Abstract
Acute pain arises from activation of myelinated (A delta) and unmyelinated (C) nociceptive afferents, leading to 1st (A-fiber) or 2nd (C-fiber) pain sensations. The current study sought to investigate first and second pain within glabrous and hairy skin sites in human upper-limbs. Fifty healthy adults (25 males/25 females, 18-30 yrs: mean = 20.5 ± 1.4) participated in a psychophysical study investigating electronically rated, thermal first and second pain sensations within the glabrous skin at the palm and hairy skin of the forearm. Repeated measures ANOVA indicated that the threshold for first pain was lower (more sensitive) than for second pain (p = .004), for both glabrous and hairy skin, and thresholds at glabrous skin were higher than for hairy skin (p = .001). Hairy skin presented a steeper slope for testing, whereas there were no differences in slope between first and second pain. The study findings support assumptions associated with mechanistic differences between first and second pain sensations, while offering a novel method for producing first and second pain with the same thermal stimulus. Efforts to understand abnormalities among people with clinical pain and development of new therapeutic agents will benefit from specific psychophysical methods.
Keywords: First Pain, Second Pain, Pain, Stimulus Response Curves, Pain Sensations
Introduction
The experience of acute pain arises from activation of a variety of thermal, mechanical and chemical receptors, transmitting non-uniformly across A and C-polymodal nociceptive afferents5, 7, 10, 17, 18, 22, 43, 44. Activation of the fast conducting myelinated A-fibers produces a short latency and duration response described as sharp and localized11, 26, 33, 37. In contrast, activation of slow conducting unmyelinated C-fibers produces a long latency and duration response described as a burning or throbbing sensation3, 6, 20, 26, 31, 37, 44. Importantly, these phenomena can occur in response to a single stimulus; however, mechanistically they remain distinctly separate2, 24, 26. For example, morphine administration in both primates and humans preferentially blocks C-fiber input to the CNS while preserving A-fiber activity -- demonstrating a clear mechanistic distinction in pain processing of A and C-fiber activation16, 46, 47. Moreover, neuroanatomical research has identified individualized afferent pathways and terminations of each fiber type1. These individual characteristics have necessitated the advent of the first pain (A-fiber) and second pain (C-fiber) classifications.
Through the use of psychophysical testing, first pain has been deemed an indication of sensitivity to brief nociceptive stimulation (i.e. pain threshold and tolerance), and nociceptive reflexes are elicited by activation of myelinated afferents. Conversely, second pain can be prolonged, outlasting stimulation, and it temporally summates (i.e. central sensitization). Moreover, second pain has become associated with chronic pain syndromes32, 38. This is demonstrable in people with fibromyalgia syndrome (FMS), as temporal summation of second pain and associated after-sensations provide a clear indication of altered pain processing40. Thus, separate assessment of first and second pain can be fruitful; it has remained elusive due to the testing methodologies employed. For example, increasing levels of electrocutaneous stimulation activate A-beta and then both A-beta and A-delta afferents19, while cutaneous application of capsaicin preferentially activates C nociceptors27, 30. A direct comparison of sensitivity to these procedures is confounded by phasic vs. tonic stimulation, leaving no direct way to compare stimulus-response functions for electrical and chemical stimulation. Similarly, painful thermal stimulation is routinely delivered by ramp-and hold progressions from a neutral temperature to levels of nociceptive heat, which activate both A-delta and C afferents to unknown relative extents.
The current study sought to directly compare first and second pain sensations evoked by heat within and across the glabrous skin (palm) and hairy skin (forearm). This aim was completed by direct comparison of both pain sensation thresholds and stimulus-response functions. The differential innervation of A-delta type II nociceptors at these sites and fiber properties provide a basis for these comparisons29, 45. Specifically, we hypothesized that first pain will demonstrate a stronger responses relative to second pain. Mechanistically these effects could result from inhibition of second pain by activation of A-delta nociceptors12, 25. The results of the current study may provide the means for mechanism-specific pain diagnoses, as multiple pain abnormalities (i.e. hyperalgesia, allodynia, abnormal temporal summation) have the propensity to develop from differing mechanisms34.
Materials and Methods
Participants
Fifty healthy adults (25 males/25 females) between the ages of 18-30 years (mean = 20.5, SD = 1.4) were recruited using flyers and word of mouth to participate in a study investigating psychophysical differences between glabrous and hairy skin. Subjects were given $25 in Visa Debit cards per session for their participation. Exclusion criteria included the inability to reliably rate pain intensity, uncontrolled hypertension defined as resting blood pressure greater than 150/99, any systemic disease that restricts normal daily activities, neurological disorders relating to changes within somatosensory perception, any uncontrolled psychiatric conditions, or daily narcotic medication use. The sample was recruited as part of a larger study examining age-related changes in pain inhibitory and facilitatory function. The University of Florida Institutional Review Board approved this protocol in accordance with the Declaration of Helsinki.
Experimental Protocol
Apparatus and testing environment
All subjects were tested in the Pain Clinical Research Unit of the University of Florida Pain Research and Intervention Center of Excellence, in isolated, climate-controlled rooms under the supervision of professional pain investigators. Thermal stimuli were delivered using a computer-controlled Medoc Pathway Thermal Sensory Analyzer (Ramat Yishai, Israel) using the CHEPS 32mm thermode set with a 40 °C/sec ramping rate. Pain was measured with an electronic visual analog scale (COVAS). The COVAS consisted of a low-friction sliding potentiometer of 100 mm travel. The left endpoint of the scale was identified as “no pain”; while the right endpoint was defined as “intolerable pain” with nine hash marks between these two anchors (see Figure 1a). The position of the slider was electronically converted into a pain rating between 0 and 100%. The visual analog scale and electronic scoring were utilized as a reliable and valid method, allowing the subject to fully concentrate on the task and give continuous real-time ratings35. All testing was conducted with the subject seated upright, upper extremity supported, and the COVAS placed on a lap table directly in front of the subject.
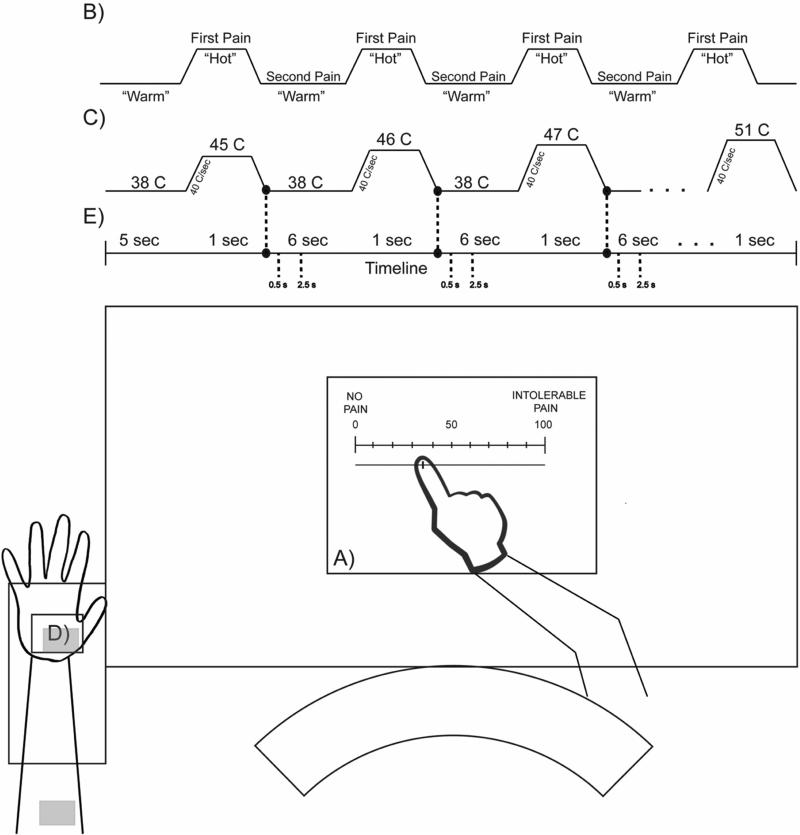
Figure 1.
Graphical representation of the experimental setup representing A) electronic score slider with 0 equal to “no pain”, 100 equal to “intolerable pain”, B) example of the visual aid presented to subjects during testing, C) stimulation profile, D) arm placement on thermode dependent on site tested, and E) timeline. This figure is an example only and not exact.
Study procedures
Individuals who were interested in the study were provided information about the procedures, and privacy regulations, and they reviewed and signed an Informed Consent Form. Eligibility for the study was determined after subjects completed a health history questionnaire, supplemented by an interview and blood pressure measurement.
As part of the orientation, subjects watched a PowerPoint presentation with imbedded video and audio overlay that described use of a 0 -100 pain scale and the temporal nature of first and second pain. Subjects were also shown a visual representation (see Figure 1b), in which there would be alternating periods of neutral “warm” stimulation (38° Celsius) and “hot”. Subjects were given familiarization trials that consisted of a series of six heat pulses ascending from 45 to 51°C where they rated either first or second pain (see Figure 1c). For first pain, they were instructed to rate the pain during the period of “hot” stimulation before the stimulus disappeared. For second pain, subjects were instructed to rate the pain after the “hot” stimulation period disappeared. Practice trials were administered at both the glabrous skin of the palm and hairy skin on the volar forearm (see Figure 1d shaded areas). Subjects were instructed to remove their arm/palm from the thermode should the pain become too intense. Testing began immediately following orientation.
A testing trial consisted of ascending heat pulses ramping from a baseline temperature of 38°C to a targeted temperature in an ascending series from 45°C, increasing in +1°C increments, to a maximum of 51°C with a 1-second pulse time and a 6-second inter-pulse-interval. The ramping portions preceding and following the stimulation demonstrated a range of timings with ascending to 45 °C from 38 °C lasted on average 184.5 ± 4.7 msec and descending on average at 219.0 ± 7 msec. Additionally, ascending to 51 °C from 38 °C lasted on average 324.0 ± 4.4 msec and descending on average at 374.0 ± 5.4 msec. The stimulation profiles were computer controlled, exactly matching the stimulation profiles for both first and second pain trials, which ensured the ability for direct comparisons. Participants were not informed that the temperature would ascend in a sequential manner (see Figure 1c). The order was randomized across four conditions in a testing session, site (glabrous and hairy skin) × pain rating (first and second pain). Subjects were reminded to focus on either first or second pain before each trial as appropriate, and to leave the COVAS slider in place until the next stimulation. To reduce sensitization across multiple trials at each site within the session, each ascending series was terminated if a subject rated greater than 60 (0-100 scale) before the maximum of 51°C was reached.
Data management
All data were exported from the laboratory computer and subsequently analyzed in MATLAB (Mathworks, MA, USA). The Pathway processor records multiple lines of data per second for both thermode temperatures and COVAS ratings in real time. The data were reviewed and the onset of each pulse (stimulation event) was defined by the thermode temperature ascending through a threshold of 38.3°C (+0.3°C change from baseline), while the end point of each pulse was defined by descending through 38.3°C (see Figure 1c). The single rating for each pulse, for data analysis, was determined by adding a defined period of time to the end. This time period was based on conduction velocities of the specific afferents as well as the sampling rate for the testing protocol (first pain: 33 data points = 0.5 sec; second pain: 156 data points = 2.5 secs) (see Figure 1e)32. Pain ratings below five (0-100 scale) were considered a “non-score” or a zero to eliminate occasional unintended movement of the COVAS pointer from the left end point.
Missing Data Imputation
Periodically, subject's trials were terminated before the maximum of 51°C, because of the 60+ pain rating termination rule. Consequently, a validated method of multiple imputation was used to produce estimates for these missing data points. Estimated values greater than 100 were scored as 100 (maximum possible score). This methodology allowed for a complete sample analysis with seven valid data points39. A curve fitting procedure using each subject's pain ratings for 45 to 51°C was used to estimate pain ratings for any missing scores at the higher temperatures. As expected, all subjects did not rate similarly throughout the temperature range tested. For example, a subset of subjects rated a high pain intensity for the initial stimulation with others not rating till later temperatures. Therefore, the individualized estimated temperature on the Y-axis where the curve passed through 0 represented pain threshold.
Tests of study aims
The primary aims of this study, testing for differences between first and second pain responses evoked within glabrous skin (palm) and hairy skin (forearm) were evaluated using repeated measures 2×2 ANOVA. The independent variables were “pain type” (first and second pain) and “site” (glabrous and hairy skin). The first dependent variable was “THRESHOLD”, defined as the predicted temperature associated with a pain score of 0, based on procedures described above. The second dependent variable was “SLOPE”, defined as the difference between the final pain ratings (51°C) and the first pain ratings (45°C) divided by 7. The SLOPE variable represents the increase in pain ratings (0-100 scale) associated with a 1°C increase in thermode temperature. An initial set of analyses included sex as a covariate; however sex was not significant in any analysis and was subsequently dropped from the final reported models. Significant differences were determined at p ≤ 0.05. Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 23.0. (Armonk, NY: IBM Corp.)
Results
Descriptive data on the 50 subjects for each temperature across all four sessions are presented within Table 1 and displayed in Figure 2. The percentage of missing data points imputed per series as well as the mean and standard deviations for the SLOPE and THRESHOLD variables across series are presented in Table 2. Post-hoc analysis looking at Pearson's product-moment correlation coefficients resulted in a weak-to-moderate relationship within all comparisons with the strongest correlation occurring between 1st and 2nd pain thresholds (0.593).
Table 1.
Mean and standard deviation pain intensity for each temperature for each of the site x pain type stimulus curves.
| 45°c Mean (SD) | 46°c Mean (SD) | 47°c Mean (SD) | 48°c Mean (SD) | 49°c Mean (SD) | 50°c Mean (SD) | 51°c Mean (SD) | |
|---|---|---|---|---|---|---|---|
| Glabrous skin (palm), 1st pain | 13.4 (6.8) | 20.2 (9.7) | 26.9 (13.7) | 33.7 (17.9) | 40.2 (21.3) | 46.4 (24.4) | 52.1 (26.6) |
| Glabrous skin (palm), 2nd pain | 5.4 (3.3) | 11.3 (5.2) | 17.4 (8.6) | 23.6 (12.6) | 29.8 (16.8) | 35.9 (21.0) | 41.3 (23.3) |
| Hairy skin (forearm), 1st pain | 23.3 (11.5) | 32.3 (15.1) | 41.3 (20.3) | 50.3 (25.7) | 58.3 (28.5) | 64.1 (28.6) | 69.0 (28.2) |
| Hairy skin (forearm), 2nd pain | 15.2 (8.4) | 24.1 (13.2) | 32.9 (18.3) | 41.7 (23.5) | 49.5 (26.3) | 56.4 (28.2) | 61.9 (28.5) |
Figure 2.
Figure representing the pain ratings for both first and second pain for glabrous skin (black lines) and hairy skin (gray lines). Dotted lines represent 1st pain ratings with the solid line representing 2nd pain ratings.
Table 2.
Missing data points imputed and descriptive statistics for THRESHOLD and SLOPE by site and pain type
| Stimulus Response Seriesa | SLOPEb Mean (SD) | THRESHOLDc Mean (SD) | Missing data per seriesd |
|---|---|---|---|
| Glabrous skin (palm), 1st pain | 5.6 (3.3) | 43.1°C (2.7) | n=38 (13%) |
| Glabrous skin (palm), 2nd pain | 5.2 (3.3) | 43.9°C (2.5) | n=51 (17%) |
| Hairy skin (forearm), 1st pain | 6.9 (2.6) | 42.2°C (2.6) | n=35 (12%) |
| Hairy skin (forearm), 2nd pain | 6.7 (3.2) | 42.9°C (2.1) | n=42 (14%) |
A series is defined as defined as a set of seven consecutive temperatures (e.g., 45° C, 46° C 47° C, 48° C, 49° C, 50° C etc.).
The variable SLOPE represents the change in pain rating (0-100 scale) for each change in temperature of 1°C.
The THRESHOLD variable was determined individually for each subject, by a curve fitting procedure and represents the temperature in degrees C where the curve passes through the 0 on the Y axis (i.e., pain rating).
The number of data points out of 300 per series that were imputed for subjects terminating before 51° C.
Pain type differences in pain threshold by site
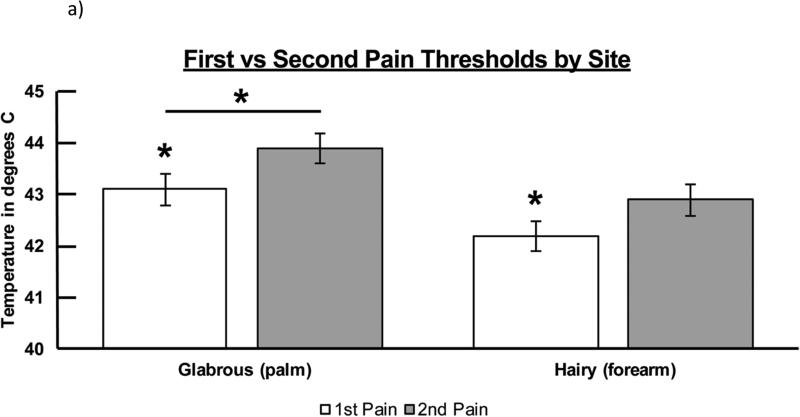
Figure 3a presents the threshold for first and second pain by site. Repeated measures ANOVA indicated that, on the THRESHOLD variable, there were significant differences for both pain type (F1,49 = 9.140, p = .004) and site (F1,49 = 11.809, p = .001). The pain × site interaction term was not significant (P = .801) indicating that the magnitude of differences between thresholds for first and second pain was not dependent upon site. Inspection of the marginal means indicated that thresholds for first pain were significantly lower (more sensitive) than for second pain at each site, and thresholds in glabrous skin were significantly higher (less sensitive) at glabrous skin than at hairy skin for both pain types.
Figure 3a.
THRESHOLD response for both first and second pain across both tested sites. Significant differences were noted for both pain type (F1,49 = 9.140, p = .004) and tested site (F1,49 = 11.809, p = .001). Significant differences were determined at the p < .05 level and denoted by asterisks.
Site differences in stimulus response gradients
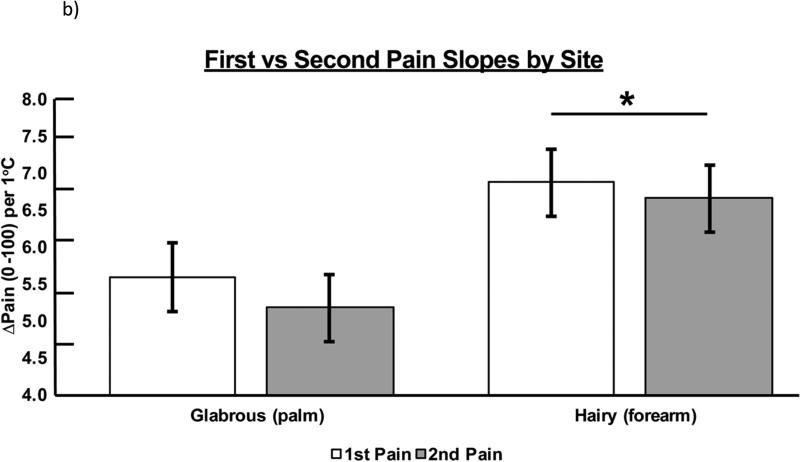
Figure 3b presents the slopes of stimulus response gradients for first pain and second pain by site. Repeated measures ANOVA indicated that, on the SLOPE variable, there was a significant difference for site (F1,49 = 9.008, p = .004), but not pain type (F1,49 = .994, p = .324). The pain × site interaction term was not significant (P = .784). Inspection of the marginal means indicated that hairy skin had a significantly steeper slope than glabrous skin for both pain types.
Figure 3b.
SLOPE of response for both first and second pain across both tested sites. Significant differences were noted for tested site (F1,49 = 9.008, p = .004). Significant differences were determined at the p < .05 level and denoted by asterisks.
Discussion
The current study utilized psychophysical techniques to investigate differences in first and second heat pain sensations within and across different testing sites (glabrous and hairy skin) in the human upper-limb. The employed methodology allowed for a direct comparison of pain sensations, with a stimulus duration of one second that permits rating of second pain following abatement of first pain. This approach is novel, as direct comparison of first and second pain sensitivity typically is confounded by variable stimulation methodologies, as previously stated. The ability to directly compare different pain sensations becomes warranted as better assessments of pain abnormalities is paramount to clinical conditions.
First pain thresholds were lower than second pain thresholds within both glabrous and hairy skin. More specifically, a relative lack of low threshold A-delta nociceptors in glabrous skin provides a potential explanation for this difference with stimulation of the hand21. Also, A-delta nociceptor activity can partially inhibit slower conducting input from C nociceptors at spinal and cortical levels; as cerebral cortical input from C-nociceptors is inhibitory to activation of the primary somatosensory cortex by A-beta and A-delta afferent input 8, 9, 12, 25, 41, 42, 46. This endogenous inhibition could account for lower first pain thresholds with stimulation of glabrous and hairy skin. Additionally, the thick epidermal layer paired with a higher activation thresholds of A-delta nociceptors in glabrous skin likely contributes to lower stimulus-response slopes. Spatial radiation of heat to sub-epidermal receptors and radially from the heated probe is especially reduced in glabrous skin, decreasing the population of A-delta and C nociceptors activated and attenuating the cumulative response of the afferent population responding to thermal stimulation, particularly for higher stimulus intensities14, 15. It remains unlikely these perceptual differences are a result of stimulation artifact due to the level of spatial radiation, as seen in perception and brain evoked potentials21. This is due to the 6-second recovery period utilized within the current methodology, which was previous demonstrated to allow for the stimulated skin to return to baseline temperatures28. Therefore, the differences in threshold and slope by site can be utilized to confirm the reliability of each subject's psychophysical performance.
Implications
With clear instructions subjects were able to separately rate first and second pain sensations in response to thermal heat stimuli of one-second duration, similar to previous reports4, 13. This investigation provides the basis for direct comparison of first and second pain sensations within and across upper-limb testing sites. Clinically, this provides the means to further investigate pain disorders. For example, pain abnormalities such as hyperalgesia, allodynia, and heightened temporal summation all have the propensity to develop from different mechanisms34, such as enhanced sensitivity to input from myelinated or unmyelinated nociceptors, accompanied by reduced inhibitory interaction between these input sources. Therefore, the ability to detect abnormalities within the same stimulation methodology allows for more specific pain diagnoses and evaluation of treatments. For example, patients with chronic pain associated with abnormal activation of C-nociceptors, such as fibromyalgia, could exhibit enhanced second pain but inhibition of first pain, reversing the relative sensitivities of normal subjects to first and second pain that were observed in the present investigation36.
Strength and Limitations
The current study demonstrates a novel methodological approach to directly compare first and second pain sensations within and across testing sites. Strengths of this method include a within-subjects design paired with an exacting quantification of self-reported pain scores. The use of young healthy subjects provides a clear normative picture of first and second pain characteristics within upper-limb testing sites. However, the current study fails to address issues such as day-to-day variation in pain scores along with life span development changes, such as aging, as well as abnormalities that may exist within clinical populations. Therefore, interpretation should be cautiously framed in the context of young, healthy adults exposed to ramping, constant contact, heat stimuli.
Seven levels of thermal stimulation were presented in order to confidently describe stimulus-response functions over a range of heat pain intensities ranging from mild to moderate or high, depending on individual sensitivities. Based upon parallel stimulus-response functions for first and second pain at each site (see Figure 3) pain thresholds provided a conservative means of comparing differences in sensitivity that were maintained through all temperatures. Also, pain ratings for the lowest and highest stimulus intensities provided good estimates of slope for the linear stimulus-response functions. However, pain thresholds were estimated rather than directly assessed using typical approaches (e.g. method of limits), and the values may not be comparable to thresholds determined using more direct methods. Also, these methods of analysis might not be appropriate for subject populations with altered (e.g., nonlinear) stimulus-response functions. Thus, it would seem advantageous to simplify the testing paradigm with fewer stimulus intensities, but this could be misleading if the form of the stimulus-response function is altered for some individuals. Additionally, it should be noted that interpretation of sited differences must be framed in the context of primary afferent innervations at each respective site. For example, glabrous skin has a higher density of nerve endings when compared to hairy skin23. Site differences in threshold and slope may partially be explain due to this factor as well as differences in activation thresholds in A-fiber types. However, while being noted, the current study methodology was not designed to specifically answer this question.
Conclusions
The current study sought to investigate first and second pain thresholds and gradients of pain intensity as a function of temperature. Results demonstrated significant differences across pain types and glabrous vs. hairy skin, revealing mechanistic properties of each pain sensation. The addition of this methodology will allow for future investigations to provide direct comparisons of first and second pain sensations across different clinical conditions, thereby improving current diagnostic and treatment strategies.
Perspective.
This article presents a novel method for directly comparing first and second pain within the same thermal stimulus. The ability to directly compare first and second pain sensations can aid in understanding pain abnormalities with clinical pain and development of therapeutic aids.
Highlights.
Methodology directly comparing first and second pain sensations is proposed.
Differences were noted in stimulus response thresholds, slopes, and sites tested.
Methodology may improve current pain diagnostic and treatment strategies.
Acknowledgements
This research was supported by the National Institutes of Health grants [NIA R01AG039659, 1T32AG049673]. The authors of this work would also like to thank the professional pain investigators of the Pain Research and Intervention Center of Excellence (PRICE) at the University of Florida. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: No authors present any conflicts of interests within this report.
References
- 1.Almeida TF, Roizenblatt S, Tufik S. Afferent pain pathways: a neuroanatomical review. Brain Research. 2004;1000:40–56. doi: 10.1016/j.brainres.2003.10.073. [DOI] [PubMed] [Google Scholar]
- 2.Barrell J, Price D. The perception of first and second pain as a function of psychological set. Perception & Psychophysics. 1975;17:163–166. [Google Scholar]
- 3.Beck PW, Handwerker HO, Zimmermann M. Nervous outflow from the cat's foot during noxious radiant heat stimulation. Brain Research. 1974;67:373–386. doi: 10.1016/0006-8993(74)90488-0. [DOI] [PubMed] [Google Scholar]
- 4.Beissner F, Brandau A, Henke C, Felden L, Baumgärtner U, Treede R-D, Oertel BG, Lötsch J. Quick discrimination of A delta and C fiber mediated pain based on three verbal descriptors. PloS one. 2010;5:e12944. doi: 10.1371/journal.pone.0012944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beitel RE, Dubner R. Response of unmyelinated (C) polymodal nociceptors to thermal stimuli applied to monkey's face. Journal of Neurophysiology. 1976;39:1160–1175. doi: 10.1152/jn.1976.39.6.1160. [DOI] [PubMed] [Google Scholar]
- 6.Beitel RE, Dubner R. Sensitization and depression of C-polymodal nociceptors by noxious heat applied to the monkey's face. Advances in pain research and therapy. 1976;1:149–153. [Google Scholar]
- 7.Bessou P, Perl ER. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. Journal of neurophysiology. 1969;32:1025–1043. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- 8.Bromm B, Treede R-D. Human cerebral potentials evoked by CO2 laser stimuli causing pain. Experimental Brain Research. 1987;67:153–162. doi: 10.1007/BF00269463. [DOI] [PubMed] [Google Scholar]
- 9.Bromm B, Treede RD. Pain Related Cerebral Potentials: Late and Ultralate Components. International Journal of Neuroscience. 1987;33:15–23. doi: 10.3109/00207458708985926. [DOI] [PubMed] [Google Scholar]
- 10.Burgess PR, Per ER. Somatosensory System. First Edition Vol. 2. Springer-Verlag; Heidelberg: 1973. Handbook of Sensory Physiology. [Google Scholar]
- 11.Campbell JN, LaMotte RH. Latency to detection of first pain. Brain Research. 1983;266:203–208. doi: 10.1016/0006-8993(83)90650-9. [DOI] [PubMed] [Google Scholar]
- 12.Chung J, Lee K, Hori Y, Endo K, Willis W. Factors influencing peripheral nerve stimulation produced inhibition of primate spinothalamic tract cells. Pain. 1984;19:277–293. doi: 10.1016/0304-3959(84)90005-8. [DOI] [PubMed] [Google Scholar]
- 13.Churyukanov M, Plaghki L, Legrain V, Mouraux A. Thermal detection thresholds of Aδ-and C-fibre afferents activated by brief CO 2 laser pulses applied onto the human hairy skin. PloS one. 2012;7:e35817. doi: 10.1371/journal.pone.0035817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen RH, Vierck CJ. Population estimates for responses of cutaneous mechanoreceptors to a vertically indenting probe on the glabrous skin of monkeys. Experimental Brain Research. 1993;94:105–119. doi: 10.1007/BF00230474. [DOI] [PubMed] [Google Scholar]
- 15.Cohen RH, Vierck CJ. Relationships between touch sensations and estimated population responses of peripheral afferent mechanoreceptors. Experimental Brain Research. 1993;94:120–130. doi: 10.1007/BF00230475. [DOI] [PubMed] [Google Scholar]
- 16.Cooper BY, Vierck CJ, Jr, Yeomans DC. Selective reduction of second pain sensations by systemic morphine in humans. Pain. 1986;24:93–116. doi: 10.1016/0304-3959(86)90030-8. [DOI] [PubMed] [Google Scholar]
- 17.Dubner R, Beitel RE. Peripheral neural correlates of escape behavior in rhesus monkey to noxious heat applied to the face. Advances in pain research and therapy. 1976;1:155–160. [Google Scholar]
- 18.Georgopoulos AP. Functional properties of primary afferent units probably related to pain mechanisms in primate glabrous skin. Journal of Neurophysiology. 1976;39:71–83. doi: 10.1152/jn.1976.39.1.71. [DOI] [PubMed] [Google Scholar]
- 19.Greenspan JD, Taylor DJ, McGillis SL. Body site variation of cool perception thresholds, with observations on paradoxical heat. Somatosens Mot Res. 1993;10:467–474. doi: 10.3109/08990229309028851. [DOI] [PubMed] [Google Scholar]
- 20.Gybels J, Handwerker HO, Van Hees J. A comparison between the discharges of human nociceptive nerve fibres and the subject's ratings of his sensations. The Journal of Physiology. 1979;292:193–206. doi: 10.1113/jphysiol.1979.sp012846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iannetti G, Zambreanu L, Wise RG, Buchanan T, Huggins J, Smart T, Vennart W, Tracey I. Pharmacological modulation of pain-related brain activity during normal and central sensitization states in humans. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18195–18200. doi: 10.1073/pnas.0506624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iggo A, Ogawa H. Primate cutaneous thermal nociceptors. The Journal of physiology. 1971;216:77P. [PubMed] [Google Scholar]
- 23.Johansson RS, Vallbo ÅB. Tactile sensibility in the human hand: relative and absolute densities of four types of mechanoreceptive units in glabrous skin. The Journal of physiology. 1979;286:283–300. doi: 10.1113/jphysiol.1979.sp012619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones MH. Evidence for a Double Peripheral Pathway for Pain. Science. 1958;128:713–714. doi: 10.1126/science.128.3326.713. [DOI] [PubMed] [Google Scholar]
- 25.Lee KH, Chung JM, Willis WD., Jr Inhibition of primate spinothalamic tract cells by TENS. Journal of neurosurgery. 1985;62:276–287. doi: 10.3171/jns.1985.62.2.0276. [DOI] [PubMed] [Google Scholar]
- 26.Lewis T, Pochin EE. The double pain response of the human skin to a single stimulus. Clin Sci. 1937;3:67–76. [Google Scholar]
- 27.Madsen C, Johnsen B, Fuglsang-Frederiksen A, Jensen T, Finnerup N. The effect of nerve compression and capsaicin on contact heat-evoked potentials related to Aδ-and C-fibers. Neuroscience. 2012;223:92–101. doi: 10.1016/j.neuroscience.2012.07.049. [DOI] [PubMed] [Google Scholar]
- 28.Mauderli AP, Vierck CJ, Cannon RL, Rodrigues A, Shen C. Relationships Between Skin Temperature and Temporal Summation of Heat and Cold Pain. Journal of Neurophysiology. 2003;90:100–109. doi: 10.1152/jn.01066.2002. [DOI] [PubMed] [Google Scholar]
- 29.McGlone F, Olausson H, Boyle JA, Jones-Gotman M, Dancer C, Guest S, Essick G. Touching and feeling: differences in pleasant touch processing between glabrous and hairy skin in humans. European Journal of Neuroscience. 2012;35:1782–1788. doi: 10.1111/j.1460-9568.2012.08092.x. [DOI] [PubMed] [Google Scholar]
- 30.Neubert JK, Rossi HL, Malphurs W, Vierck CJ, Caudle RM. Differentiation between capsaicin-induced allodynia and hyperalgesia using a thermal operant assay. Behavioural brain research. 2006;170:308–315. doi: 10.1016/j.bbr.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Price DD. Characteristics of second pain and flexion reflexes indicative of prolonged central summation. Experimental Neurology. 1972;37:371–387. doi: 10.1016/0014-4886(72)90081-7. [DOI] [PubMed] [Google Scholar]
- 32.Price DD, Dubner R. Mechanisms of First and Second Pain in the Peripheral and Central Nervous Systems. J Investig Dermatol. 1977;69:167–171. doi: 10.1111/1523-1747.ep12497942. [DOI] [PubMed] [Google Scholar]
- 33.Price DD, Hull CD, Buchwald NA. Intracellular responses of dorsal horn cells to cutaneous and sural nerve A and C fiber stimuli. Experimental Neurology. 1971;33:291–309. doi: 10.1016/0014-4886(71)90022-7. [DOI] [PubMed] [Google Scholar]
- 34.Price DD, Long S, Huitt C. Sensory testing of pathophysiological mechanisms of pain in patients with reflex sympathetic dystrophy. Pain. 1992;49:163–173. doi: 10.1016/0304-3959(92)90139-3. [DOI] [PubMed] [Google Scholar]
- 35.Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17:45–56. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- 36.Price DD, Staud R, Robinson ME, Mauderli AP, Cannon R, Vierck CJ. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain. 2002;99:49–59. doi: 10.1016/s0304-3959(02)00053-2. [DOI] [PubMed] [Google Scholar]
- 37.Price DD, Wagman IH. Physiological roles of A and C fiber inputs to the spinal dorsal horn of Macaca mulatta. Experimental Neurology. 1970;29:383–399. doi: 10.1016/0014-4886(70)90066-x. [DOI] [PubMed] [Google Scholar]
- 38.Rolke R, Magerl W, Campbell KA, Schalber C, Caspari S, Birklein F, Treede RD. Quantitative sensory testing: a comprehensive protocol for clinical trials. European Journal of Pain. 2006;10:77–77. doi: 10.1016/j.ejpain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Rubin DB. Multiple imputation for nonresponse in surveys. John Wiley & Sons; 2004. [Google Scholar]
- 40.Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165–175. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 41.Tommerdahl M, Delemos K, Favorov OV, Metz C, Vierck C, Whitsel B. Response of anterior parietal cortex to different modes of same-site skin stimulation. Journal of Neurophysiology. 1998;80:3272–3283. doi: 10.1152/jn.1998.80.6.3272. [DOI] [PubMed] [Google Scholar]
- 42.Tommerdahl M, Delemos K, Vierck C, Favorov OV, Whitsel B. Anterior parietal cortical response to tactile and skin-heating stimuli applied to the same skin site. Journal of Neurophysiology. 1996;75:2662–2670. doi: 10.1152/jn.1996.75.6.2662. [DOI] [PubMed] [Google Scholar]
- 43.Torebjörk HE. Afferent G Units Responding to Mechanical, Thermal and Chemical Stimuli in Human Non-Glabrous Skin. Acta Physiologica Scandinavica. 1974;92:374–390. doi: 10.1111/j.1748-1716.1974.tb05755.x. [DOI] [PubMed] [Google Scholar]
- 44.Torebjörk HE, Hallin RG. Identification of afferent C units in intact human skin nerves. Brain Research. 1974;67:387–403. doi: 10.1016/0006-8993(74)90489-2. [DOI] [PubMed] [Google Scholar]
- 45.Treede RD, Meyer RA, Raja SN, Campbell JN. Evidence for two different heat transduction mechanisms in nociceptive primary afferents innervating monkey skin. The Journal of Physiology. 1995;483:747–758. doi: 10.1113/jphysiol.1995.sp020619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vierck CJ, Cooper BY, Cohen RH, Yeomans DC, Franzen O. Somatosensory Mechanisms. MacMillan Press; London: 1984. Effects of systemic morphine on monkeys and man: generalized suppression of behavior and preferential inhibition of pain elicited by unmyelinated nociceptors. pp. 309–323. [Google Scholar]
- 47.Yeomans DC, Cooper BY, Vierck CJ., Jr Effects of systemic morphine on responses of primates to first or second pain sensations. Pain. 1996;66:253–263. doi: 10.1016/0304-3959(96)03082-5. [DOI] [PubMed] [Google Scholar]