Abstract
Estrogens have been implicated as complete carcinogens for breast and other tissues through mechanisms involving increased cell proliferation, oxidative stress and DNA damage. Because of their potent antioxidant activity and other effects, tocopherols have been shown to exert anti-tumor activities in various cancers. However, limited information is available on the effect of different forms of tocopherols in estrogen-mediated breast cancer. To address this, we examined the effects of α-, γ- and δ-tocopherols as well as a natural γ-tocopherol rich mixture of tocopherols, γ-TmT, on estrogen-stimulated MCF-7 cells in vitro and in vivo. For the in vivo studies, MCF-7 cells were injected into the mammary fat pad of immunodeficient mice previously implanted with estrogen pellets. Mice were then administered diets containing 0.2% α-, γ-, δ-tocopherol or γ-TmT for 5 weeks. Treatment with α-, γ-, δ-tocopherols and γ-TmT reduced tumor volumes by 29% (p<0.05), 45% (p<0.05), 41% (p<0.05) and 58% (p<0.01), as well as tumor weights by 20%, 37% (p<0.05), 39% (p<0.05) and 52% (p<0.05), respectively. γ- and δ-Tocopherols and γ-TmT inhibited the expression of cell proliferation-related genes such as cyclin D1 and c-Myc, and estrogen-related genes such as TFF/pS2, cathepsin D and progesterone receptor in estrogen-stimulated MCF-7 cells in vitro. Further, γ- and δ-tocopherols decreased the levels of estrogen-induced oxidative stress and nitrosative stress markers, 8-hydroxy-2’-deoxyguanosine and nitrotyrosine, as well as the DNA damage marker, γ-H2AX. Our results suggest that γ- and δ-tocopherols and the γ-tocopherol rich mixture are effective natural agents for the prevention and treatment of estrogen-mediated breast cancer.
Introduction
Extended exposure to estrogen is epidemiologically and experimentally associated with an increased risk of developing breast cancer (1). This increased risk appears to be related to the role of estrogen in the regulation of genes involved in breast cell proliferation and is mediated through the estrogen receptor (ER) (2). In addition to ER-dependent effects, there are several ER-independent pathways that have been proposed for biological responses to estrogen including carcinogenesis (1, 3). The ER-independent mechanisms are mediated through the metabolism of estrogen to generate genotoxic molecules, free radicals and reactive oxygen species (ROS) that could be involved in the pathogenesis of breast cancer (1, 4). Oxidative stress exerted by the metabolites of estrogen is also thought to act in concert with ER signaling to promote genetic alterations, DNA damage and deregulated cell proliferation (2, 5). As a result of these findings, considerable effort has been made to develop strategies to selectively block the effect of overexposure to estrogen as an approach to prevent and treat human breast cancers.
One area of investigational focus has been the use of dietary components to inhibit breast carcinogenesis (6). Many natural products and bioactive compounds found in foods have demonstrated inhibitory effects on breast carcinogenesis via the reduction of estrogen-induced oxidative stress, as well as downregulation of ER signaling, without excessive toxicity (7–9). Our laboratory and others have examined the potential use of tocopherols, which are potent antioxidants in the diet, in prevention and treatment of breast and other cancers (10–13).
Tocopherols, the major forms of vitamin E, are a family of fat soluble phenolic compounds consisting of a chromanol ring system and a 16-carbon side chain (14). Specific forms of tocopherol are distinguished by the number and position of methyl groups on the chromanol ring, and exist as α-, β-, γ-, or δ-tocopherol (14). Many studies have shown that tocopherols inhibit cancer formation and development due to their strong antioxidant properties (12, 15, 16). α-Tocopherol is considered to be the classic form of vitamin E as it is the major form of tocopherol found in blood and tissues (17). As a result, α-tocopherol has been the most widely used form of tocopherol for cancer prevention studies. However, large-scale human trials with α-tocopherol did not find a cancer preventive effect (18–20).
γ-Tocopherol is the most abundant tocopherol in the U.S. diet, found in vegetable oils, such as soybean, corn and cottonseed (17). Treatment with γ- and δ-tocopherols were shown to inhibit mammary tumor growth in N-methyl-N-nitrosourea (NMU)-induced Sprague Dawley (SD) rats, but did not prevent human epidermal growth factor receptor 2 (HER2/neu)-dependent tumorigenesis (10). γ-TmT, a byproduct of refined vegetable oil, is a naturally occurring tocopherol mixture rich in γ-tocopherol (21, 22). γ-TmT has been shown to inhibit tumorigenesis in ER-positive breast and other cancer models (11, 21, 23, 24). γ-TmT suppresses NMU-induced mammary tumor growth in SD rats by downregulation of ERα, Akt and activation of PPARγ (25). In addition, dietary supplementation with γ-TmT reduced oxidative and nitrosative stress and upregulated Nrf2-dependent antioxidant response in estrogen-induced early mammary hyperplasia in ACI rats (21). Treatment with γ-TmT suppressed inflammatory markers, inhibited estrogen-induced cell proliferation, and regulated the expression of nuclear receptors ERα and PPARγ in ACI rats (26).
We have recently demonstrated that dietary administration of a natural mixture of γ-TmT suppresses estrogen-mediated mammary tumor growth in both ACI rats and MCF-7 xenografted immunodeficient mice (11). In the present study, we investigated the relative activities of different forms of tocopherols in estrogen-mediated mammary tumor growth, expression of cell proliferation and estrogen-related genes, as well as estrogen-induced oxidative/nitrosative stress and DNA damage in breast cancer.
Materials and Methods
Reagents and cell culture
γ-TmT (Covi-ox T-90 containing 56.1% γ-tocopherol, 22.3% δ-tocopherol, 11.5% α-tocopherol, and 1.2% β-tocopherol) were obtained from BASF Corporation (Florham Park, NJ; Covi-ox T-90, Batch number 0008778732). γ-Tocopherol was purified from a γ-T-rich mixture of tocopherols (obtained from BASF Corporation) to ≥97% purity with no detectable α- and δ-tocopherol. α- and δ-Tocopherols were purified from commercial grade α-tocopherol (T3634) and δ-tocopherol (T2028) (obtained from Sigma-Aldrich, St. Louis, MO), to ≥97% purity with no other detectable forms of tocopherol. A CombiFlash Companion XL automated flash chromatographic system (Teledyne ISCO, Lincoln, NE) with a RediSep Rf Gold high performance flash silica gel column (20–40 µm in particle size) was used for the purification. Each tocopherol was dissolved in dimethyl sulfoxide (Sigma-Aldrich) for cell culture studies. The MCF-7 breast cancer cell line was acquired from American Type Culture Collection (ATCC, Manassas, VA) and authenticated by short tandem repeat profiling at ATCC. MCF-7 cells were grown in DMEM/F12 medium, 10% fetal bovine serum and 1% penicillin/streptomycin a 37°C and 5% CO2. When noted, MCF-7 cells were treated in 10% charcoal stripped FBS/phenol red free RPMI medium to examine effects of tocopherols on estrogen-stimulated events. MDA-MB-231 cells were maintained in DMEM medium, 10% fetal bovine serum, 1% penicillin/streptomycin and 1% glutamine at 37°C and 5% CO2 in a humidified atmosphere.
Animals and experimental procedures
Female nu/nu mice were obtained from Charles River Breeding Laboratories (Kingston, NY). After 2 weeks of acclimatization, the mice (7–8 weeks old) were implanted subcutaneously with 17β-estradiol pellets (0.72 mg, 90 days release; Innovative Research of America, Sarasota, FL). Two days following estradiol implantation, MCF-7 cells (5 × 106) were orthotopically injected into the mouse mammary fat pad, and the mice then fed with different tocopherol diets, containing 0.2% α-tocopherol, γ-tocopherol, δ-tocopherol or γ-TmT, or control diet throughout the remainder of the study. Tumors were palpated twice per week and body weights were measured weekly. Tumors were measured with a vernier caliper and tumor volume (V, cm3) was calculated using the equation V = D*d2/2, where D (cm) and d (cm) are the largest and smallest perpendicular diameters. All animal studies were approved by the Institutional Review Board for the Animal Care and Facilities Committee at Rutgers, the State University of New Jersey (Protocol Number: 04-001).
Diets for animal experiment
Semi-purified AIN-93M obtained from Research Diets, Inc. (New Brunswick, NJ) was used as the control diet. The test diets were prepared by adding 0.2% of each tocopherol to the AIN-93M diet. The diets were stored in sealed containers at 4°C, and the food cups were replenished with fresh food twice weekly.
Analysis of tocopherol levels in mouse serum
Mouse serum was collected at termination, and the levels of α-, γ- and δ-tocopherols in the serum were analyzed by high performance liquid chromatography using a previously described procedure (21, 27).
Western blot analysis
The detailed procedures have been described previously (25). The primary antibody detecting c-Myc (1:1000, 5605P), TFF/pS2 (1:1000, 12419S), cathepsin D (1:1000, 2284S) and progesterone receptor (PGR) (1:500, 8757S) were from Cell Signaling Technology (Danvers, MA); Cyclin D1 antibody was from Santa Cruz Biotechnology (1:500, sc-718; Santa Cruz, CA); β-actin antibody was from Sigma-Aldrich (1:2000, A1978; St. Louis, MO). Secondary antibodies were from Santa Cruz Biotechnology.
Quantitative polymerase chain reaction analysis
The procedure was described previously (24); the labeled primers, including MYC (Hs00153708), CCND1 (Hs0076553), TFF1 (Hs00907239), CTSD (Hs00157205), PGR (Hs01556702), SERPINA1 (Hs00165475) and CITED1 (Hs00918445) were obtained from Applied Biosystems (Foster City, CA).
Fluorescence Microscopy
MCF-7 cells were seeded into 6-well plates at a density of 8 × 104 cells per well and then treated with 1 nM estrogen and 10 µM tocopherols. After 24 h or 48 h, cells were fixed with 4% paraformaldehyde for 15 min at room temperature. Fixed cells were incubated with PBS containing 10% goat serum to block non-specific binding for 1 h, and then incubated overnight at 4°C with primary antibodies to 8-hydroxy-2’-deoxyguanosine (8-oxo-dG) (1:100, N45.1; JaICA/GENOX Corporation, Baltimore, MD), nitrotyrosine (1:100, MAB5404; Millipore, Billerica, MA) or γ-H2AX (1:100, 2577; Cell signaling Technology, Beverly, MA). Samples were then incubated with fluorophore-conjugated secondary antibody (Alexa Fluor 488; Invitrogen, Carlsbad, CA) and TO-PRO3 iodide nuclear stain (Invitrogen, 1 µM) for 60 and 15 min, respectively. The images were taken using a confocal microscope with laser filters at 488 nm for 8-oxo-dG, nitrotyrosine and γ-H2AX, and 644 nm for TO-PRO3. The fluorescence was analyzed using Image J software (NIH, Bethesda, MD) (http://rsbweb.nih.gov/ij).
Statistical analysis
The significance of the difference between control or individual treatment groups and the estrogen-treated groups was evaluated by the Student’s t-test or one-way analysis of variance (ANOVA) followed by Dunnett’s test. The estrogen-treated group was compared to the negative control (represented as “a”) and tocopherol groups were compared to the estrogen group (represented as “b”). P-values <0.05 were considered significant.
Results
Dietary administration of tocopherols inhibits growth of estrogen-supplemented MCF-7 xenografts
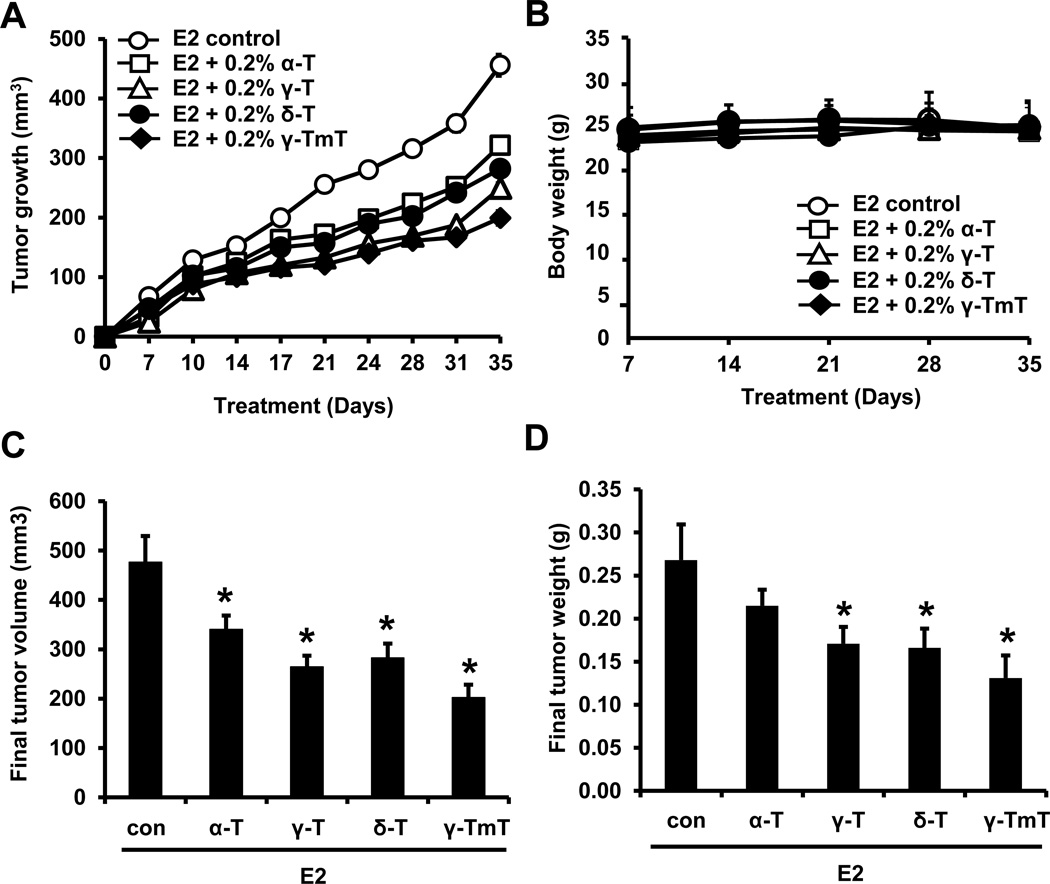
We first tested the effects of individual forms of dietary tocopherols on the growth of mammary tumors in the estrogen-induced MCF-7 xenograft model. Nu/nu mice were implanted with estradiol pellets, orthotopically injected with MCF-7 cells and fed pure 0.2% α-, γ-, δ-tocopherol or γ-TmT in AIN-93M diet for 5 weeks. No difference in body weight was observed among the different tocopherol treatment groups (Fig. 1B). Starting from day 7 after the MCF-7 cell injection, mammary tumors became palpable, and the volume of mammary tumors was measured two times per week. Mammary tumors continued to grow in the estradiol control group, whereas the tumor growth was inhibited in groups fed with tocopherols (Fig. 1A). As compared with the estrogen only group, the final tumor volume of α-, γ-, δ-tocopherol and γ-TmT groups was decreased by 29% (p<0.05), 45% (p<0.05), 41% (p<0.05) and 58% (p<0.01), respectively (Fig. 1C). As shown in Fig. 1D, tumor weight at termination in α-, γ-, δ-tocopherol and γ-TmT groups was decreased by 20%, 37% (p<0.05), 39% (p<0.05) and 52% (p<0.05), respectively.
Figure 1.
Tocopherols inhibit estrogen (E2)-induced mammary tumor growth in MCF-7 xenografted mice. Nu/nu mice were implanted with estrogen pellets (0.72 mg). After 2 days, MCF-7 cells (5 × 106 cells/mouse) were injected into the mammary fat pad and the mice fed with control or tocopherol diet for 5 weeks. A. Average tumor size at time points for the different treatment groups. B. Average body weight at time points for the different treatment groups. C. Average tumor volume (mm3) at necropsy. D. Average tumor weight at necropsy. Data are represented as mean ± S.E. (n=10 per group). Statistical significance, *p <0.05, **p<0.01. P-values are compared to the estrogen control.
Measurement of the levels of tocopherols in the serum of MCF-7 xenografted mice fed various tocopherol diets
To understand the bioavailability of dietary tocopherols in the experimental animals, we determined the serum levels of α-, γ-, and δ-tocopherols in the treatment groups. As shown in Table 1, the serum α-tocopherol level was increased from 11.7 µM in estrogen control group to 34.1 µM by dietary supplementation with 0.2% α-tocopherol. Serum γ-tocopherol levels were, as expected, increased from 0.230 µM in estrogen control group to 2.523 µM by γ-tocopherol and 0.464 µM by γ-TmT. Serum δ-tocopherol levels were increased from 0.043 µM in estrogen control group to 1.479 µM by δ-tocopherol and 0.148 µM by γ-TmT. Interestingly, in animals fed α-tocopherol, serum γ-tocopherol was considerably decreased.
Table 1.
Analysis of α-, γ-, and δ-tocopherols in the serum of xenografted mice.
| Treatment | α-tocopherol (µM) | γ-tocopherol (µM) | δ-tocopherol (µM) |
|---|---|---|---|
| E2 control | 11.7 ± 2.4 | 0.230 ± 0.088 | 0.043 ± 0.041 |
| E2 + 0.2% α-tocopherol | 34.1 ± 9.9** | 0.054 ± 0.055** | 0.051 ± 0.060 |
| E2 + 0.2% γ-tocopherol | 10.8 ± 3.3 | 2.523 ± 0.497** | 0.068 ± 0.074 |
| E2 + 0.2% δ-tocopherol | 13.1 ± 3.1 | 0.176 ± 0.056 | 1.479 ± 0.653** |
| E2 + 0.2% γ-TmT | 14.7 ± 4.6 | 0.464 ± 0.126* | 0.148 ± 0.044* |
The data are presented as mean ± S.E. (n=6 analyzed serum samples per group).
p<0.05,
p<0.01,
P-values are compared to the estrogen (E2) control.
Tocopherols down-regulate the levels of cyclin D1 and c-Myc in estrogen-treated MCF-7 cells
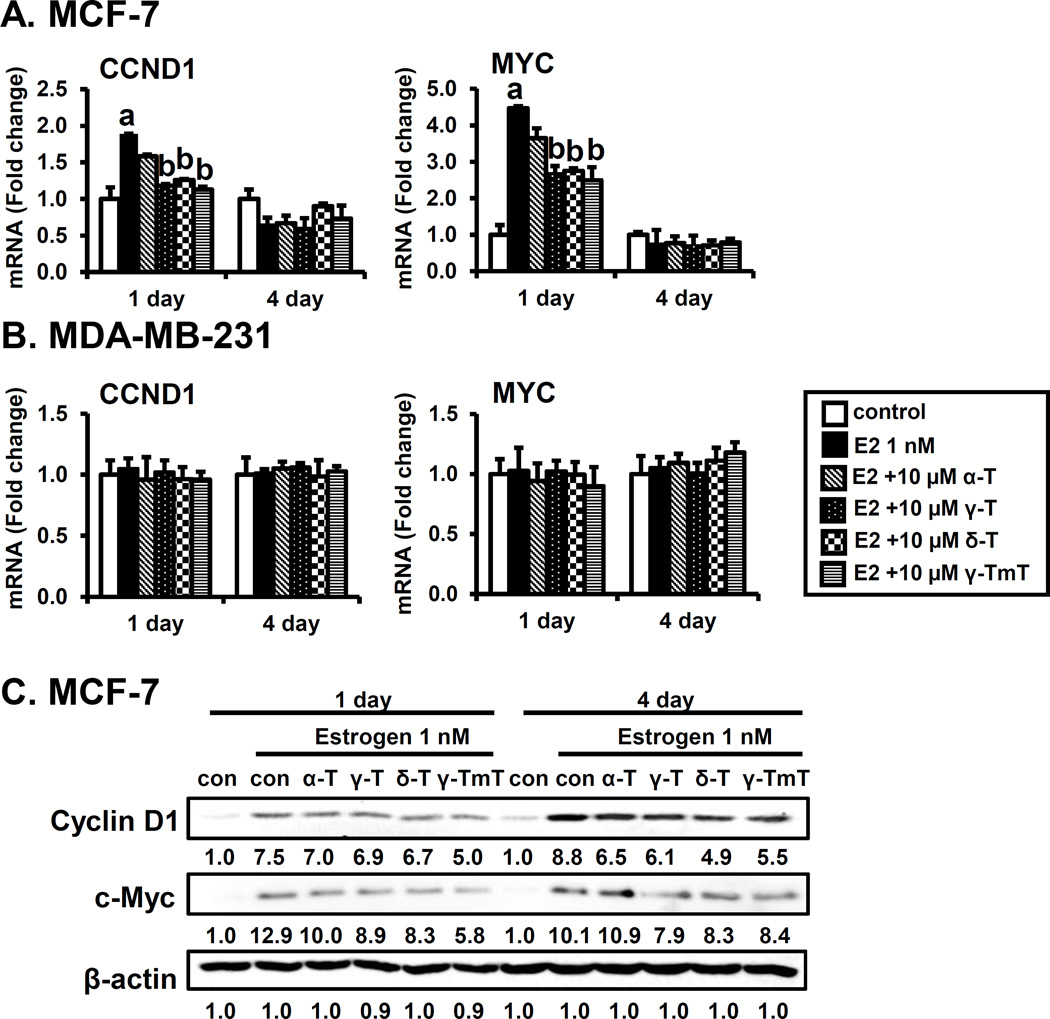
It has been shown that the expression of cyclin D1 and c-Myc is increased by estrogen-induced cell progression through the G1 phase of the cell cycle (28). Thus, we investigated whether α, γ, δ-tocopherols and γ-TmT modulate the expression of estrogen-mediated cell proliferation genes, cyclin D1 and c-Myc in MCF-7 cells and MDA-MB-231 cells in vitro. As shown in Fig. 2A, treatment with estrogen increased the expression of cyclin D1 and c-Myc mRNAs at day 1, but expression returned to the basal level by day 4. Treatment with γ, δ-tocopherols and γ-TmT significantly reduced estrogen-induced cyclin D1 and c-Myc mRNA levels on day 1, whereas α-tocopherol had little or no significant effect on mRNA levels of these genes. In contrast to MCF-7 cells, mRNA levels of cyclin D1 and c-Myc were not changed by the treatment with estrogen and tocopherols in MDA-MB-231 cells (Fig. 2B). The protein levels of cyclin D1 and c-Myc were increased by estrogen at both day 1 and day 4 time points in MCF-7 cells, as expected. α-Tocopherol had little or no significant effect on protein levels, while γ, δ-tocopherols and γ-TmT modestly reduced estrogen stimulation of cyclin D1 and c-Myc levels on day 1 and 4 (Fig. 2C). Protein levels of cyclin D1 and c-Myc were not changed by the treatment with estrogen and tocopherols in MDA-MB-231 cells (Supplementary Fig. S1). These results suggest that inhibition of cell proliferation caused by tocopherols is dependent on the presence of ER.
Figure 2.
Effects of tocopherols on expression of cell proliferation-related genes encoding Cyclin D1 and c-Myc in MCF-7 cells and MDA-MB-231 cells. A. qPCR analysis of the effects of tocopherols (10 µM) on MCF-7 in the presence of estrogen (E2, 1 nM) at 1 and 4 days. MCF-7 cells were plated at a density of 8 × 104 cells/mL in 6-well plates and incubated with estrogen for 1 and 4 days. The data are represented as mean ± standard deviation (SD). n=3 independent experiments, a,b Significantly different from the control and estrogen control, respectively (p<0.05). Cycle numbers for genes related to cyclin D1 and c-Myc for the estrogen treated group at Day 1 were 20 and 23, respectively. B. qPCR analysis of the effects of tocopherols (10 µM) on MDA-MB-231 cells in the presence of estrogen (E2, 1 nM) at 1 and 4 days. MDA-MB-231 cells were plated at a density of 1 × 105 cells/mL in 6-well plates and incubated with estrogen for 1 and 4 days. The data are represented as mean ± standard deviation (SD). n=3 independent experiments, a,b Significantly different from the control and estrogen control, respectively (p<0.05). Cycle numbers for genes related to cyclin D1 and c-Myc for the estrogen treated group at Day 1 were 19 and 21, respectively. C. Western blot analysis of effects of tocopherols (10 µM) in MCF-7 in the presence of estrogen (1 nM) at 1 and 4 days. Three independent experiments were performed. β-Actin was used as a loading control.
Tocopherols down-regulate the levels of estrogen responsive genes in estrogen-treated MCF-7 cells
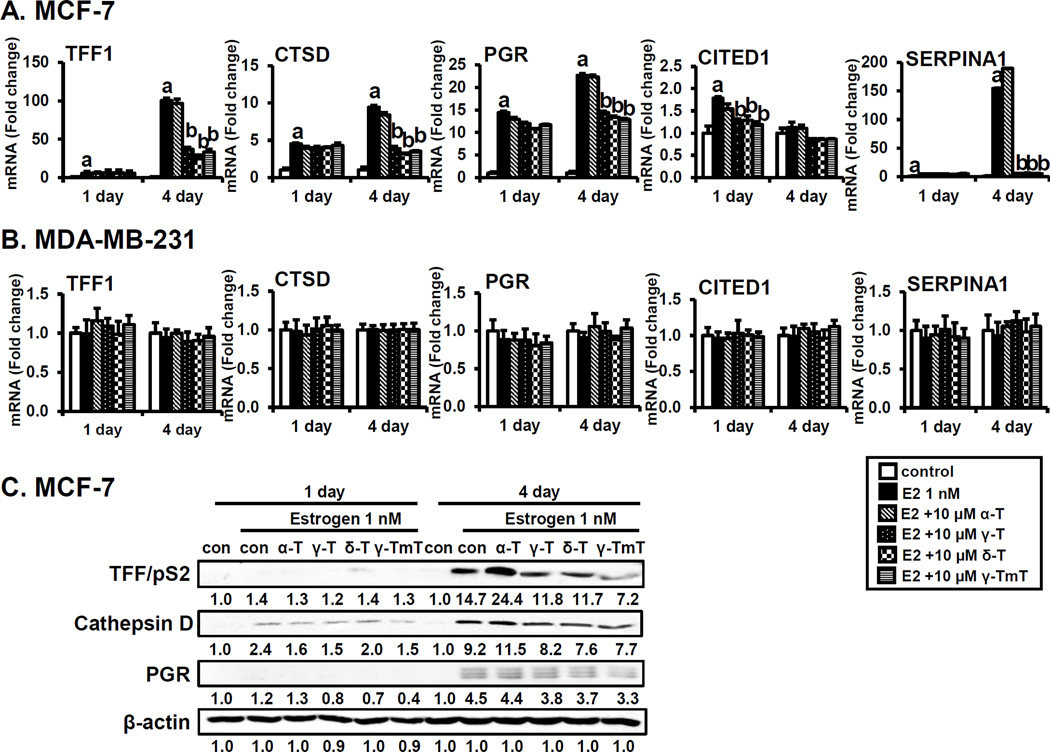
Since tocopherols have anti-proliferative effects in the presence of estrogen, we investigated the effects of individual tocopherols on the modulation of the estrogen-related genes TFF/pS2, cathepsin D, PGR, SERPINA1 and CITED1 in estrogen-treated MCF-7 cells, in comparison to MDA-MB-231 cells. A number of estrogen target genes have been shown to be involved in regulating the proliferation of ER-positive breast cancer cells and modulating estrogen sensitivity in a gene-specific manner (29–31). As shown in Fig. 3A, the mRNA levels of TFF/pS2, cathepsin D and PGR were significantly up-regulated by estrogen treatment in a time-dependent manner. Treatment with γ-, δ-tocopherols and γ-TmT decreased the levels of these mRNAs at 4 days. Furthermore, mRNA levels of CITED1 and SERPINA1 were increased by estrogen, and this stimulation was inhibited by γ-, δ-tocopherols and γ-TmT. α-Tocopherol had little or no effect on mRNA levels of estrogen target genes indicated. In contrast to MCF-7 cells, the mRNA levels of TFF/pS2, cathepsin D, PGR, CITED1 and SERPINA1 were unaffected by treatment with estrogen and tocopherols in MDA-MB-231 cells (Fig. 3B). In MCF-7 cells, the protein levels of TFF/pS2, cathepsin D and PGR were increased by estrogen, and γ-, δ-tocopherols and γ-TmT down-regulated estrogen-stimulated levels of these markers. Interestingly, α-tocopherol had little or no effect on the protein levels and even increased levels of TFF/pS2 and cathepsin D protein on day 4 (Fig. 3C). Protein levels of estrogen-downstream molecules were not changed by the treatment with estrogen and tocopherols in MDA-MB-231 cells (Supplementary Fig. S2).
Figure 3.
Effects of tocopherols on expression of estrogen-related genes encoding TFF1, cathepsin D, PGR, SERPINA1 and CITED1 in MCF-7 cells and MDA-MB-231 cells. A. qPCR analysis of effects of tocopherols (10 µM) in MCF-7 in the presence of estrogen (E2, 1 nM) at 1 and 4 days. MCF-7 cells were plated at a density of 8 × 104 cells/mL in 6-well plates and incubated with estrogen for 1 and 4 days. The data are represented as mean ± standard deviation (SD). n=3 independent experiments, a,b Significantly different from the control and estrogen control, respectively (p<0.05). Cycle numbers for genes related to TFF, CTSD, PGR, CITED1 and SERPINA1 for estrogen group at Day 4 were 16, 18, 24, 31 and 31, respectively. B. qPCR analysis of effects of tocopherols (10 µM) in MDA-MB-231 cells in the presence of estrogen (E2, 1 nM) at 1 and 4 days. MDA-MB-231 cells were plated at a density of 1 × 105 cells/mL in 6-well plates and incubated with estrogen for 1 and 4 days. The data are represented as mean ± standard deviation (SD). n=3 independent experiments, a,b Significantly different from the control and estrogen control, respectively (p<0.05). Cycle numbers for genes related to TFF, CTSD, PGR, CITED1 and SERPINA1 for estrogen group at Day 4 were 31, 18, 35, 29 and 24, respectively. C. Western blot analysis of effects of tocopherols (10 µM) in MCF-7 in the presence of estrogen (1 nM) at 1 and 4 days. Three independent experiments were performed. β-Actin was used as a loading control.
Tocopherols reduce estrogen-induced 8-oxo-dG in MCF-7 cells
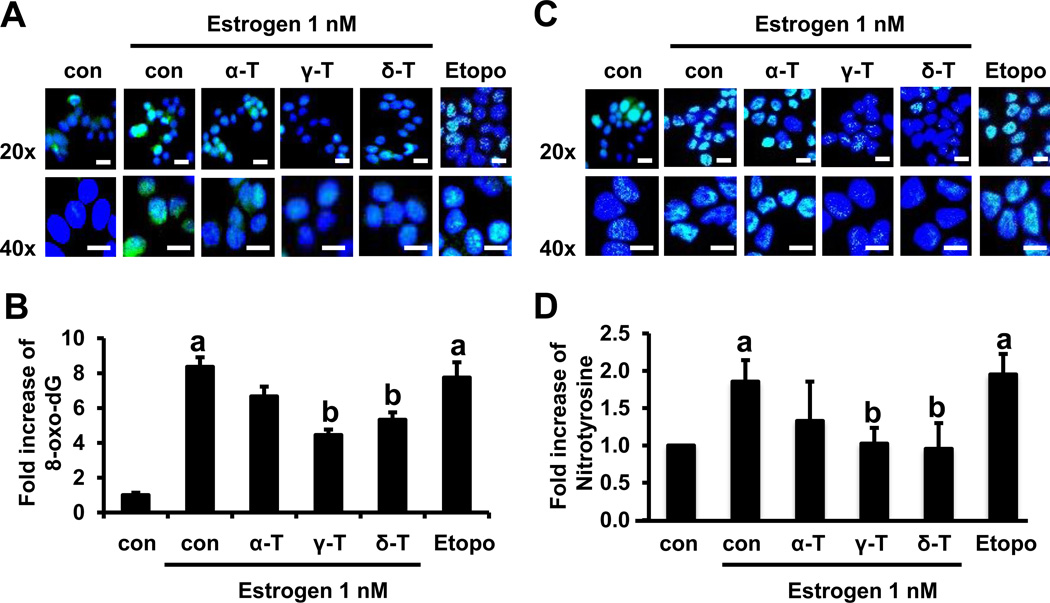
Next, we investigated whether α-, γ- and δ-tocopherols inhibit estrogen-mediated oxidative stress. 8-oxo-dG is one of the most commonly formed DNA lesions produced in response to estrogen-induced oxidative stress and oxidative DNA damage (32, 33). Therefore, we assessed the effects of tocopherols on 8-oxo-dG levels in MCF-7 cells induced with estrogen. Etoposide was used a positive control for induction of oxidative stress (34). As shown in Fig. 4A, the level of 8-oxo-dG staining was increased by 1 nM estrogen (8.36 fold, p<0.05) compared with untreated control in MCF-7 cells. α-Tocopherol demonstrated a weak inhibitory effect on the formation of 8-oxo-dG. However, treatment with 10 µM γ- and δ-tocopherols attenuated the estrogen-induced increase in 8-oxo-dG levels by 47% (p<0.05) and 37% (p<0.05), respectively (Fig. 4B). These results show that tocopherols have a protective effect against estrogen-induced oxidative stress, and that γ- and δ-tocopherols are more potent than α-tocopherol in MCF-7 cells. In contrast to MCF-7 cells, estrogen did not induce the level of 8-oxo-dG in MDA-MB-231 cells (Supplementary Fig. S3A and S3B).
Figure 4.
Effects of tocopherols on estrogen-induced oxidative and nitrosative stress in MCF-7 cells. A. Representative immunofluorescent staining for 8-oxo-dG. Cells were plated at a density of 8 × 104 cells/mL in 35 mm confocal plates and incubated with tocopherols (10 µM) and estrogen (1 nM) for 48 h. Scale bars: 20 µm. B. Quantification of 8-oxo-dG (green) in the cell nucleus (blue), calculated by ImageJ program. Four images with 20x magnification from each group were selected randomly and analyzed for the expression of 8-oxo-dG. The data are represented as mean ± standard deviation (SD). n=4 independent experiments, a,b Significantly different from the control and estrogen control, respectively (p<0.05). C. Representative immunofluorescent staining for nitrotyrosine. Cells were plated at a density of 8 × 104 cells/mL in 35 mm confocal plates and incubated with tocopherols (10 µM) and estrogen (1 nM) for 24 h. Scale bars: 20 µm D. Quantification of nitrotyrosine (green) in the cell nucleus (blue) by ImageJ program. Four images with 20x magnification from each group were selected at random and analyzed for the expression of nitrotyrosine. Etoposide (10 µM) is used as a positive control. The data are represented as mean ± standard deviation (SD). n=4 independent experiments, a,b Significantly different from the control and estrogen control, respectively (p<0.05).
Tocopherols reduce estrogen-induced nitrotyrosine in MCF-7 cells
To evaluate the effects of tocopherols on nitrosative stress in estrogen-treated MCF-7 cells, analysis of nitrotyrosine, a marker of nitrosative stress, was performed using immunofluorescence (Fig. 4C). The levels of nitrotyrosine staining in the estrogen-treated group increased by 1.96-fold (p<0.05), compared with the vehicle control. Similar results were obtained in cells treated with etoposide (10 µM), which was used as a positive control. Treatment with 10 µM γ- and δ-tocopherols decreased the levels of nitrotyrosine by 48% (p<0.05) and 52% (p<0.05), whereas α-tocopherol did not (Fig. 4C and 4D). In contrast to MCF-7 cells, estrogen did not induce the level of nitrotyrosine in MDA-MB-231 cells (Supplementary Fig. S3C and S3D).
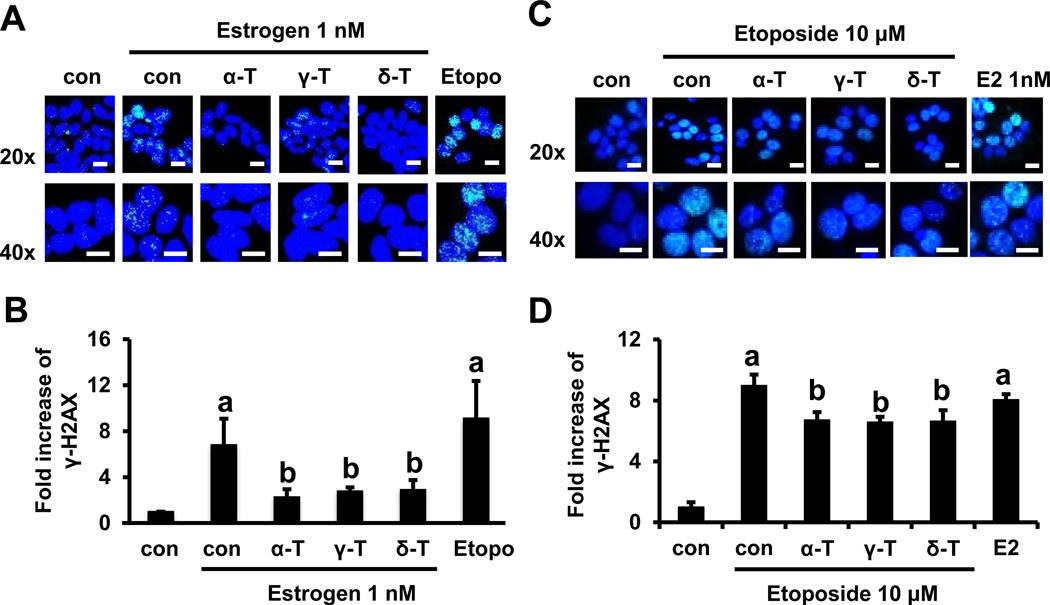
Tocopherols reduce estrogen-and etoposide-induced γ-H2AX formation in MCF-7 cells
Estrogen induces reactive oxygen and nitrogen species as well as DNA double-stranded breaks (2, 5). Therefore, we examined the inhibitory effect of tocopherols on DNA double-stranded breaks, as measured by the formation of γ-H2AX foci. Histone H2AX has been implicated in the maintenance of genomic stability by participating in the repair of DNA damage (35). H2AX is phosphorylated to its activated form, γ-H2AX, which then rapidly forms foci in response to DNA double-strand breaks resulting in replication arrest (35). As shown in Fig. 5A and 5B, exposure to estrogen resulted in a 6.81-fold (p<0.05) increase in the number of γ-H2AX foci compared with untreated control cells. Etoposide (10 µM), which causes high levels of DNA damage (36), significantly increased the number of γ-H2AX foci (9.14-fold, p<0.05) over the vehicle control in MCF-7 cells. Compared with the estrogen control, treatment with 10 µM α-, γ- and δ-tocopherol significantly decreased focus formation by 67% (p<0.05), 60% (p<0.05) and 58% (p<0.05), respectively. In contrast to MCF-7 cells, estrogen did not induce formation of γ-H2AX foci in MDA-MB-231 cells (Supplementary Fig. S4A and S4B). To further investigate whether tocopherols protect DNA damage from stimuli other than estrogen, the formation of γ-H2AX was analyzed in etoposide-stimulated MCF-7 cells (Fig. 5C and 5D). Treatment with etoposide (10 µM) resulted in 8.98-fold (p<0.05) increase in the formation of γ-H2AX foci, compared with the vehicle control. Treatment with α-, γ- and δ-tocopherol showed a modest decrease of the formation in MCF-7 cells by 25% (p<0.05), 27% (p<0.05) and 26% (p<0.05), respectively. In MDA-MB-231 cells, the formation of γ-H2AX was induced by etoposide (5.24-fold, p<0.05), and it was moderately attenuated by treatment with α-, γ- and δ-tocopherol (Supplementary Fig. S4C and S4D). Although tocopherols inhibited both estrogen- and etoposide-induced DNA double-stranded breaks, the effects of tocopherols are most significant in estrogen-mediated and ER-dependent events.
Figure 5.
Effects of tocopherols on estrogen- and etoposide-induced γ-H2AX formation in MCF-7 cells. A. Representative immunofluorescent staining for γ-H2AX in estrogen-treated MCF-7 cells. Cells were plated at a density of 8 × 104 cells/mL in 35 mm confocal plates and incubated with tocopherols (10 µM) and estrogen (1 nM) for 24 h. Scale bars: 20 µm. B. Quantification of γ-H2AX (green) in the cell nucleus (blue) by ImageJ program. Four images with 20x magnification from each group were selected at random and analyzed for the expression of γ-H2AX. Etoposide (10 µM) is used as a positive control. The data are represented as mean ± standard deviation (SD). n=4 independent experiments, a,b Significantly different from the control and estrogen control, respectively (p<0.05). C. Representative immunofluorescent staining for γ-H2AX in etoposide-treated MCF-7 cells. Cells were plated at a density of 8 × 104 cells/mL in 35 mm confocal plates and incubated with tocopherols (10 µM) and etoposide (10 µM) for 24 h. Scale bars: 20 µm. D. Quantification of γ-H2AX (green) in the cell nucleus (blue) by ImageJ program. Two images with 20x magnification from each group were selected at random and analyzed for the expression of γ-H2AX. Estrogen (1 nM) is used as a control. The data are represented as mean ± standard deviation (SD). n=2 independent experiments, a,b Significantly different from the control and estrogen control, respectively (p<0.05).
Discussion
The finding that estrogen-induced breast carcinogenesis could at least in part be mediated through generation of free radicals, oxidative stress and genotoxicity led to considerable interest in use of natural antioxidants to interfere with this process. Much attention was focused on the tocopherols, vitamins with potent antioxidant properties. The clinical trials were carried out with α-tocopherol"classical vitamin E” and the most abundant form in human tissues and plasma, but results have been disappointing. Recent epidemiological and preclinical studies have indicated that γ- and δ-tocopherols may be more effective because they are more efficient traps for reactive oxygen and nitrogen species (13, 19, 37). To better understand the biological activities of the different forms of tocopherols as potential inhibitors of estrogen-mediated breast cancer, we examined the effects of α-, γ-, δ-tocopherols and γ-TmT on MCF-7 cells in vitro and in vivo in xenograft tumors.
Our previous studies have shown that 0.3% and 0.5% dietary levels of a natural mixture, γ-TmT, were effective in inhibiting mammary tumor growth in estrogen-treated MCF-7 xenografted mice (11). To compare relative activities of individual tocopherols, 0.2% of purified α-, γ-, δ-tocopherols as well as γ-TmT in diet were used in our present in vivo study. Tocopherols significantly inhibited tumor growth, in the order: γ-TmT > γ-tocopherol > δ-tocopherol > α-tocopherol. Many studies have demonstrated that γ-TmT inhibits carcinogenesis in animal models of colon (23), lung (38), prostate (39, 40), and breast cancer (11). With regard to the relative activity of different forms of tocopherols, several studies reported the superiority of γ- and δ-tocopherols to α-tocopherol: γ- and δ-tocopherol had inhibitory effects in azoxymethane-induced colon carcinogenesis (13) and PhIP-induced colon cancer (12); δ-tocopherol was more active than γ-tocopherol in inhibiting lung xenograft tumor growth, whereas α-tocopherol was not effective (37); dietary administration of γ- and δ-tocopherols, but not α-tocopherol, inhibited the NMU-induced mammary tumorigenesis (10).
The greater activity of γ-, δ-tocopherol and γ-TmT in the inhibition of tumor growth corresponded well with its ability to down-regulate the expression of cell proliferation and estrogen-related genes. Estrogen stimulates cell proliferation due to activation of ER transcriptional activity and regulation of cell proliferation-related genes such as c-Myc and cyclin D1 (28). Previously, we reported that γ- and δ-tocopherol inhibited estrogen-induced cell proliferation in both MCF-7 and T47D cells (25). In the present study, estrogen increased the mRNA levels of cyclin D1 and c-Myc within 24 h of exposure in ER-positive MCF-7 cells, but not in ER-negative MDA-MB-231 cells. Treatment with γ-, δ-tocopherol and γ-TmT markedly down-regulated this estrogen-induced expression of cyclin D1 and c-Myc at both the RNA and protein levels in MCF-7 cells, indicating that the inhibition of cell proliferation by tocopherols is dependent on the ER.
Additionally, estrogen significantly upregulated the expression of TFF/pS2, cathepsin D and PGR in a time-dependent manner in MCF-7 cells, but not in MDA-MB-231 cells. Treatment with γ-, δ-tocopherol and γ-TmT, but not α-tocopherol, down-regulated the mRNA and protein levels of these genes. Progesterone plays an important role in mammary gland pathophysiology, and PGR, TFF/pS2 as well as cathepsin D have been used as indicators of breast cancer progression and as a predictor for tamoxifen resistance of breast tumors (41, 42). Several in vitro and in vivo studies have reported that a number of natural products have inhibitory activity against ER-positive breast cancers, due to their ability to regulate estrogen-responsive genes (7, 8, 43). Our studies suggest that tocopherol may likewise suppress ER-positive tumor growth by inhibiting the cellular response to estrogen, and that γ-, δ-tocopherol and γ-TmT are more active than α-tocopherol.
We further determined the inhibitory effects of α-, γ- and δ-tocopherols on estrogen-mediated oxidative/nitrosative stress as well as DNA damage in MCF-7 cells. Reactive oxygen and nitrogen species promote the initiation and progression of tumor by causing genomic instability, mutations, and deregulated cell proliferation (44). Metabolites of estrogen are implicated in oxidative stress and DNA damage (2). 8-oxo-dG is an oxidized derivative of deoxyguanosine, and is a frequent product of DNA oxidation and the cause of mutagenic lesions in DNA. During DNA replication, unrepaired 8-oxo-dG lesions induce G-T transversions, which may contribute to carcinogenesis (45). A number of recent studies have demonstrated that 8-oxo-dG, likely induced by estrogen metabolism-mediated oxidative stress, is formed after estrogen exposure and suggested that 8-oxo-dG may be associated with estrogen-induced breast carcinogenesis (32, 33, 46). In addition, reactive nitrogen species also affect the redox status of cells, which can alter cellular macromolecule structure and function and cause cancer. Elevated levels of nitrotyrosine, a biomarker of nitrosative stress, has been shown to induce DNA damage (47). In agreement with our results, some studies have demonstrated that DNA damage induced by estrogen is dependent on ER: the ER antagonist tamoxifen inhibits estrogen effects in MCF-7 cells, but not in MDA-MB-231 cells (48). In the current study, DNA double-stranded breaks measured by γ-H2AX formation were inhibited by α-, γ- and δ-tocopherols in estrogen-stimulated MCF-7 cells. Estrogen-induced 8-oxo-dG and nitrotyrosine levels were also decreased by γ- and δ-tocopherols, but not by α-tocopherol in MCF-7 cells (Fig. 4). Therefore, overall reduction of estrogen-induced oxidative and nitrosative stress as well as DNA damage by γ- and δ-tocopherol treatment may contribute to lowering the risk of mammary carcinogenesis via ER-dependent pathway.
Our study found that administration of 0.2% γ-, δ-tocopherol and γ-TmT in the diet significantly inhibit tumor growth and final tumor weight in an estrogen-induced MCF-7 xenograft model. In vitro studies showed that γ-, δ-tocopherol and γ-TmT have the ability to protect cells against estrogen-induced effects by regulating the expression of cell proliferation and estrogen-related genes and inhibiting oxidative and nitrosative stress as well as DNA damage in MCF-7 cells. The ineffectiveness of α-tocopherol was an important finding, and could be due to the presence of the 5-methyl group on the chromanol ring, which is less active in quenching reactive oxygen and nitrogen species than γ- and δ-tocopherols with their 5-position unmethylated. The difference in the chromanol ring structure may, in part, contribute to different anti-estrogenic activities of each form. Natural tocopherols, such as γ- and δ-tocopherols and γ-tocopherol rich mixture, are safe and effective dietary factors useful for the prevention and treatment of estrogen-mediated breast cancer. Previous clinical trials with α-tocopherol have proved negative in protection against cancer. The current results suggest a mechanistic explanation and that further tests of γ-, δ-tocopherol and γ-TmT in estrogen-induced or stimulated breast cancers are warranted.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Philip Furmanski for his helpful suggestions. Research reported in this publication was supported by the National Center for Complementary and Integrative Health of the National Institutes of Health under Award Number R01 AT007036, the National Institute of Environmental Health Sciences grant ES005022, Charles and Johanna Busch Memorial Fund at Rutgers University, the Trustees Research Fellowship Program at Rutgers, and the New Jersey Commission on Cancer Research Postdoctoral Fellowship to Soumyasri Das Gupta. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure of potential conflicts of interest: No potential conflicts of interest were disclosed.
References
- 1.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. The New England journal of medicine. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 2.Williamson LM, Lees-Miller SP. Estrogen receptor alpha-mediated transcription induces cell cycle-dependent DNA double-strand breaks. Carcinogenesis. 2011;32:279–285. doi: 10.1093/carcin/bgq255. [DOI] [PubMed] [Google Scholar]
- 3.Yue W, Yager JD, Wang JP, Jupe ER, Santen RJ. Estrogen receptor-dependent and independent mechanisms of breast cancer carcinogenesis. Steroids. 2013;78:161–170. doi: 10.1016/j.steroids.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Cribb AE, Knight MJ, Dryer D, Guernsey J, Hender K, Tesch M, et al. Role of polymorphic human cytochrome P450 enzymes in estrone oxidation. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15:551–558. doi: 10.1158/1055-9965.EPI-05-0801. [DOI] [PubMed] [Google Scholar]
- 5.Belous AR, Hachey DL, Dawling S, Roodi N, Parl FF. Cytochrome P450 1B1-mediated estrogen metabolism results in estrogen-deoxyribonucleoside adduct formation. Cancer research. 2007;67:812–817. doi: 10.1158/0008-5472.CAN-06-2133. [DOI] [PubMed] [Google Scholar]
- 6.Bak MJ, Das Gupta S, Wahler J, Suh N. Role of dietary bioactive natural products in estrogen receptor-positive breast cancer. Seminars in cancer biology. 2016;40–41:170–191. doi: 10.1016/j.semcancer.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shao ZM, Shen ZZ, Liu CH, Sartippour MR, Go VL, Heber D, et al. Curcumin exerts multiple suppressive effects on human breast carcinoma cells. International journal of cancer. 2002;98:234–240. doi: 10.1002/ijc.10183. [DOI] [PubMed] [Google Scholar]
- 8.Meng Q, Yuan F, Goldberg ID, Rosen EM, Auborn K, Fan S. Indole-3-carbinol is a negative regulator of estrogen receptor-alpha signaling in human tumor cells. The Journal of nutrition. 2000;130:2927–2931. doi: 10.1093/jn/130.12.2927. [DOI] [PubMed] [Google Scholar]
- 9.Sreeja S, Santhosh Kumar TR, Lakshmi BS. Pomegranate extract demonstrate a selective estrogen receptor modulator profile in human tumor cell lines and in vivo models of estrogen deprivation. The Journal of nutritional biochemistry. 2012;23:725–732. doi: 10.1016/j.jnutbio.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Smolarek AK, So JY, Burgess B, Kong AN, Reuhl K, Lin Y, et al. Dietary administration of delta- and gamma-tocopherol inhibits tumorigenesis in the animal model of estrogen receptor-positive, but not HER-2 breast cancer. Cancer Prev Res (Phila) 2012;5:1310–1320. doi: 10.1158/1940-6207.CAPR-12-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das Gupta S, Sae-tan S, Wahler J, So JY, Bak MJ, Cheng LC, et al. Dietary gamma-Tocopherol-Rich Mixture Inhibits Estrogen-Induced Mammary Tumorigenesis by Modulating Estrogen Metabolism, Antioxidant Response, and PPARgamma. Cancer Prev Res (Phila) 2015;8:807–816. doi: 10.1158/1940-6207.CAPR-15-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen JX, Liu A, Lee MJ, Wang H, Yu S, Chi E, et al. delta- and gamma-tocopherols inhibit phIP/DSS-induced colon carcinogenesis by protection against early cellular and DNA damages. Molecular carcinogenesis. 2017;56:172–183. doi: 10.1002/mc.22481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan F, Li G, Liu AB, Lee MJ, Yang Z, Chen YK, et al. delta- and gamma-tocopherols, but not alpha-tocopherol, inhibit colon carcinogenesis in azoxymethane-treated F344 rats. Cancer Prev Res (Phila) 2012;5:644–654. doi: 10.1158/1940-6207.CAPR-11-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Constantinou C, Papas A, Constantinou AI. Vitamin E and cancer: An insight into the anticancer activities of vitamin E isomers and analogs. International journal of cancer. 2008;123:739–752. doi: 10.1002/ijc.23689. [DOI] [PubMed] [Google Scholar]
- 15.Li G, Lee MJ, Liu AB, Yang Z, Lin Y, Shih WJ, et al. The antioxidant and anti-inflammatory activities of tocopherols are independent of Nrf2 in mice. Free radical biology & medicine. 2012;52:1151–1158. doi: 10.1016/j.freeradbiomed.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida Y, Niki E, Noguchi N. Comparative study on the action of tocopherols and tocotrienols as antioxidant: chemical and physical effects. Chemistry and physics of lipids. 2003;123:63–75. doi: 10.1016/s0009-3084(02)00164-0. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Q, Christen S, Shigenaga MK, Ames BN. gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. The American journal of clinical nutrition. 2001;74:714–722. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 18.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) Jama. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das Gupta S, Suh N. Tocopherols in cancer: An update. Molecular nutrition & food research. 2016;60:1354–1363. doi: 10.1002/mnfr.201500847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaziano JM, Glynn RJ, Christen WG, Kurth T, Belanger C, MacFadyen J, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians' Health Study II randomized controlled trial. Jama. 2009;301:52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das Gupta S, So JY, Wall B, Wahler J, Smolarek AK, Sae-Tan S, et al. Tocopherols inhibit oxidative and nitrosative stress in estrogen-induced early mammary hyperplasia in ACI rats. Molecular carcinogenesis. 2015;54:916–925. doi: 10.1002/mc.22164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suh N, Paul S, Lee HJ, Ji Y, Lee MJ, Yang CS, et al. Mixed tocopherols inhibit N-methyl-N-nitrosourea-induced mammary tumor growth in rats. Nutrition and cancer. 2007;59:76–81. doi: 10.1080/01635580701419022. [DOI] [PubMed] [Google Scholar]
- 23.Ju J, Hao X, Lee MJ, Lambert JD, Lu G, Xiao H, et al. A gamma-tocopherol-rich mixture of tocopherols inhibits colon inflammation and carcinogenesis in azoxymethane and dextran sulfate sodium-treated mice. Cancer Prev Res (Phila) 2009;2:143–152. doi: 10.1158/1940-6207.CAPR-08-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee HJ, Liu H, Goodman C, Ji Y, Maehr H, Uskokovic M, et al. Gene expression profiling changes induced by a novel Gemini Vitamin D derivative during the progression of breast cancer. Biochemical pharmacology. 2006;72:332–343. doi: 10.1016/j.bcp.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 25.Lee HJ, Ju J, Paul S, So JY, DeCastro A, Smolarek A, et al. Mixed tocopherols prevent mammary tumorigenesis by inhibiting estrogen action and activating PPAR-gamma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:4242–4249. doi: 10.1158/1078-0432.CCR-08-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smolarek AK, So JY, Thomas PE, Lee HJ, Paul S, Dombrowski A, et al. Dietary tocopherols inhibit cell proliferation, regulate expression of ERalpha, PPARgamma, and Nrf2, and decrease serum inflammatory markers during the development of mammary hyperplasia. Molecular carcinogenesis. 2013;52:514–525. doi: 10.1002/mc.21886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y, Lee MJ, Cheung C, Ju JH, Chen YK, Liu B, et al. Analysis of multiple metabolites of tocopherols and tocotrienols in mice and humans. Journal of agricultural and food chemistry. 2010;58:4844–4852. doi: 10.1021/jf904464u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mawson A, Lai A, Carroll JS, Sergio CM, Mitchell CJ, Sarcevic B. Estrogen and insulin/IGF-1 cooperatively stimulate cell cycle progression in MCF-7 breast cancer cells through differential regulation of c-Myc and cyclin D1. Molecular and cellular endocrinology. 2005;229:161–173. doi: 10.1016/j.mce.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Lee YM, Kim JB, Bae JH, Lee JS, Kim PS, Jang HH, et al. Estrogen-like activity of aqueous extract from Agrimonia pilosa Ledeb. in MCF-7 cells. BMC complementary and alternative medicine. 2012;12:260. doi: 10.1186/1472-6882-12-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan HJ, Li H, Liu Z, Yuan YC, Mortimer J, Chen S. SERPINA1 is a direct estrogen receptor target gene and a predictor of survival in breast cancer patients. Oncotarget. 2015;6:25815–25827. doi: 10.18632/oncotarget.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yahata T, Shao W, Endoh H, Hur J, Coser KR, Sun H, et al. Selective coactivation of estrogen-dependent transcription by CITED1 CBP/p300-binding protein. Genes & development. 2001;15:2598–2612. doi: 10.1101/gad.906301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh B, Chatterjee A, Ronghe AM, Bhat NK, Bhat HK. Antioxidant-mediated up-regulation of OGG1 via NRF2 induction is associated with inhibition of oxidative DNA damage in estrogen-induced breast cancer. BMC cancer. 2013;13:253. doi: 10.1186/1471-2407-13-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montano MM, Chaplin LJ, Deng H, Mesia-Vela S, Gaikwad N, Zahid M, et al. Protective roles of quinone reductase and tamoxifen against estrogen-induced mammary tumorigenesis. Oncogene. 2007;26:3587–3590. doi: 10.1038/sj.onc.1210144. [DOI] [PubMed] [Google Scholar]
- 34.Leung T, Rajendran R, Singh S, Garva R, Krstic-Demonacos M, Demonacos C. Cytochrome P450 2E1 (CYP2E1) regulates the response to oxidative stress and migration of breast cancer cells. Breast cancer research : BCR. 2013;15:R107. doi: 10.1186/bcr3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tu WZ, Li B, Huang B, Wang Y, Liu XD, Guan H, et al. gammaH2AX foci formation in the absence of DNA damage: mitotic H2AX phosphorylation is mediated by the DNA-PKcs/CHK2 pathway. FEBS letters. 2013;587:3437–3443. doi: 10.1016/j.febslet.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 36.Walles SA, Zhou R, Liliemark E. DNA damage induced by etoposide; a comparison of two different methods for determination of strand breaks in DNA. Cancer letters. 1996;105:153–159. doi: 10.1016/0304-3835(96)04266-8. [DOI] [PubMed] [Google Scholar]
- 37.Li GX, Lee MJ, Liu AB, Yang Z, Lin Y, Shih WJ, et al. delta-tocopherol is more active than alpha - or gamma -tocopherol in inhibiting lung tumorigenesis in vivo. Cancer Prev Res (Phila) 2011;4:404–413. doi: 10.1158/1940-6207.CAPR-10-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu G, Xiao H, Li GX, Picinich SC, Chen YK, Liu A, et al. A gamma-tocopherol-rich mixture of tocopherols inhibits chemically induced lung tumorigenesis in A/J mice and xenograft tumor growth. Carcinogenesis. 2010;31:687–694. doi: 10.1093/carcin/bgp332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng X, Cui XX, Khor TO, Huang Y, Dipaola RS, Goodin S, et al. Inhibitory Effect of a gamma-Tocopherol-Rich Mixture of Tocopherols on the Formation and Growth of LNCaP Prostate Tumors in Immunodeficient Mice. Cancers. 2011;3:3762–3772. doi: 10.3390/cancers3043762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen JX, Li G, Wang H, Liu A, Lee MJ, Reuhl K, et al. Dietary tocopherols inhibit PhIP-induced prostate carcinogenesis in CYP1A-humanized mice. Cancer letters. 2016;371:71–78. doi: 10.1016/j.canlet.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao Y, Zhou Q. A novel antiestrogen agent Shikonin inhibits estrogen-dependent gene transcription in human breast cancer cells. Breast cancer research and treatment. 2010;121:233–240. doi: 10.1007/s10549-009-0547-2. [DOI] [PubMed] [Google Scholar]
- 42.Johnston SR, Saccani-Jotti G, Smith IE, Salter J, Newby J, Coppen M, et al. Changes in estrogen receptor, progesterone receptor, and pS2 expression in tamoxifen-resistant human breast cancer. Cancer research. 1995;55:3331–3338. [PubMed] [Google Scholar]
- 43.Devipriya S, Ganapathy V, Shyamaladevi CS. Suppression of tumor growth and invasion in 9,10 dimethyl benz(a) anthracene induced mammary carcinoma by the plant bioflavonoid quercetin. Chemico-biological interactions. 2006;162:106–113. doi: 10.1016/j.cbi.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer cell. 2006;10:175–176. doi: 10.1016/j.ccr.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 45.Arsova-Sarafinovska Z, Eken A, Matevska N, Erdem O, Sayal A, Savaser A, et al. Increased oxidative/nitrosative stress and decreased antioxidant enzyme activities in prostate cancer. Clinical biochemistry. 2009;42:1228–1235. doi: 10.1016/j.clinbiochem.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 46.Singh B, Bhat NK, Bhat HK. Partial inhibition of estrogen-induced mammary carcinogenesis in rats by tamoxifen: balance between oxidant stress and estrogen responsiveness. PloS one. 2011;6:e25125. doi: 10.1371/journal.pone.0025125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roy D, Cai Q, Felty Q, Narayan S. Estrogen-induced generation of reactive oxygen and nitrogen species, gene damage, and estrogen-dependent cancers. Journal of toxicology and environmental health Part B, Critical reviews. 2007;10:235–257. doi: 10.1080/15287390600974924. [DOI] [PubMed] [Google Scholar]
- 48.Mobley JA, Brueggemeier RW. Estrogen receptor-mediated regulation of oxidative stress and DNA damage in breast cancer. Carcinogenesis. 2004;25:3–9. doi: 10.1093/carcin/bgg175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.