Abstract
Painful events early in life have been shown to increase the incidence of interstitial cystitis/painful bladder syndrome in adulthood. However, the intrinsic mechanism is not well studied. We previously reported that neonatal bladder inflammation causes chronic visceral hypersensitivity along with molecular disruption of spinal GABAergic system in rats. The present study investigates whether these molecular changes affect the integrative function and responses of bladder-sensitive primary afferent and spinal neurons. Neonatal bladder inflammation was induced by intravesicular injection of zymosan during postnatal (P) days 14 to 16. In adulthood (P60), the viscero-motor response (VMR) to visceral stimuli was significantly inhibited by intrathecal (i.t) HZ166 (GABAAα-2 agonist) only in neonatally saline-treated, but not in neonatally zymosan-treated rats. HZ166 significantly inhibited the responses of bladder responsive lumbosacral (LS) spinal neurons to urinary bladder distension (UBD) and slow infusion (SI) in neonatally saline-treated rats. Similar results were also observed in naïve adult rats where HZ166 produced significant inhibition of bladder responsive spinal neurons. However, HZ166 did not inhibit responses of UBD-responsive spinal neurons from neonatally zymosan-treated rats. The drug did not attenuate the responses of UBD-sensitive pelvic nerve afferent (PNA) fibers to UBD and SI in either group of rats tested. Immunohistochemical studies showed a significantly lower level of GABAAα-2 receptor expression in the LS spinal cord of neonatally zymosan treated rats compared to saline treated rats. These findings indicate that neonatal bladder inflammation leads to functional and molecular alteration of spinal GABAAα-2 receptor subtypes, which may result in chronic visceral hyperalgesia in adulthood.
Keywords: GABAA, interstitial cystitis, neonatal bladder injury, painful bladder syndrome, rat, visceral hypersensitivity
INTRODUCTION
Painful bladder syndrome (PBS)/ interstitial cystitis (IC) is characterized by mild to severe pain in the lower abdomen (Driscoll and Teichman, 2001; Berry et al., 2011; Nickel et al., 2014). In spite of several years of research, the etiology of this syndrome is not clearly understood. Pre-clinical studies have shown that adverse events including pain and inflammation during the neonatal stage can alter the functional development of sensory pathways, leading to long-term changes in pain threshold (Davis et al., 2005; Fillingim and Edwards, 2005; Fitzgerald and Walker, 2009; Low and Schweinhardt, 2012; Walker, 2013). For example, repeated gastric or colonic irritation in neonatal rats has been reported to alter their responses to innocuous and noxious stimuli in adulthood (Al-Chaer et al., 2000; Lin and Al-Chaer, 2003; Smith et al., 2007; Liu et al., 2008; Wang et al., 2008). In addition, traumatic psychological stress like maternal separation of neonatal rats causes visceral hyperalgesia during their adulthood (Coutinho et al., 2002; Wouters et al., 2012). Nickel and Tripp (2015) reported a close correlation between IC/PBS patients and childhood traumatic events like sexual abuse and severe illness. Similarly, recurrent urinary tract infection during childhood was reported to correlate with chronic pelvic pain, which often overlaps with irritable bowel syndrome (Peters et al., 2009).
Over the past decade several animal models have been used to fully understand the impact of the neonatal painful experience on the pain perception in adulthood. It was reported that transient inflammation of the rat bladder produced by instilling zymosan during the neonatal period produces significant bladder hypersensitivity in adulthood (Blatt et al., 2009). We have previously documented that zymosan-induced neonatal bladder inflammation also produces colonic hypersensitivity in adulthood, which is a model for cross organ hypersensitivity (Miranda et al., 2011). However, the exact mechanism by which neonatal bladder inflammation causes heightened pain sensation in adulthood is not fully understood, although multiple mechanisms both at the peripheral and central nervous system have been proposed (Randich et al., 2006; DeBerry et al., 2010). Our previous study reported a significant upregulation of micro-RNA (miR-181a) in the lumbo-sacral (LS) spinal dorsal horn neurons in neonatally zymosan-treated adult rats. The study documents that an increase in miR-181a can lead to a significant downregulation of GABAAα–1 receptor subtype in adult spinal cords of neonatally zymosan-treated rats (Sengupta et al., 2013). However, the functional effect of these molecular alterations caused by neonatal zymosan treatment has not been investigated systematically. The present study investigates whether the molecular alterations of GABAergic system including receptor expression and transmission following neonatal zymosan-induced bladder inflammation can alter the response characteristics of bladder distension-sensitive pelvic nerve afferent (PNA) fibers and lumbosacral (LS) spinal neurons receiving synaptic projection from the bladder PNA fibers. We used a highly selective GABAAα-2 receptor agonist, HZ166 to test its visceral analgesic effect on bladder pain and its efficacy in our model of chronic visceral hyperalgesia caused by zymosan-induced bladder inflammation in rats.
EXPERIMENTAL PROCEDURES
Animal care approval
All experimental procedures performed in this study were approved by Medical College of Wisconsin's Institutional Animal Care and Use Committee (AUA 000355). In addition, we followed the guidelines directed by the International Association for the Study of Pain (IASP) and extreme care was taken to minimize suffering to the animals during the study.
Animal housing and handling
The study was performed in 84 female Sprague-Dawley rats (8-10 weeks old). A total of 33 adult and 51 neonatally treated rats were used for this study. Timed-pregnant female Sprague-Dawley rats (n=12) were obtained from Taconic Biosciences (Indiana, IN, USA) and maintained in separate cages. Rats were kept under controlled conditions with a 12 hours light/dark schedule and had free access to both food and water. Rat pups were born in our animal care facility. On postnatal day 10 (P10), the sex of the pups was determined and female pups were marked by punching the ear. The pups remained housed with their mothers until they were weaned on P21. For adult, naïve rats, age matched females were obtained from the same vendor and maintained under same conditions. Twelve hours before the behavioral and electrophysiology experiments rats were deprived food, but not water.
Drugs and chemicals
A 1% zymosan A (Sigma-Aldrich, USA) in sterile saline was made freshly before transurethral instillation. HZ166 (ethyl 8-ethynyl-6-(pyridin-2-yl)-4H-benzo[f]imidazo[1,5-a][1,4]diazepine-3-carboxylate), a GABAAα-2 selective agonist was obtained from Dr. James Cook, Department of Chemistry, University of Wisconsin, Milwaukee. The dose of HZ166 was determined based on a pilot dose response study and also from previously published reports (Di Lio et al., 2011; Paul et al., 2014). In VMR experiments, HZ166 was injected intrathecally (i.t.) in a bolus (10 μg in 5μl/animal) dose followed by 5-10 μl saline to clear the indwelling epidural catheter. In electrophysiology experiments, a dose of 10 mg/kg, (i.v.) was injected based on previously study (Paul et al., 2014).
Induction of transient bladder inflammation in P14-P16 rats
Out of the 66 female pups obtained from 12 timed-pregnant rats, 30 pups were used for saline treatment and 36 were used for zymosan treatment. Bladder inflammation was produced by intravesicular injection of zymosan (1% in sterile saline) into the bladder. Female pups (n=36) at P14 were lightly anesthetized with isoflurane (3% induction and 1.5% maintenance with flow rate 1 ml/min), the vaginal area was swabbed clean with betadine and 100μl of zymosan was transurethrally injected into the bladder by gently inserting a shielded intravenous (i.v.) catheter (BD Insyte-N Autoguard, 24GA). The solution was left inside the bladder for 30 minutes followed by slow aspiration. Control rats (n=30) were subjected to the same protocol and received 100μl of sterile saline transurethrally injected into the bladder. This procedure was repeated for three consecutive days (P14 thru P16). Following saline/zymosan treatment, the pups were returned to their mothers until they were weaned on P21. All behavioral and electrophysiology studies were carried out at P60 and was performed by an investigator not blinded to treatment groups. At P60 we had 23 rats in the neonatally saline treated group and 28 rats in the neonatally zymosan treated group. Seven saline- and 8 zymosan-treated rats were rendered unusable either due to poor weight gain, loss of EMG electrode, misplaced catheter and post-surgical death.
Surgical procedure for recording EMG and intrathecal (i.t.) drug delivery
Behavioral measurement of visceral pain was performed on adult naïve and neonatally saline- and zymosan-treated rats. The rats (n=27) were anesthetized by injecting sodium pentobarbital (50 mg/kg. i.p) and pair of teflon-coated electrodes (Cooner Wire, Part No. A5631, Chatsworth, CA, USA) stripped at the tip were implanted into the external oblique muscle of the abdomen 1-1.5 cm apart to record the electrical activity of the muscles. For intrathecal (i.t.) drug administration at the lumbo-sacral segment of the spinal cord, a polyethylene catheter (PE-10, ~8 cm long) was chronically implanted into the epidural space following our previous procedure (Mickle et al., 2010; Kannampalli et al., 2014). Briefly, following the EMG electrode implantation, the head was stabilized by placing it on a stereotactic head holder (David Kopf, CA, USA). The i.t. catheter was advanced rostro-caudally through an incision made at the atlanto-occipital foramen. The EMG recording electrodes and the i.t. catheter were tunneled through the dorsal aspect of the skin and externalized dorsally near the neck. Electrodes and catheter were secured in place by suturing it to the neck muscle and guarded by a silastic tubing (~1cm long). The skin around the tubing was closed using 3-0 silk suture.
The placement of the i.t. catheter in the lumbo-sacral segment of the spinal cord was confirmed by injecting 20μl of sterile lidocaine (2%) after the completion of experiment. Rats having right placement of the i.t. catheter exhibited a transient hind limb paralysis following lidocaine injection. Rats that did not exhibit paralysis were excluded from the study. Following the placement of intrathecal catheter, rats were closely monitored for any signs of hind limb paralysis due to spinal injury. After the completion of experiments rats were euthanized by injecting Beuthanasia-D (Schering-Plough Animal Health Corp, NJ, USA).
EMG recordings from the abdominal muscles during colon or bladder distension
Seventy-two hours after the electrode implantation into the external oblique muscle of the abdomen, rats were placed inside the plexiglass restraining tubes for 2 hours/day for three days in order to acclimatize them to experimental conditions. VMR was measured by recording EMG to colorectal distension (CRD) of the hind gut. On the day of VMR recordings, rats were placed in restraining tubes and a highly compliant latex balloon (~5 cm long and 3.5 cm OD when fully inflated) coated with non-reactive bacteriostatic lubricant (Surgilube, Savage Laboratories, Melville, USA) was inserted into the colon through the anal opening for colon distension. A stimulus-response function (SRF) to graded intensities of CRD (10, 20, 30, 40, 60 mmHg) was recorded. The duration of distension was 30s with a 180s inter-stimulus interval between two intensities of distensions.
For urinary bladder distension (UBD), a PE-50 catheter was inserted transurethrally into the bladder and secured with a suture under isoflurane anesthesia. After the recovery from the anesthesia, rats were placed inside the restraining tube for at least 30 min before testing the VMR to UBD. For the measurement of VMR to UBD, graded volume (0.2, 0.4, 0.6 and 0.8 ml) of sterile saline was injected into the bladder. Similar to CRD, the bladder distending pressure was maintained for 30s and the responses to different volumes of distension were measured with a 180s inter-distension interval. The EMG signal was amplified using the amplifier (A-M System, model 1700, Carlsborg, WA, USA). Data were recorded real-time using the Spike 4/CED 1401 data acquisition program (CED 1401; Cambridge Electronic Design, Cambridge, UK).
Electrophysiology
Recording from UBD-sensitive pelvic nerve afferent (PNA) fibers
Recordings from PNA fibers innervating the urinary bladder were performed in (1) adult naïve (n=8), (2) neonatally saline (n=5) and (3) neonatally zymosan-treated (n=5) rats. Rats were anesthetized with urethane (1.5 g/kg, i.p) and maintained with supplemental doses as required. The trachea was intubated to mechanically ventilate the rat. The femoral artery and vein were cannulated for recording blood pressure and injecting drug, respectively. The rat was paralyzed with gallamine triethiodide (1 mg/kg, i.v.) and mechanically ventilated (55-60 strokes/min and 3-4 ml stroke volume). Supplemental doses of gallamine triethiodide were given as required to maintain paralysis during the recording process. A midline laparotomy was performed to expose the urinary bladder. The urine was carefully aspirated with a syringe and a small incision was made on the dome of the bladder. A PE-100 catheter for UBD and a pressure transducer (Millar Mikro-Tip®, Model # MPC-500) probe were inserted into the bladder through the incision and tightly secured to the bladder without any leakage. The external urethral orifice was sealed with a tissue adhesive (3M™ Vetbond™). The abdominal incision was sutured in layers with 4-0 silk sutures. A laminectomy was performed to expose the lumbo-sacral (T13-S2) segment of the spinal cord and the rat was stabilized by clamping the thoracic vertebra and hip joint. The dorsal incised skin was tied laterally to the spinal post to make a pool. The dura membrane was removed and the spinal cord was covered with warm mineral oil (37°C). Recordings were made from the distal cut end of the central processes of the L6 dorsal root as described in our previous study (Sengupta et al., 2002). The distension-sensitive PNA fibers innervating the bladder were identified by an UBD (0.4 ml). A stimulus-response function (SRF) curve was constructed by applying graded UBD (0.1, 0.2, 0.3, 0.4, 0.6 and 0.8 ml). The action potentials were amplified through a low-noise AC differential amplifier (model 3000; A-M Systems) and displayed on an oscilloscope. The nerve action potentials were processed through the window discriminator and the firing frequency histogram of the identified nerve fiber was counted (1s binwidth) on-line using the spike2/CED 1401 data acquisition software program.
Recording from UBD-sensitive lumbo-sacral (LS) spinal neurons
Recordings from lumbo-sacral (LS) spinal neurons were performed in (1) naïve adult (n=10), (2) neonatally saline-treated (n=6), and (3) neonatal zymosan-treated (n=11) rats as previously described (Kannampalli et al., 2014). Rats were anesthetized, paralyzed and the head was stabilized on a stereotaxic head holder. Blood vessel cannulation, tracheal intubation and bladder cannulation were performed as described in the previous section. For cervical (C1-C2) spinal transection, a laminectomy was performed to expose the cervical spinal cord. The dura membrane was gently removed and 10-15μl of 2% lidocaine was applied to the dorsal surface of the exposed spinal cord. After 10 min, the spinal cord was completely transected. The transected area was covered with a small piece of gelfoam soaked in warm saline. Initially, animals exhibited a vasodepressor response following spinal transection, but recovered after 15-30 minutes. Spinal recordings were made at least one hour following the spinal transection. The LS spinal cord was exposed by laminectomy (T13-S2) and the rat was suspended from the thoracic vertebrae with ischial spinal clamps. The dorsal incised skin was tied laterally as described in earlier section to make a pool for mineral oil. The dura was carefully removed. The pool was covered with 1.75% agar in saline and after hardening of the agar, a small window was cut to expose the spinal cord. Single barrel carbon fiber filled glass microelectrode (0.4-0.8 mΩ, CARBOSTAR-1, Kation Scientific, MN) was used for extracellular recording. The placement of the electrode was 0.1-0.5 mm lateral from the spinal midline and recordings were done in depth between 800-1000 μm from the dorsal surface of the spinal cord. The SRF to UBD was constructed following the same protocol as used for PNA recordings.
Experimental protocol
Behavioral study (EMG recording)
The effect of HZ166 was tested on VMRs to CRD or UBD in (1) naïve adult, (2) neonatally saline-treated and (3) neonatally zymosan-treated rats. Following a baseline SRF to either CRD or UBD, rats were administered HZ166 (10 μg bolus, i.t.) and the VMR was repeated 5 minutes after the drug administration.
Electrophysiology study
Similar to VMR study protocol, electrophysiology experiments were undertaken in (1) naïve adult, (2) neonatally saline-treated and (3) neonatally zymosan-treated rats. Both in PNA fiber and spinal recordings, following the identification UBD-sensitive neuron, a baseline SRF was constructed to graded UBD (0.1 to 0.8ml) and was repeated 5 minutes after the injection of HZ166 (10 mg/kg, i.v.). The spinal neuron recordings were done in spinal transected (C1-C2) rats to eliminate supraspinal descending influence. Additionally, slow infusion (SI) of saline (flow rate: 0.05 ml/min for 15 min) was performed in the same rat to test the response of UBD-responsive afferents/neurons to slow filling of the bladder. Baseline recordings were done beginning with a pre-SI phase (60s), SI phase (15 min) and finally a post-SI phase (60s). Similar to phasic graded UBD, following a baseline recording of the response of the PNA/ LS neuron to SI, the same protocol was repeated 5 minutes after the injection of HZ166.
Immunostaining for GABAAα–2 in L6-S1 DRGs and LS spinal dorsal horn neurons
For immunohistochemical studies, rats (n=12; 6 each from saline and zymosan treated group) at P60 were deeply anesthetized with pentobarbital sodium (50 mg/kg, i.p.) and the chest cavity was opened by a midsternal incision. Animals were perfused transcardially with cold phosphate buffer solution followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The lumbosacral spinal cord and L6-S1 DRGs were collected bilaterally and stored in 4% paraformaldehyde overnight at 4°C followed by transferring the tissues to 20% sucrose solution for 24 hours. Spinal cord (20 μm) sections were cut and collected in a 24-well plate containing the cryoprotectant. For DRGs, sections (20μm) were cut and collected on to sterile glass slide. The sections were allowed to reach room temperature and washed twice with 1X PBS (phosphate buffered saline) for 5 min each. The sections were then incubated in blocking solution (0.1 M PBS + 0.25% Triton-X-100 + 10% normal goat serum) at room temperature for 1h followed by incubation with anti-GABAAα-2 receptor antibody (EMD Millipore Chemicals; Cat # MABN1724) at a dilution of 1:200 for 48 hrs at 4° C. For DRG sections, the slides were incubated overnight at 4 °C in a humid chamber with anti-GABAAα-2 receptor antibody (Alomone labs, Cat #: AGA-002) at a dilution of 1:250. Thereafter, the sections were washed (15 min/wash × 6) with wash buffer and incubated with goat anti-rabbit secondary antibody (Alexa Fluor 568, Invitrogen # A11036,) at RT for 2h. The sections were washed as mentioned above and mounted using mounting medium (VECTASHIELD®; Vector Laboratories, Inc, CA, USA). Slides were examined under a fluorescence microscope (Nikon Eclipse 50i) using narrow band cubes Alexa 568 (DM 568, excitation filter 540-560, barrier filter 575-645 nm). Images were captured with a Spot II high-resolution digital camera (Diagnostic Instruments Inc., Sterling Heights, MI, USA). To maintain the consistency of image capturing, we used the same time exposure, gain and gamma adjustment for the all samples. Tissue sections obtained from 6 separate animals from each treatment (saline and zymosan) groups were examined for the expression of GABAAα-2 receptor in the spinal cord and DRGs.
Data analysis
VMR analysis
All statistical analysis was performed using SigmaStat (V2.03, SPSS, Inc., Chicago, IL). An SRF to graded CRD/UBD was constructed to test the intensity dependent increase in EMG activity. The EMG response to each distension pressure or volume was divided by the EMG response to the highest distension volume (0.8ml) or pressure (60mmHg) in order to obtain normalized percentage values. Comparison was performed between pre- and post-drug responses to respective intensity of distending volume or pressure. Similarly, group comparison was made between saline- and zymosan-treated rats to confirm the visceral hypersensitivity. Statistical analysis was determined by two way repeated measure of ANOVA followed by Holm-Sidak test for multiple comparisons. The data was also subjected to the normality and equal variance test with Shapiro-Wilk test. Values are expressed as mean ± S.E.M. and p≤0.05 were considered to be significant.
Electrophysiology analysis
For analysis of PNA fibers and LS spinal neuron's responses to UBD, the total number of action potentials over a 30s resting period prior to bladder distension and during the distension period (30s) was counted and represented as impulses/s. To measure the actual changes in response of the neurons to UBD, the mean firing frequency during the resting period (30s pre-distension) was subtracted from the mean firing frequency during bladder distension (30s distension). The difference was calculated for each distension volume tested (0.1-0.8 ml) and the response was divided by the response of the neuron to the highest distension volume (0.8 ml; 100%) in order to obtain normalized percentage values. The baseline spontaneous firing response was calculated from the mean firing frequency during a 60s resting period devoid of any distension and represented as impulses/s. Statistical analysis was determined by two way repeated measure of ANOVA followed by Holm-Sidak test for multiple comparisons. The data was also subjected to the normality and equal variance test with Shapiro-Wilk test. Values are expressed as mean ± S.E.M. and p≤0.05 were considered to be significant.
For slow infusion (SI) studies, the action potentials of PNA fibers/ LS spinal neurons pre-SI, SI and post-SI was calculated for a period of 60s. Following administration of HZ166, the action potentials of PNA fibers and LS spinal neurons pre-SI, SI and post-SI was calculated for a period of 60s. Statistical comparison was performed using Student ‘t’ test between the pre and post-HZ166 responses during the SI phase. The data was also subjected to the normality and equal variance test. Values are presented as mean impulses/min and p≤0.05 were considered to be significant.
Immunohistochemical analysis
Six animals each from saline- and zymosan-treated groups were used for quantifying the expression of GABAAα–2 in the LS spinal cord and DRGs. We selected one section per animal from both groups and the image at 20 × magnifications was opened under image J program (NIH, Bethesda, MD). The freehand tool was used to trace the outline of all the cells on the field and the number of positively stained cells from 6 sections per group was counted by an investigator blinded to the groups. The average number of positively stained cells from 6 sections (1 per rat) from both the group was determined. The number of GABAAα–2 positive cells from zymosan-treated rats were compared with saline-treated controls using Student ‘t’ test. Values are presented as mean ± S.E.M and p≤0.05 were considered to be significant.
RESULTS
Behavioral Pain response
VMR and effect of HZ166 in naïve adult rats
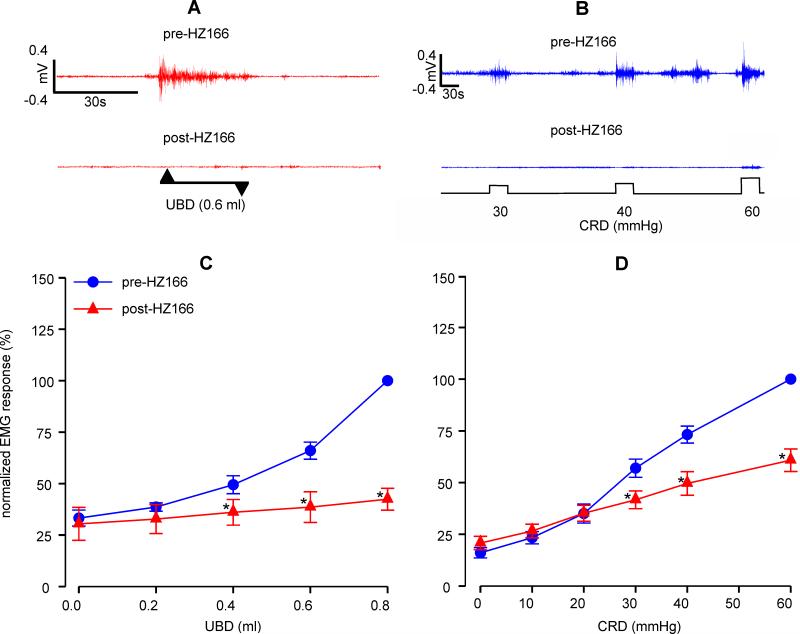
HZ166, a selective GABAAα-2 agonist, exhibit analgesic effect in somatic pain model (Paul et al., 2014). However, its effect on a model of visceral pain has not yet been studied. In naïve adult rats, i.t. injection of HZ166 (10 μg/animal) produced a significant (n=6, F4, 20 = 10.10, p<0.001 vs pre-HZ166) decrease in VMR to phasic isovolumic UBD from 0.4 to 0.8ml (figs 1A and C). Similarly, HZ166 also produced a significant (n = 9, F5, 40 = 12.45, p<0.001 vs pre-HZ166) decrease in VMR to graded CRD between distension pressure of 30 mmHg to 60 mmHg (figs 1B and D). Unlike muscimol, a GABAA agonist, HZ166 did not produce any hind limb paralysis or motor deficit in our study (unpublished observation).
Fig. 1.
Effect of HZ166 (10 μg/animal) on the VMR of naïve adult rats. Representative EMG tracing pre- and post- intrathecal (i.t) administration of HZ166 to 0.6 ml of UBD (A) and 30-60 mmHg of CRD (B). VMR is represented as percentage normalized EMG response to either to phasic (0.2-0.8 ml) UBD or graded (10–60 mmHg) CRD. (C) Intrathecal administration of HZ166 significantly (n=6, p<0.001 vs pre-HZ166) decreased the VMR to UBD from 0.4 ml onwards. (D) Similarly, i.t administration of HZ166 also significantly (n=9, p<0.001 vs pre-HZ166) decreased the VMR to CRD from 20 mmHg pressure onwards. Values are expressed as mean ± S.E.M of ‘n’ animals in each group. p≤0.05 was considered significant. * compared with pre-HZ166 baseline.
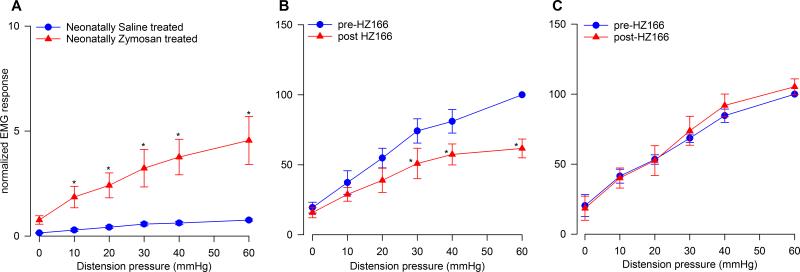
VMR and effect of HZ166 in neonatally zymosan- and saline-treated rats
Neonatally saline- and zymosan-treated rats were tested for VMR at P60 when these rats were fully adult. The zymosan-treated rats exhibited visceral hyperalgesia characterized by significantly increased VMR (n = 6, F5, 25 = 8.587, p<0.001 vs pre-HZ166, fig 2A) compared to saline-treated rats. Injection of HZ166 (10 μg/animal, i.t.) to the neonatally saline-treated rats produced a significant (n = 6, F5, 25 = 4.77, p<0.001 vs pre-HZ166, fig 2B) decrease in the VMR to graded CRD similar to that observed in adult naïve rats (fig 1D). However, in neonatally zymosan-treated rats that exhibited visceral hyperalgesia, administration of HZ166 did not produce any significant decrease (n = 6, F5, 25 = 0.893, p = 0.847 vs pre-HZ166, fig 2C) in the VMR to graded CRD.
Fig. 2.
Effect of HZ166 on the VMR to CRD of neonatal saline- and zymosan-treated rats tested at P60. (A) Neonatal zymosan-treated rats exhibited visceral hypersensitivity characterized by significantly (n=6, p<0.001 vs neonatal saline- treated rats) increased VMR to CRD from 10 mmHg onwards. (B) Administration of HZ166 (10 μg/animal, i.t) to neonatal saline- treated rats produced a significant (n=6, p<0.001 vs pre-HZ166) decrease in their VMR to graded CRD from distension pressure 30 mmHg onwards. (C) However, in neonatal zymosan-treated rats, the same dose of HZ166 did not produce any inhibition of the VMR to CRD. Values expressed as mean ± S.E.M of ‘6’ animals in each group. p≤0.05 was considered significant. * compared with neonatal saline-treated or pre-HZ166 baseline.
Electrophysiology studies
Effect of HZ166 on responses of UBD-sensitive PNAs in naïve adult rats
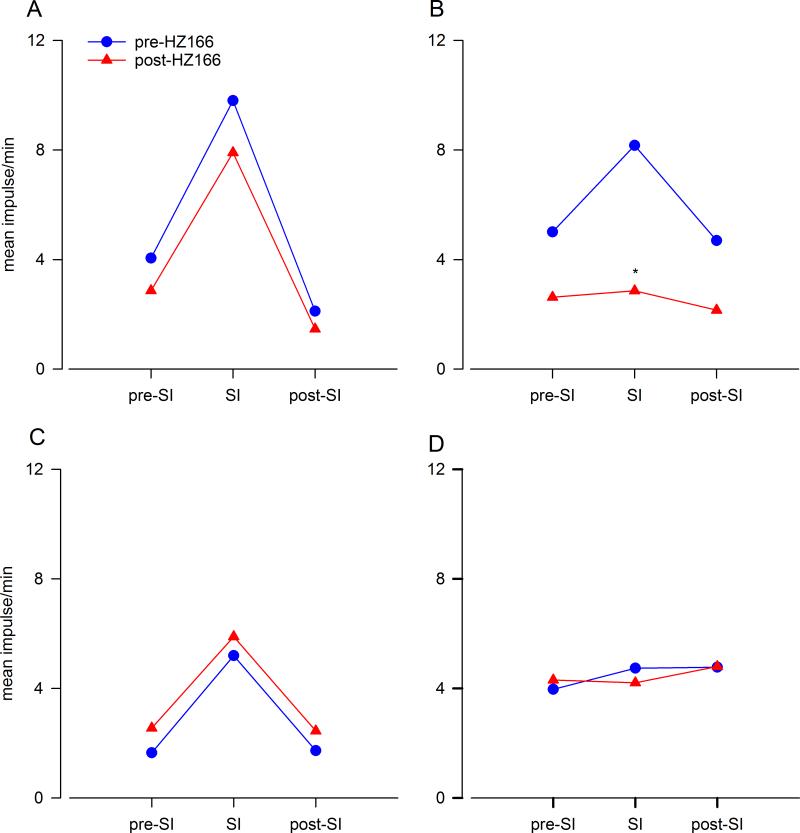
To examine whether HZ166 has any effect on the mechanotransduction of UBD-sensitive PNAs in adult naïve rats, we have recorded the responses of these afferent nerve fibers before and after HZ166 injection (10 mg/kg, i.v.). Figures 3A and B show examples of volume-dependent responses of one UBD-sensitive PNA fiber before and after injection of HZ166 in a naïve adult rat. A total of 8 PNA fibers from 8 adult naive rats were tested. Results show that HZ166 does not modulate both the intensity dependent increase to phasic UBD (n = 8, F5, 35 = 0.925, p = 0.845 vs pre-HZ166, fig 3C) and the spontaneous resting firing (n = 8, p = 0.495 vs pre-HZ166) of these afferent fibers (fig 3D).
Fig. 3.
Example of typical response of a UBD-sensitive PNA fiber from an adult naive rat pre- (A) and post (B) administration of HZ166 to phasic bladder distension. In all panels, the top trace shows the response to UBD represented as a frequency histogram (1s binwidth), the middle trace is the neuron action potential and the bottom trace is the distension volume. (C) Administration of HZ166 (10 mg/kg, b.w. i.v) did not alter the response of these fibers to phasic UBD. (D) The spontaneous firing of the PNAs was not significantly altered following HZ166 administration. Values expressed as normalized mean ± S.E.M of 8 neurons tested.
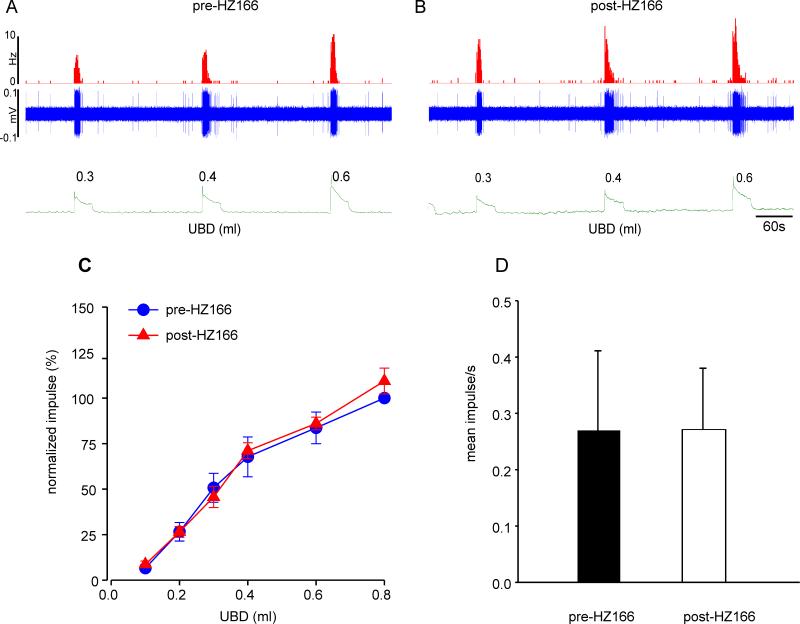
Effect of HZ166 on responses of PNAs from neonatally saline- and zymosan-treated rats
In the second set of experiments, we tested the effect of HZ166 on UBD-sensitive PNA fibers from neonatally saline- and zymosan-tread rats. Figures 4A and B show examples of typical response characteristics of one fiber from neonatally saline-treated rats before and after injection of HZ166. A total of 5 PNA fibers from 5 neonatally saline treated rats were tested. Similar to that observed in adult rats (fig 3), HZ166 did not attenuate the responses of PNA fibers from neonatally saline-treated rats as the mean SRFs of these fibers before and after HZ166 are basically superimposed (n = 5, F5, 20 = 1.11, p = 0.382 vs pre-HZ166, fig 4C). The spontaneous resting firing of these fibers also remained unaltered following HZ166 treatment (n = 5, p = 0.151, fig 4D).
Fig. 4.
Effect of HZ166 on the mechanotransduction of PNA fibers from neonatal saline-treated rats. Example of the typical response of a UBD-sensitive PNA fiber to bladder distention pre- (A) and post- (B) administration of HZ166. In all panels, the top trace shows the response to UBD represented as a frequency histogram (1s binwidth), the middle trace is the neuron action potential and the bottom trace is the distension volume. (C) Administration of HZ166 (10 mg/kg, b.w. i.v) did not affect the mechanotransduction properties of these fibers to phasic UBD. (D) The drug also did not significantly attenuate the spontaneous firing of PNAs. Values expressed as normalized mean ± S.E.M of 5 neurons.
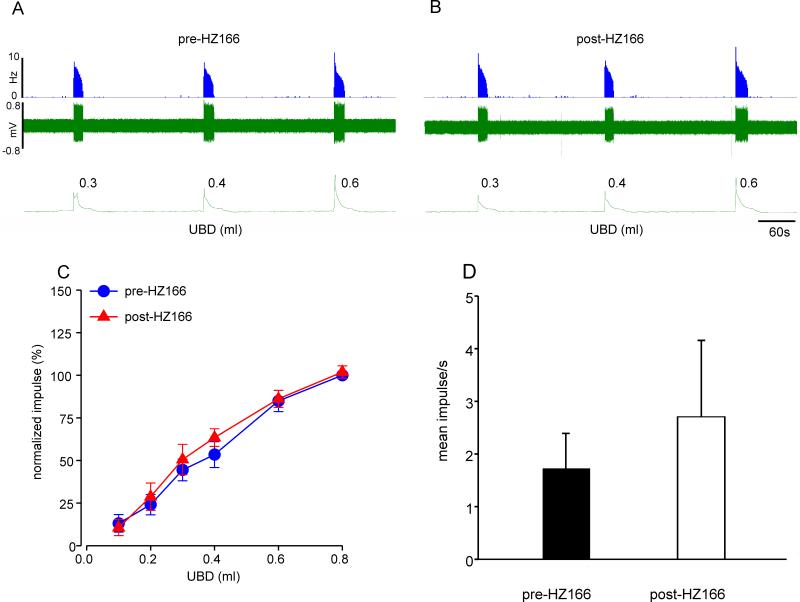
Similarly, HZ166 did not attenuate mechanotransduction of UBD-sensitive PNA afferent fibers from neonatally zymosan-treated rats. Figures 5A and B show examples of mechanosensitive responses of one UBD-sensitive PNA afferent to UBD before and after HZ166 injection. A total of 5 PNA fibers from 5 neonatally zymosan treated rats were tested. Data shows that the mean SRFs remained unaltered (n = 5, F5, 20 = 0.731, p = 0.608 vs pre-HZ166, fig 5C) however the spontaneous firing of these fibers showed a significant increase (n = 5, p<0.05 vs pre-HZ166, fig 5D) following HZ166 administration.
Fig 5.
Effect of HZ166 on the mechanotransduction of PNA fibers from neonatal zymosan-treated rats. Example of the typical response of an UBD-sensitive PNA fiber pre- (A) and post- (B) HZ166 administration. In all panels, the top trace shows the response to UBD represented as a frequency histogram (1s binwidth), the middle trace is the neuron action potential and the bottom trace is the distension volume. (C) Administration of HZ166 (10 mg/kg, b.w. i.v) did not alter the mechanotransduction properties of these fibers to phasic UBD. (D) However, HZ166 produced a significant (p<0.05 vs pre-HZ166) increase in the spontaneous firing of PNA. Values expressed as normalized mean ± S.E.M of 5 neurons. p≤0.05 was considered significant. * compared with pre-HZ166 baseline.
Effect of HZ166 on UBD-responsive L6 spinal neurons from adult naïve rats
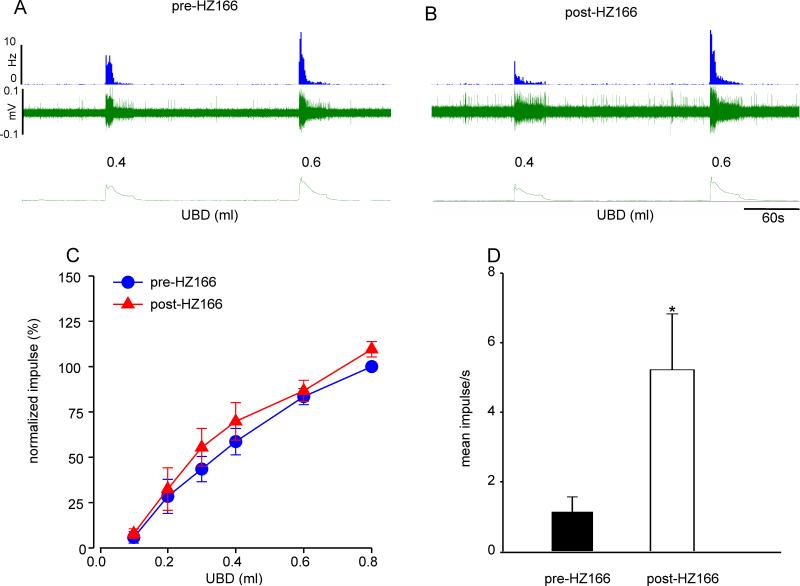
We studied a total of 10 UBD sensitive LS spinal neurons from 10 adult naive rats for their responses to HZ166. Unlike the effect of HZ166 on UBD-sensitive PNA fibers, the drug produced a significant inhibition of responses of UBD-responsive spinal neurons at every volume of UBD ≥ 0.2 ml. Figure 6A and 6B shows typical responses of one UBD-responsive neuron to incremental volume of UBD before and after injection of HZ166 (10 mg/kg, i.v.). However, the responses of the neuron were markedly inhibited following the injection of HZ166. The mean SRFs show that HZ166 significantly (n=10, F5, 45 = 8.542, p<0.001 vs pre-HZ166, fig 6C) inhibits the responses of UBD-sensitive LS spinal neurons. HZ166 did not modulate the spontaneous resting firing of the spinal neurons tested (n = 10, p = 0.471, fig 6D).
Fig. 6.
Effect of HZ166 on the UBD-responsive L6 spinal neurons from adult naïve. Example of the typical response of an UBD-sensitive L6 spinal neuron pre- (A) and post- (B) HZ166 administration. In all panels, the top trace shows the response to UBD represented as a frequency histogram (1s binwidth), the middle trace is the neuron action potential and the bottom trace is the distension volume. (C) Administration of HZ166 (10 mg/kg, b.w. i.v) significantly (n=10, p<0.001 vs pre-HZ166) decreased the response of these neurons to phasic UBD. (D) HZ166 did not attenuate the spontaneous firing of these spinal neurons. Values expressed as normalized mean impulse ± S.E.M of 10 neurons. p≤0.05 was considered significant. * compared with pre-HZ166 baseline.
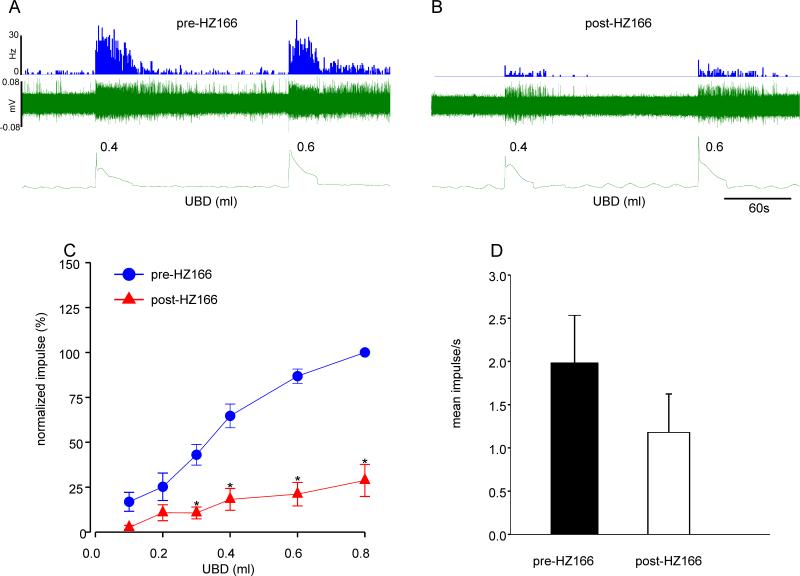
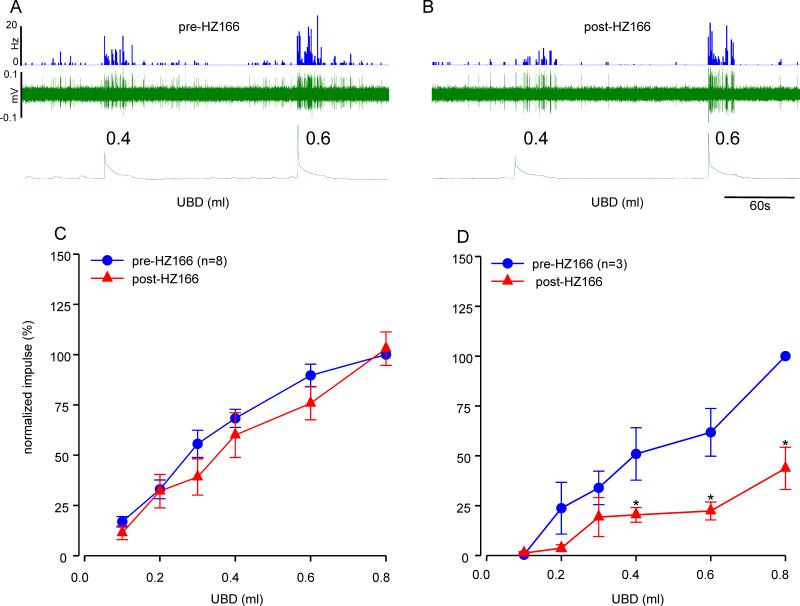
Effect of HZ166 on UBD-sensitive spinal neurons from neonatally saline- and zymosan-treated rats
Figures 7A and 7B illustrates the responses of one UBD-responsive neuron from neonatally saline-treated rats before and after injection of HZ166 (10 mg/kg, i.v.), respectively. Six UBD sensitive LS spinal neurons from 6 neonatally saline treated rats were tested. Similar to that observed in adult naïve rats, HZ166 produced a significant inhibition of responses of spinal neurons at every volume of UBD ≥ 0.2ml onwards (n = 5, F5, 25 = 5.127, p<0.001 vs pre-HZ166 fig. 7C). Although the drug produced a marked reduction of spontaneous resting firing the neurons, it was not statistically significant (n = 6, p = 0.116, fig. 7D).
Fig. 7.
Effect of HZ166 on the UBD-responsive L6 spinal neurons from neonatal saline-treated rats measured at P60. Example of the typical response of an UBD-sensitive L6 spinal neuron pre- (A) and post- (B) HZ166 administration. In all panels, the top trace shows the response to UBD represented as a frequency histogram (1s binwidth), the middle trace is the neuron action potential and the bottom trace is the distension volume. (C) Administration of HZ166 (10 mg/kg, b.w. i.v) significantly (p<0.001 vs pre-HZ166) decreased the response of these neurons to phasic UBD from 0.3 ml onwards. (D) HZ166 did not significantly attenuate the spontaneous firing of these spinal neurons. Values expressed as normalized mean impulse ± S.E.M of 6 neurons. p≤0.05 was considered significant. * compared with pre-HZ166 baseline.
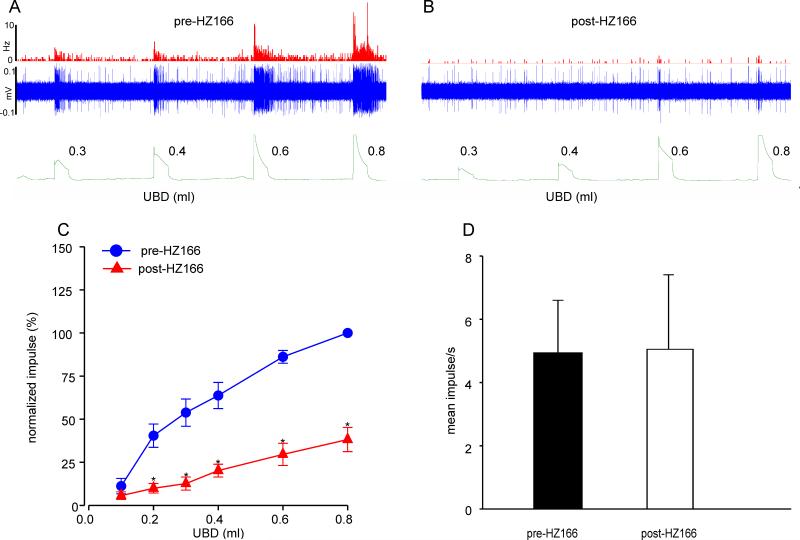
In a second set of experiment, we tested the effect of HZ166 on 11 UBD-responsive spinal neurons from 11 neonatally zymosan-treated rats. The drug failed to produce marked inhibition of UBD-induced excitation of the spinal neuron (figs. 8A & B). The inhibitory effect of HZ166 on the excitation of UBD-responsive neurons was not observed in 73% (8/11) neurons. The mean SRFs of these neurons before after the injection of the drug were identical (n = 8, F5, 35 = 0.657, p = 0.658 vs pre-HZ166 fig 8C). However, responses of 27% (3/11) neurons were significantly (n = 3, F5, 10 = 4.484, p<0.001 vs pre-HZ166, fig 8D) inhibited by HZ166.
Fig. 8.
Effect of HZ166 on the UBD-responsive L6 spinal neurons from neonatal zymosan-treated rats at P60. Example of the typical response of an UBD-sensitive L6 spinal neuron pre- (A) and post- (B) HZ166 administration. In all panels, the top trace shows the response to UBD represented as a frequency histogram (1s binwidth), the middle trace is the neuron action potential and the bottom trace is the distension volume. (C) Administration of HZ166 (10 mg/kg, b.w. i.v) did not decrease the response in 8 out of the 11 neurons tested to phasic UBD. (D) In 3 out of 11 neurons tested, the same dose of HZ166 significantly (p<0.001 vs pre-HZ166) attenuated the response to UBD from 0.4 ml distension onwards. . Values expressed as normalized mean impulse ± S.E.M of ‘n’ neurons in each panel. . p≤0.05 was considered significant. * compared with pre-HZ166 baseline.
Effect of HZ166 on responses of UBD-sensitive PNAs and L6 spinal neurons to slow infusion (SI) in adult naïve, neonatally saline- and zymosan-treated rats
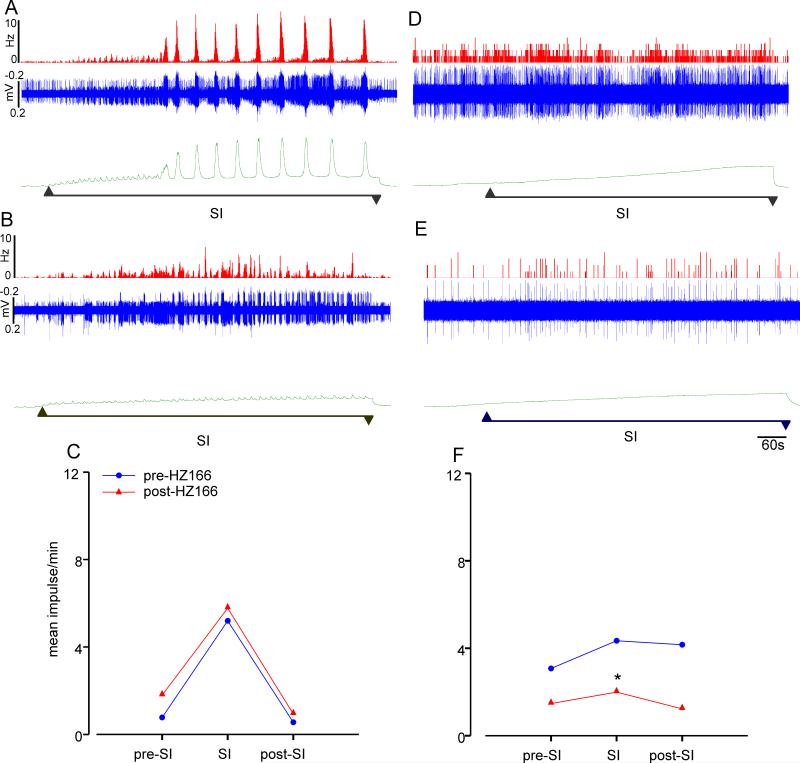
The animals used in electrophysiology studies were also subjected to the slow infusion protocol. In adult naïve rats, slow infusion (SI) of saline into the bladder produced voiding contraction dependent simultaneous increase in the firing frequency of bladder sensitive PNA fibers. Administration of HZ166 (10 mg/kg, i.v.) inhibited the voiding contraction, but it did not significantly inhibit the PNAs (n = 6, p = 0.325, figs. 9A, B and C). In spinal transected rats, slow infusion of saline into the urinary bladder did not produce any infusion-dependent voiding contraction due to lack of supra-spinal motor influence on bladder contraction. However, administration of HZ166 produced a significant decrease in the firing frequency of the bladder-responsive neurons. (n = 8, p <0.01, figs. 9D, E and F).
Fig. 9.
Effect of HZ166 on responses of UBD-sensitive PNAs and L6 spinal neurons to slow infusion (SI) in adult naïve rats. Example of the typical response of an UBD-sensitive PNA fiber pre- (A) and post- (B) HZ166 administration to SI of saline. In all panels, the top trace shows the response to UBD represented as a frequency histogram (1s binwidth), the middle trace is the neuron action potential and the bottom trace indicates the cystometrogram following slow infusion of saline. (C) Slow infusion of saline into the bladder produced voiding contraction dependent simultaneous increase in the firing frequency of bladder sensitive PNA fibers. However, administration of HZ166 (10 mg/kg, b.w. i.v) did not significantly inhibit the firing frequency of the PNAs tested (n=6). Example of a typical response of an UBD-sensitive L6 spinal neuron pre- (D) and post- (E) HZ166 administration to SI of saline. Cervical spinal transection resulted in the absence of the characteristic voiding contraction during SI (F). Administration of HZ166 significantly (n=10, p <0.01 vs pre-HZ166) inhibited the firing frequency of these neurons to SI of saline into the bladder. Values expressed as mean impulse of 6 or 8 neurons tested. p≤0.05 was considered significant. * compared with corresponding pre-HZ166 baseline.
Similar to naïve adult rats, neonatally saline-treated rats exhibited infusion induced voiding contraction and a simultaneous increase in firing frequency of PNAs to slow infusion of saline. Administration of HZ166 did not affect the responses of UBD-sensitive PNA fibers to slow infusion (n = 3, p = 0.306, fig 10A). However, the drug significantly inhibited the responses of LS spinal neurons (n = 4; p<0.05, fig. 10B). This distinct differential effect is similar to that observed in adult naïve rats (fig 9). Slow infusion of saline in neonatally zymosan-treated rats did not produce the characteristic intensity-dependent voiding contractions typically observed in adult naïve and neonatally saline treated rats. HZ166 was found to be ineffective against both UBD-sensitive PNA fibers (n = 3; p = 0.349, fig. 10C) and spinal neurons (n = 10; p = 0.166, fig. 10D) during slow infusion.
Fig. 10.
Effect of HZ166 on responses of UBD-sensitive PNAs and L6 spinal neurons to slow infusion (SI) in neonatally saline- and zymosan-treated rats. In neonatally saline-treated rats, administration of HZ166 (10 mg/kg, i.v.) did not significantly inhibit the PNAs at P60 (n=3, A); however the drug significantly (n=4, p<0.05 vs pre-HZ166) inhibited the response of UBD-responsive L6 spinal neuron (B). In neonatally zymosan-treated rats, administration of the same dose of HZ166 did not significantly inhibit the PNAs (n=3, C). HZ166 was found to be ineffective against UBD-sensitive spinal neurons during slow infusion in neonatally zymosan-treated rats (n=10, D). Values expressed as mean impulse/min of the neurons tested. p≤0.05 was considered significant. * compared with corresponding pre-HZ166 baseline.
Levels of GABAAα-2 expression in the LS spinal cord and DRGs of neonatally saline and zymosan- treated rats
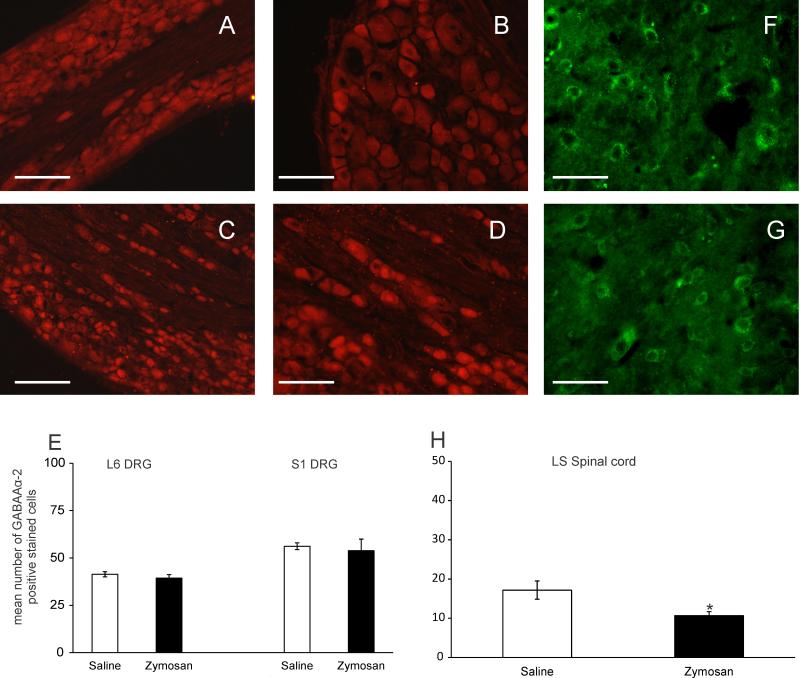
DRGs from both neonatally saline- (fig 11A and B) and zymosan-treated (fig 11C and D) rats exhibited strong expression for GABAAα-2 receptors. Immunohistochemical quantification of the number of GABAAα-2 positive stained cells showed no significantdifference at both L6 (n = 6, p = 0.309) and S1 DRGs (n = 6, p = 0.371) in the expression between the neonatally saline- and zymosan-treated groups (fig 11E). Fig 11F and G shows the expression of GABAAα-2 receptors in the LS spinal cord from both neonatally saline- and zymosan-treated rats, respectively. Immunohistochemical quantification showed that neonatally zymosan treated rats exhibit a significant decrease in the number of GABAAα-2 positive stained cells in the LS spinal cord. (n = 6, p<0.05, fig 11H)
Fig. 11.
Photomicrograph of GABAAα-2 positive stained DRGs from the L6 and S1 DRG from neonatally saline- (A and B) and zymosan-treated rats (C-D). (E) Quantitative analysis of the number of GABAAα-2 immunoreactive cells from both L6 and S1 DRGs indicate that there was no significant difference between neonatally saline- and zymosan- treated rats. LS spinal cord from neonatally saline- (F) and zymosan-treated rats (G) showed a significant (n=6, p<0.05 vs saline) decrease in the number of GABAAα-2 positive stained cells (H). Scale bars represent 50μm. Values expressed as mean ± S.E.M of the number of GABAAα-2 positive cells from 6 animals in each group. p≤0.05 was considered significant. * compared with neonatally saline treated group.
DISCUSSION
There is a growing interest to understand how painful events in the neonatal period affect the developing nociceptive neural circuitry and how it alters pain perception in adulthood. Pre-clinical studies have clearly demonstrated that exposure to noxious stimuli early in life causes long-term alterations in sensory processing (Grunau et al., 1994; Ren et al., 2004; Laprairie and Murphy, 2009; Schwaller and Fitzgerald, 2014). Increasing evidence from clinical studies also indicate that the pain experience of children has a significant impact on their pain responses in adulthood (Jones et al., 2009; Walker et al., 2009a; Walker et al., 2009b; Ranger and Grunau, 2014). However, the underlying mechanism of neonatal injury-induced alteration in pain processing is yet to be elucidated.
Previous studies have reported that neonatal intravesical zymosan leads to bladder hypersensitivity in adulthood characterized with an increased baseline micturition frequency and heightened EMG activity to UBD (Randich et al., 2006). We previously reported that intravesical zymosan during the neonatal period also produces cross-organ visceral hypersensitivity characterized by an increase in VMR to CRD without any pathological changes (Miranda et al., 2011). Expanding on these findings, we reported that neonatally zymosan treated rats show significant downregulation of GABAAα–1 receptor expression in adult spinal cords (Sengupta et al., 2013). Based on these observations, we hypothesized that the loss of spinal GABAergic inhibition might be responsible for the visceral hypersensitivity observed in these rats (Sengupta et al., 2013). However, whether the molecular alterations following neonatal zymosan treatments directly affects the functional characteristics of peripheral (i.e., bladder-sensitive PNA fibers) and the LS spinal neurons receiving synaptic input from the bladder is not known. The objective of the present study was to undertake a systematic electrophysiological recording from bladder responsive PNA fibers and LS spinal cord neurons from both neonatally saline- and zymosan-treated rats and compare their responses to a selective GABAAα–2 agonist (HZ166). A comparative result between the groups will delineate the outcome of painful sensations that these animals experience following early life painful insult in the viscera.
In our initial studies, we tested the effect of HZ166 in adult naïve rats to establish the visceral analgesic effect of the drug. In these rats, i.t. injection of HZ166 produced a significant decrease in VMR to UBD and CRD indicating its spinally mediated analgesic effect to painful visceral stimuli. HZ166, a preferential GABAAα-2 and GABAAα-3 receptors agonist, has previously been reported to produce analgesic effect in mouse models of somatic pain (Di Lio et al., 2011). Our result further expands the analgesic effect of HZ166 in a rat model of visceral pain. We extended our study to establish the proof of concept that if neonatal painful insult in the bladder affects the normal development of inhibitory GABAergic mechanism in the spinal cord and modulates the pain signaling pathway via altered expression of GABAA receptors, this specific agonist will fail to produce analgesic effect in neonatally zymosan-treated rats. To prove our concept and to validate our previously published observations (Sengupta et al., 2013), we tested the effect of HZ166 in neonatally bladder inflammation-induced chronic visceral hypersensitivity model. HZ166 produced a robust spinally mediated visceral analgesic effect in neonatally saline-treated rats similar to that observed in naïve adult rats. However, in neonatally zymosan-treated rats, HZ166 did not produce analgesic effect to noxious visceral stimuli. This observation is consistent with our previous report documenting a lack of effect for muscimol (a non-specific GABAA receptor agonist) on the VMR to CRD in neonatally-zymosan treated rats (Sengupta et al., 2013). However, a major limitation of our previous study was that muscimol produced motor deficit in rats at analgesic doses tested and this has also been reported by several investigators (Hara et al., 2004). To address this issue, in the present study we decided to use HZ166, which was reported not to produce any motor deficits in rats at analgesic doses (Ralvenius et al., 2015).
In electrophysiology studies, HZ166 did not inhibit either the spontaneous firing or the responses of UBD-sensitive PNA fibers from naïve adult, saline- and zymosan-treated rats to UBD suggesting that the drug does not attenuate mechanotransduction functions of UBD-sensitive primary sensory afferents. Although immunohistochemistry revealed strong expression of GABAAα-2 receptors in the L6-S1 DRGs from both groups, HZ166 was not found to be effective on the PNAs. This indicates a lack of role for these receptors in the modulation of mechanotransduction of PNA fibers innervating the urinary bladder. However, HZ166 significantly inhibited the responses of bladder responsive LS spinal neurons in naïve adult and neonatally saline-treated rats. Since all spinal recordings were done on cervical spinal transected rats, the inhibitory effect of HZ166 can be attributed to its direct effect on the spinal neurons rather than its action at supra-spinal sites to exert descending inhibitory influence. In somatic pain models, HZ166 mediates its analgesic effect predominantly by its action on spinal GABAAα-2 receptors without the involvement of receptors at the supraspinal sites (Paul et al., 2014). This report reinforces our present results from the naïve adult and neonatally saline-treated rats. In slow infusion studies, experiments from spinally intact rats, injection of HZ166 produced inhibition of the urinary bladder contractions without inhibiting the firing of PNA fibers, suggesting that HZ166 might have an effect on bladder contraction. However, our study does not address whether this effect is a direct inhibitory effect on the detrusor muscles or through attenuation of extrinsic supraspinal motor control. In spite of the lack of baseline spontaneous bladder contraction following cervical spinal transection, HZ166 significantly inhibited the firing of bladder sensitive LS spinal neuron to passive phasic UBD, further confirming its direct inhibitory effect of the spinal neurons. These findings are in accordance with the earlier report indicating a lack of peripheral effects for muscimol and baclofen (GABAB agonist) on the lower urinary tract and the observed decrease in micturition rate is a centrally mediated effect (Igawa et al., 1993).
Our result also shows that HZ166 produced a similar effect in neonatally saline treated rats viz a strong inhibitory effect on the UBD-responsive LS spinal neurons and a lack of effect on the attenuation of mechanotransduction of PNA fibers to both phasic UBD and SI of the bladder. In contrast, in neonatally zymosan-treated rats, HZ166 did not produce any inhibition of the UBD-responsive spinal neurons to both phasic UBD and SI of the bladder. This can be attributed to the molecular downregulation of the GABAAα-2 receptors in the spinal cord of neonatally zymosan-treated rats. A similar lack of GABA function in the spinal cord characterized by alterations in GABAA receptor expression has also been reported in rodent models of diabetic neuropathy (Jolivalt et al., 2008) and spinal cord injury (Gwak et al., 2006). The results of our VMR and electrophysiology studies indicate that the lack of effect of HZ166 in neonatally zymosan-treated rats and correlates with the decreased expression of GABAAα-2 observed in IHC experiments. Taken together, it can be postulated that neonatal bladder inflammation resulted in a decrease in inhibitory tone in the spinal cord, which subsequently unmasked the excitatory transmission leading to the observed chronic visceral hypersensitivity in adulthood.
Under normal conditions, it has been reported that supraspinal activation of GABAergic neurons is pronociceptive, while spinal activation of GABAergic neurons can produce an anti-nociceptive effect (Orii et al., 2003). However, an imbalance in the GABAergic system is implicated in several pathological conditions including a number of pain states (Enna and McCarson, 2006). For example, diminished pain control by spinal glycinergic and GABAergic neurons was found to be a major factor for chronic inflammatory and neuropathic pain (Zeilhofer and Zeilhofer, 2008). In addition, loss or dysfunction of GABAergic interneurons is also reported in several neurological and psychiatric disorders (Hori and Hoshino, 2012; Le Magueresse and Monyer, 2013; Shetty and Bates, 2016). Several preclinical evidence support that impairment of GABAergic transmission both in early-life and in adulthood can cause stress related disorders. For example, maternal separation of rat pups was found to alter the GABAA receptor-mediated modulation of norepinephrine release in adult rats (Sterley et al., 2013). The results of our study suggest that alterations in GABAergic signaling system following neonatal bladder inflammation might be one of the potential mechanisms responsible for the visceral hypersensitivity observed in conditions like IC/PBS. However, our study did not examine several other components of the GABAergic system including levels of GABA synthesizing enzyme GAD65 (Eaton et al., 1998; Moore et al., 2002), functional expression of the GABA transporter GAT-1 (Stiller et al., 1996; Eaton et al., 1998; Daemen et al., 2008) and GABA associated ion transporters KCC2 and NKCC2 (Coull et al., 2003; Price et al., 2005; Morgado et al., 2011), all of which are widely implicated in various pathological pain conditions. Another limitation of the current study is that we did not investigate the effect of neonatal bladder inflammation on the supra-spinal GABAergic neurotransmission. It has been shown that in the rat hippocampus, fast GABAergic transmission is excitatory at birth and shifts to become inhibitory soon after first postnatal week (Ben-Ari et al., 1989; Cherubini et al., 1991). These components are worth investigating in our model of chronic visceral pain and form the basis of future studies. Nevertheless, strategies that will enhance inhibitory neurotransmission may serve as potential targets in alleviating several disorders including pain (Shetty and Bates, 2016).
In summary, inflammation of the urinary bladder in the neonatal period leads to the loss of GABA-mediated inhibitory tone at the spinal cord resulting in neuronal hyper-excitability resulting in chronic visceral hypersensitivity in adulthood. This study provides behavioral and electrophysiological evidence for the loss of spinal inhibitory tone and confirms our previous findings. It is highly possible that these changes are evident in a subset of IC/PBS patients. Understanding the role of GABAergic system in mechanism of hypersensitivity induced by neonatal inflammatory events will provide useful insights into the future treatment strategies for chronic bladder pain conditions.
HIGHLIGHTS.
➢ Neonatal inflammation of the urinary bladder leads to chronic visceral hypersensitivity in adulthood.
➢ Loss of spinal GABA-mediated inhibitory tone results in neuronal hyper-excitability and during bladder pain.
➢ This study provides behavioral and electrophysiological evidence in the development of chronic visceral pain.
➢ Understanding the mechanism of hypersensitivity induced by neonatal injury provide treatment strategies for bladder pain.
ACKNOWLEDGMENT
This work was supported by NIH R01DK099201-01A1 grant awarded to Drs. J. N. Sengupta and B. Banerjee. We thank Dr. Anjishnu Banerjee from the Biostatistics division of the Medical College of Wisconsin for his assistance with the statistical analysis. This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1TR001436. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
No conflicts of interest exist with any of the authors.
ETHICS APPROVAL
All experiments were performed according to the approved guidelines of the Institutional Animal care and Use committee at the Medical college of Wisconsin (approval # AUA355) and International Association for the Study of Pain (IASP) for humane use of laboratory animals.
REFERENCES
- Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterol. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J. Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P, Nyberg L, Clemens JQ. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186:540–544. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt LK, Lashinger ES, Laping NJ, Su X. Evaluation of pressor and visceromotor reflex responses to bladder distension in urethane anesthetized rats. Neurourol Urodyn. 2009;28:442–446. doi: 10.1002/nau.20650. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sík A, De Koninck P, DeKoninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- Coutinho SV, Plotsky PM, Sablad M, Miller JC, Zhou H, Bayati AI, McRoberts JA, Mayer EA. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G307–316. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- Daemen MA, Hoogland G, Cijntje JM, Spincemaille GH. Upregulation of the GABA-transporter GAT-1 in the spinal cord contributes to pain behavior in experimental neuropathy. Neurosci Lett. 2008;444:112–115. doi: 10.1016/j.neulet.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Davis DA, Luecken LJ, Zautra AJ. Are reports of childhood abuse related to the experience of chronic pain in adulthood? A meta-analytic review of the literature. Clin J Pain. 2005;21:398–405. doi: 10.1097/01.ajp.0000149795.08746.31. [DOI] [PubMed] [Google Scholar]
- DeBerry J, Randich A, Shaffer AD, Robbins MT, Ness TJ. Neonatal bladder inflammation produces functional changes and alters neuropeptide content in bladders of adult female rats. J Pain. 2010;11:247–255. doi: 10.1016/j.jpain.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lio A, Benke D, Besson M, Desmeules J, Daali Y, Wang ZJ, Edwankar R, Cook JM, Zeilhofer HU. HZ166, a novel GABAA receptor subtype-selective benzodiazepine site ligand, is antihyperalgesic in mouse models of inflammatory and neuropathic pain. Neuropharmacology. 2011;60:626–632. doi: 10.1016/j.neuropharm.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll A, Teichman JMH. How do patients with interstitial cystitis present? J Urol. 2001;166:2118–2120. [PubMed] [Google Scholar]
- Eaton MJ, Plunkett JA, Karmally S, Martinez MA, Montanez K. Changes in GAD- and GABA-immunoreactivity in the spinal dorsal horn after peripheral nerve injury and promotion of recovery by lumbar transplant of immortalized serotonergic precursors. J Chem Neuroanat. 1998;16:57–72. doi: 10.1016/s0891-0618(98)00062-3. [DOI] [PubMed] [Google Scholar]
- Enna SJ, McCarson KE. The role of GABA in the mediation and perception of pain. Adv Pharmacol. 2006;54:1–27. doi: 10.1016/s1054-3589(06)54001-3. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Edwards RR. Is self-reported childhood abuse history associated with pain perception among healthy young women and men? Clin J Pain. 2005;21:387–397. doi: 10.1097/01.ajp.0000149801.46864.39. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Walker SM. Infant pain management: A developmental neurobiological approach. Nat Clin Pract Neurol. 2009;5:35–50. doi: 10.1038/ncpneuro0984. [DOI] [PubMed] [Google Scholar]
- Grunau RV, Whitfield MF, Petrie JH, Fryer EL. Early pain experience, child and family factors, as precursors of somatization: a prospective study of extremely premature and full-term children. Pain. 1994;56:353–359. doi: 10.1016/0304-3959(94)90174-0. [DOI] [PubMed] [Google Scholar]
- Gwak YS, Tan HY, Nam TS, Paik KS, Hulsebosch CE, Leem JW. Activation of spinal GABA receptors attenuates chronic central neuropathic pain after spinal cord injury. J Neurotrauma. 2006;23:1111–1124. doi: 10.1089/neu.2006.23.1111. [DOI] [PubMed] [Google Scholar]
- Hara K, Saito Y, Kirihara Y, Sakura S. The interaction between gamma-aminobutyric acid agonists and diltiazem in visceral antinociception in rats. Anesth Analg. 2004;98:1380–1384. doi: 10.1213/01.ane.0000107935.84035.48. [DOI] [PubMed] [Google Scholar]
- Hori K, Hoshino M. GABAergic neuron specification in the spinal cord, the cerebellum, and the cochlear nucleus. Neural Plast. 2012;2012:9217–9232. doi: 10.1155/2012/921732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igawa Y, Mattiasson A, Andersson KE. Effects of GABA-receptor stimulation and blockade on micturition in normal rats and rats with bladder outflow obstruction. Urol. 1993;150:537–542. doi: 10.1016/s0022-5347(17)35542-8. [DOI] [PubMed] [Google Scholar]
- Jolivalt CG, Lee CA, Ramos KM, Calcutt NA. Allodynia and hyperalgesia in diabetic rats are mediated by GABA and depletion of spinal potassium-chloride co-transporters. Pain. 2008;140:48–57. doi: 10.1016/j.pain.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GT, Power C, Macfarlane GJ. Adverse events in childhood and chronic widespread pain in adult life: Results from the 1958 British Birth Cohort Study. Pain. 2009;143:92–96. doi: 10.1016/j.pain.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Kannampalli P, Pochiraju S, Bruckert M, Shaker R, Banerjee B, Sengupta JN. Analgesic effect of minocycline in rat model of inflammation-induced visceral pain. Eur J Pharmacol. 2014;727:87–98. doi: 10.1016/j.ejphar.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprairie JL, Murphy AZ. Neonatal injury alters adult pain sensitivity by increasing opioid tone in the periaqueductal gray. Front Behav Neurosci. 2009;3:31. doi: 10.3389/neuro.08.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Magueresse C, Monyer H. GABAergic interneurons shape the functional maturation of the cortex. Neuron. 2013;77:388–405. doi: 10.1016/j.neuron.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Lin C, Al-Chaer ED. Long-term sensitization of primary afferents in adult rats exposed to neonatal colon pain. Brain Res. 2003;971:73–82. doi: 10.1016/s0006-8993(03)02358-8. [DOI] [PubMed] [Google Scholar]
- Liu LS, Winston JH, Shenoy MM, Song GQ, Chen JD, Pasricha PJ. A rat model of chronic gastric sensorimotor dysfunction resulting from transient neonatal gastric irritation. Gastroenterol. 2008;134:2070–2079. doi: 10.1053/j.gastro.2008.02.093. [DOI] [PubMed] [Google Scholar]
- Low LA, Schweinhardt P. Early life adversity as a risk factor for fibromyalgia in later life. Pain Res Treat. 2012;2012:140832. doi: 10.1155/2012/140832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickle A, Sood M, Zhang Z, Shahmohammadi G, Sengupta JN, Miranda A. Antinociceptive effects of melatonin in a rat model of post-inflammatory visceral hyperalgesia: a centrally mediated process. Pain. 2010;149:555–564. doi: 10.1016/j.pain.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda A, Mickle A, Schmidt J, Zhang Z, Shaker R, Banerjee B, Sengupta JN. Neonatal cystitis-induced colonic hypersensitivity in adult rats: a model of viscero-visceral convergence. Neurogastroenterol Motil. 2011;23:683–e281. doi: 10.1111/j.1365-2982.2011.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgado C, Pereira-Terra P, Cruz CD, Tavares I. Minocycline completely reverses mechanical hyperalgesia in diabetic rats through microglia-induced changes in the expression of the potassium chloride co-transporter 2 (KCC2) at the spinal cord. Diabetes Obes Metab. 2011;13:150–159. doi: 10.1111/j.1463-1326.2010.01333.x. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Lewis-Sides A, Castroman P. Characterization of pressor and visceromotor reflex responses to bladder distension in rats: sources of variability and effect of analgesics. J Urol. 2001;165:968–974. [PubMed] [Google Scholar]
- Nickel JC, Irvine-Bird K, Jianbo L, Shoskes DA. Phenotype-directed management of interstitial cystitis/bladder pain syndrome. Urology. 2014;84:175–179. doi: 10.1016/j.urology.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Nickel JC, Tripp DA. International Interstitial Cystitis Study Group. Clinical and psychological parameters associated with pain pattern phenotypes in women with interstitial cystitis/bladder pain syndrome. J Urol. 2015;193:138–144. doi: 10.1016/j.juro.2014.07.108. [DOI] [PubMed] [Google Scholar]
- Orii R, Ohashi Y, Halder S, Giombini M, Maze M, Fujinaga M. GABAergic interneurons at supraspinal and spinal levels differentially modulate the antinociceptive effect of nitrous oxide in Fischer rats. Anesthesiology. 2003;98:1223–1230. doi: 10.1097/00000542-200305000-00026. [DOI] [PubMed] [Google Scholar]
- Paul J, Yévenes GE, Benke D, Di Lio A, Ralvenius WT, Witschi R, Scheurer L, Cook JM, Rudolph U, Fritschy JM, Zeilhofer HU. Antihyperalgesia by 2-GABAA receptors occurs via a genuine spinal action and does not involve supraspinal sites. Neuropsychopharmacology. 2014;39:477–487. doi: 10.1038/npp.2013.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters KM, Killinger KA, Ibrahim IA. Childhood symptoms and events in women with interstitial cystitis/painful bladder syndrome. Urology. 2009;73:258–262. doi: 10.1016/j.urology.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Price TJ, Cervero F, de Koninck Y. Role of cation-chloride-cotransporters (CCC) in pain and hyperalgesia. Curr Top Med Chem. 2005;5:547–555. doi: 10.2174/1568026054367629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralvenius WT, Benke D, Acuña MA, Rudolph U, Zeilhofer HU. Analgesia and unwanted benzodiazepine effects in point-mutated mice expressing only one benzodiazepine-sensitive GABAA receptor subtype. Nat Commun. 2015;6:6803. doi: 10.1038/ncomms7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randich A, Uzzell T, DeBerry JJ, Ness TJ. Neonatal urinary bladder inflammation produces adult bladder hypersensitivity. J Pain. 2006;7:469–479. doi: 10.1016/j.jpain.2006.01.450. [DOI] [PubMed] [Google Scholar]
- Ranger M, Grunau RE. Early repetitive pain in preterm infants in relation to the developing brain. Pain Manag. 2014;4:57–67. doi: 10.2217/pmt.13.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, Anseloni V, Zou SP, Wade EB, Novikova SI, Ennis M, Traub RJ, Gold MS, Dubner R, Lidow MS. Characterization of basal and re-inflammation-associated long-term alteration in pain responsivity following short-lasting neonatal local inflammatory insult. Pain. 2004;110:588–596. doi: 10.1016/j.pain.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Schwaller F, Fitzgerald M. The consequences of pain in early life: injury-induced plasticity in developing pain pathways. Eur J Neurosci. 2014;39:344–352. doi: 10.1111/ejn.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta JN, Medda BK, Shaker R. Effect of GABA(B) receptor agonist on distension-sensitive pelvic nerve afferent fibers innervating rat colon. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1343–G1351. doi: 10.1152/ajpgi.00124.2002. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Pochiraju S, Kannampalli P, Bruckert M, Addya S, Yadav P, Miranda A, Shaker R, Banerjee B. MicroRNA-mediated GABA A -1 receptor subunit down-regulation in adult spinal cord following neonatal cystitis-induced chronic visceral pain in rats. Pain. 2013;154:59–70. doi: 10.1016/j.pain.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Bates A. Potential of GABA-ergic cell therapy for schizophrenia, neuropathic pain, and Alzheimer's and Parkinson's diseases. Brain Res. 2016;1638:74–87. doi: 10.1016/j.brainres.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Nordstrom E, Sengupta JN, Miranda A. Neonatal gastric suctioning results in chronic visceral and somatic hyperalgesia: role of corticotrophin releasing factor. Neurogastroenterol Motil. 2007;19:692–699. doi: 10.1111/j.1365-2982.2007.00949.x. [DOI] [PubMed] [Google Scholar]
- Sterley TL, Howells FM, Russell VA. Maternal separation increases GABA(A) receptor-mediated modulation of norepinephrine release in the hippocampus of a rat model of ADHD, the spontaneously hypertensive rat. Brain Res. 2013;1497:23–31. doi: 10.1016/j.brainres.2012.12.029. [DOI] [PubMed] [Google Scholar]
- Stiller CO, Cui JG, O'Connor WT, Brodin E, Meyerson BA, Linderoth B. Release of gamma-aminobutyric acid in the dorsal horn and suppression of tactile allodynia by spinal cord stimulation in mononeuropathic rats. Neurosurgery. 1996;39:367–375. doi: 10.1097/00006123-199608000-00026. [DOI] [PubMed] [Google Scholar]
- Walker SM. Biological and neurodevelopmental implications of neonatal pain. Clin Perinatol. 2013;40:471–91. doi: 10.1016/j.clp.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Walker SM, Franck LS, Fitzgerald M, Myles J, Stocks J, Marlow N. Long-term impact of neonatal intensive care and surgery on somatosensory perception in children born extremely preterm. Pain. 2009a;141:79–87. doi: 10.1016/j.pain.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Walker SM, Tochiki KK, Fitzgerald M. Hindpaw incision in early life increases the hyperalgesic response to repeat surgical injury: critical period and dependence on initial afferent activity. Pain. 2009b;147:99–106. doi: 10.1016/j.pain.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Wang J, Gu C, Al-Chaer E. Altered behaviour and digestive outcomes in adult male rats primed with minimal colon pain as neonates. Behav Brain Funct. 2008;4:28. doi: 10.1186/1744-9081-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters MM, Van Wanrooy S, Casteels C, Nemethova A, de Vries A, Van Oudenhove L, Van den Wijngaard RM, Van Laere K, Boeckxstaens G. Altered brain activation to colorectal distention in visceral hypersensitive maternal-separated rats. Neurogastroenterol Motil. 2012;24:678–685. doi: 10.1111/j.1365-2982.2012.01919.x. [DOI] [PubMed] [Google Scholar]
- Zeilhofer HU, Zeilhofer UB. Spinal dis-inhibition in inflammatory pain. Neurosci Lett. 2008;437:170–174. doi: 10.1016/j.neulet.2008.03.056. [DOI] [PubMed] [Google Scholar]