Abstract
The CCAAT/Enhancer Binding Protein β (C/EBPβ) transcription factor is activated by multiple inflammatory stimuli, including IL-17 and LPS, and C/EBPβ itself regulates numerous genes involved in inflammation. However, the role of C/EBPβ in driving autoimmunity is not well understood. Here, we demonstrate that Cebpb−/− mice are resistant to EAE. Cebpb−/− mice exhibited reduced lymphocyte and APC infiltration into CNS following EAE induction. Furthermore, MOG-induced Th17 cytokine production was impaired in draining LN, indicating defects in Th17 cell priming. In vitro Th17 polarization studies indicated that T cell responses are not inherently defective, instead supporting the known roles for C/EBPβ in myeloid lineage cell activation as the likely mechanism for defective Th17 priming in vivo. However, we did uncover an unexpected role for C/EBPβ in regulating ll23r expression in APCs. ChIP assays confirmed that C/EBPβ binds directly to the Il23r gene promoter in dendritic cells and Th17 cells. These data establish C/EBPβ as a key driver of autoimmune inflammation in EAE, and propose a novel role for C/EBPβ in regulation of IL-23R expression.
Keywords: C/EBPβ, transcription factor, EAE, IL-17, Th17, IL-23R
Introduction
The NIH estimates that 5–8% of the US population suffers from an autoimmune disorder [1, 2]. Despite improvements in treatment due in part to anti-cytokine biologic drugs, the underlying etiology of autoimmunity remains poorly understood [3]. A major advance in understanding autoimmune pathogenesis came in 2005 with the discovery of Th17 cells, a new class of CD4+ T helper cells. Characterized by expression of IL-17 (IL-17A), IL-17F, IL-22 and GM-CSF, these cells play a non-redundant function in immunity to extracellular microbes, particularly fungi [4]. However, Th17 cells are dysregulated in many autoimmune diseases. Th17 cells differentiate from naïve Th0 cells under conditions of inflammation, driven by signals from TGFβ, IL-6, IL-1 and IL-21 [5]. During differentiation, Th17 cells express the IL-23 receptor (IL-23R), and signals from IL-23 are necessary for these cells to be pathogenic [6–9]. Indeed, antibodies against IL-17A and IL-23 are effective in treating autoimmunity, particularly plaque psoriasis [10, 11]. These biologics are also under evaluation for ankylosing spondylitis, uveitis and multiple sclerosis [12–14].
The IL-17 cytokine family is the newest and least well-understood of the cytokine subclasses [15, 16]. IL-17 and IL-17F signal through a heterodimeric receptor composed of IL-17RA and IL-17RC, and the primary cellular targets of IL-17 are non-hematopoietic cells [16]. Upon engagement with IL-17, the receptor recruits the adaptor Act1 (also known as CIKS), which activates the classical NF-κB pathway, MAPK pathways, and a TRAF2/5-dependent cascade that promotes mRNA stability [3, 17]. In addition, IL-17 activates the CCAAT/Enhancer Binding Protein (C/EBP) family of transcription factors, particularly C/EBPβ [18–24], but the biological role of this pathway is not well defined. Cebpb−/− mice are highly susceptible to systemic Candida albicans and Listeria monocytogenes infections, clearance of which requires IL-17 [25, 26]. In contrast, Cebpb−/− mice were found to be resistant to oral candidiasis, showing increased susceptibility only under conditions of steroid-induced immunosuppression [27]. Hence, it does not appear that all immune responses are defective in the absence of C/EBPβ, and the requirement for C/EBPβ in IL-17 signaling is not absolute in all circumstances.
C/EBPβ plays diverse roles in inflammation through a variety of cell types [28, 29]. For example, C/EBPβ is a central regulator of adipocyte differentiation [30]. Additionally, studies in vivo have identified especially important functions in macrophage populations, [31] [26, 32]. However, to date its function in autoimmunity is not well defined.
The experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis is frequently employed for defining parameters of T cell-driven autoimmunity, particularly Th17 cells [33, 34]. Mice deficient in IL-17, IL-23 or the associated receptors are resistant to EAE [35]. IL-17 promotes expression of chemokines that recruit myeloid cells to the CNS, and also acts directly to damage CNS-resident cells such as oligodendrocytes. IL-23 also promotes Th17 production of GM-CSF that activates recruited macrophages as well as CNS-resident microglia to further promote tissue inflammation [36–38]. Indeed, macrophage recruitment and activation as well as DC presentation of myelin antigens are required for development of EAE [39]. Notably, the CCAAT/Enhancer Binding Protein (C/EBPβ) transcription factor (TF) is upregulated in human MS tissue samples [40]. A recent GWAS study of MS patients identified a risk allele in a C/EBPβ binding element in the promoter of CBL-B (Casitas B-lineage lymphoma proto-oncogene b) [41]. To date, however, the role of C/EBPβ) in EAE has not been assessed.
In this study, we assessed the role of C/EBPβ in Th17-dependent autoimmunity in EAE. We found that CNS in Cebpb−/− mice was associated with decreased infiltration of lymphocytes and APCs as well as reduced expression of Th17 cytokines in the CNS. LN expression of the IL-23 receptor (IL-23R) was reduced in Cebpb−/− mice during EAE, and ChIP analyses confirmed that C/EBPβ binds to the Il23r promoter in Th17 cells and bone marrow derived myeloid cells. Thus, we conclude that C/EBPβ contributes to Th17-dependent regulation of inflammation in EAE.
Materials and Methods
Mice
Cebpbtm1Vpo/J+/+ mice from The Jackson Laboratory (JAX, Bar Harbor ME) were bred to generate Cebpb−/− and Cebpb+/+ littermate controls. C57BL/6 mice were also from JAX.
EAE
Cebpb−/− and littermates were immunized s.c. with 100 μg MOG peptide (residues 35–55) in CFA containing 500 μg heat killed Mycobacterium tuberculosis as described [42]. Mice received 100 ng pertussis toxin (List Biological Laboratories, Campbell CA) i.p. on days 0 and 2. Disease severity was evaluated using the the following scale: 1: flaccid tail; 2: impaired righting reflex and hindlimb weakness; 3: partial hindlimb paralysis; 4: complete hindlimb paralysis; 5: hindlimb paralysis with partial forelimb paralysis; 6: moribund. All procedures were approved by the University of Pittsburgh IACUC.
qPCR
RNA was isolated with RNAeasy Kits (Qiagen, Gaithersburg, MD), and cDNA was created with the SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad CA). Quantification was determined by real-time PCR with SYBR Green (Quanta BioSciences, Beverly MA) normalized to Gapdh. Primers were from Qiagen. Results were analyzed on a 7300 Real Time PCR System (Applied Biosystems, Carlsbad, CA).
Cell cultures, Flow cytometry, ELISAs
Bone marrow-derived myeloid cells were generated by cultured bone marrow cells with GM-CSF (10ng/ml) for 6-8 d. When indicated, BM-derived myeloid cells were stimulated with lipopolysaccharide (lps) (100ng/ml), TNFα (10ng/ml) or GM-CSF (10ng/ml) for 24 h. CD4+ T cells were isolated from LN and spleen of naïve animals by positive selection using Miltenyi Biotec (San Diego, CA) CD4 (L3T4) MicroBeads and LS columns. 106 CD4+ cells were stimulated with plate bound anti-CD3 (5ug/ml, Bio X Cell, Lebanon, NH) for 3 days. Differentiation conditions were: Th0, anti-IFNγ (10ug/ml); Th17, TGFβ (2.5ng/ml), IL-6 (20ng/ml), IL-1β (20ng/ml), IL-23 (50ng/ml), anti-IFNγ (10ug/ml) and anti-IL-2 (10ug/ml) unless otherwise described. Cytokines were from R&D Systems (Minneapolis, MN). Differentiated cells were stimulated with 50ng/ml PMA and 500ng/ml Ionomycin (Sigma, St Louis, MO) for 4 hours with Golgi Plug (BD Biosciences). After stimulation, cells were stained for CD4 (RPA-T4, RM4-5), IL-17 (TC11-18H10). Dead cells were excluded with Ghost Dye Violet 510 (Tonbo Biosciences, San Diego CA). Intracellular cytokine staining was performed with the Cytofix Cytoperm kit (BD Biosciences). CNS preparations were stained with the following Abs: CD45 (30-F11), CD4 (RPA-T4, RM4-5), Gr1 (RB6-8C5), CD11b (M1/70), CD11c (HL3), IA/IE (2G9), CD80 (16-10A1), CD86 (GL1). Data were acquired on a FACS ARIA II or BD Fortessa (BD Biosciences) and analyzed using FlowJo (Ashland, OR). For ELISAs, supernatants were analyzed in duplicate with kits from eBiosciences.
Promoter Analysis
Promoter sequences, defined as 1.5 kb upstream of the transcription start site (TSS) for the human and murine IL-23 receptor genes, were obtained from the UCSC genome browser (University of California, Santa Cruz, CA). Conservation was assessed using LALIGN and CLC Main Workbench 6 software (Qiagen). C/EBPβ binding sites were identified using Match software (Biobase Biological Databases, Qiagen).
Chromatin immunoprecipitation
Cell suspensions were sonicated for 15 cycles at 15% amplitude (BM-myeloid cells) or 15–20 cycles at 30% amplitude (CD4+ T cells) (30 secs ON/30 secs OFF) using the Epishear Probe Sonicator (Active Motif Carlsbad, CA). ChIP was performed using the EZ-Magna ChIP A/G Chromatin Immunoprecipitation Kit (EMD Millipore, Billerica, MA). Cross-linked chromatin was immunoprecipitated with anti-C/EBPβ Abs (C19 sc-150; Santa Cruz Biotechnology, Santa Cruz CA) or non-immune rabbit IgG. Primers at positions -284 (IL-23R 01A) and -1296 (IL-23R 02A) upstream of the TSS of the Il23r promoter (Qiagen) were used for qPCR.
Statistics
At least 2 independent replicates were performed for all experiments. Data were compared by unpaired Student’s t test or ANOVA and Mann-Whitney using GraphPad Prism (v. 4). P values < 0.05 were considered significant.
Results
C/EBPβ deficient mice are resistant to EAE
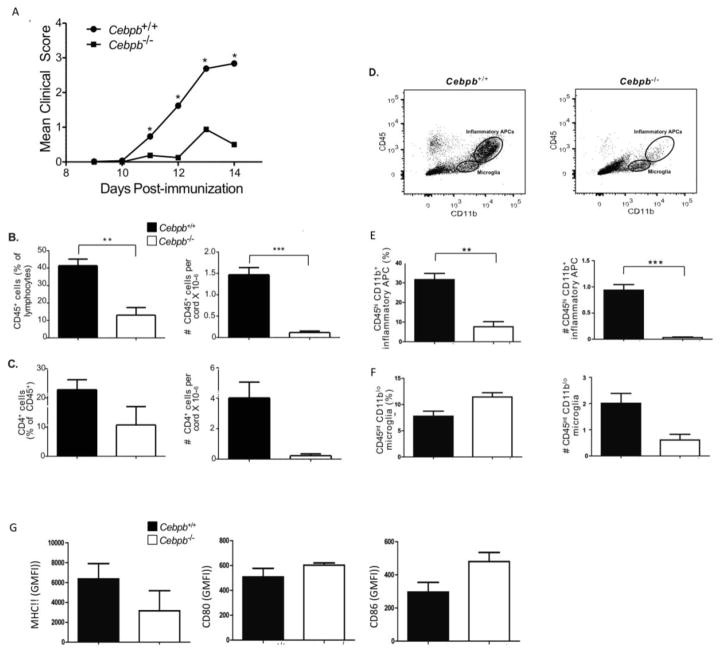
Cebpb−/− and Cebpb+/+ controls on a mixed 129S/SvEv-Gpi1 background were immunized with MOG(35–55) in CFA to induce EAE. Cebpb+/+ mice developed a typical onset and clinical course of EAE, peaking at 13–14 days post immunization, with an incidence and disease severity similar to typical EAE in WT C57BL/6 mice (Fig 1A and data not shown). At peak of disease, Cebpb−/− mice showed markedly reduced EAE disease severity compared to WT Cebpb+/+ littermate controls (Fig 1A). Concomitant with reduced disease scores, there was a significant reduction in the percentage and absolute numbers of CD45+ infiltrating mononuclear cells and in the CNS of Cebpb−/− compared to WT mice at the peak of disease following EAE induction (Fig 1B). Of the infiltrating cell populations, both frequencies and absolute numbers of CD4+ T cells (Fig 1C) and CD45hiCD11bhi macrophages were reduced (Fig 1D–F). Given that C/EBPβ is a key transcription factor for macrophage activation [31], it was perhaps surprising to note that expression of class II MHC and costimulatory molecules CD80 and CD86 by infiltrating macrophages in the CNS were not significantly different in Cebpb−/− mice (Fig 1G). Hence we concluded that Cebpb is required for full induction of the inflammatory response in autoimmune attack of the CNS during EAE and that reduced disease severity is associated with reduced recruitment of inflammatory cells to the CNS. Nonetheless, we have not ruled out a delayed onset of clinical signs in Cebpb−/− mice.
Figure 1. Cebpb−/− mice are resistant to EAE.
Cebpb+/+ and Cebpb−/− mice were immunized with MOG to induce EAE. A. Clinical EAE scores of Cebpb+/+ (n =52) and Cebpb−/− (n=9) mice, data are pooled from multiple experiments, statistical significance of EAE scores was assessed by Mann Whitney separately for each timepoint (* P <0.05). B. Percentage (left) and absolute numbers (right) of CD45+ cells gated from FSC/SSC lymphocyte gate in the CNS on day 14, Cebpb+/+ (n=31), Cebpb−/− (n=6). C. Percentage (left) and absolute numbers (right) of CD4+ cells in the CNS on day 14. Cebpb+/+ (n=15), Cebpb−/− (n=2). (D) Representative plots showing gating strategy to identify microglia and infiltrating macrophages from the CNS of C/EBPβ mice on day 14; (E) percentage (left) and absolute numbers (right) of CD45highCD11b+ infiltrating macrophages and (F) percentage (left) and absolute numbers (right) of CD45intCD11bint microglia. (G) MFI of MHC Class II (IA/IE), CD80, CD86 in CD45highCD11b+ macrophages, Cebpb+/+ (n=23–31) and Cebpb−/− (n=3–5). Data pooled from more than 3 independent experiments, except C is pooled from 2 experiments. Bars represent mean and SEM. **P<0.005 and ***P<0.0005 by student unpaired t-test.
C/EBPβ−/− T cells have impaired induction of MOG-reactive Th17 responses
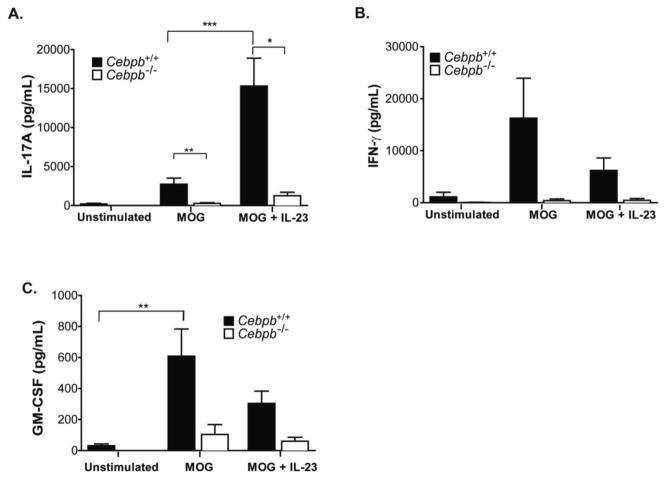
To determine whether defects in autoreactive Th17 cell priming contributed to the resistance of Cebpb−/− mice to EAE induction, we tested the LN MOG(35–55) response in immunized Cebpb−/− mice. MOG(35–55)-induced IL-17 production was markedly reduced in Cebpb−/− mice compared to WT littermates (Fig 2A). Although addition of IL-23 enhanced expression of IL-17 in WT cultures, this response was impaired in Cebpb−/− mice (Fig 2A). Production of IFNγ and GM-CSF showed a similar trend to reduced expression in Cebpb−/− compared to Cebpb+/+ mice (Fig 2B–C). Thus, T cell effector cytokines are reduced with C/EBPβ deficiency.
Figure 2. MOG-reactive IL-17 responses are reduced in Cebpb−/− LN during EAE.
Cebpb+/+ and Cebpb−/− mice were immunized with MOG to induce EAE, and day 14 draining LN cells were cultured with MOG(35–55) with or without IL-23 for 3 days before supernatants were assessed by ELISA for IL-17A (A), IFN-γ (B), and GM-CSF (C). Data are from 5 independent experiments, with Cebpb+/+ n=17–22, Cebpb−/− n=5. Bars show mean and SEM. *P<0.05, **P<0.005 and ***P<0.0005 by one-way ANOVA.
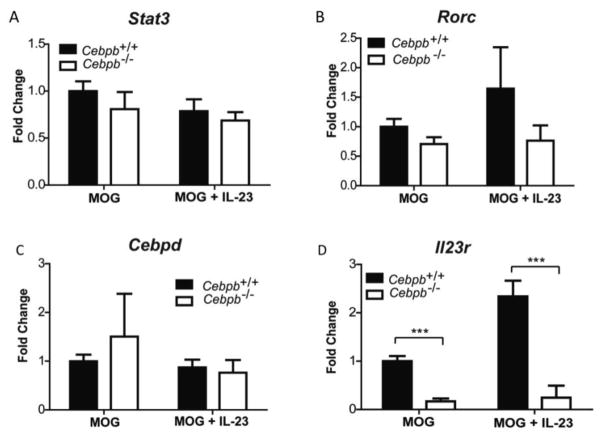
We next evaluated gene expression associated with EAE to determine which, if any, aspects of Th17 cell development were impaired in the absence of C/EBPβ. Gene expression analysis from EAE LN cultures indicated that mRNA expression of putative Th17 transcription factors STAT3 and RORγt were not significantly different in absence of C/EBPβ (Fig 3A,B). It should be noted that gene expression does not necessarily indicate function of transcription factors, which depend on post-transcriptional modifications and nuclear localization for activity, and it is not known whether C/EBPβ could also regulate these. C/EBPδ has also been shown to contribute to EAE [43], and C/EBPδ regulates C/EBPβ expression in adipocytes [20, 30]. However, there was no difference in C/EBPδ expression (Fig 3C), indicating that this did not account for the impact of C/EBPβ in this setting. Unexpectedly, expression of Il23r, a receptor required for effector Th17 proliferation and function, was significantly reduced in Cebpb−/− cultures (Fig 3D). Collectively, these data indicate that MOG-reactive Th17 cell cytokine production is impaired in absence of C/EBPβ.
Figure 3. Il23r is reduced in Cebpb−/− LN during EAE.
Cebpb+/+ and Cebpb−/− mice were immunized with MOG to induce EAE, and draining LN cells were isolated and stimulated as described for Figure 2. Gene expression of (A) Stat3 (B) Rorc (C) Cebpd and (D) Il23r were assessed by qPCR in triplicate. Data are presented as fold change compared to C/EBPβ+/+ stimulated with MOG(35–55). Data are pooled from 3 independent experiments, with Cebpb+/+ (n=7), Cebpb−/− (n=3). Bars show mean and SEM. ***P<0.0005 by one-way ANOVA.
Il23r expression is reduced in innate cells from Cebpb deficient mice
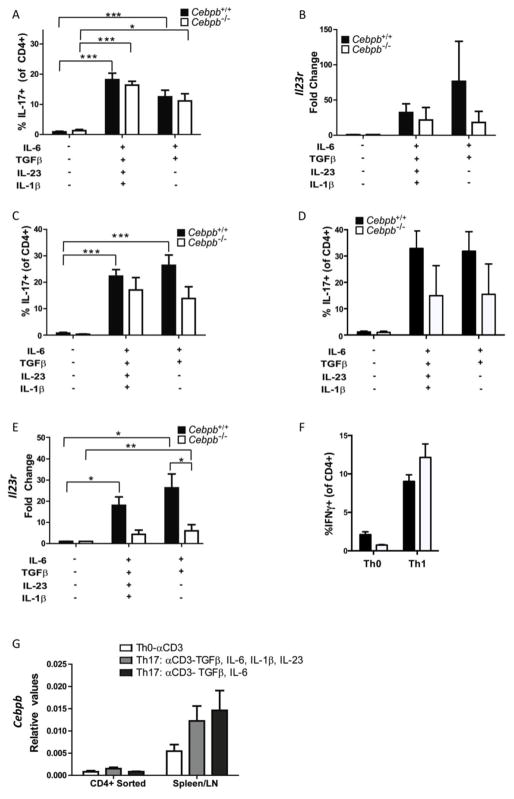
As C/EBPβ is mostly thought to act in myeloid lineage cells, it was intriguing that Th17 responses and particularly IL-23R were defective, raising the question of T cell intrinsic activities of C/EBPβ. To determine whether C/EBPβ was acting directly in T cells, CD4+ T cells were isolated from Cebpb−/− or Cebpb+/+ littermates and differentiated in vitro with anti-CD3 Abs in the absence of APCs. Induction of IL-17-producing cells under these conditions occurred independently of C/EBPβ (Fig 4A). There was a trend but no significant decrease in IL-23R in Th17 cells activated by anti-CD3 Abs (Fig 4B). In total splenocyte cultures, which contain myeloid lineage cells including DCs and macrophages, there was also a non-significant trend towards reduced IL-17 levels in Cebpb−/− T cells (Fig 4C). Addition of anti-CD28 to the cultures boosted IL-17 but did not change the overall trend towards decreased IL-17 in Cebpb−/− cells (Fig 4D). However, there was a clear and significant reduction in IL-23R mRNA expression in Cebpb−/− splenocyte cultures compared to WT controls (Fig 4E). In contrast to Th17 conditions, differentiation of Cebpb−/− Th1 cells by culture with IL-12 showed no difference from WT cells (Fig 4F), suggesting that global T cell differentiation is not impaired in Cebpb−/− cells. Finally, analysis of Cebpb expression in CD4+ T cells compared to splenocytes confirmed that C/EBPβ is not highly expressed in T cells (Fig 4E). Rather, total splenocyte cultures showed high levels of C/EBPβ (Fig 4E), consistent with a requirement for C/EBPβ for optimal IL-23R expression in that population. Albeit indirect evidence, these data suggest a novel role for C/EBPβ in regulating IL-23R, in addition to its known functions in myeloid cell activation.
Figure 4. Il23r expression is reduced in Cebpb deficient mice.
A–B: CD4+ T cells were isolated from Cebpb+/+and Cebpb−/− mice and activated with anti-CD3 in presence or absence of Th17-inducing cytokines for 3 days before analysis of IL-17A by flow cytometry (A) and expression of Il23r by qPCR (B). C–D: Total splenocytes/lymph node cells from Cebpb+/+and Cebpb−/− mice were activated with anti-CD3 in presence or absence of Th17-inducing cytokines for 3 days (C) or Th17-inducing cytokines and anti-CD28 (D) before analysis of IL-17A by flow cytometry and expression of Il23r by qPCR (E). F: Total splenocytes/lymph node cells from Cebpb+/+and Cebpb−/− mice were activated with anti-CD3 in presence or absence of IL-12 to induce Th1 cells for 3 days before analysis of IFNγ by flow cytometry. G: Cebpb expression in WT T cells and total splenocyte/lymph node cell cultures. Data are pooled from 2 independent experiments. Bars on graphs show mean and SEM. *P<0.05, **P<0.005 and ***P<0.0005 by ANOVA.
C/EBPβ binds directly to the Il23r promoter
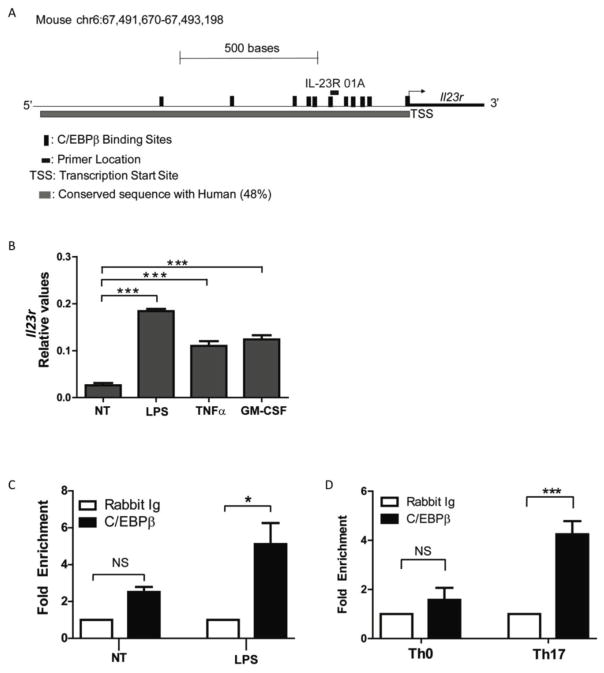
C/EBPβ has multiple activities that could lead to regulation of IL-23R expression. Using computational analysis, we identified specific C/EBPβ binding sites within the human and mouse Il23r promoter sequences, with the promoter defined as the first 1.5 kb upstream of the predicted transcriptional start site (Fig 5A). These promoters are 48% identical and indeed encode several putative C/EBPβ DNA binding elements (Fig 5A). Since IL-23R expression was clearly defective in cultures that contained innate myeloid cells, we first confirmed that bone-marrow derived myeloid cells express IL-23R in response to pro-inflammatory cytokines present in Th17 cultures: TNF and GMCSF. They also induced IL-23R in response to LPS (Fig 5B). Chromatin immunoprecipitation (ChIP) was performed with anti-C/EBPβ Abs, and genomic DNA was analyzed by qPCR for the conserved proximal promoter region of the IL-23R that contains C/EBPβ recognition elements (IL-23R-01A)) (primer position indicated in Fig 5A). The Il23r promoter sequence was enriched following C/EBPβ ChIP in LPS-stimulated myeloid cells compared to untreated cells (Fig 5C). Similarly, C/EBPβ associated with the Il23r promoter in Th17 cells more than in Th0 cells (Fig 5D). Together, these data indicate that C/EBPβ can occupy the proximal Il23r promoter, suggesting a direct role in regulating IL-23R expression.
Figure 5. C/EBPβ occupies the Il23r promoter following inflammatory stimuli.
(A) Diagram of mouse Il23r promoter. Predicted C/EBPβ binding sites and location of the Il23r qPCR primer are indicated. (B) BM-derived myeloid cells from WT mice were stimulated with lps, TNFα or GM-CSF for 24 hours. Il23r expression was assessed by qPCR in triplicate. Data are representative of 2 independent experiments. (C–D) ChIP with Abs to C/EBPβ (black bars) or IgG (white bars) was performed on genomic DNA from BM-myeloid cells from WT mice treated with lps for 24 hours (C) and from T cells differentiated for 3 days in presence or absence of Th17-inducing cytokines (D). Fold enrichment was calculated as 2−(C/EBPβ IP) − (Isotype IP)). Data are pooled from 2 (A,C) and 4 (D) independent experiments. Bars show mean + SEM. *P<0.05, and ***P<0.0005 by unpaired student t-test and ANOVA.
Discussion
Cebpb mRNA is upregulated in the brain tissue of MS patients [40]. In relapsing-remitting patients, enhanced binding of C/EBPβ to a risk-associated CBL-B allele is observed, correlating with reduced CBL-B expression in CD4+ T cells [41]. However, no mechanistic studies to date have assessed the contribution of C/EBPβ to CNS inflammation. The C/EBP transcription factors regulate numerous genes impacting inflammation and immunity [29]. Our prior observations indicated that C/EBPβ is regulated by IL-17 in multiple ways [3]. For example, Cebpb is part of the characteristic IL-17-induced gene signature [20, 22, 44, 45]. IL-17 promotes alternative translation of C/EBPβ from its dominant “LAP” (liver activated protein) isoform into the LAP* and LIP (liver inhibitory protein) species [44]. Additionally, C/EBPβ is phosphorylated following IL-17 stimulation, influencing its transcriptional activity [21, 24].
These connections led us to evaluate the role of C/EBPβ in IL-17-driven autoimmunity in the mouse model of multiple sclerosis, EAE. Indeed, we observed a reduced susceptibility of Cebpb−/− mice to EAE. The associated defect in MOG-reactive Th17 cell priming suggested a role for C/EBPβ in APC-mediated Th17 cell activation. This was further supported by the finding that T cells isolated from Cebpb−/− mice were able to differentiate Th17 cells to a similar level as WT cells when stimulated with anti-CD3 and exogenous cytokines in the absence of APC; these data also confirmed that T cells from these mice are not inherently defective. C/EBPβ is already known to regulate expression of several factors that contribute to Th17 priming, including IL-6 [28, 46, 47] hence to some extent these observations are in line with previously published actions of C/EBPβ and we did not focus our studies on these mechanisms.
One unexpected finding was reduced expression of IL-23R in Cebpb−/− cells, uncovering an additional novel mechanism through which C/EBPβ can contribute to inflammatory responses in a Th17 setting. IL-23R is not expressed on naïve T cells, but is induced by inflammatory signals from cytokines on T cells, including IL-6, IL-1, and enhanced by IL-23 itself. In the EAE model, IL-23 is required to promote late effector Th17 cell generation, including production of GM-CSF[48]. We also observed a decrease in IFNγ production. Although historically associated with Th1 responses in the EAE model, fate-tracking reporter mice have demonstrated that the majority of IFNγ in EAE actually is produced by cells of Th17 origin[49]. Furthermore, conversion to IFNγ producing cells actually requires signaling from IL-23[8, 49, 50]. For innate cells, there are a paucity of studies on the roles of IL-23R in macrophages and dendritic cells, although it has been shown to be upregulated in models of EAE, psoriasis and atopic dermatitis [51–53]. Accordingly, the regulation and function of IL-23R expression in myeloid cells is less clear than for T cells, but it is also likely to be induced by inflammatory stimuli through both cytokines and also pattern recognition receptors as we have shown here for LPS. In a model of atopic dematitis, IL-23 was shown to stimulate IL-23R+ skin dendritic cells to enhance their production of IL-23 in a feed-forward loop that potentiated local inflammation through activation of Th22 cells[53]. In EAE, IL23R+ myeloid cells were reported to express Th17-associated factors including RORγt[51–53], although the functional significance of this finding has not been clarified. Our study indicates a direct role for C/EBPβ in promoting Il23r gene expression, through binding to the proximal region of the Il23r promoter following inflammatory signals from LPS (dendritic cells) and cytokines (Th17 cells). In Th17 cells, deficiency of C/EBPβ did not appear to strongly affect the early cytokine-driven IL-23R expression in vitro, which would be expected since other transcription factors including STAT3 are strongly activated by these signals and known to also directly regulate Il23r gene expression [54]. However, this does not rule out a role for CEBPβ-23R expression in later effector Th17 cells.
It is likely that there are additional roles for C/EBPβ acting downstream of IL-17 signaling in the CNS, for example to promote expression of chemokines for recruitment of inflammatory myeloid cells, which contribute to the resistance of Cebpb−/− mice to EAE. Since these events occur after Th17 cell activation, which was also defective in the absence of C/EBPβ, the experiments performed here do not provide a conclusive answer to this question. Because Cebpb−/− mice have developmental defects that include low viability of pups, they have to be maintained on a mixed genetic background [26, 27]. Although more representative of an outbred human population, this mixed background made it impossible to perform the ‘typical’ cross-transfer experiments that one would perform to distinguish whether immune cells (and which ones) versus tissue-resident cells require C/EBPβ for EAE. Nevertheless, they provide further basis for consideration of C/EBPβ functions in Th17-mediated disease.
In addition to its impact on C/EBPβ, IL-17 induces expression of the related transcription factor C/EBPδ. In mesenchymal cell types, C/EBPβ and C/EBPδ redundantly activate target genes downstream of IL-17 such as Il6 and Cxcl5 [20, 55]. However, such functional overlap does not appear to occur in EAE, as Cebpb−/− and Cebpδ−/− mice are both resistant to disease [43]. During adipogenesis, C/EBPβ and C/EBPδ co-regulate one another [23]; however, the current study showed that Cebpd expression is normal in Cebpb−/− mice subjected to EAE, suggesting that the resistant phenotype in C/EBPβ−/− mice is not secondary to C/EBPδ-deficiency.
In conclusion, our data reveal that C/EBPβ plays a vital role in the development of CNS inflammation in the EAE model, with impaired development of myelin-reactive Th17 cells and reduced recruitment of inflammatory infiltrating cells to the CNS in Cebpb−/− mice. These results are in accordance with previous studies highlighting the role of C/EBPβ in promoting a cycle of inflammatory responses to promote recovery from infection, but have not been shown before for autoimmune disease. Furthermore, we have uncovered an unexpected role for C/EBPβ in regulating expression of IL-23R, a key lynchpin in Th17 autoimmune disease, and raise the possibility of a pathogenic role for C/EBPβ in other IL-23-dependent autoimmune settings.
HIGHLIGHTS.
Cebpb−/− mice are resistant to EAE
In vivo Th17 responses are impaired in absence of C/EBPβ
Th17 differentiation is normal in APC-free cultures
C/EBPβ binds to the IL-23R in myeloid and Th17 cells
Acknowledgments
We thank Bianca Coleman for excellent technical assistance and Drs. Dhan Kalvakolanu and Partha Biswas for helpful suggestions. SLG was supported by the NIH (DE022550, AI107825). MJM was supported by AI110822-01. MRSA was supported by the NIH (F32-AI098243) and the Arthritis Foundation. This work is solely the responsibility of the authors and does not necessarily reflect the views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cooper GS, Bynum ML, Somers EC. Recent insights in the epidemiology of autoimmune diseases: improved prevalence estimates and understanding of clustering of diseases. Journal of autoimmunity. 2009;33(3–4):197–207. doi: 10.1016/j.jaut.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NIH Progress in Autoimmune Diseases Research. National Institute of Health Publication; 2005. [Google Scholar]
- 3.Gaffen SL, Jain R, Garg A, Cua D. IL-23-IL-17 immune axis: From mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14(9):585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conti HR, Gaffen SL. IL-17-Mediated Immunity to the Opportunistic Fungal Pathogen Candida albicans. J Immunol. 2015;195(3):780–8. doi: 10.4049/jimmunol.1500909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annual review of immunology. 2009;27:485–518. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 6.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O’Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10(3):314–24. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, Wu C, Kleinewietfeld M, Kunder S, Hafler DA, Sobel RA, Regev A, Kuchroo VK. Induction and molecular signature of pathogenic TH17 cells. Nature immunology. 2012;13(10):991–9. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haines CJ, Chen Y, Blumenschein WM, Jain R, Chang C, Joyce-Shaikh B, Porth K, Boniface K, Mattson J, Basham B, Anderton SM, McClanahan TK, Sadekova S, Cua DJ, McGeachy MJ. Autoimmune memory T helper 17 cell function and expansion are dependent on interleukin-23. Cell Rep. 2013;3(5):1378–88. doi: 10.1016/j.celrep.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 9.Jain R, Chen Y, Kanno Y, Joyce-Shaikh B, Vahedi G, Hirahara K, Blumenschein WM, Sukumar S, Haines CJ, Sadekova S, McClanahan TK, McGeachy MJ, O’Shea JJ, Cua DJ. Interleukin-23-Induced Transcription Factor Blimp-1 Promotes Pathogenicity of T Helper 17 Cells. Immunity. 2016;44(1):131–42. doi: 10.1016/j.immuni.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Sanford M, McKeage K. Secukinumab: first global approval. Drugs. 2015;75(3):329–38. doi: 10.1007/s40265-015-0359-0. [DOI] [PubMed] [Google Scholar]
- 11.Teng MW, Bowman EP, McElwee JJ, Smyth MJ, Casanova JL, Cooper AM, Cua DJ. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nature medicine. 2015;21(7):719–29. doi: 10.1038/nm.3895. [DOI] [PubMed] [Google Scholar]
- 12.Patel DD, Lee DM, Kolbinger F, Antoni C. Effect of IL-17A blockade with secukinumab in autoimmune diseases. Annals of the rheumatic diseases. 2013;72(Suppl 2):116–23. doi: 10.1136/annrheumdis-2012-202371. [DOI] [PubMed] [Google Scholar]
- 13.Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11(10):763–76. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 14.Baeten D, Sieper J, Braun J, Baraliakos X, Dougados M, Emery P, Deodhar A, Porter B, Martin R, Andersson M, Mpofu S, Richards HB, Group MS, Group MS. Secukinumab, an Interleukin-17A Inhibitor, in Ankylosing Spondylitis. N Engl J Med. 2015;373(26):2534–48. doi: 10.1056/NEJMoa1505066. [DOI] [PubMed] [Google Scholar]
- 15.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Song X, Qian Y. The activation and regulation of IL-17 receptor mediated signaling. Cytokine. 2013;62(2):175–82. doi: 10.1016/j.cyto.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 18.Shen F, Hu Z, Goswami J, Gaffen SL. Identification of common transcriptional regulatory elements in interleukin-17 target genes. The Journal of biological chemistry. 2006;281:24138–48. doi: 10.1074/jbc.M604597200. [DOI] [PubMed] [Google Scholar]
- 19.Patel DN, King CA, Bailey SR, Holt JW, Venkatachalam K, Agrawal A, Valente AJ, Chandrasekar B. Interleukin-17 stimulates C-reactive protein expression in hepatocytes and smooth muscle cells via p38 MAPK and ERK1/2-dependent NF-kappaB and C/EBPbeta activation. The Journal of biological chemistry. 2007;282(37):27229–38. doi: 10.1074/jbc.M703250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruddy MJ, Wong GC, Liu XK, Yamamoto H, Kasayama S, Kirkwood KL, Gaffen SL. Functional cooperation between interleukin-17 and tumor necrosis factor-a is mediated by CCAAT/enhancer binding protein family members. The Journal of biological chemistry. 2004;279(4):2559–2567. doi: 10.1074/jbc.M308809200. [DOI] [PubMed] [Google Scholar]
- 21.Shen F, Li N, Gade P, Kalvakolanu DV, Weibley T, Doble B, Woodgett JR, Wood TD, Gaffen SL. IL-17 Receptor Signaling Inhibits C/EBP{beta} by Sequential Phosphorylation of the Regulatory 2 Domain. Sci Signal. 2009;2(59):ra8. doi: 10.1126/scisignal.2000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiricozzi A, Nograles KE, Johnson-Huang LM, Fuentes-Duculan J, Cardinale I, Bonifacio KM, Gulati N, Mitsui H, Guttman-Yassky E, Suarez-Farinas M, Krueger JG. IL-17 induces an expanded range of downstream genes in reconstituted human epidermis model. PLoS One. 2014;9(2):e90284. doi: 10.1371/journal.pone.0090284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed M, Gaffen SL. IL-17 inhibits adipogenesis in part via C/EBPα, PPARγ and Kruppel-like factors. Cytokine. 2013;61:898–905. doi: 10.1016/j.cyto.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maekawa T, Hosur K, Abe T, Kantarci A, Ziogas A, Wang B, Van Dyke TE, Chavakis T, Hajishengallis G. Antagonistic effects of IL-17 and D-resolvins on endothelial Del-1 expression through a GSK-3beta-C/EBPbeta pathway. Nat Commun. 2015;6:8272. doi: 10.1038/ncomms9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meeks KD, Sieve AN, Kolls JK, Ghilardi N, Berg RE. IL-23 is required for protection against systemic infection with Listeria monocytogenes. J Immunol. 2009;183(12):8026–34. doi: 10.4049/jimmunol.0901588. [DOI] [PubMed] [Google Scholar]
- 26.Screpanti I, Romani L, Musiani P, Modesti A, Fattori E, Lazzaro D, Sellitto C, Scarpa S, Bellavia D, Lattanzio G, et al. Lymphoproliferative disorder and imbalanced T-helper response in C/EBP beta-deficient mice. EMBO J. 1995;14(9):1932–41. doi: 10.1002/j.1460-2075.1995.tb07185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simpson-Abelson MR, Childs EE, Ferreira MC, Bishu S, Conti HR, Gaffen SL. C/EBPbeta Promotes Immunity to Oral Candidiasis through Regulation of beta-Defensins. PLoS One. 2015;10(8):e0136538. doi: 10.1371/journal.pone.0136538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsukada J, Yoshida Y, Kominato Y, Auron PE. The CCAAT/enhancer (C/EBP) family of basic-leucine zipper (bZIP) transcription factors is a multifaceted highly-regulated system for gene regulation. Cytokine. 2011;54(1):6–19. doi: 10.1016/j.cyto.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J. 2002;365(Pt 3):561–575. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmed M, Gaffen SL. IL-17 in obesity and adipogenesis. Cytokine Growth Factor Rev. 2010;21(6):449–53. doi: 10.1016/j.cytogfr.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huber R, Pietsch D, Panterodt T, Brand K. Regulation of C/EBPbeta and resulting functions in cells of the monocytic lineage. Cell Signal. 2012;24(6):1287–96. doi: 10.1016/j.cellsig.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Uematsu S, Kaisho T, Tanaka T, Matsumoto M, Yamakami M, Omori H, Yamamoto M, Yoshimori T, Akira S. The C/EBP beta isoform 34-kDa LAP is responsible for NF-IL-6-mediated gene induction in activated macrophages, but is not essential for intracellular bacteria killing. J Immunol. 2007;179(8):5378–86. doi: 10.4049/jimmunol.179.8.5378. [DOI] [PubMed] [Google Scholar]
- 33.McGeachy MJ, Cua DJ. The link between IL-23 and Th17 cell-mediated immune pathologies. Semin Immunol. 2007;19(6):372–6. doi: 10.1016/j.smim.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448(7152):484–7. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, Sudo K, Nakae S, Sasakawa C, Iwakura Y. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30(1):108–19. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421(6924):744–8. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 37.Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nature immunology. 2011;12(6):560–7. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 38.El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nature immunology. 2011;12(6):568–75. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rangachari M, Kuchroo VK. Using EAE to better understand principles of immune function and autoimmune pathology. Journal of autoimmunity. 2013;45:31–9. doi: 10.1016/j.jaut.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nature medicine. 2002;8(5):500–8. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 41.Sturner KH, Borgmeyer U, Schulze C, Pless O, Martin R. A multiple sclerosis-associated variant of CBLB links genetic risk with type I IFN function. J Immunol. 2014;193(9):4439–47. doi: 10.4049/jimmunol.1303077. [DOI] [PubMed] [Google Scholar]
- 42.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8(12):1390–7. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 43.Tsai VW, Mohammad MG, Tolhurst O, Breit SN, Sawchenko PE, Brown DA. CCAAT/enhancer binding protein-delta expression by dendritic cells regulates CNS autoimmune inflammatory disease. J Neurosci. 2011;31(48):17612–21. doi: 10.1523/JNEUROSCI.3449-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maitra A, Shen F, Hanel W, Mossman K, Tocker J, Swart D, Gaffen SL. Distinct functional motifs within the IL-17 receptor regulate signal transduction and target gene expression. Proc Natl Acad Sci, USA. 2007;104:7506–11. doi: 10.1073/pnas.0611589104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conti H, Shen F, Nayyar N, Stocum E, JNS, Lindemann M, Ho A, Hai J, Yu J, Jung J, Filler S, Masso-Welch P, Edgerton M, Gaffen S. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. The Journal of experimental medicine. 2009;206(2):299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fattori E, Sellitto C, Cappelletti M, Lazzaro D, Bellavia D, Screpanti I, Gulino A, Costantini F, Poli V. Functional analysis of IL-6 and IL-6DBP/C/EBP beta by gene targeting. Ann N Y Acad Sci. 1995;762:262–73. doi: 10.1111/j.1749-6632.1995.tb32331.x. [DOI] [PubMed] [Google Scholar]
- 47.Camporeale A, Poli V. IL-6, IL-17 and STAT3: A holy trinity in auto-immunity? Front Biosci. 2012;17:2306–26. doi: 10.2741/4054. [DOI] [PubMed] [Google Scholar]
- 48.McGeachy MJ. GM-CSF: the secret weapon in the T(H)17 arsenal. Nat Immunol. 2011;12(6):521–2. doi: 10.1038/ni.2044. [DOI] [PubMed] [Google Scholar]
- 49.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, Stockinger B. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12(3):255–63. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duhen R, Glatigny S, Arbelaez CA, Blair TC, Oukka M, Bettelli E. Cutting edge: the pathogenicity of IFN-gamma-producing Th17 cells is independent of T-bet. J Immunol. 2013;190(9):4478–82. doi: 10.4049/jimmunol.1203172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Awasthi A, Riol-Blanco L, Jager A, Korn T, Pot C, Galileos G, Bettelli E, Kuchroo VK, Oukka M. Cutting edge: IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J Immunol. 2009;182(10):5904–8. doi: 10.4049/jimmunol.0900732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP, Lubberts E. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182(9):5836–45. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 53.Yoon J, Leyva-Castillo JM, Wang G, Galand C, Oyoshi MK, Kumar L, Hoff S, He R, Chervonsky A, Oppenheim JJ, Kuchroo VK, van den Brink MR, de Malefyt RW, Tessier PA, Fuhlbrigge R, Rosenstiel P, Terhorst C, Murphy G, Geha RS. IL-23 induced in keratinocytes by endogenous TLR4 ligands polarizes dendritic cells to drive IL-22 responses to skin immunization. J Exp Med. 2016;213(10):2147–66. doi: 10.1084/jem.20150376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O’Shea JJ. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006;103(21):8137–42. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruddy MJ, Shen F, Smith J, Sharma A, Gaffen SL. Interleukin-17 regulates expression of the CXC chemokine LIX/CXCL5 in osteoblasts: Implications for inflammation and neutrophil recruitment. J Leukoc Biol. 2004;76:135–144. doi: 10.1189/jlb.0204065. [DOI] [PubMed] [Google Scholar]