Abstract
Context
Routine symptom assessment represents the cornerstone of symptom management. ESAS is one of the first quantitative symptom assessment batteries that allows for simple and rapid documentation of multiple patient-reported symptoms at the same time.
Objective
To discuss the historical development of ESAS, its current uses in different settings, and future developments.
Methods
Narrative review
Results
Since its development in 1991, ESAS has been psychometrically validated and translated into over 20 languages. We will discuss the variations, advantages and limitations with ESAS. From the clinical perspective, ESAS is now commonly used for symptom screening and longitudinal monitoring in patients seen by palliative care, oncology, nephrology, and other disciplines in both inpatient and outpatient settings. From the research perspective, ESAS has offered important insights into the nature of symptom trajectory, symptom clusters and symptom modulators. Furthermore, multiple clinical studies have incorporated ESAS as a study outcome and documented the impact of various interventions on symptom burden. On the horizon, multiple groups are actively investigating further refinements to ESAS, such as incorporating it in electronic health records, employing ESAS as a trigger for palliative care referral, and coupling ESAS with personalized symptom goals to optimize symptom response assessment.
Conclusion
ESAS has evolved over the past 25 years to become an important symptom assessment instrument in both clinical practice and research. Future efforts are needed to standardize this tool and explore its full potential to support symptom management.
Keywords: clinical trial, dyspnea, fatigue, surveys and questionnaires, symptom assessment, personalized medicine, neoplasms, pain, palliative care
A. Introduction
Patients with advanced diseases experience significant symptom burden from the time of diagnosis, which often increases in intensity over time.(1, 2) In cross sectional studies, the average cancer patient reports 8–12 symptoms, with fatigue, pain, anorexia, cachexia, dyspnea, anxiety and depression being particularly common.(3–5) These symptoms are often multi-dimensional in nature, and can negatively impact patients’ quality of life and function while increasing caregiver burden.(6)
Over the past decades, the specialty of palliative care has acquired substantial expertise in symptom management.(7) One of the most critical aspects of symptom management is routine symptom assessment and re-assessment—which allows symptoms to be recognized, diagnosed, treated and monitored over time. Theoretical frameworks such as the symptom expression pathway have formed the basis for multidimensional symptom management guided by patient-reported outcomes instead of clinician-based assessments.(8) The symptom transduction cascade illustrates why patients often present with multiple symptoms at the same time, and support the need for symptom assessment batteries that document multiple symptoms.(9)
The Edmonton Symptom Assessment System (ESAS) represents one of the first symptom batteries in palliative care, and has since been validated by multiple groups, translated into over 20 languages, and adopted in both clinical practice and research to support symptom assessment in many centers worldwide. The year 2016 marks the 25th anniversary of ESAS. In this review, we shall examine the historical development of ESAS, its current uses in different settings, and future developments of this tool.
B. Past Developments
Derivation
ESAS was initially developed by Bruera and colleagues as a clinical tool to document the symptom burden in patients with advanced cancer admitted to a palliative care unit.(10) The initial version consisted of 8 horizontal 0–100 mm visual analog scales (VAS) assessing pain, activity, nausea, depression, anxiety, drowsiness, appetite, and sensation of well-being. A ninth VAS was added to document “a less frequent symptom that might be important for a given patient”. ESAS was completed by patients, relatives and/or nurses twice daily at 10 am and 6 pm. Although not explicitly stated, the original version was intended to examine symptom intensity at the moment of assessment. The investigators proposed a symptom distress score based on the total score of 8 symptoms (range 0–800). Among the 101 consecutive patients admitted to the palliative care unit in Edmonton, the mean symptom distress score was 410 on day 1 and 362 on day 5. The authors concluded that ESAS was a “simple and useful method for the regular assessment of symptom distress in the palliative care setting”.
Validation and Modifications
ESAS has been validated by multiple research groups. In 1993, Bruera et al. found that ESAS had good test-retest reliability among 34 hospitalized patients, and correlated with Support Team Assessment Schedule (STAS).(11) Philip et al. assessed the validity of a slightly modified version of ESAS assessing symptoms “now”, in which “activity” was replaced with “weakness” in 80 patients with cancer from Australia. ESAS had satisfactory to good correlation with Brief Pain Inventory and Rotterdam Symptom Checklist, with weighted kappas between 0.46 and 0.61.(12) In a prospective study involving 240 cancer patients from the US, Chang et al. reported ESAS (9 items, VAS) to have good internal reliability (Cronbach alpha 0.79), test-retest reliability (Spearman correlation coefficient 0.86 on day 2 and 0.45 on day 7) and convergent validity (correlation coefficient 0.85 with FACT pain, 0.83 with MSAS pain, 0.56 with BPI worst pain).(13) The psychometric validation of ESAS has been reviewed in detail by others.(14, 15) More recently, several investigators have also examined ESAS’s predictive validity. Specifically, higher ESAS symptom burden was associated with more emergency room visits in the next 7 days and a shorter survival.(16–18)
Over the years, ESAS has evolved from VASs to 11-point numeric rating scales (NRS) ranging from 0 (no symptom) to 10 (worst possible). NRS was easier to complete and report, and the findings generally corresponded with VAS.(19) The items were also revised: “activity” was replaced with “tiredness/fatigue”; “shortness of breath” was added as a standard item; and “constipation”, “insomnia”, “spiritual distress”, “financial distress” and several other symptoms have been proposed as additional items for assessment.(20–22) When ESAS was used daily, the time frame of assessment was modified to examine the average symptom intensity over the past 24 hours instead of “now” to better capture the fluctuating nature of many symptoms.(23) Several studies have examined patients’ perception of ESAS and highlighted opportunities for improvement. In a prospective study of 60 patients seen at an outpatient palliative care clinic, Garyali et al. found that the items of appetite and sleep were sometimes misinterpreted, resulting in reversed scoring.(23) Watanabe et al. conducted a think-aloud study asking 20 patients about their perception of ESAS, and reported that some patients had difficulty understanding the terms depression, anxiety, appetite and well being, while others found it challenging to distinguish between tiredness and drowsiness.(24)
These findings led to the proposal of a revised ESAS numeric rating scale (ESAS-r) consisting of 9 core symptoms (pain, tiredness, nausea, depression, anxious, drowsiness, appetite, feeling of well being, shortness of breath) and an optional 10th symptom.(25) Specifically, ESAS-r (1) stated the time frame of symptom assessment as “now”, (2) added brief explanations for tiredness (“lack of energy”), drowsiness (“feeling sleepy”), depression (“feeling sad”) and anxiety (“feeling nervous”) and well-being (“how you feel overall”), (3) changed “appetite” to “lack of appetite”, (4) adjusted the order of symptoms, (5) removed the horizontal line over the numbers and shaded alternate items in gray for readability, and (6) suggested constipation as the tenth item. A study comparing the two versions of ESAS in 160 cancer patients reported that ESAS-r was easier to understand (P=0.008).(25) More recently, Hannon et al. assessed the validity of the original versus revised version of ESAS with constipation and sleep added (ESAS-CS) among 202 ambulatory patients with advanced cancer. Both NRSs were found to be reliable and valid. A greater proportion of patients found the wording in ESAS-r-CS to be easier to understand than ESAS-CS (44% vs. 11%), but more preferred the 24 hour time frame in ESAS-CS over “right now” in ESAS-r-CS (53% vs. 21%).(20)
To date, many permutations of ESAS exist. The version used by the Supportive Care team at MD Anderson Cancer Center is shown in Figure 1. It consists of 10 items with “sleep” replacing “other symptom”, and asks about the average symptom intensity over the past 24 hours.
Figure 1. Edmonton Symptom Assessment Scale.
The current version used at MD Anderson Cancer Center uses 24 hour as the time frame anchor for the 0–10 numeric rating scales.
Translation
ESAS has been translated professionally by Mapi Research Trust into over 20 languages and is freely available (Table 1). Multiple research groups have further validated ESAS both linguistically and psychometrically in Chinese,(26) Flemish,(27) French,(28) German,(29) Icelandic,(30) Italian,(31), Japanese,(32) Korean,(33) Portugese,(35) Spanish,(34) Thai,(36) and Turkish.(37) An Arabic variation of ESAS is also available.(38)
Table 1.
Language Availability for the Edmonton Symptom Assessment System
| Country | Language | Psychometrically validated in language (reference) | Linguistically validated by Mapi Research Institute |
|---|---|---|---|
| Argentina | Spanish | - | ✓ |
| Australia | English | (12) | ✓ |
| Belgium | Flemish | (27) | - |
| Brazil | Portugese | (35) | ✓ |
| China | Chinese | (26) | ✓ |
| Canada | English French |
(10, 11) - |
✓ ✓ |
| Denmark | Danish | - | ✓ |
| France | French | (28) | ✓ |
| Germany | German | (29) | ✓ |
| Hungary | Hungarian | - | ✓ |
| Iceland | Icelandic | (30) | - |
| Israel | Hebrew Russian Arabic |
- - - |
✓ ✓ ✓ |
| Italy | Italian | (31) | ✓ |
| Japan | Japanese | (32) | ✓ |
| Korean | Korean | (33). | |
| Netherlands | Dutch | - | ✓ |
| New Zealand | English | - | ✓ |
| Portugal | Portugese | - | ✓ |
| Poland | Polish | - | ✓ |
| Russia | Russian | - | ✓ |
| Saudi Arabia | Arabic | (38) | - |
| South Africa | English Afrikaans |
- |
✓ ✓ |
| Spain | Spanish | (34) | ✓ |
| Sweden | Swedish | - | ✓ |
| Thailand | Thai | (36) | |
| Turkey | Turkish | (37) | ✓ |
| United Kingdom | English | - | ✓ |
| United States | English Spanish |
(13) - |
✓ ✓ |
Score Interpretation
Some investigators have examined how the 0–10 was interpreted by patients. Specifically, what cutoffs within the 0–10 NRS represent none, mild, moderate and severe symptom burden? In a prospective study involving 400 cancer patients, Selby et al. reported that 7 was the optimal cutoff for severe pain, depression, anxiety, drowsiness, appetite and well-being, 8 was the optimal cutoff for severe fatigue, and 6 was the optimal cutoff for dyspnea.(39) Oldenmenger conducted a systematic review of cutoffs for ESAS numeric rating scale. Among 18 studies, the cutoffs for moderate symptom intensity was generally between 4 and 5, and the cutoffs for severe symptom burden varied between 7 and 8.(40) A recent study found similar cutoffs for moderate (i.e. 3–4) and severe symptoms (i.e. 5–7) for the Japanese version of ESAS-r, despite differences in culture, language and patient populations.(41) In summary, ESAS scores of 0, 1–3, 4–6 and 7–10 are generally considered as none, mild, moderate and severe in clinical practice,(42) although there may be significant variations in how the individual patient interprets the scores.(43)
Responsiveness and Minimal Clinically Important Difference (MCID)
Another aspect of ESAS relates to its responsiveness to change and what is the smallest magnitude of change that is clinically significant. Hui et al. conducted a prospective multicenter study specifically designed to identify the MCID for each of the 10 ESAS symptoms.(44) 796 patients with cancer were enrolled from 6 centers. Patients were asked about their average ESAS symptom intensity over the past 24 hours at the first clinic visit and then a subsequent visit approximately 3 weeks later. They were also asked to provide the global assessment of change (better, same or worse) for each symptom which was used as an anchor for MCID determination. The area under the receiver-operating characteristic curves ranged between 0.70 and 0.87, suggesting that ESAS had good discrimination for symptom change.(44) Interestingly, a change of 1 point was found to be the optimal cutoff for both improvement and deterioration for all of the 10 symptoms using a sensitivity-specificity approach (Table 2). This finding was consistent with additional analyses employing other anchor-based and distribution-based approaches in the same data set. A retrospective analysis using change in ESAS well being categories as an anchor also found similar magnitude of change to be the MCID.(45, 46)
Table 2.
Minimal Clinically Important Differences for Edmonton Symptom Assessment System (ESAS) Individual Items and Total Scores (44, 51)
| Improvement | Deterioration | |||||
|---|---|---|---|---|---|---|
| Symptom | Optimal Cutoff* | Sensitivity | Specificity | Optimal Cutoff* | Sensitivity | Specificity |
| Pain | ≥+1 | 0.727 | 0.739 | ≤−1 | 0.731 | 0.849 |
| Fatigue | ≥+1 | 0.727 | 0.694 | ≤−1 | 0.733 | 0.805 |
| Nausea | ≥+1 | 0.593 | 0.841 | ≤−1 | 0.856 | 0.851 |
| Depression | ≥+1 | 0.639 | 0.758 | ≤−1 | 0.780 | 0.813 |
| Anxiety | ≥+1 | 0.681 | 0.711 | ≤−1 | 0.595 | 0.805 |
| Drowsiness | ≥+1 | 0.599 | 0.732 | ≤−1 | 0.728 | 0.733 |
| Appetite | ≥+1 | 0.673 | 0.765 | ≤−1 | 0.790 | 0.765 |
| Well being | ≥+1 | 0.664 | 0.689 | ≤−1 | 0.642 | 0.743 |
| Dyspnea | ≥+1 | 0.658 | 0.743 | ≤−1 | 0.722 | 0.842 |
| Sleep | ≥+1 | 0.728 | 0.693 | ≤−1 | 0.677 | 0.765 |
| Physical score† | ≥+3 | 0.630 | 0.697 | ≤−4 | 0.598 | 0.804 |
| Emotional score‡ | ≥+2 | 0.585 | 0.742 | ≤−1 | 0.611 | 0.752 |
| Total symptom distress scoreψ | ≥+3 | 0.683 | 0.622 | ≤−4 | 0.590 | 0.776 |
The optimal cutoff for sensitivity and specificity was determined based on the Youden’s J method and top left method. A positive value indicates improvement, while a negative value indicates deterioration.
Combined score based on ESAS pain, fatigue nausea, drowsiness, appetite, and dyspnea. The total ranges from 0 to 60, with a higher score indicating higher physical symptom burden.
Combined score based on ESAS anxiety and depression. The total ranges from 0 to 20, with a higher score indicating higher emotional symptom burden.
Combined score based on ESAS physical score, ESAS emotional score and ESAS well being. The total ranges from 0 to 90, with a higher score indicating higher total symptom burden.
ESAS Physical, Emotional and Total Symptom Distress Scores
A symptom distress score (SDS) was proposed by Bruera et al. by adding the 8 VAS in the original ESAS (total score 0–800). Since then, ESAS has undergone significant modifications, although most versions of ESAS retain 6 physical symptoms (pain, tiredness, nausea, drowsiness, appetite), 2 emotional symptoms (depression, anxiety) and one global item (well being). This led some investigators to propose the ESAS physical score (total of physical 6 symptoms, score range 0–60), ESAS emotional score (total of 2 emotional symptoms, score range 0–60) and ESAS total symptom distress score (physical score + emotional score + well being).(47) Indeed, the ESAS physical and emotional symptoms form 2 separate groups in cluster analysis.(48, 49) Furthermore, higher ESAS physical and total symptom distress scores were associated with shortened survival.(50)
A recent study identified the MCID cutoffs for symptom improvement was ≥+3/60, ≥+2/20 and ≥+3/90 for ESAS physical, emotional and total symptom distress scores, respectively, and ≤− 4/60, ≤−1/20 and ≤−4/90 for deterioration.(51)
C. Present Applications
The ability of ESAS to quantify multiple symptoms efficiently and systematically has revolutionized symptom assessment in both clinical practice and research, resulting in its widespread adoption. The advantages and limitations of ESAS are shown in Table 3. ESAS is currently used for symptom screening and monitoring in different palliative care settings, including inpatients,(52–54) outpatients,(55–58) and home care.(17) Within other branches of oncology, ESAS has been used by medical oncologists,(59, 60) radiation oncologists,(61) surgical oncologists,(62, 63) and gynecological oncologists.(64, 65) Outside of oncology, ESAS has also been adopted for symptom assessment in patients with kidney diseases,(66, 67) heart failure,(68, 69) pulmonary disorders,(70) hepatic diseases,(71) and sickle cell anemia.(72)
Table 3.
Strengths and Limitations of the Edmonton Symptom Assessment Scale
| Strengths | Limitations |
|---|---|
|
|
Clinical Applications: Symptom Screening
In the clinical setting, ESAS is most often used to identify patients’ unmet needs by systematic screening. Since 2006, Cancer Care Ontario (CCO) has adopted the ESAS for routine symptom assessment in a province-wide Palliative Care Integration Project (PCIP).(73–75) Patients rated their symptom intensity using the ESAS at ambulatory clinics at 14 Regional Cancer Centers. Data was predominantly captured electronically using Interactive Symptom Assessment and Collection (ISAAC) with touch-screen kiosks.(60) In 2014, 2 million symptom data points had been captured from 280,000 patients. The target symptom screening rate was 70%. Over 28,000 patients providing their symptom rating using ESAS each month.(75) A patient satisfaction survey involving 3660 patients in Ontario reported that a vast majority of patients (92%) agreed that the ESAS was important “as it helped their healthcare team to know their symptoms and severity”.(76)
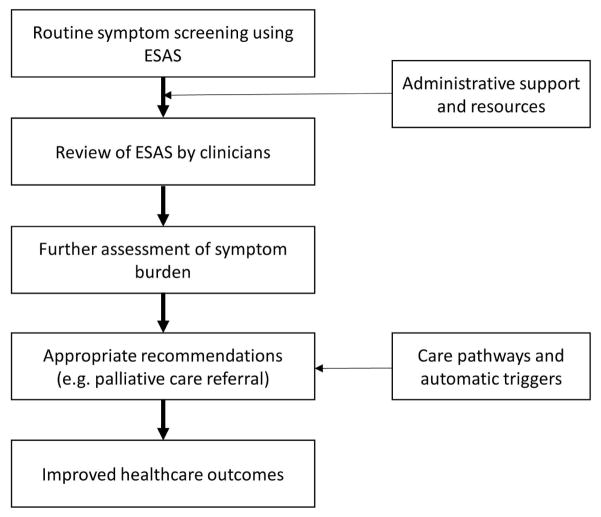
Routine collection of symptom data needs to be coupled with clinician endorsement and proper action plan to have a meaningful impact on patient care (Figure 2). In a survey of 40 physicians from a single center in Ottawa, the respondents found ESAS to be helpful and should be completed at every visit.(77) A subsequent survey of 2806 oncology professionals in Ontario (response rate 38%) also found that a majority of physicians (67%) and nurses (85%) perceived ESAS to be a useful starting point to assess patients’ symptoms.(76) 79% of physicians reported that they reviewed the ESAS scores at visits either “always” or “often”. However, a separate chart audit found that only 29% of patients with moderate-to-severe pain and 6% of patients with moderate-to-severe dyspnea had clinical actions documented in the chart, suggesting the need to strengthen the downstream actions from symptom screening through clinician education, resource allocation, and care pathways.(42) We shall discuss the use of ESAS as automatic trigger later in this manuscript.
Figure 2. Use of ESAS to Trigger Palliative Care Referral.
Routine symptom assessment needs to be endorsed by clinicians and coupled with action plans to improve clinical outcomes. A recent international consensus identified severe symptom distress as a criteria for palliative care referral, although this threshold may need to be refined at each institution.(128)
Clinical Applications: Longitudinal Symptom Monitoring
Because symptoms often fluctuate time, it is important to follow patients longitudinally and document their symptom improvement and/or deterioration.(78) As such, ESAS can be administered at every clinic visit to capture symptom changes. In a study that included 1612 patients with cancer seen at an outpatient palliative care clinic reported the change in symptom scores by baseline symptom intensity (absent/mild NRS ≤3 vs. moderate/severe NRS ≥4). The average symptom intensity worsened among patients with absent/mild baseline symptom intensity (−3.04 to 0.12) but generally improved among those with moderate/severe intensity (− 0.2 to 3.86). Overall, between 52% and 74% of patients with moderate/severe symptoms reported an improvement. This study highlights the fluctuating nature of symptom intensity, which is related to disease trajectory, effectiveness of symptom management strategies, and variations in symptom expression. It further illustrates why it is important to document baseline symptoms even in patients who have low symptom burden because they are likely to experience concerns in the future.(55)
Research Applications: Symptom Trajectory
Cummings et al. conducted a bibliometric analysis of ESAS between 1991 and 2006, and documented the rapid uptake of this tool in the global literature, particularly in general medicine and oncology journals.(79) By facilitating the documentation of multiple symptoms systematically, longitudinally and universally, ESAS has contributed to advancing multiple aspects of symptom research, including symptom trajectory, symptom clusters, symptom modulators and interventions for symptom management.(48, 49, 80–84)
As mentioned above, Ontario has a rich and growing dataset of over 4 million ESAS scores, providing some unique insights into symptom trajectory. Seow et al. documented the intensity of 9 ESAS symptoms in the last 6 months of life. Fatigue, appetite, drowsiness, shortness of breath, and well being worsened over time while nausea, depression, anxiety, and pain remained mostly stable.(80) Jia et al. recently reported the use of Markov Multistate Models to examine the symptom trajectory in patients with cancer. 280,000 assessments were collected among 55883 patients. They reported that fatigue and well being deteriorated rapidly over time.(81)
Research Applications: Symptom Cluster Studies
The assessment of multiple symptoms at the same time has allowed researchers to gain insights into symptom clusters. Symptoms often have similar etiology (e.g. inflammation), modulators (e.g. alcoholism) and may contribute to each other (e.g. dyspnea may worsen anxiety and vice versa). Multiple investigators have examined symptom clusters within ESAS. In the outpatient palliative care setting, 2 main symptom clusters had been identified (physical and emotional).(48, 49) Chen et al. examined symptom clusters among 1296 patients with advanced cancer seen at palliative radiation oncology clinics using three statistical approaches (i.e. principal component analysis, hierarchical cluster analysis, and exploratory factor analysis). Depression and anxiety consistently formed a cluster, while fatigue, drowsiness and dyspnea formed another cluster.(85) Using a version of ESAS that included 22 different items, Jimenez et al. reported 4 clusters (cognitive impairment, agitation and urinary incontinence; anxiety, depression and insomnia; anorexia, weight loss and tiredness; and nausea and vomiting) among 437 hospitalized patients with advanced cancer.(86) The variations in symptom clusters among different studies is likely related to differences in statistical techniques, patient populations and ESAS versions.(87) Further studies are needed to better understanding the evolving nature of symptom clusters. More recently, ESAS has also been used to assess symptom clusters among patients with advanced heart failure.(88)
Research Applications: Symptom Modulators
The examination of ESAS symptoms with other factors enabled the identification of various symptom modulators—variables that are consistently associated with the expression of one or more symptoms. For example, Parsons et al. identified that a history of alcoholism (assessed by on CAGE questionnaire positivity) was associated with elevated symptom expression in multiple ESAS items.(89) Similarly, a history of smoking correlated with an increased expression of multiple symptoms.(82, 83) Spiritual distress, depression and anxiety were also found to be important modulators of symptom expression.(90, 91) These insights into symptom modulators have substantial implications for symptom management. For example, a patient with high pain expression and severe depression and spiritual distress would mandate concurrent interdisciplinary management of his emotional and spiritual concerns rather than continual escalation of opioid doses.(8)
Research Applications: Assessing the Effect of Various Symptom Control Interventions
Because symptoms are often associated with each other, interventions targeting one symptom may also have an impact on others. Over the years, ESAS has been incorporated as an outcome to assess symptom response in multiple observational studies, open-label studies and randomized controlled trials.(92–101) This has facilitated the documentation of the treatment effect on multiple symptoms simultaneously. For example, in double-blind, randomized controlled trial of dexamethasone for cancer-related fatigue, ESAS-dyspnea as one of the secondary outcomes and showed a trend towards improvement with dexamethasone.(102) More recently, a separate randomized placebo-controlled trial that incorporated ESAS dyspnea as the primary outcome confirmed this observation.(103)
Several investigators have also used total ESAS scores to examine the effect of specialty palliative care versus usual oncologic care on symptom burden. In a single-blinded cluster randomized trial, Zimmermann et al. found that timely involvement of palliative care was associated with symptom improvement, while the symptoms worsened in the usual care group, with a statistically significant difference between the two study arms at 4 months.(104) Based on an MCID of 3 points for total symptom distress, this magnitude could be considered to be clinically significant.(51) Bakitas et al. also examined the effect of a nurse-led palliative care program, although there was an improvement in quality of life and depression, ESAS total burden did not change significantly.(105)
D. Future Developments
As ESAS continues to be used by a growing number of clinics, hospitals, jurisdictions and countries, multiple groups are actively examining how ESAS can be applied to further augment clinical practice and research. We shall discuss standardization and further validation of ESAS, incorporation of ESAS in the electronic health records, the use of ESAS to trigger clinical actions and the use personalized symptom goals.
Standardization and Further Validation
As highlighted in Table 3, there are several barriers to the use of ESAS. Going forward, it would be ideal to standardize ESAS item description and layout to facilitate combination and comparison of data across studies. Although symptom intensity over the past 24 hour is associated with symptom intensity “now”, there are important differences given that symptom burden fluctuates over time. ESAS “now” may be particularly useful to assess interventions with a rapid onset (i.e. effect of intravenous opioids on dyspnea “now”), while ESAS “24 hour” may be more suited for everyday clinical practice. At a minimum, investigators should consistently report which version of ESAS they are using in the publications and clearly state the time frame anchor. Further efforts are also needed to standardize the administration of ESAS to optimize accuracy.(106) As in many aspects of palliative care, precise definitions for specific terms are needed.(107–109) ESAS-r has contributed to improving the clarity for several items. However, some terms such as depression and well being may benefit from further research to examine their construct validity.(110–112) Further studies to compare the use of ESAS to other PROs would also be useful.
Incorporation of ESAS in Electronic Medical Records
In the era of information technology, patient reported outcomes are increasingly being captured, stored and displayed electronically. As mentioned above, Ontario has been systematically collecting ESAS via kiosks.(75) Several groups have also published their experience capturing ESAS using mobile device or computer.(113, 114) Strasser et al. reported a cluster randomized controlled trial comparing provision of symptom data to oncologists immediately following electronic symptom assessment versus no provision of data. The intervention arm was associated with a statistically and clinically significant improvement in ESAS symptom distress score (reduction of 5.4 points vs. worsening by 2.1 points, P=0.003).(115)
Electronic data capture has some advantages, including reduced missing data during the data entry process, the ease of completing the questionnaires at home, the possibility for computerized adaptive testing, rapid data access while minimizing the need for data entry manually, immediate display and scoring, and the ability to incorporate patient alerts and automatic triggers.(113, 116) However, there are some barriers to implementation, including the upfront cost of building a system for data entry, storage, display, integration, and protection and the financial burden for maintaining and updating, lack of familiarity with electronic interface among some patients and healthcare professionals, the training, the need to address security concerns, and the need to build a system that can be incorporated into the clinical work flow. Although the advantages of incorporating ESAS and other health outcomes electronically outweigh the disadvantages, each institution would need to customize this process in the clinical workflow.
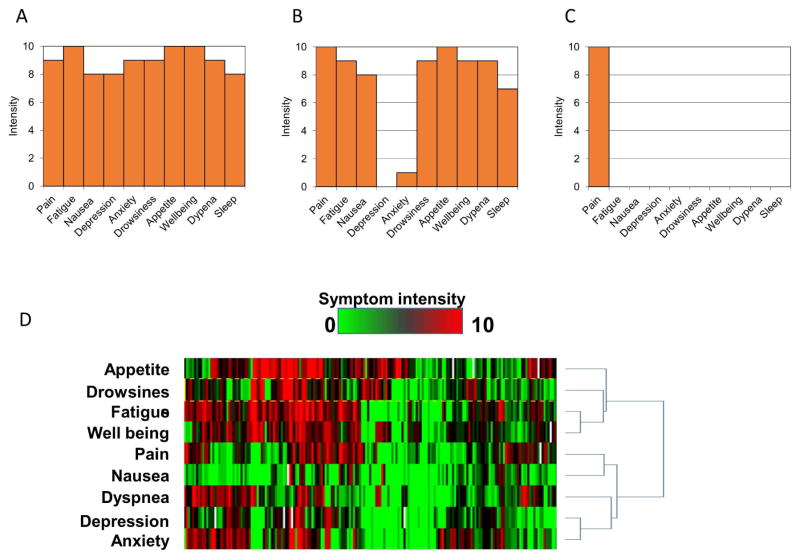
Electronic data capture could also facilitate data display and interpretation. ESAS can be plotted graphically using bar graphs, with some specific patterns that may augment symptom assessment (Figure 3A–C). More recently, our group has piloted the use of symptom expression arrays to display the individual data for large number of patients (Figure 3D).
Figure 3. ESAS Displays.
ESAS can be graphically displayed, and the pattern of symptom expression can be highly informative. (A) Globally elevated symptom expression – this pattern may suggest the presence of symptom modulators such as depression or anxiety. These modulators would need to be properly addressed as part of the symptom management plan. (B) U-shape distribution – some patients may under-report their level of anxiety and depression even though they may be contributing to their high physical symptom expression. These patients may benefit from assessment of their emotional status even if they do not report any. (C) Solitary pain – some patients have very high pain expression but no other associated symptoms which is atypical. The clinician may want to carefully characterize the patient’s pain history and ensure safe opioid use. (D) ESAS symptom expression array – each column represents one ESAS assessment for an individual patient, each row represents one ESAS symptom, and the colour represents symptom intensity (green = none, red = worst). This novel display may be generated by a computer program to illustrate the ESAS symptoms for multiple patients at the same time, or for the same patient over time. The example here displays ESAS scores on admission for patients at an acute palliative care unit. Symptoms cluster can be clearly detected (fatigue, appetite, drowsiness). Nausea had low expression. The expression of dyspnea was also associated with anxiety.
Use of ESAS to Trigger Clinical Actions
ESAS is increasingly used to trigger specific clinical actions, such as referral to a palliative care team (Figure 2).(117) The American College of Surgeons Commission on Cancer mandates distress screening as a criterion for accreditation.(118) ESAS has been proposed as tool for such purpose.(119) In a systematic review of the literature to characterize referral criteria to outpatient palliative care for patients with cancer, 13 of 21 included studies specified symptom distress as a reason for referral.(120) Among these studies, ESAS was the most commonly used symptom assessment scale, with 7 of the 9 studies that reported the use of a validated scale employing ESAS.(121–127) However, only one study stated a symptom intensity cutoff of ≥6/10 was needed to trigger a referral.(122)
More recently, 60 international experts reached consensus on 11 major criteria for outpatient palliative care referral for patients with advanced cancer, in which fulfillment of any one major criteria is sufficient to initiate a referral. The level of agreement was highest for severe physical distress (i.e. NRS ≥7/10, agreement 100%) and severe emotion distress (i.e. NRS ≥7/10, agreement 97%).(128) These ESAS cutoffs may vary somewhat at each institution by the resource availability of specialty palliative care and the level of interest among oncologists to provide basic symptom management.(129, 130) Importantly, any automatic referral should complement rather than override clinician judgement. Future studies should determine what proportion of patients fulfill these criteria,(117) how patients, families and clinicians perceive the use of ESAS to trigger a referral, and whether it would improve healthcare outcomes compared to clinician-based referral alone.(131)
ESAS may also trigger clinical actions other than a palliative care referral. Dhiliwal et al. described the use of ESAS to triage patients for the intensity of home-based palliative care visits. Among the 506 patients included, 6% had high symptom burden (any ESAS ≥7), 21% had moderate burden (any ESAS 4–6), and 73% had low symptom burden (ESAS scores 0–3). These three groups were seen within an average of 2.6, 7 and 10.5 working days of referral. Comparing to data a year ago, implementation of this triaging system was associated with a decrease in hospital deaths (19% vs. 27%).(132)
Personalized symptom goals
Although 0, 1–3, 4–6, and 7–10 points on a scale of 0 to 10 generally correspond to none, mild, moderate, and severe symptom burden, there is significant variation in how each patient interprets the scale. For example, one patient may consider a pain score of 6/10 to be agonizing, while another may consider this to be her baseline and appears to be comfortable. Furthermore, a change in 1 point (i.e. MCID of ESAS) may or may not be representative of a meaningful change for the individual patient.
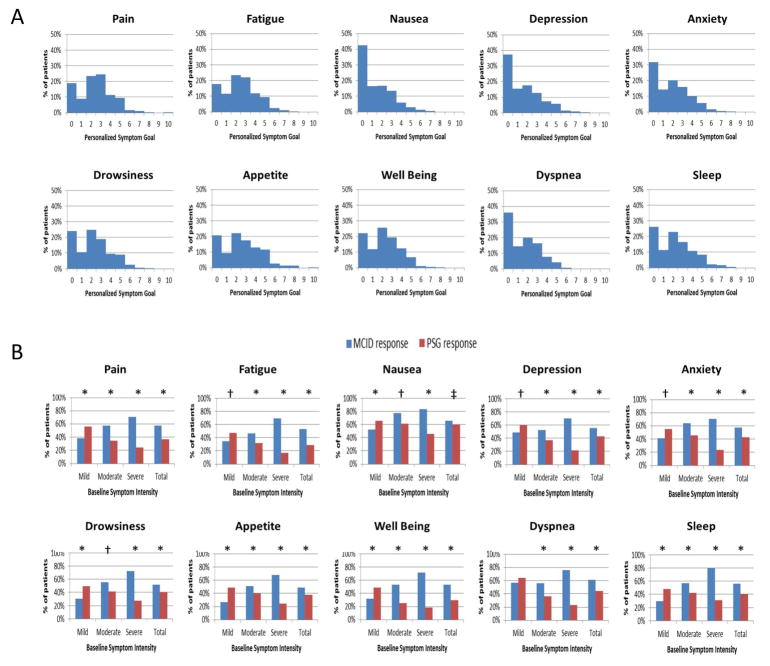
Personalized symptom goal (PSG) represents an innovative approach to address these issues. By asking patients “Using the same 0–10 scale, at what level of (specific symptom) would you feel comfortable?” clinicians can better appreciate how each patient interprets the NRS, while establishing an individualized treatment target at the same time.(133) Our research group conducted a multicenter study involving 728 patients with advanced cancer seen at palliative care clinics.(43) A majority reported a PSG of 3 or less for each ESAS symptom (Figure 4A). The median PSG was 1 for nausea, 2 for depression, anxiety, drowsiness, well being, dyspnea and sleep, and 3 for pain, fatigue, and appetite. Between 33% and 73% of patients achieved their PSG by the second palliative care clinic visit. PSG also addresses a concern with the MCID criterion to assess response—that patients with higher symptom intensity were more likely to achieve a response, when many patients who “responded” continue to have suboptimal symptom control above their PSG (Figure 4B). PSG may be applied in clinical practice (e.g. one assessment at consultation) or research studies to personalize the symptom treatment goal.
Figure 4. Symptom Response Criteria.
(A) Distribution of Personalized Symptom Goal for 10 Symptoms. A majority of patients reported a personalized symptom goal of 3 or less. (B) Response Rates Differences by Baseline Symptom Intensity and Response Criteria. We plotted the response rates by two criteria (minimal clinically important difference [MCID] and personalized symptom goal [PSG]) according to baseline symptom intensity (i.e. mild 1–3, moderate 4–6, and severe 7–10). Using the MCID criteria, patients with higher baseline symptom intensity were more likely to achieve a response and vice versa; in contrast, the personalized symptom response criteria resulted in the opposite conclusion. P-values were computed based on the McNemer test (* P<0.0001, † P<0.001, ‡ P<0.05). Reprinted with permissions from the American Cancer Society.(43)
E. Summary
Over 25 years, ESAS has evolved to become one of the most commonly used patient reported outcomes for symptom assessment in palliative care, oncology and beyond. ESAS has been psychometrically validated, translated into numerous languages, and is freely available. By enabling rapid, pragmatic assessment of multiple symptoms simultaneously, ESAS is used extensively in the clinical setting for symptom screening and monitoring worldwide. As one of the first symptom batteries ever developed, ESAS has also transformed the symptom research paradigm, contributing to major insights into symptom prevalence, trajectory, clusters, modulators and interventions. Active work is ongoing to help standardize the administration of ESAS, integrate it into electronic health records, link it to clinical actions, and couple it to personalized symptom goals.
Acknowledgments
Funding: DH and EB are supported in part by an American Cancer Society Mentored Research Scholar Grant in Applied and Clinical Research (MRSG-14-1418-01-CCE) and a National Institutes of Health grant (R21CA186000-01A1). DH is also partly supported by the Andrew Sabin Family Fellowship.
We would like to thank Ms. Ganiraju Manyam and Dr. John Weinstein for their assistance in generating the symptom expression array.
Footnotes
Conflict of interest: None reported
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage. 2006;31:58–69. doi: 10.1016/j.jpainsymman.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Teunissen SC, Wesker W, Kruitwagen C, de Haes HC, Voest EE, de Graeff A. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage. 2007;34:94–104. doi: 10.1016/j.jpainsymman.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Portenoy RK, Thaler HT, Kornblith AB, et al. Symptom prevalence, characteristics and distress in a cancer population. Qual Life Res. 1994;3:183–189. doi: 10.1007/BF00435383. [DOI] [PubMed] [Google Scholar]
- 4.Chang VT, Hwang SS, Feuerman M, Kasimis BS. Symptom and quality of life survey of medical oncology patients at a veterans affairs medical center: a role for symptom assessment. Cancer. 2000;88:1175–1183. doi: 10.1002/(sici)1097-0142(20000301)88:5<1175::aid-cncr30>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 5.Walsh D, Donnelly S, Rybicki L. The symptoms of advanced cancer: relationship to age, gender, and performance status in 1,000 patients. Support Care Cancer. 2000;8:175–179. doi: 10.1007/s005200050281. [DOI] [PubMed] [Google Scholar]
- 6.Dionne-Odom JN, Hull JG, Martin MY, et al. Associations between advanced cancer patients’ survival and family caregiver presence and burden. Cancer medicine. 2016;5:853–862. doi: 10.1002/cam4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruera E, Hui D. Palliative care research: Lessons learned by our team over the last 25 years. Palliat Med. 2013;27:939–951. doi: 10.1177/0269216313477177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hui D, Bruera E. A personalized approach to assessing and managing pain in patients with cancer. J Clin Oncol. 2014;32:1640–1646. doi: 10.1200/JCO.2013.52.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hui D, Bruera E. Supportive and Palliative Oncology: A New Paradigm for Comprehensive Cancer Care. Hematology & Oncology Review. 2013;9:68–74. [Google Scholar]
- 10.Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 11.Bruera E, MacDonald S. Audit Methods: The Edmonton Symptom Assessment System. In: Higginson I, editor. Clinical Audit in Palliative Care. Oxford: Radcliffe Medical Press; 1993. pp. 61–77. [Google Scholar]
- 12.Philip J, Smith WB, Craft P, Lickiss N. Concurrent validity of the modified Edmonton Symptom Assessment System with the Rotterdam Symptom Checklist and the Brief Pain Inventory. Support Care Cancer. 1998;6:539–541. doi: 10.1007/s005200050212. [DOI] [PubMed] [Google Scholar]
- 13.Chang VT, Hwang SS, Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer. 2000;88:2164–2171. doi: 10.1002/(sici)1097-0142(20000501)88:9<2164::aid-cncr24>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Nekolaichuk C, Watanabe S, Beaumont C. The Edmonton Symptom Assessment System: a 15-year retrospective review of validation studies (1991–2006) Palliat Med. 2008;22:111–122. doi: 10.1177/0269216307087659. [DOI] [PubMed] [Google Scholar]
- 15.Richardson LA, Jones GW. A review of the reliability and validity of the Edmonton Symptom Assessment System. Curr Oncol. 2009;16:55. doi: 10.3747/co.v16i1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng L, Zhang L, Culleton S, et al. Edmonton symptom assessment scale as a prognosticative indicator in patients with advanced cancer. J Palliat Med. 2011;14:337–342. doi: 10.1089/jpm.2010.0438. [DOI] [PubMed] [Google Scholar]
- 17.Mercadante S, Valle A, Porzio G, Aielli F, Adile C, Casuccio A. Prognostic factors of survival in patients with advanced cancer admitted to home care. J Pain Symptom Manage. 2013;45:56–62. doi: 10.1016/j.jpainsymman.2011.12.288. [DOI] [PubMed] [Google Scholar]
- 18.Barbera L, Atzema C, Sutradhar R, et al. Do patient-reported symptoms predict emergency department visits in cancer patients? A population-based analysis. Ann Emerg Med. 2013;61:427–437. e425. doi: 10.1016/j.annemergmed.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Hjermstad MJ, Fayers PM, Haugen DF, et al. Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. 2011;41:1073–1093. doi: 10.1016/j.jpainsymman.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Hannon B, Dyck M, Pope A, et al. Modified Edmonton Symptom Assessment System including constipation and sleep: validation in outpatients with cancer. J Pain Symptom Manage. 2015;49:945–952. doi: 10.1016/j.jpainsymman.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Delgado-Guay MO, Chisholm G, Williams J, Frisbee-Hume S, Ferguson AO, Bruera E. Frequency, intensity, and correlates of spiritual pain in advanced cancer patients assessed in a supportive/palliative care clinic. Palliat Support Care. 2016;14:341–348. doi: 10.1017/S147895151500108X. [DOI] [PubMed] [Google Scholar]
- 22.Delgado-Guay M, Ferrer J, Rieber AG, et al. Financial Distress and Its Associations With Physical and Emotional Symptoms and Quality of Life Among Advanced Cancer Patients. Oncologist. 2015;20:1092–1098. doi: 10.1634/theoncologist.2015-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garyali A, Palmer JL, Yennurajalingam S, Zhang T, Pace EA, Bruera E. Errors in symptom intensity self-assessment by patients receiving outpatient palliative care. J Palliat Med. 2006;9:1059–1065. doi: 10.1089/jpm.2006.9.1059. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe S, Nekolaichuk C, Beaumont C, Mawani A. The Edmonton symptom assessment system--what do patients think? Support Care Cancer. 2009;17:675–683. doi: 10.1007/s00520-008-0522-1. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe SM, Nekolaichuk C, Beaumont C, Johnson L, Myers J, Strasser F. A multicenter study comparing two numerical versions of the Edmonton Symptom Assessment System in palliative care patients. J Pain Symptom Manage. 2011;41:456–468. doi: 10.1016/j.jpainsymman.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 26.Dong Y, Chen H, Zheng Y, et al. Psychometric Validation of the Edmonton Symptom Assessment System in Chinese Patients. J Pain Symptom Manage. 2015;50:712–717. e712. doi: 10.1016/j.jpainsymman.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 27.Claessens P, Menten J, Schotsmans P, Broeckaert B. Development and validation of a modified version of the Edmonton Symptom Assessment Scale in a Flemish palliative care population. Am J Hosp Palliat Care. 2011;28:475–482. doi: 10.1177/1049909111400724. [DOI] [PubMed] [Google Scholar]
- 28.Pautex S, Berger A, Chatelain C, Herrmann F, Zulian GB. Symptom assessment in elderly cancer patients receiving palliative care. Crit Rev Oncol Hematol. 2003;47:281–286. doi: 10.1016/s1040-8428(03)00043-x. [DOI] [PubMed] [Google Scholar]
- 29.Stiel S, Matthes ME, Bertram L, Ostgathe C, Elsner F, Radbruch L. Validation of the new version of the minimal documentation system (MIDOS) for patients in palliative care: the German version of the edmonton symptom assessment scale (ESAS) Schmerz (Berlin, Germany) 2010;24:596–604. doi: 10.1007/s00482-010-0972-5. [DOI] [PubMed] [Google Scholar]
- 30.Gretarsdottir H, Fridriksdottir N, Gunnarsdottir S. Psychometric Properties of the Icelandic Version of the Revised Edmonton Symptom Assessment Scale. J Pain Symptom Manage. 2016;51:133–137. doi: 10.1016/j.jpainsymman.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Moro C, Brunelli C, Miccinesi G, et al. Edmonton symptom assessment scale: Italian validation in two palliative care settings. Support Care Cancer. 2006;14:30–37. doi: 10.1007/s00520-005-0834-3. [DOI] [PubMed] [Google Scholar]
- 32.Yokomichi N, Morita T, Nitto A, et al. Validation of the Japanese Version of the Edmonton Symptom Assessment System-Revised. J Pain Symptom Manage. 2015;50:718–723. doi: 10.1016/j.jpainsymman.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 33.Kwon JH, Nam SH, Koh S, et al. Validation of the Edmonton Symptom Assessment System in Korean patients with cancer. J Pain Symptom Manage. 2013;46:947–956. doi: 10.1016/j.jpainsymman.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carvajal A, Centeno C, Watson R, Bruera E. A comprehensive study of psychometric properties of the Edmonton Symptom Assessment System (ESAS) in Spanish advanced cancer patients. Eur J Cancer. 2011;47:1863–1872. doi: 10.1016/j.ejca.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 35.Paiva CE, Manfredini LL, Paiva BS, Hui D, Bruera E. The Brazilian Version of the Edmonton Symptom Assessment System (ESAS) Is a Feasible, Valid and Reliable Instrument for the Measurement of Symptoms in Advanced Cancer Patients. PLoS ONE. 2015;10:e0132073. doi: 10.1371/journal.pone.0132073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chinda M, Jaturapatporn D, Kirshen AJ, Udomsubpayakul U. Reliability and validity of a Thai version of the edmonton symptom assessment scale (ESAS-Thai) J Pain Symptom Manage. 2011;42:954–960. doi: 10.1016/j.jpainsymman.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 37.Yeşilbalkan ÖU, Özkütük N, Karadakovan A, Turgut T, Kazgan B. Validity and Reliability of the Edmonton Symptom Assessment Scale in Turkish Cancer Patients. Turk J Cancer. 2008;38:62–67. [Google Scholar]
- 38.Al-Shahri MZ, Al-Zahrani AS, Alansari A, et al. Validation of an Arabic Questionnaire for Symptom Assessment. Am J Hosp Palliat Care. 2016 doi: 10.1177/1049909115624654. (in press) [DOI] [PubMed] [Google Scholar]
- 39.Selby D, Cascella A, Gardiner K, et al. A single set of numerical cutpoints to define moderate and severe symptoms for the Edmonton Symptom Assessment System. J Pain Symptom Manage. 2010;39:241–249. doi: 10.1016/j.jpainsymman.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 40.Oldenmenger WH, de Raaf PJ, de Klerk C, van der Rijt CC. Cut points on 0–10 numeric rating scales for symptoms included in the Edmonton Symptom Assessment Scale in cancer patients: a systematic review. J Pain Symptom Manage. 2013;45:1083–1093. doi: 10.1016/j.jpainsymman.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi T, Morita T, Nitto A, et al. Establishing Cutoff Points for Defining Symptom Severity Using the Edmonton Symptom Assessment System-Revised Japanese Version. J Pain Symptom Manage. 2016;51:292–297. doi: 10.1016/j.jpainsymman.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 42.Seow H, Sussman J, Martelli-Reid L, Pond G, Bainbridge D. Do high symptom scores trigger clinical actions? An audit after implementing electronic symptom screening. Journal of oncology practice/American Society of Clinical Oncology. 2012;8:e142–148. doi: 10.1200/JOP.2011.000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hui D, Park M, Shamieh O, et al. Personalized symptom goals and response in patients with advanced cancer. Cancer. 2016;22:1774–1781. doi: 10.1002/cncr.29970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hui D, Shamieh O, Paiva C, et al. Minimal Clinically Important Differences in the Edmonton Symptom Assessment Scale in Cancer Patients: A Prospective Study. Cancer. 2015;121:3027–3035. doi: 10.1002/cncr.29437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bedard G, Zeng L, Zhang L, et al. Minimal Clinically Important Differences in the Edmonton Symptom Assessment System in Patients With Advanced Cancer. J Pain Symptom Manage. 2012 doi: 10.1016/j.jpainsymman.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 46.Hui D, Bruera E. Minimal clinically important differences in the edmonton symptom assessment system: the anchor is key. J Pain Symptom Manage. 2013;45:e4–5. doi: 10.1016/j.jpainsymman.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 47.Zimmermann C, Burman D, Bandukwala S, et al. Nurse and physician inter-rater agreement of three performance status measures in palliative care outpatients. Support Care Cancer. 2010;18:609–616. doi: 10.1007/s00520-009-0700-9. [DOI] [PubMed] [Google Scholar]
- 48.Yennurajalingam S, Kwon JH, Urbauer DL, Hui D, Reyes-Gibby CC, Bruera E. Consistency of symptom clusters among advanced cancer patients seen at an outpatient supportive care clinic in a tertiary cancer center. Palliat Support Care. 2013;11:473–480. doi: 10.1017/S1478951512000879. [DOI] [PubMed] [Google Scholar]
- 49.Cheung WY, Le LW, Zimmermann C. Symptom clusters in patients with advanced cancers. Support Care Cancer. 2009;17:1223–1230. doi: 10.1007/s00520-009-0577-7. [DOI] [PubMed] [Google Scholar]
- 50.Cheung WY, Barmala N, Zarinehbaf S, Rodin G, Le LW, Zimmermann C. The association of physical and psychological symptom burden with time to death among palliative cancer outpatients. J Pain Symptom Manage. 2009;37:297–304. doi: 10.1016/j.jpainsymman.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 51.Hui D, Shamieh O, Paiva CE, et al. Minimal Clinically Important Difference in the Physical, Emotional, and Total Symptom Distress Scores of the Edmonton Symptom Assessment System. J Pain Symptom Manage. 2016;51:262–269. doi: 10.1016/j.jpainsymman.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yennurajalingam S, Urbauer DL, Casper KL, et al. Impact of a Palliative Care Consultation Team on Cancer-Related Symptoms in Advanced Cancer Patients Referred to an Outpatient Supportive Care Clinic. J Pain Symptom Manage. 2010;41:49–56. doi: 10.1016/j.jpainsymman.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 53.Hui D, Dos Santos R, Chisholm G, Bruera E. Symptom Expression in the Last 7 Days of Life among Cancer Patients Admitted to Acute Palliative Care Units. J Pain Symptom Manage. 2015;50:488–494. doi: 10.1016/j.jpainsymman.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Modonesi C, Scarpi E, Maltoni M, et al. Impact of palliative care unit admission on symptom control evaluated by the edmonton symptom assessment system. J Pain Symptom Manage. 2005;30:367–373. doi: 10.1016/j.jpainsymman.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 55.Kang JH, Kwon JH, Hui D, Yennurajalingam S, Bruera E. Changes in Symptom Intensity Among Cancer Patients Receiving Outpatient Palliative Care. J Pain Symptom Manage. 2013;46:652–660. doi: 10.1016/j.jpainsymman.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 56.Yennurajalingam S, Kang JH, Hui D, Kang DH, Kim SH, Bruera E. Clinical response to an outpatient palliative care consultation in patients with advanced cancer and cancer pain. J Pain Symptom Manage. 2012;44:340–350. doi: 10.1016/j.jpainsymman.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 57.Kim Y, Yen IH, Rabow MW. Comparing Symptom Burden in Patients with Metastatic and Nonmetastatic Cancer. J Palliat Med. 2016;19:64–68. doi: 10.1089/jpm.2011.0456. [DOI] [PubMed] [Google Scholar]
- 58.Paiva CE, Faria CB, Nascimento MS, et al. Effectiveness of a palliative care outpatient programme in improving cancer-related symptoms among ambulatory Brazilian patients. Eur J Cancer Care (Engl) 2012;21:124–130. doi: 10.1111/j.1365-2354.2011.01298.x. [DOI] [PubMed] [Google Scholar]
- 59.Thomas S, Walsh D, Shrotriya S, et al. Symptoms, Quality of Life, and Daily Activities in People With Newly Diagnosed Solid Tumors Presenting to a Medical Oncologist. Am J Hosp Palliat Care. 2016 doi: 10.1177/1049909116649948. (in press) [DOI] [PubMed] [Google Scholar]
- 60.Barbera L, Seow H, Howell D, et al. Symptom burden and performance status in a population-based cohort of ambulatory cancer patients. Cancer. 2010;116:5767–5776. doi: 10.1002/cncr.25681. [DOI] [PubMed] [Google Scholar]
- 61.Bradley N, Davis L, Chow E. Symptom distress in patients attending an outpatient palliative radiotherapy clinic. J Pain Symptom Manage. 2005;30:123–131. doi: 10.1016/j.jpainsymman.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 62.Aigner CJ, Hernandez M, Koyyalagunta L, Novy D. The association of presurgery psychological symptoms with postsurgery pain among cancer patients receiving implantable devices for pain management. Support Care Cancer. 2014;22:2323–2328. doi: 10.1007/s00520-014-2219-y. [DOI] [PubMed] [Google Scholar]
- 63.Chu L, Hawley P, Munk P, Mallinson P, Clarkson P. Minimally invasive palliative procedures in oncology: a review of a multidisciplinary collaboration. Support Care Cancer. 2015;23:1589–1596. doi: 10.1007/s00520-014-2509-4. [DOI] [PubMed] [Google Scholar]
- 64.Lefkowits C, MWR, AES, et al. Predictors of high symptom burden in gynecologic oncology outpatients: who should be referred to outpatient palliative care? Gynecol Oncol. 2014;132:698–702. doi: 10.1016/j.ygyno.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 65.Spoozak L, Seow H, Liu Y, Wright J, Barbera L. Performance status and symptom scores of women with gynecologic cancer at the end of life. Int J Gynecol Cancer. 2013;23:971–978. doi: 10.1097/IGC.0b013e318291e5ef. [DOI] [PubMed] [Google Scholar]
- 66.Davison SN, Jhangri GS, Johnson JA. Longitudinal validation of a modified Edmonton symptom assessment system (ESAS) in haemodialysis patients. Nephrol Dial Transplant. 2006;21:3189–3195. doi: 10.1093/ndt/gfl380. [DOI] [PubMed] [Google Scholar]
- 67.Flythe JE, Powell JD, Poulton CJ, et al. Patient-Reported Outcome Instruments for Physical Symptoms Among Patients Receiving Maintenance Dialysis: A Systematic Review. Am J Kidney Dis. 2015;66:1033–1046. doi: 10.1053/j.ajkd.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shah AB, Udeoji DU, Baraghoush A, Bharadwaj P, Yennurajalingam S, Schwarz ER. An evaluation of the prevalence and severity of pain and other symptoms in acute decompensated heart failure. J Palliat Med. 2013;16:87–90. doi: 10.1089/jpm.2012.0248. [DOI] [PubMed] [Google Scholar]
- 69.Udeoji DU, Shah AB, Bharadwaj P, Katsiyiannis P, Schwarz ER. Evaluation of the prevalence and severity of pain in patients with stable chronic heart failure. World journal of cardiology. 2012;4:250–255. doi: 10.4330/wjc.v4.i8.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walke LM, Gallo WT, Tinetti ME, Fried TR. The burden of symptoms among community-dwelling older persons with advanced chronic disease. Arch Intern Med. 2004;164:2321–2324. doi: 10.1001/archinte.164.21.2321. [DOI] [PubMed] [Google Scholar]
- 71.Poonja Z, Brisebois A, van Zanten SV, Tandon P, Meeberg G, Karvellas CJ. Patients with cirrhosis and denied liver transplants rarely receive adequate palliative care or appropriate management. Clin Gastroenterol Hepatol. 2014;12:692–698. doi: 10.1016/j.cgh.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 72.Lopez G, Liles DK, Knupp CL. Edmonton Symptom Assessment System for outpatient symptom monitoring of sickle cell disease. South Med J. 2014;107:768–772. doi: 10.14423/SMJ.0000000000000209. [DOI] [PubMed] [Google Scholar]
- 73.Dudgeon DJ, Knott C, Eichholz M, et al. Palliative Care Integration Project (PCIP) quality improvement strategy evaluation. J Pain Symptom Manage. 2008;35:573–582. doi: 10.1016/j.jpainsymman.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dudgeon D, King S, Howell D, et al. Cancer Care Ontario’s experience with implementation of routine physical and psychological symptom distress screening. Psychooncology. 2012;21:357–364. doi: 10.1002/pon.1918. [DOI] [PubMed] [Google Scholar]
- 75.Pereira J, Green E, Molloy S, et al. Population-based standardized symptom screening: Cancer Care Ontario’s Edmonton Symptom Assessment System and performance status initiatives. Journal of oncology practice/American Society of Clinical Oncology. 2014;10:212–214. doi: 10.1200/JOP.2014.001390. [DOI] [PubMed] [Google Scholar]
- 76.Pereira JL, Chasen MR, Molloy S, et al. Cancer Care Professionals’ Attitudes Toward Systematic Standardized Symptom Assessment and the Edmonton Symptom Assessment System After Large-Scale Population-Based Implementation in Ontario, Canada. J Pain Symptom Manage. 2016;51:662–672. e668. doi: 10.1016/j.jpainsymman.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 77.Chasen M, Bhargava R, Dalzell C, Pereira JL. Attitudes of oncologists towards palliative care and the Edmonton Symptom Assessment System (ESAS) at an Ontario cancer center in Canada. Support Care Cancer. 2015;23:769–778. doi: 10.1007/s00520-014-2411-0. [DOI] [PubMed] [Google Scholar]
- 78.Shamieh O, Khamash O, Khraisat M, et al. Impact of outpatient palliative care (PC) on symptom burden in patients with advanced cancer at a tertiary cancer center in Jordan. Support Care Cancer. 2016 doi: 10.1007/s00520-016-3395-8. (in press) [DOI] [PubMed] [Google Scholar]
- 79.Cummings G, Biondo PD, Campbell D, et al. Can the global uptake of palliative care innovations be improved? Insights from a bibliometric analysis of the Edmonton Symptom Assessment System. Palliat Med. 2011;25:71–82. doi: 10.1177/0269216310381449. [DOI] [PubMed] [Google Scholar]
- 80.Seow H, Barbera L, Sutradhar R, et al. Trajectory of performance status and symptom scores for patients with cancer during the last six months of life. J Clin Oncol. 2011;29:1151–1158. doi: 10.1200/JCO.2010.30.7173. [DOI] [PubMed] [Google Scholar]
- 81.Jia J, Barbera L, Sutradhar R. Using Markov Multistate Models to Examine the Progression of Symptom Severity Among an Ambulatory Population of Cancer Patients: Are Certain Symptoms Better Managed Than Others? J Pain Symptom Manage. 2016;51:232–239. doi: 10.1016/j.jpainsymman.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 82.Dev R, Parsons HA, Palla S, Palmer JL, Del Fabbro E, Bruera E. Undocumented alcoholism and its correlation with tobacco and illegal drug use in advanced cancer patients. Cancer. 2011;117:4551–4556. doi: 10.1002/cncr.26082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim YJ, Dev R, Reddy A, et al. Association Between Tobacco Use, Symptom Expression, and Alcohol and Illicit Drug Use in Advanced Cancer Patients. J Pain Symptom Manage. 2016;51:762–768. doi: 10.1016/j.jpainsymman.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 84.Hui D, Kilgore K, Park M, Williams J, Liu D, Bruera E. Impact of Prophylactic Fentanyl Pectin Nasal Spray on Exercise-Induced Episodic Dyspnea in Cancer Patients: A Double- Blind, Randomized Controlled Trial. J Pain Symptom Manage. 2016 doi: 10.1016/j.jpainsymman.2016.05.013. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen E, Nguyen J, Cramarossa G, et al. Symptom clusters in patients with advanced cancer: sub-analysis of patients reporting exclusively non-zero ESAS scores. Palliat Med. 2012;26:826–833. doi: 10.1177/0269216311420197. [DOI] [PubMed] [Google Scholar]
- 86.Jimenez A, Madero R, Alonso A, et al. Symptom clusters in advanced cancer. J Pain Symptom Manage. 2011;42:24–31. doi: 10.1016/j.jpainsymman.2010.10.266. [DOI] [PubMed] [Google Scholar]
- 87.Dong ST, Butow PN, Costa DS, Lovell MR, Agar M. Symptom clusters in patients with advanced cancer: a systematic review of observational studies. J Pain Symptom Manage. 2014;48:411–450. doi: 10.1016/j.jpainsymman.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 88.Yu DS, Chan HY, Leung DY, Hui E, Sit JW. Symptom clusters and quality of life among patients with advanced heart failure. Journal of geriatric cardiology: JGC. 2016;13:408–414. doi: 10.11909/j.issn.1671-5411.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Parsons HA, Delgado-Guay MO, El Osta B, et al. Alcoholism screening in patients with advanced cancer: impact on symptom burden and opioid use. J Palliat Med. 2008;11:964–968. doi: 10.1089/jpm.2008.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hui D, de la Cruz M, Thorney S, Parsons HA, Delgado-Guay M, Bruera E. The frequency and correlates of spiritual distress among patients with advanced cancer admitted to an acute palliative care unit. Am J Hosp Palliat Care. 2011;28:264–270. doi: 10.1177/1049909110385917. [DOI] [PubMed] [Google Scholar]
- 91.Delgado-Guay M, Parsons HA, Li Z, Palmer JL, Bruera E. Symptom distress in advanced cancer patients with anxiety and depression in the palliative care setting. Support Care Cancer. 2009;17:573–579. doi: 10.1007/s00520-008-0529-7. [DOI] [PubMed] [Google Scholar]
- 92.Centeno C, Sanz A, Cuervo MA, et al. Multicentre, double-blind, randomised placebo-controlled clinical trial on the efficacy of methylphenidate on depressive symptoms in advanced cancer patients. BMJ supportive & palliative care. 2012;2:328–333. doi: 10.1136/bmjspcare-2011-000093. [DOI] [PubMed] [Google Scholar]
- 93.Salas S, Frasca M, Planchet-Barraud B, et al. Ketamine analgesic effect by continuous intravenous infusion in refractory cancer pain: considerations about the clinical research in palliative care. J Palliat Med. 2012;15:287–293. doi: 10.1089/jpm.2011.0353. [DOI] [PubMed] [Google Scholar]
- 94.Del Fabbro E, Dev R, Hui D, Palmer L, Bruera E. Effects of melatonin on appetite and other symptoms in patients with advanced cancer and cachexia: a double-blind placebo-controlled trial. J Clin Oncol. 2013;31:1271–1276. doi: 10.1200/JCO.2012.43.6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Del Fabbro E, Garcia JM, Dev R, et al. Testosterone replacement for fatigue in hypogonadal ambulatory males with advanced cancer: a preliminary double-blind placebo-controlled trial. Support Care Cancer. 2013;21:2599–2607. doi: 10.1007/s00520-013-1832-5. [DOI] [PubMed] [Google Scholar]
- 96.Bandieri E, Romero M, Ripamonti CI, et al. Randomized Trial of Low-Dose Morphine Versus Weak Opioids in Moderate Cancer Pain. J Clin Oncol. 2016;34:436–442. doi: 10.1200/JCO.2015.61.0733. [DOI] [PubMed] [Google Scholar]
- 97.Bruera E, Moyano JR, Sala R, et al. Dexamethasone in addition to metoclopramide for chronic nausea in patients with advanced cancer: a randomized controlled trial. J Pain Symptom Manage. 2004;28:381–388. doi: 10.1016/j.jpainsymman.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 98.Bruera E, El Osta B, Valero V, et al. Donepezil for cancer fatigue: a double-blind, randomized, placebo-controlled trial. J Clin Oncol. 2007;25:3475–3481. doi: 10.1200/JCO.2007.10.9231. [DOI] [PubMed] [Google Scholar]
- 99.Bruera E, Hui D, Dalal S, et al. Parenteral hydration in patients with advanced cancer: a multicenter, double-blind, placebo-controlled randomized trial. J Clin Oncol. 2013;31:111–118. doi: 10.1200/JCO.2012.44.6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Follwell M, Burman D, Le LW, et al. Phase II study of an outpatient palliative care intervention in patients with metastatic cancer. J Clin Oncol. 2009;27:206–213. doi: 10.1200/JCO.2008.17.7568. [DOI] [PubMed] [Google Scholar]
- 101.Hui D, Glitza I, Chisholm G, Yennu S, Bruera E. Attrition rates, reasons, and predictive factors in supportive care and palliative oncology clinical trials. Cancer. 2012;119:1098–1105. doi: 10.1002/cncr.27854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yennurajalingam S, Frisbee-Hume S, Palmer JL, et al. Reduction of cancer-related fatigue with dexamethasone: a double-blind, randomized, placebo-controlled trial in patients with advanced cancer. J Clin Oncol. 2013;31:3076–3082. doi: 10.1200/JCO.2012.44.4661. [DOI] [PubMed] [Google Scholar]
- 103.Hui D, Kilgore K, Frisbee-Hume S, et al. Dexamethasone for Dyspnea in Cancer Patients: A Pilot Double-Blind, Randomized, Controlled Trial. J Pain Symptom Manage. 2016;52:8–16. doi: 10.1016/j.jpainsymman.2015.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383:1721–1730. doi: 10.1016/S0140-6736(13)62416-2. [DOI] [PubMed] [Google Scholar]
- 105.Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302:741–749. doi: 10.1001/jama.2009.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Carli Buttenschoen D, Stephan J, Watanabe S, Nekolaichuk C. Health care providers’ use and knowledge of the Edmonton Symptom Assessment System (ESAS): is there a need to improve information and training? Support Care Cancer. 2014;22:201–208. doi: 10.1007/s00520-013-1955-8. [DOI] [PubMed] [Google Scholar]
- 107.Hui D, Mori M, Parsons H, et al. The Lack of Standard Definitions in the Supportive and Palliative Oncology Literature. J Pain Symptom Manage. 2012;43:582–592. doi: 10.1016/j.jpainsymman.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hui D, De La Cruz M, Mori M, et al. Concepts and definitions for “supportive care,” “best supportive care,” “palliative care,” and “hospice care” in the published literature, dictionaries, and textbooks. Support Care Cancer. 2013;21:659–685. doi: 10.1007/s00520-012-1564-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hui D, Nooruddin Z, Didwaniya N, et al. Concepts and definitions for “actively dying,” “end of life,” “terminally ill,” “terminal care,” and “transition of care”: a systematic review. J Pain Symptom Manage. 2014;47:77–89. doi: 10.1016/j.jpainsymman.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bush SH, Parsons HA, Palmer JL, Li Z, Chacko R, Bruera E. Single- vs. multiple-item instruments in the assessment of quality of life in patients with advanced cancer. J Pain Symptom Manage. 2010;39:564–571. doi: 10.1016/j.jpainsymman.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 111.Lien K, Zeng L, Zhang L, et al. Predictive factors for well-being in advanced cancer patients referred for palliative radiotherapy. Clin Oncol (R Coll Radiol) 2012;24:443–451. doi: 10.1016/j.clon.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 112.Harrison LD, Zhang-Salomons J, Mates M, Booth CM, King WD, Mackillop WJ. Comparing effectiveness with efficacy: outcomes of palliative chemotherapy for non-small-cell lung cancer in routine practice. Current oncology (Toronto, Ont) 2015;22:184–191. doi: 10.3747/co.22.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schick-Makaroff K, Molzahn A. Strategies to use tablet computers for collection of electronic patient-reported outcomes. Health Qual Life Outcomes. 2015;13:2. doi: 10.1186/s12955-014-0205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kallen MA, Yang D, Haas N. A technical solution to improving palliative and hospice care. Support Care Cancer. 2012;20:167–174. doi: 10.1007/s00520-011-1086-z. [DOI] [PubMed] [Google Scholar]
- 115.Strasser F, Blum D, von Moos R, et al. The effect of real-time electronic monitoring of patient-reported symptoms and clinical syndromes in outpatient workflow of medical oncologists: E-MOSAIC, a multicenter cluster-randomized phase III study (SAKK 95/06) Ann Oncol. 2016;27:324–332. doi: 10.1093/annonc/mdv576. [DOI] [PubMed] [Google Scholar]
- 116.Wagner LI, Schink J, Bass M, et al. Bringing PROMIS to practice: brief and precise symptom screening in ambulatory cancer care. Cancer. 2015;121:927–934. doi: 10.1002/cncr.29104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nguyen J, Di Giovanni J, Zhang L, et al. Projected referral to other healthcare services in an outpatient palliative radiotherapy clinic. Expert Rev Pharmacoecon Outcomes Res. 2012;12:237–243. doi: 10.1586/erp.12.4. [DOI] [PubMed] [Google Scholar]
- 118.Pirl WF, Fann JR, Greer JA, et al. Recommendations for the implementation of distress screening programs in cancer centers: report from the American Psychosocial Oncology Society (APOS), Association of Oncology Social Work (AOSW), and Oncology Nursing Society (ONS) joint task force. Cancer. 2014;120:2946–2954. doi: 10.1002/cncr.28750. [DOI] [PubMed] [Google Scholar]
- 119.Watanabe SM, Nekolaichuk CL, Beaumont C. The Edmonton Symptom Assessment System, a proposed tool for distress screening in cancer patients: development and refinement. Psychooncology. 2012;21:977–985. doi: 10.1002/pon.1996. [DOI] [PubMed] [Google Scholar]
- 120.Hui D, Meng YC, Bruera S, et al. Referral Criteria for Outpatient Palliative Cancer Care: A Systematic Review. Oncologist. 2016;21:895–901. doi: 10.1634/theoncologist.2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Strasser F, Sweeney C, Willey J, Benisch-Tolley S, Palmer JL, Bruera E. Impact of a half-day multidisciplinary symptom control and palliative care outpatient clinic in a comprehensive cancer center on recommendations, symptom intensity, and patient satisfaction: a retrospective descriptive study. Journal of pain and symptom management. 2004;27:481–491. doi: 10.1016/j.jpainsymman.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 122.Riechelmann RP, Krzyzanowska MK, O’Carroll A, Zimmermann C. Symptom and medication profiles among cancer patients attending a palliative care clinic. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. 2007;15:1407–1412. doi: 10.1007/s00520-007-0253-8. [DOI] [PubMed] [Google Scholar]
- 123.Follwell M, Burman D, Le LW, et al. Phase II study of an outpatient palliative care intervention in patients with metastatic cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:206–213. doi: 10.1200/JCO.2008.17.7568. [DOI] [PubMed] [Google Scholar]
- 124.Wentlandt K, Krzyzanowska MK, Swami N, Rodin GM, Le LW, Zimmermann C. Referral practices of oncologists to specialized palliative care. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30:4380–4386. doi: 10.1200/JCO.2012.44.0248. [DOI] [PubMed] [Google Scholar]
- 125.Watanabe SM, Fairchild A, Pituskin E, Borgersen P, Hanson J, Fassbender K. Improving access to specialist multidisciplinary palliative care consultation for rural cancer patients by videoconferencing: report of a pilot project. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. 2013;21:1201–1207. doi: 10.1007/s00520-012-1649-7. [DOI] [PubMed] [Google Scholar]
- 126.Lefkowits C, Rabow WM, Sherman EA, et al. Predictors of high symptom burden in gynecologic oncology outpatients: who should be referred to outpatient palliative care? Gynecologic oncology. 2014;132:698–702. doi: 10.1016/j.ygyno.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 127.Wentlandt K, Krzyzanowska MK, Swami N, et al. Referral practices of pediatric oncologists to specialized palliative care. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. 2014;22:2315–2322. doi: 10.1007/s00520-014-2203-6. [DOI] [PubMed] [Google Scholar]
- 128.Hui D, Masanori M, Watanabe S, et al. Referral Criteria for Outpatient Specialty Palliative Cancer Care: An International Consensus. Lancet Oncol. 2016 doi: 10.1016/S1470-2045(16)30577-0. (in press) [DOI] [PubMed] [Google Scholar]
- 129.Wentlandt K, Krzyzanowska MK, Swami N, Rodin GM, Le LW, Zimmermann C. Referral practices of oncologists to specialized palliative care. J Clin Oncol. 2012;30:4380–4386. doi: 10.1200/JCO.2012.44.0248. [DOI] [PubMed] [Google Scholar]
- 130.Schenker Y, Crowley-Matoka M, Dohan D, et al. Oncologist Factors That Influence Referrals to Subspecialty Palliative Care Clinics. Journal of oncology practice/American Society of Clinical Oncology. 2013;10:e37–44. doi: 10.1200/JOP.2013.001130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hui D, Bruera E. Integrating palliative care into the trajectory of cancer care. Nat Rev Clin Oncol. 2016;13:159–171. doi: 10.1038/nrclinonc.2015.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dhiliwal S, Salins N, Deodhar J, Rao R, Muckaden MA. Pilot Testing of Triage Coding System in Home-based Palliative Care Using Edmonton Symptom Assessment Scale. Indian journal of palliative care. 2016;22:19–24. doi: 10.4103/0973-1075.173943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dalal S, Hui D, Nguyen L, et al. Achievement of personalized pain goal in cancer patients referred to a supportive care clinic at a comprehensive cancer center. Cancer. 2012;118:3869–3877. doi: 10.1002/cncr.26694. [DOI] [PMC free article] [PubMed] [Google Scholar]