. doi: 10.1107/S1399004713026795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderstrom K, Johnson F. Cannabinoid exposure alters learning of zebra finch vocal patterns. Dev Brain Res. 2003;142:215–217. doi: 10.1016/s0165-3806(03)00061-0. [DOI] [PubMed] [Google Scholar]
- Soderstrom K, Poklis JL, Lichtman AH. Cannabinoid exposure during zebra finch sensorimotor vocal learning persistently alters expression of endocannabinoid signaling elements and acute agonist responsiveness. BMC Neurosci. 2011;12:1. doi: 10.1186/1471-2202-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderstrom K, Tian Q. Developmental pattern of CB1 cannabinoid receptor immunoreactivity in brain regions important to zebra finch (Taeniopygia guttata) song learning and control. J Comp Neurol. 2006;496:739–758. doi: 10.1002/cne.20963. [DOI] [PubMed] [Google Scholar]
- Soderstrom K, Wilson AR. Developmental pattern of diacylglycerol lipase-α (DAGLα) immunoreactivity in brain regions important for song learning and control in the zebra finch (Taeniopygia guttata) J Chem Neuroanat. 2013;53:41–59. doi: 10.1016/j.jchemneu.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Nordeen EJ, Nordeen KW. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav Neural Biol. 1990;53:51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR, Piomelli D. The endocannabinoid system in brain reward processes. Brit J Pharmacol. 2008;154:369–383. doi: 10.1038/bjp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Yasar S, Goldberg SR. Endocannabinoid system involvement in brain reward processes related to drug abuse. Pharmacol Res. 2007;56:393–405. doi: 10.1016/j.phrs.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossinka R, Böhner J. Song types in the zebra finch Poephila guttata castanotis. Z Tierpsychol. 1980;53:123–132. [Google Scholar]
- Szabo B, Siemes S, Wallmichrath I. Inhibition of GABAergic neurotransmission in the ventral tegmental area by cannabinoids. Eur J Neurosci. 2002;15:2057–2061. doi: 10.1046/j.1460-9568.2002.02041.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Lupica CR. Release of endogenous cannabinoids from ventral tegmental area dopamine neurons and the modulation of synaptic processes. Prog Neuro-Psychoph. 2014;52:24–27. doi: 10.1016/j.pnpbp.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Wilson-Poe AR, Morgan MM, Aicher SA, Hegarty DM. Distribution of CB1 cannabinoid receptors and their relationship with mu-opioid receptors in the rat periaqueductal gray. Neuroscience. 2012;213:191–200. doi: 10.1016/j.neuroscience.2012.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]


