Abstract
Recent findings have elucidated roles for mitochondrial uncoupling proteins (UCPs) in neuronal plasticity and resistance to metabolic and oxidative stress. UCPs are induced by bioenergetic challenges such as caloric restriction and exercise, and may protect neurons against dysfunction and degeneration. The pharmacological uncoupler 2,4-dinitrophenol (DNP), which was once prescribed to over 100,000 people as a treatment for obesity, stimulates several adaptive cellular stress response signaling pathways in neurons including those involving the neurotrophic factor BDNF, the transcription factor CREB, and autophagy. Preclinical data show that low doses of DNP can protect neurons and improve functional outcome in animal models of Alzheimer’s and Parkinson’s diseases, epilepsy and cerebral ischemic stroke. Repurposing of DNP and the development of novel uncoupling agents with hormetic mechanisms of action provide opportunities for new breakthrough therapeutic interventions in a range of acute and chronic insidious neurodegenerative/neuromuscular conditions, all paradoxically at body weight-preserving doses.
Bioenergetic Challenges, Hormesis and Neuroprotection
Three lifestyle factors that promote optimal brain function and neuronal resistance to injury and age-related neurodegenerative disorders are exercise, restriction of dietary energy intake, and regular engagement in social interactions and intellectual challenges [1–4]. For example, intermittent fasting and exercise can protect neurons against dysfunction and degeneration in animal models of Alzheimer’s disease (AD), Parkinson’s disease (PD) and stroke [5–11]. Conversely, individuals who are sedentary, possess the over-nourished metabolic phenotype and are not intellectually engaged (the so-called ‘couch potato’ lifestyle) are at increased risk of AD and stroke and a host of other comorbidities [4, 12, 13].
Neurons regularly experience metabolic and oxidative stress, which results in part from increased activation of excitatory (glutamatergic) synapses. Emerging findings suggest that the cellular and molecular mechanisms by which dietary energy restriction, exercise and intellectual challenges bolster neuronal health and resilience involve activation of cellular signaling pathways that counteract metabolic, oxidative and proteotoxic stress [14]. The pathways include those involving transcription factors such as cyclic AMP response element-binding protein (CREB), NF-κB and Nrf-2 [15–17]. Gene targets of these transcription factors include neurotrophic factors such as brain-derived neurotrophic factor (BDNF), DNA repair enzymes, antioxidant enzymes, and lysine deacetylases [18–20]. In addition, mild bioenergetic challenges stimulate the removal of damaged proteins and mitochondria via autophagy/mitophagy [21, 22].
Cells, organ systems and organisms have evolved multiple integrated signaling mechanisms by which they respond adaptively to stress so as to enhance their ability to tolerate more severe stress. A major driving force for evolution of nervous systems has been the need to obtain food/energy to support survival and reproduction. Accordingly, brains and bodies have evolved so as to function well when the individual is hungry, thereby maximizing their chances of outwitting their competitors [4]. Studies of laboratory animals and human subjects support the notion that intermittent bioenergetic challenges, such as extended periods of time without food and vigorous physical exertion, bolster brain function and stress resistance [1]. This is a prominent example of the biological principle of hormesis, which is characterized by a biphasic dose response curve wherein low to moderate levels of a potentially damaging agent or condition trigger beneficial physiological responses [23] (Figure 1). It is well known that exposure of cultured cells and animals to conditions that cause mild metabolic stress can increase their resistance not only to more severe metabolic stress, but also to oxidative stress and other types of stress. For example, exposure of cultured neurons and mice to 2-deoxyglucose, a non-metabolizable analog of glucose that limits cellular glucose availability, protects neurons against oxidative stress, excitotoxicity and ischemic stroke [24, 25]. The neuroprotective action of 2-deoxyglucose involves induction of expression of the protein chaperones GRP-78 (glucose regulated protein 78) and HSP-70 (heat shock protein 70) [24].
Figure 1.
Neuronal responses to mitochondrial uncoupling are consistent with a hormesis-based mechanism of action. As is true with many processes in biology and medicine, the dose – response curve for mitochondrial uncoupling is biphasic, with mild uncoupling eliciting beneficial adaptive responses and high levels of uncoupling causing cell damage and death. Mild uncoupling may enhance neuronal resilience by activating signaling pathways that promote synaptic plasticity, reduce oxidative damage, increase autophagy and bolster bioenergetics. Some of the proteins that may mediate the hormetic effects of mild uncoupling are brain derived neurotrophic factor (BDNF), cyclic AMP response element-binding protein (CREB), superoxide dismutase 2 (SOD2) and glucose transporter 3 (GLUT3). Excessive sustained uncoupling can trigger cell death which is mediated by pro-apoptotic proteins such as p53 and BAX.
Mitochondria not only generate ATP, but also play important roles in synaptic signaling and plasticity, and as mediators of adaptive responses to cellular stress [26]. Among the signaling pathways that affect mitochondrial function are those that play fundamental roles in synaptic plasticity and learning and memory, including glutamate and BDNF (both a neurotrophin and a myokine). For example, glutamate-induced Ca2+ influx results in mitochondrial Ca2+ uptake which in turn affects electron transport chain activity and can stimulate superoxide production [27, 28]. In addition, activation of transcription factors such as CREB and PGC-1α by glutamate and BDNF signaling can stimulate mitochondrial biogenesis and the expression of SIRT3, a lysine deacetylase that protects mitochondria against oxidative and metabolic stress [20, 29]. The latter studies provided evidence that mitochondrial biogenesis plays a critical role in the formation and maintenance of synapses, and that SIRT3 mediates beneficial effects of exercise (running) on neuronal resilience. Conditions which result in mild bioenergetic and oxidative stress in mitochondria can protect neurons against more severe stress. For example, inhibition of the mitochondrial ATP synthase (Complex V) results in resistance of neurons to excitotoxic damage [30]. Neuroprotective effects of metabolic and oxidative preconditioning also involve enhanced mitochondrial robustness which is associated with increased expression of multiple mitochondrial stress resistance proteins including SOD2 and Bcl-2 [31]. These kinds of findings suggest that bolstering mitochondrial stress resistance may be an effective therapeutic avenue for preventing neuronal death and improving function outcome in a range of neurodegenerative disorders that involve metabolic and oxidative cellular stress.
Mitochondrial Uncoupling Proteins and Neuroprotection
Animals have evolved nuclear DNA-encoded mitochondrial uncoupling proteins (UCPs), which may serve multiple roles in regulating cellular metabolism and signaling processes. The first UCP discovered, UCP1, is expressed in brown fat cells where its function is to cause heat production in mammals living in cold environments [32]. However, humans have ~50-fold less brown fat than rodents, and UCP1 therefore plays a negligible role in thermogenesis in human [13]. However the UCP1 homologues, UCP2, UCP3, UCP4 and UCP5 (BMCP1), are more widely expressed and have roles in the mitochondria related to cellular stress adaptation [33]. Neurons express little or no UCP1 and, instead, express UCP2, UCP4 and UCP5 [34]. The latter UCPs are activated by fatty acid and free radicals, inhibited by purine nucleotides, and their expression can be induced by a variety of metabolic and oxidative challenges including exercise and dietary energy restriction [34, 35]. The transcription factors that regulate the expression of neuronal UCPs have not been established; cyclic AMP response element-binding protein (CREB) is a likely candidate as it mediates induction of UCP1 expression in adipocytes [36], and because it mediates adaptive responses of neurons to metabolic and excitatory challenges [37].
Cell culture and in vivo studies have provided evidence that UCP2, UCP4 and UCP5 play important roles in adaptive responses of neurons to bioenergetic and oxidative stress, and that these UCPs can prevent the death of neurons in experimental models relevant to both acute brain injuries and neurodegenerative disorders. Cultured mouse cortical neurons overexpressing UCP2 exhibited resistance to death induced by deprivation of oxygen and glucose, and transgenic mice overexpressing UCP2 exhibited less brain damage and improved functional recovery in models of ischemic stroke and traumatic brain and peripheral nerve injury [38, 39]. Ischemic preconditioning induced the expression of UCP2 in brain cells and this was associated with neuroprotection in an animal model of ischemia/reperfusion brain injury [40]. Data further suggest that UCP2 can protect neurons against excitotoxicity and traumatic injury [41–43]. A study of peripheral sensory neurons showed that hyperglycemia down-regulates UCP3 expression, and that overexpression of UCP3 or UCP1 protected the neurons against damage caused by chronic hyperglycemia, suggesting a potential role for UCPs in diabetic peripheral neuropathy [44]. Transgenic mice overexpressing UCP2 selectively in catecholaminergic neurons exhibited resistance of neurons in their substantia nigra to death induced by the neurotoxin MPTP/MPP+ in a model of Parkinson’s disease [45]. Similarly, overexpression of UCP4 [46] or UCP5 [47] protects dopaminergic neurons against MPP+ toxicity by a mechanism involving reduced oxidative stress and preservation of mitochondrial membrane potential. Collectively these findings document roles for UCPs in protecting neurons against metabolic, oxidative and excitotoxic stress.
The actions of UCPs in mitochondria can influence multiple signaling pathways. Dysregulation of cellular Ca2+ signaling and homeostasis is believed to play a major role in the early synaptic dysfunction and subsequent neuronal degeneration that occurs in both acute brain injuries (e.g., stroke and traumatic brain injury) and in Alzheimer’s and Parkinson’s diseases [48]. Neural cells overexpressing UCP4 exhibit reduced Ca2+ accumulation in the cytosol when challenged with thapsigargin, an agent that causes Ca2+ release from the endoplasmic reticulum [49]. The latter study showed that UCP4 attenuates mitochondrial Ca2+ accumulation and oxidative stress, which contributes to neuroprotection. One mechanism by which UCPs may regulate mitochondrial Ca2+ dynamics is by interacting with the mitochondrial Ca2+ uniporter, as suggested by experiments showing that UCP2 increases uniporter Ca2+ currents [50]. In addition to affects on cellular Ca2+ signaling, neuronal UCPs may also influence other prominent signaling pathways including the cyclic AMP pathway. Thus, overexpression of UCP2 protected cultured primary dopaminergic neurons against the mitochondrial Complex I inhibitor rotenone, by a mechanism involving cyclic AMP-dependent protein kinase [51].
Although the activation of UCPs can result in a decrease in the amount of ATP produced by the individual mitochondrion, overall cellular ATP pools may be maintained or even increased. Several factors explain this bioenergetic adaptation. First, UCPs are typically upregulated/activated by free fatty acids (e.g., docosohexanoid acid, palmitoleic acid and butyric) that are mobilized in response to fasting and related metabolic conditions in which ketones such as β-hydroxybutyrate are also elevated [52]. The metabolism of free fatty acids and β-hydroxybutyrate to acetyl CoA results in generation of ATP via the citric acid cycle.
Second, conditions that enhance mitochondrial uncoupling also stimulate mitochondrial biogenesis resulting in an increase in the number of mitochondria in the cell [53]. In neurons, increased mitochondrial biogenesis may enable the formation and maintenance of new synapses in response to bioenergetic challenges such as exercise and fasting [29]. Thus, by lessening activity of the mitochondrial electron transport chain, and increasing ATP generation via alternative pathways, UCPs can reduce mitochondrial free radical production will sustaining cellular bioenergetics. Interestingly, it was shown in a model of Alzheimer’s disease that a rise of isoprostane levels, a biomarker of membrane-associated oxidative stress, was significantly elevated in blood/urine 4-months prior to that of plaque formation, suggesting that mitochondrial oxidative stress occurs early and upstream of amyloid pathology [54].
Chemical uncouplers and neuroprotection
Emerging findings have revealed the therapeutic potential of pharmacological agents that induce mild mitochondrial uncoupling (release of protons into the mitochondrial matrix) in a range of acute and chronic neurodegenerative conditions. The most widely studied and consistently effective uncoupling agent in experimental models of neurodegenerative conditions is 2,4-dinitrophenol (DNP). Treatment of cultured cortical neurons with low levels of DNP (1 – 3 μM) protected them against oxygen and glucose deprivation [38]. Enhancing respiratory rates by mild uncoupling: 1) reduces the formation of superoxide radical anions (O2−) by decreasing O2 tension in the microenvironment; 2) favors an oxidized state of respiratory chain intermediates, such those in Complexes I and III (the major sites of O2−. production);, 3) suppresses NADH levels and thereby prevents ROS formation by mitochondrial matrix flavoproteins; and 4) lowers membrane potential, which inhibits reverse flow of electrons from Complex II to I [55]. The reduction in mitochondrial membrane potential in response to DNP transiently increases cytosolic, Ca2+ levels but lowers intra-mitochondrial Ca2+ levels, thereby reducing levels of oxidative stress in cultured rat cerebral cortical neurons [56]. Increased intra-mitochondrial Ca2+ levels is associated with the loss of dystrophin in children with Duchenne’s muscular dystrophy and diseases associated with the endoplasmic reticulum unfolded protein stress response, which can result in opening of the mitochondrial transition pore and consequent apoptosis [57, 58].
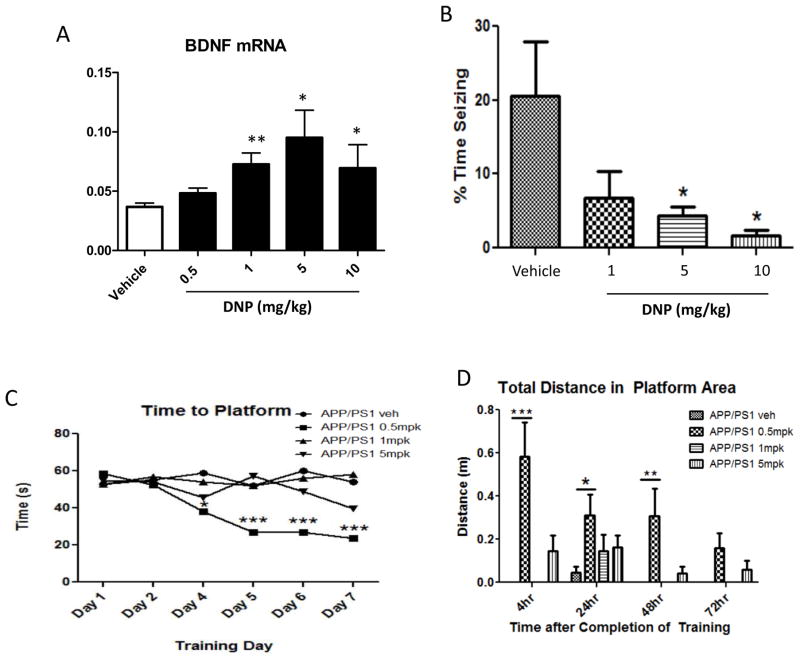
The ability of DNP to lower mitochondrial free radical generation and prevent mitochondrial Ca2+ accumulation could provide a therapeutic benefit in disorders involving cellular calcium overload. Preconditioning of the isolated perfused heart with DNP protected myocardial cells against ischemic injury [59, 60]. Administration of only one dose of DNP (5 mg/kg) to rats after 2 hours of middle cerebral artery occlusion and a subsequent 1 hour of reperfusion to mimic stroke in humans, resulted in a 40% reduction of infarct volume and bolstered indicators of mitochondrial health [61]. This is an example where mild pharmacological uncoupling protects threatened tissue in the ischemica penumbra by bolstering their stress resistance, and could also be beneficial for preserving tissue in burn victims [62]. When pretreated with DNP (100 nM), cultured rat substantia nigra dopaminergic neurons exhibited resistance to the toxicity of the mitochondrial Complex I inhibitor rotenone [63]. When mice were treated with DNP (5 mg/kg) once each day for 14 days, their performance on a learning and memory test was significantly enhanced compared to vehicle-treated control mice [56]. DNP induced a dose-dependent increase in levels of the mRNA encoding BDNF in the hippocampus of mice, with 1 and 5 mg/kg being the most effective doses (Figure 3A). Low concentrations of DNP stimulated cyclic AMP (cAMP) production, Tau expression and neurite outgrowth in cultured neural cells [64]. Analyses of gene expression in the cerebral cortex of mice treated with 5 mg/kg DNP demonstrated induction of the immediate early gene Arc, and genes involved in Ca2+/calmodulin, cAMP and CREB signaling, BDNF signaling and autophagy [57]. In addition, data in the latter study suggest that DNP treatment reduces activities of the mTOR and insulin signaling pathways. Collectively, these findings are entirely consistent with a hormesis-based neuroprotective mechanism of action of DNP in which mild mitochondrial uncoupling stimulates a coordinated adaptive molecular response that bolsters neuronal resistance to metabolic, oxidative and excitotoxic stress (Figure 2).
Figure 3.
Treatment of mice with low doses of the uncoupler DNP results in increased expression of BDNF in the brain and improves functional outcomes in models of epileptic seizures and Alzheimer’s disease (AD). A. Mice were administered the indicated doses of DNP or vehicle by oral gavage once daily for 14-days. BDNF mRNA levels in cortex tissue samples were measured by semi-quantitative PCR (qPCR). Values are the mean and SEM of measurements made on samples from 8 mice/group. *p<0.05, **p<0.01 compared to the value for vehicle-treated mice. B. Mice were administered the indicated doses of DNP or vehicle by oral gavage once daily for 7-days. Mice were then administered the seizure-inducing excitotoxin kainic acid by direct injection into the dorsal hippocampus [99]. Seizures were evaluated during the ensuing 4 hours using a semi-quantitative rating scale as described previously [100]. Values are the mean and SEM (6 mice/group). *p<0.01 compared to the value for vehicle-treated mice. C and D. APP/PS1 double mutant transgenic mice (an animal model of AD) were administered the indicated doses of DNP (mpk, mg/kg) or vehicle by oral gavage once daily for 4 months. Hippocampus-dependent spatial learning and memory were then evaluated in a water maze test in which the time taken to locate a submerged platform in the pool (goal latency; memory acquisition) was measured daily for 7 days of training (C). The platform was then removed from the pool and the total distance the mouse swam in the specific area where the platform had been was determined at 4, 24, 48 and 72 hours (an indicator of memory retention) (D). See ref. 101 for methods. Values are the mean and SEM of measurements made on samples from 5 or 6 mice/group. *p<0.01, **p<0.01, ***p<0.001.
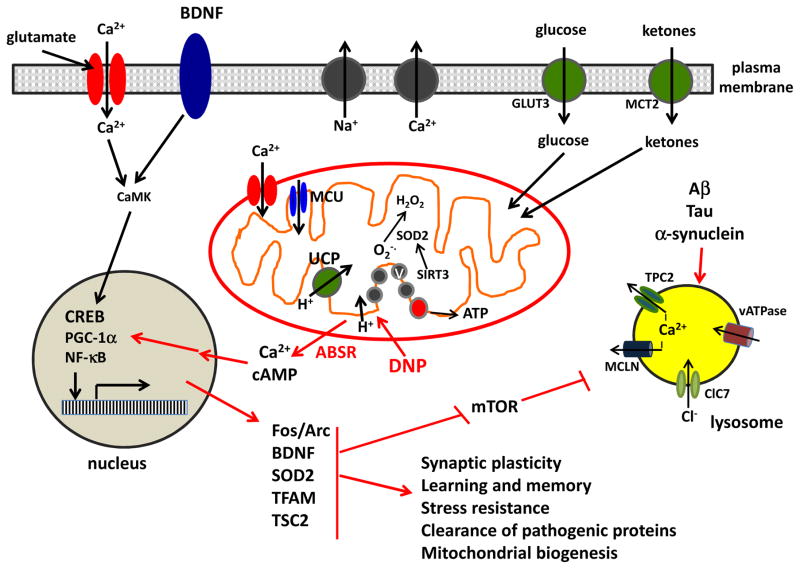
Figure 2.
Mechanisms by which mild mitochondrial uncoupling protects neurons against oxidative stress, excitotoxicity and the accumulation of disease-related self-aggregating proteins such as amyloid β-peptide, Tau and α-synuclein. Mild uncoupling resulting from activation of endogenous uncoupling proteins (UCP) or pharmacological agents such as 2,4-dinitrophenol (DNP) triggers an adaptive bioenergetic stress response (ABSR) involving multiple signaling pathways and organelles. The ABSR involves activation of kinases, and transcription factors such as CREB, PGC-1α and NF-kB which, in turn, induce the expression of genes encoding proteins that enhance stress resistance and neuroplasticity including: the immediate early gene products Fos and Arc; the neurotrophic factor BDNF; the antioxidant enzyme SOD2; the regulator of mitochondrial biogenesis TFAM; an inhibitor of the mTOR pathway (TSC2). CamK, calcium/calmodulin-dependent kinase; ClC7, chloride channel 7; GLUT3, glucose transporter 3; MCLN, mucolipin; TPC2, two pore channel 2; MCT2, monocarboxylic acid transporter 2; vATPase, vesicular ATPase.
Other uncoupling agents that have been reported to exhibit neuroprotective efficacy in one or more models include FCCP and diazoxide [65–67]. Treatment of cultured cerebellar granule neurons to a low concentration of FCCP (100 nM) increased glucose transport, activated AMPK and conferred resistance of the cells to excitotoxicity [68]. Low doses of FCCP were also reported to protect cardiac cells against ischemic injury [69]. Diazoxide, an agent that opens mitochondrial ATP-sensitive K+ channels has been reported to be neuroprotective in experimental models of Alzheimer’s disease and stroke, by hormesis-based mechanisms [65, 70]. A component of diazoxide’s neuroprotective action may involve mitochondrial uncoupling [71, 72].
The relative merits of interventions that up-regulate UCP expression versus treatment with pharmacological uncouples will be important to evaluate in future studies. Several interventions have been shown to upregulate the expression of one or more UCPs in brain cells. Running wheel exercise induced the expression of UCP2 in the hippocampus of rats [73]. Studies of UCP2 knockout mice showed that UCP2 is required for exercise to increase the number of synapses on dendrites of CA1 pyramidal neurons and dentate granule neurons in the hippocampus [74]. Caloric restriction and fasting also stimulate the expression of UCPs. For example, caloric restriction increased the expression of UCP4 in the cerebral cortex of adult rats [35]. Consistent with the general notion that UCP expression is increased in response to physiological metabolic challenges such as exercise and dietary energy restriction, exposure of animals to mild hypoxia increases the expression of UCP2 in the cerebral cortex and hippocampus [75]. A ketogenic diet has also been reported to increase UCP2 expression in the brain [76]. Interestingly, as is the case with UCP1 in brown fat cells, cold temperatures induce the expression of UCP4 in neurons [35]. There are certainly advantages of exercise and energy restriction as a means of promoting mild mitochondrial uncoupling in neurons. Exercise and energy restriction have far-reaching beneficial effects on organ systems that involve multiple highly integrated, evolutionarily-conserved cellular and molecular mechanisms [4]. With regards to brain function and neurological disorders, there is abundant evidence that exercise and energy restriction improve cognitive function and mood, and may reduce the risk of AD, PD and stroke [1]. However, many individuals live a sedentary overindulgent lifestyle and are unwilling or no longer physically able to commit to more healthy lifestyles. It is for such individuals that pharmacological approaches to inducing mild mitochondrial uncoupling, as with low doses of DNP, that treatment may be particularly beneficial.
The Phoenix of Uncoupling Agents: Development of DNP as a Neurohormetic Drug
Several commonly prescribed drugs and some dietary phytochemicals are toxic and even lethal when ingested in high doses. For example, prescription opioid overdoses caused nearly 15,000 deaths in the United States in 2008, and there has been an alarming increase in opioid overdose deaths in the past few years [77, 78]. Many patients also die from overdoses of other types of drugs including benzodiazepines [79]. Even widely consumed phytochemicals such as caffeine can be toxic and lethal when consumed in high amounts, an emerging health concern resulting from the proliferation of caffeine-laden “high energy” sports drinks and supplements [80]. It is therefore widely understood that most drugs and many natural products exhibit a ‘therapeutic window’ for clinical efficacy that, if exceeded, can result in adverse events, including death. Here we summarize the human experience with DNP, the notorious weight loss agent from the 1930’s that acts as a protonophore that allows H+ to leak across the inner mitochondrial membrane, and is therefore a “mitochondrial uncoupler” [13].
Before considering the past and potential future clinical applications of DNP, it is instructive to consider a different example of the development of a widely used and effective drug that exhibits serious adverse effects at high doses. The origin of warfarin as a possible anti-coagulant can be traced to the 1920’s when entire farms lost their herds due to consuming spoiled silage called “sweet clover”, which resulted in uncontrolled bleeding after common procedures such as dehorning [81]. In the 1930s the ingredient causing the bleeding, warfarin, was extracted and then used for rodent control. However, in the 1950s, warfarin was brought forward as a new treatment as a blood thinner, but it was not an easy path to the clinic due to the many years prior being used as a rat killer. Dr. Link writes “the transition to a substance originally promoted to exterminate rats and mice was a bit more than they (clinicians) could accept with real enthusiasm”. The fortunate fate in 1951 of a newly enlisted soldier’s failed attempt to commit suicide by ingesting a concentrated form of warfarin [82], provided the catalyst to finally move forward into the clinic using moderate doses of warfarin as a blood thinner [83]. Warfarin (Coumadin) became an approved drug and is still used today as the mainline therapy for protection against stroke. The Warfarin story provides a compelling example of the repositioning of an old drug with a tainted past just by reducing the dose to an amount that is within the disease-modifying hormetic range. Another example is the case of repositioning of the notorious drug, thalidomide. Thalidomide was provided as an unapproved “experimental” drug to completely uninformed pregnant women as a sleep/nausea aide, and caused horrible birth defects [84]. Now thalidomide and related analogs are effective disease-modifying treatments for cancer and other serious indications [85].
In an article published in 1891, Gibbs and Reichert reported that a high dose of DNP (300 mg/kg) was toxic to dogs, which was associated with pyrexia and rapid postmortem onset of rigor mortis (Figure 3). DNP was used in the manufacture of explosives during World War I and clinical observations and studies of animal models established that it induced heat production [86]. It was reported in 1933 by Cutting et al. that once daily oral doses of 3 or 5 mg/kg DNP increased the metabolic rate of overweight patients by 40%; all subjects lost weight (typically 2–3 pounds/week) with no evidence of adverse effects during a 3 month treatment period [87]. Within one year of that report, DNP had been used by more than 100,000 patients in the United States. At that time only two deaths attributable to DNP had been reported, with both cases involving individuals who consumed over 10 times the recommended dose (i.e., more than 50 mg/kg). No adverse effects of therapeutic doses of DNP on the cardiovascular, renal, gastrointestinal or hepatic systems were noted by Tainter et al. [88]. Evidence that DNP promotes cataract formation and rashes then emerged [89] which, together with the potential for lethal overdose, prompted the FDA to ban its use as a prescription drug. However, DNP continues to be used by bodybuilders and some athletes to enhance fat loss [90]. In support of potential health benefits of low doses of DNP, it was recently shown that DNP can protect mice against diet-induced obesity and hepatic steatosis, while improving glucose tolerance [91].
At the time of its development as a thermogenic weight loss-promoting drug in 1930s, it was not known that mitochondrial uncoupling is a normal physiological process that mediates cellular responses to environmental challenges or that it mimics the naturally occurring phenomenon of “proton leak” by which the body loses ~30% of its energy to heat [92]. Indeed, the first mitochondrial uncoupling protein UCP1 was not discovered until four decades later [93]. Only within the most recent 20 years have the UCPs expressed by neurons been established and their roles in neuroplasticity and neuroprotection have begun to be understood (see above). In addition to reports of neuroprotection and improved functional outcome in experimental models of stroke and Parkinson’s disease [63, 65], DNP can protect mice against seizures induced by the excitotoxin kainic acid (Figure 3B). Moreover, data from a study of a mouse model of Alzheimer’s disease (APP/PS1 double mutant transgenic mice) demonstrated that daily administration of a very low dose of DNP (0.5 mg/kg) for 4 months ameliorated spatial learning and memory deficits in a water maze task, with striking results on short-term memory (Figure 3C and D). These kinds of findings suggest that DNP may have potential applications for a host of insidious neurodegenerative diseases at weight neutral doses due to its hormesis-based effects found at low doses (Figures 1 and 2).
The development of uncoupling agents with improved safety profiles are being developed for obesity, diabetes and neurodegenerative disorders. For example, a controlled release oral form of DNP that produces mild uncoupling in hepatocytes reduced insulin resistance, hyperlipidemia and hepatic steatosis in a rat model of diabetes [94]. No evidence of toxicity was detected during chronic administration of the controlled release DNP. In another study, a DNP analog (DNP-methyl ether) targeted to liver was shown to reverse insulin resistance and fatty liver in rats fed a high-fat diabetogenic diet [95]. From a drug development perspective, perhaps it is time for a paradigm shift away from the lofty goals of weight loss per se with all the historic and recent hazards, to a “wellness program” using weight neutral steady state delivery of very low doses of uncoupling agents such as DNP. Since over-nutrition is not only associated in metabolic syndrome, but also with a significant increase in the incidence of neurodegenerative diseases, an uncoupling agent that mimics caloric restriction by lowering intra-hepatic, intra-muscular and circulating lipids may provide a therapeutic benefit. As proof-of-concept it was reported that mice chronically treated for ~80 weeks with DNP provided in their drinking water at a dose of ~100 μg/kg lived longer than control mice and had reduced levels of ROS in both liver and brain, lower amounts of oxidized proteins and DNA damage, and lower circulating glucose, lipid and insulin levels [87]. To put this into perspective, this is the equivalent to humans of ~0.5 mg per day or ~600-fold lower dose than what was used in the 1930s for weight loss (300 mg per day). The intermittent drinking of water containing DNP mimicked sustained delivery, and since DNP lacks a methyl-ether that would cause it to be sequestered in the liver, the drug distributes to cells in all organs. The findings in mice suggest that a very low sustained level of DNP can attenuate the age-related accumulation intra-hepatic and intra-muscular lipids, without reducing body weight [96]. It will therefore be of considerable interest to determine whether very low doses of a mitochondrial uncoupling agent such as DNP would ameliorates metabolic morbidities resulting from excessive calorie intake and a sedentary lifestyle in human subjects.
The theme that is building is that low doses of DNP provide broad neuroprotection, perhaps due to its unique mechanism of action initiated as an adaptive stress response, and its specificity to the mitochondria, the only cellular organelle with a basic pH environment. Indeed, the target of chemical uncouplers such as DNP is not a protein, but is instead the mitochondrial membrane where they transfer protons to the matrix [13]. Although DNP’s initial uncoupling action is non-genomic, several prominent signaling cascades involved in adaptive neuroplasticity and stress resistance are activated including those involving calcium and cyclic AMP, and downstream kinases and transcription factors (Figure 2) [56, 97].
During the 80 years after the initial use of DNP in humans at very high doses for inducing weight loss in obese subjects, the knowledge base of mitochondrial bioenergetics and chemical uncoupling has expanded greatly. It was not known until recently, however, that very low doses of uncoupling agents such as DNP are effective in ameliorating disease processes and improving functional outcome in preclinical models of a range of neurological disorders that involve metabolic and oxidative stress including Alzheimer’s, Parkinson’s diseases, epilepsy and ischemic stroke [61, 65, 98] (Figure 4). The emerging findings described in this article suggest that, similar to the broadly beneficial effects of caloric restriction, even very low levels of mitochondrial uncoupling can protect multiple organ systems against dysfunction and degeneration in preclinical models of a wide range of disorders that involve dysregulation of energy metabolism metabolic and oxidative stress. Translation to humans is the next critical step.
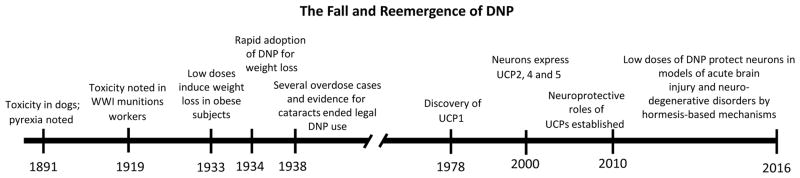
Figure 4.
Historical timeline of studies of DNP dose-dependent clinical efficacy and toxicity, mechanisms of action, and potential applications to acute and chronic neurodegenerative conditions.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Aging, and by Mitochon Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012;16:706–722. doi: 10.1016/j.cmet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnard ND, Bush AI, Ceccarelli A, Cooper J, de Jager CA, Erickson KI, Fraser G, Kesler S, Levin SM, Lucey B, Morris MC, Squitti R. Dietary and lifestyle guidelines for the prevention of Alzheimer’s disease. Neurobiol Aging. 2014;35:S74–78. doi: 10.1016/j.neurobiolaging.2014.03.033. [DOI] [PubMed] [Google Scholar]
- 3.Yaffe K, Hoang TD, Byers AL, Barnes DE, Friedl KE. Lifestyle and health-related risk factors and risk of cognitive aging among older veterans. Alzheimers Dement. 2014;10:S111–121. doi: 10.1016/j.jalz.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Mattson MP. Lifelong brain health is a lifelong challenge: from evolutionary principles to empirical evidence. Ageing Res Rev. 2015;20:37–45. doi: 10.1016/j.arr.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duan W, Mattson MP. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson’s disease. J Neurosci Res. 1999;57:195–206. doi: 10.1002/(SICI)1097-4547(19990715)57:2<195::AID-JNR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 6.Halagappa VK, Guo Z, Pearson M, Matsuoka Y, Cutler RG, Laferla FM, Mattson MP. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2007;26:212–220. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 7.Ploughman M, Attwood Z, White N, Doré JJ, Corbett D. Endurance exercise facilitates relearning of forelimb motor skill after focal ischemia. Eur J Neurosci. 2007;25:3453–3460. doi: 10.1111/j.1460-9568.2007.05591.x. [DOI] [PubMed] [Google Scholar]
- 8.Yuede CM, Zimmerman SD, Dong H, Kling MJ, Bero AW, Holtzman DM, Timson BF, Csernansky JG. Effects of voluntary and forced exercise on plaque deposition, hippocampal volume, and behavior in the Tg2576 mouse model of Alzheimer’s disease. Neurobiol Dis. 2009;35:426–432. doi: 10.1016/j.nbd.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tapia-Rojas C, Aranguiz F, Varela-Nallar L, Inestrosa NC. Voluntary running attenuates memory loss, decreases neuropathological changes and induces neurogenesis in a mouse model of Alzheimer’s disease. Brain Pathol. 2016;26:62–74. doi: 10.1111/bpa.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arumugam TV, Phillips TM, Cheng A, Morrell CH, Mattson MP, Wan R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann Neurol. 2010;67:41–52. doi: 10.1002/ana.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zigmond MJ, Smeyne RJ. Exercise: is it a neuroprotective and if so, how does it work? Parkinsonism Relat Disord. 2014;20:S123–127. doi: 10.1016/S1353-8020(13)70030-0. [DOI] [PubMed] [Google Scholar]
- 12.Akinyemi RO, Mukaetova-Ladinska EB, Attems J, Ihara M, Kalaria RN. Vascular risk factors and neurodegeneration in ageing related dementias: Alzheimer’sdisease and vascular dementia. Curr Alzheimer Res. 2013;10:642–653. doi: 10.2174/15672050113109990037. [DOI] [PubMed] [Google Scholar]
- 13.Geisler JG. Targeting energy expenditure via fuel switching and beyond. Diabetologia. 2011;54:237–244. doi: 10.1007/s00125-010-1932-4. [DOI] [PubMed] [Google Scholar]
- 14.Stranahan AM, Mattson MP. Recruiting adaptive cellular stress responses for successful brain ageing. Nat Rev Neurosci. 2012;13:209–216. doi: 10.1038/nrn3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattson MP, Meffert MK. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13:852–860. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh N, Ghosh R, Mandal SC. Antioxidant protection: A promising therapeutic intervention in neurodegenerative disease. Free Radic Res. 2011;45:888–905. doi: 10.3109/10715762.2011.574290. [DOI] [PubMed] [Google Scholar]
- 17.Cohen SM, Li B, Tsien RW, Ma H. Evolutionary and functional perspectives on signaling from neuronal surface to nucleus. Biochem Biophys Res Commun. 2015;460:88–99. doi: 10.1016/j.bbrc.2015.02.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marosi K, Mattson MP. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab. 2014;25:89–98. doi: 10.1016/j.tem.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang JL, Lin YT, Chuang PC, Bohr VA, Mattson MP. BDNF and exercise enhance neuronal DNA repair by stimulating CREB-mediated production of apurinic/apyrimidinic endonuclease 1. Neuromolecular Med. 2014;16:161–174. doi: 10.1007/s12017-013-8270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng A, Yang Y, Zhou Y, Maharana C, Lu D, Peng W, Liu Y, Wan R, Marosi K, Misiak M, Bohr VA, Mattson MP. Mitochondrial SIRT3 mediates adaptive responses of neurons to exercise and metabolic and excitatory challenges. Cell Metab. 2016;23:128–142. doi: 10.1016/j.cmet.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alirezaei M, Kemball CC, Flynn CT, Wood MR, Whitton JL, Kiosses WB. Short-term fasting induces profound neuronal autophagy. Autophagy. 2010;6:702–710. doi: 10.4161/auto.6.6.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pallanck LJ. Culling sick mitochondria from the herd. J Cell Biol. 2011;191:1225–1227. doi: 10.1083/jcb.201011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calabrese EJ, Bachmann KA, Bailer AJ, Bolger PM, Borak J, Cai L, Cedergreen N, Cherian MG, Chiueh CC, Clarkson TW, Cook RR, Diamond DM, Doolittle DJ, Dorato MA, Duke SO, Feinendegen L, Gardner DE, Hart RW, Hastings KL, Hayes AW, Hoffmann GR, Ives JA, Jaworowski Z, Johnson TE, Jonas WB, Kaminski NE, Keller JG, Klaunig JE, Knudsen TB, Kozumbo WJ, Lettieri T, Liu SZ, Maisseu A, Maynard KI, Masoro EJ, McClellan RO, Mehendale HM, Mothersill C, Newlin DB, Nigg HN, Oehme FW, Phalen RF, Philbert MA, Rattan SI, Riviere JE, Rodricks J, Sapolsky RM, Scott BR, Seymour C, Sinclair DA, Smith-Sonneborn J, Snow ET, Spear L, Stevenson DE, Thomas Y, Tubiana M, Williams GM, Mattson MP. Biological stress response terminology: integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol. 2007;222:122–128. doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Lee J, Bruce-Keller AJ, Kruman Y, Chan SL, Mattson MP. 2-Deoxy-D-glucose protects hippocampal neurons against excitotoxic and oxidative injury: evidence for the involvement of stress proteins. J Neurosci Res. 1999;57:48–61. doi: 10.1002/(SICI)1097-4547(19990701)57:1<48::AID-JNR6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 25.Yu ZF, Mattson MP. Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: evidence for a preconditioning mechanism. J Neurosci Res. 1999;57:830–839. [PubMed] [Google Scholar]
- 26.Mattson MP, Gleichmann M, Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60:748–766. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hongpaisan J, Winters CA, Andrews SB. Strong calcium entry activates mitochondrial superoxide generation, upregulating kinase signaling in hippocampal neurons. J Neurosci. 2004;24:10878–10887. doi: 10.1523/JNEUROSCI.3278-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Z, Ji G, Neugebauer V. Mitochondrial reactive oxygen species are activated by mGluR5 through IP3 and activate ERK and PKA to increase excitability of amygdala neurons and pain behavior. J Neurosci. 2011;31:1114–1127. doi: 10.1523/JNEUROSCI.5387-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng A, Wan R, Yang JL, Kamimura N, Son TG, Ouyang X, Luo Y, Okun E, Mattson MP. Involvement of PGC-1α in the formation and maintenance of neuronal dendritic spines. Nat Commun. 2012;3:1250. doi: 10.1038/ncomms2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Formentini L, Pereira MP, Sánchez-Cenizo L, Santacatterina F, Lucas JJ, Navarro C, Martínez-Serrano A, Cuezva JM. In vivo inhibition of the mitochondrial H+-ATP synthase in neurons promotes metabolic preconditioning. EMBO J. 2014;33:762–778. doi: 10.1002/embj.201386392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dirnagl U, Meisel A. Endogenous neuroprotection: mitochondria as gateways to cerebral preconditioning? Neuropharmacology. 2008;55:334–344. doi: 10.1016/j.neuropharm.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 32.Golozoubova V, Hohtola E, Matthias A, Jacobsson A, Cannon B, Nedergaard J. Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB J. 2001;15:2048–2050. doi: 10.1096/fj.00-0536fje. [DOI] [PubMed] [Google Scholar]
- 33.Giralt M, Villarroya F. Mitochondrial uncoupling and the regulation of glucose homeostasis. Curr Diabetes Rev. 2016 Feb 17; doi: 10.2174/1573399812666160217122707. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Andrews ZB, Diano S, Horvath TL. Mitochondrial uncoupling proteins in the CNS: in support of function and survival. Nat Rev Neurosci. 2005;6:829–840. doi: 10.1038/nrn1767. [DOI] [PubMed] [Google Scholar]
- 35.Liu D, Chan SL, de Souza-Pinto NC, Slevin JR, Wersto RP, Zhan M, Mustafa K, de Cabo R, Mattson MP. Mitochondrial UCP4 mediates an adaptive shift in energy metabolism and increases the resistance of neurons to metabolic and oxidative stress. Neuromolecular Med. 2006;8:389–414. doi: 10.1385/NMM:8:3:389. [DOI] [PubMed] [Google Scholar]
- 36.Rim JS, Kozak LP. Regulatory motifs for CREB-binding protein and Nfe2l2 transcription factors in the upstream enhancer of the mitochondrial uncoupling protein 1 gene. J Biol Chem. 2002;277:34589–34600. doi: 10.1074/jbc.M108866200. [DOI] [PubMed] [Google Scholar]
- 37.Bengtson CP, Bading H. Nuclear calcium signaling. Adv Exp Med Biol. 2012;970:377–405. doi: 10.1007/978-3-7091-0932-8_17. [DOI] [PubMed] [Google Scholar]
- 38.Mattiasson G, Shamloo M, Gido G, Mathi K, Tomasevic G, Yi S, Warden CH, Castilho RF, Melcher T, Gonzalez-Zulueta M, Nikolich K, Wieloch T. Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat Med. 2003;9:1062–1068. doi: 10.1038/nm903. [DOI] [PubMed] [Google Scholar]
- 39.da Costa RF, Martinez AM, Ferreira ST. 2,4-Dinitrophenol blocks neurodegeneration and preserves sciatic nerve function after trauma. J Neurotrauma. 2010;27:829–841. doi: 10.1089/neu.2009.1189. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Chen L, Xu X, Vicaut E, Sercombe R. Both ischemic preconditioning and ghrelin administration protect hippocampus from ischemia/reperfusion and upregulated uncoupling protein 2. BMC Physiol. 2009;9:17. doi: 10.1186/1472-6793-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sullivan PG, Dube C, Dorenbos KD, Steward O, Baram TZ. Mitochondrial uncoupling protein-2 protects the immature brain from excitotoxic neuronal death. Ann Neurol. 2003;53:711–717. doi: 10.1002/ana.10543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sullivan PG, Springer JE, Hall ED, Scheff SW. Mitochondrial uncoupling as a therapeutic target following neuronal injury. J Bioenerg Biomembr. 2004a;36:353–356. doi: 10.1023/B:JOBB.0000041767.30992.19. [DOI] [PubMed] [Google Scholar]
- 43.Mattiasson G, Sullivan PG. The emerging roles of UCP2 in health and disease. Antiox Redox Signal. 2006;8:1–38. doi: 10.1089/ars.2006.8.1. [DOI] [PubMed] [Google Scholar]
- 44.Vincent AM, Olzmann JA, Brownlee M, Sivitz WI, Russell JW. Uncoupling proteins prevent glucose-induced neuronal oxidative stress and programmed cell death. Diabetes. 2004;53:726–734. doi: 10.2337/diabetes.53.3.726. [DOI] [PubMed] [Google Scholar]
- 45.Conti B, Sugama S, Lucero J, Winsky-Sommerer R, Wirz SA, Maher P, Andrews Z, Barr AM, Morale MC, Paneda C, Pemberton J, Gaidarova S, Behrens MM, Beal F, Sanna PP, Horvath T, Bartfai T. Uncoupling protein 2 protects dopaminergic neurons from acute 1,2,3,6-methyl-phenyl-tetrahydropyridine toxicity. J Neurochem. 2005;93:493–501. doi: 10.1111/j.1471-4159.2005.03052.x. [DOI] [PubMed] [Google Scholar]
- 46.Chu AC, Ho PW, Kwok KH, Ho JW, Chan KH, Liu HF, Kung MH, Ramsden DB, Ho SL. Mitochondrial UCP4 attenuates MPP+ - and dopamine-induced oxidative stress, mitochondrial depolarization, and ATP deficiency in neurons and is interlinked with UCP2 expression. Free Radic Biol Med. 2009;46:810–820. doi: 10.1016/j.freeradbiomed.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 47.Kwok KH, Ho PW, Chu AC, Ho JW, Liu HF, Yiu DC, Chan KH, Kung MH, Ramsden DB, Ho SL. Mitochondrial UCP5 is neuroprotective by preserving mitochondrial membrane potential, ATP levels, and reducing oxidative stress in MPP+ and dopamine toxicity. Free Radic Biol Med. 2010;49:1023–1035. doi: 10.1016/j.freeradbiomed.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 48.Mattson MP. Excitotoxic and excitoprotective mechanisms: abundant targets for the prevention and treatment of neurodegenerative disorders. Neuromolecular Med. 2003;3:65–94. doi: 10.1385/NMM:3:2:65. [DOI] [PubMed] [Google Scholar]
- 49.Chan SL, Liu D, Kyriazis GA, Bagsiyao P, Ouyang X, Mattson MP. Mitochondrial uncoupling protein-4 regulates calcium homeostasis and sensitivity to store depletion-induced apoptosis in neural cells. J Biol Chem. 2006;281:37391–37403. doi: 10.1074/jbc.M605552200. [DOI] [PubMed] [Google Scholar]
- 50.Bondarenko AI, Parichatikanond W, Madreiter CT, Rost R, Waldeck-Weiermair M, Malli R, Graier WF. UCP2 modulates single-channel properties of a MCU-dependent Ca(2+) inward current in mitochondria. Pflugers Arch. 2015;467:2509–2518. doi: 10.1007/s00424-015-1727-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hwang RD, Wiemerslage L, LaBreck CJ, Khan M, Kannan K, Wang X, Zhu X, Lee D, Fridell YW. The neuroprotective effect of human uncoupling protein 2 (hUCP2) requires cAMP-dependent protein kinase in a toxin model of Parkinson’s disease. Neurobiol Dis. 2014;69:180–191. doi: 10.1016/j.nbd.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 52.Davis LM, Rho JM, Sullivan PG. UCP-mediated free fatty acid uncoupling of isolated cortical mitochondria from fasted animals: correlations to dietary modulations. Epilepsia. 2008;49(Suppl 8):117–119. doi: 10.1111/j.1528-1167.2008.01854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 54.Pratico D, Uryu K, Leight S, Trojanoswki JQ, Lee VM. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci. 2001;21:4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE. Mitochondria and reactive oxygen species. Free Radic Biol Med. 2009;47:333–343. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 56.Liu D, Zhang Y, Gharavi R, Park HR, Lee J, Siddiqui S, Telljohann R, Nassar MR, Cutler RG, Becker KG, Mattson MP. The mitochondrial uncoupler DNP triggers brain cell mTOR signaling network reprogramming and CREB pathway up-regulation. J Neurochem. 2015;134:677–692. doi: 10.1111/jnc.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- 58.Stutzmann GE, Mattson MP. Endoplasmic reticulum Ca(2+) handling in excitable cells in health and disease. Pharmacol Rev. 2011;63:700–727. doi: 10.1124/pr.110.003814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Minners J, van den Bos EJ, Yellon DM, Schwalb H, Opie LH, Sack MN. Dinitrophenol, cyclosporin A, and trimetazidine modulate preconditioning in the isolated rat heart: support for a mitochondrial role in cardioprotection. Cardiovasc Res. 2000;47:68–73. doi: 10.1016/s0008-6363(00)00069-9. [DOI] [PubMed] [Google Scholar]
- 60.Hausenloy D, Wynne A, Duchen M, Yellon D. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation. 2004;109:1714–1717. doi: 10.1161/01.CIR.0000126294.81407.7D. [DOI] [PubMed] [Google Scholar]
- 61.Korde AS, Pettigrew LC, Craddock SD, Maragos WF. The mitochondrial uncoupler 2,4-dinitrophenol attenuates tissue damage and improves mitochondrial homeostasis following transient focal cerebral ischemia. J Neurochem. 2005;94:1676–1684. doi: 10.1111/j.1471-4159.2005.03328.x. [DOI] [PubMed] [Google Scholar]
- 62.Righi V, Constantinou C, Mintzopoulos D, Khan N, Mupparaju SP, Rahme LG, Swartz HM, Szeto HH, Tompkins RG, Tzika AA. Mitochondria-targeted antioxidant promotes recovery of skeletal muscle mitochondrial function after burn trauma assessed by in vivo 31P nuclear magnetic resonance and electron paramagnetic resonance spectroscopy. FASEB J. 2013;27:2521–2530. doi: 10.1096/fj.12-220764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu YN, Munhall AC, Johnson SW. Mitochondrial uncoupling agents antagonize rotenone actions in rat substantia nigra dopamine neurons. Brain Res. 2011;1395:86–93. doi: 10.1016/j.brainres.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 64.Wasilewska-Sampaio AP, Silveira MS, Holub O, Goecking R, Gomes FC, Neto VM, Linden R, Ferreira ST, De Felice FG. Neuritogenesis and neuronal differentiation promoted by 2,4-dinitrophenol, a novel anti-amyloidogenic compound. FASEB J. 2005;19:1627–1636. doi: 10.1096/fj.05-3812com. [DOI] [PubMed] [Google Scholar]
- 65.Liu D, Lu C, Wan R, Auyeung WW, Mattson MP. Activation of mitochondrial ATP-dependent potassium channels protects neurons against ischemia-induced death by a mechanism involving suppression of Bax translocation and cytochrome c release. J Cereb Blood Flow Metab. 2002;22:431–443. doi: 10.1097/00004647-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 66.Pandya JD, Pauly JR, Nukala VN, Sebastian AH, Day KM, Korde AM, Springer JE, Maragos WF, Hall ED, Sullivan PG. Mitochondrial uncouplers as possible therapeutic interventions following traumatic brain injury. J Neurotrauma. 2007;24:798–811. doi: 10.1089/neu.2006.3673. [DOI] [PubMed] [Google Scholar]
- 67.Pandya JD, Pauly JR, Sullivan PG. The optimal dosage and window of opportunity to maintain mitochondrial homeostasis following traumatic brain injury using the uncoupler FCCP. Exp Neurol. 2009;218:381–389. doi: 10.1016/j.expneurol.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 68.Weisová P, Anilkumar U, Ryan C, Concannon CG, Prehn JH, Ward MW. Mild mitochondrial uncoupling’ induced protection against neuronal excitotoxicity requires AMPK activity. Biochim Biophys Acta. 2012;1817:744–753. doi: 10.1016/j.bbabio.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 69.Brennan JP, Southworth R, Medina RA, Davidson SM, Duchen MR, Shattock MJ. Mitochondrial uncoupling, with low concentration FCCP, induces ROS-dependent cardioprotection independent of KATP channel activation. Cardiovasc Res. 2006;72:313–321. doi: 10.1016/j.cardiores.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 70.Liu D, Pitta M, Lee JH, Ray B, Lahiri DK, Furukawa K, Mughal M, Jiang H, Villarreal J, Cutler RG, Greig NH, Mattson MP. The KATP channel activator diazoxide ameliorates amyloid-β and tau pathologies and improves memory in the 3xTgAD mouse model of Alzheimer’s disease. J Alzheimers Dis. 2010;22:443–457. doi: 10.3233/JAD-2010-101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holmuhamedov EL, Jahangir A, Oberlin A, Komarov A, Colombini M, Terzic A. Potassium channel openers are uncoupling protonophores: implication in cardioprotection. FEBS Lett. 2004;568:167–170. doi: 10.1016/j.febslet.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 72.Alemzadeh R, Karlstad MD, Tushaus K, Buchholz M. Diazoxide enhances basal metabolic rate and fat oxidation in obese Zucker rats. Metabolism. 2008;57:1597–1607. doi: 10.1016/j.metabol.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 73.Vaynman S, Ying Z, Wu A, Gomez-Pinilla F. Coupling energy metabolism with a mechanism with a mechanism to support brain-derived neurotrophic factor-mediated synaptic plasticity. Neuroscience. 2006;139:1221–34. doi: 10.1016/j.neuroscience.2006.01.062. [DOI] [PubMed] [Google Scholar]
- 74.Dietrich MO, Andrews ZB, Horvath TL. Exercise-induced synaptogenesis in the hippocampus is dependent on UCP2-regulated mitochondrial adaptation. J Neurosci. 2008;28:10766–10771. doi: 10.1523/JNEUROSCI.2744-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Varela L, Schwartz ML, Horvath TL. Mitochondria controlled by UCP2 determine hypoxia-induced synaptic remodeling in the cortex and hippocampus. Neurobiol Dis. 2016;90:68–74. doi: 10.1016/j.nbd.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 76.Sullivan PG, Rippy NA, Rho JM. The ketogenic diet increases mitochondrial uncoupling protein levels and activity in mouse hippocampus. Ann Neurol. 2004;55:576–580. doi: 10.1002/ana.20062. [DOI] [PubMed] [Google Scholar]
- 77.Meyer R, Patel AM, Rattana SK, Quock TP, Mody SH. Prescription opioid abuse: a literature review of the clinical and economic burden in the United States. Popul Health Manag. 2014;17:372–387. doi: 10.1089/pop.2013.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jones CM, McAninch JK. Emergency department visits and overdose deaths from combined use of opioids and benzodiazepines. Am J Prev Med. 2015;49:493–501. doi: 10.1016/j.amepre.2015.03.040. [DOI] [PubMed] [Google Scholar]
- 79.Charlson F, Degenhardt L, McLaren J, Hall W, Lynskey M. A systematic review of research examining benzodiazepine-related mortality. Pharmacoepidemiol Drug Saf. 2009;18:93–103. doi: 10.1002/pds.1694. [DOI] [PubMed] [Google Scholar]
- 80.Jabbar SB, Hanly MG. Fatal caffeine overdose: a case report and review of literature. Am J Forensic Med Pathol. 2013;34:321–324. doi: 10.1097/PAF.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 81.Link KP. The discovery of dicumarol and its sequels. Circulation. 1959;19:97–107. doi: 10.1161/01.cir.19.1.97. [DOI] [PubMed] [Google Scholar]
- 82.Holmes RW, Love J. Suicide attempt with warfarin, a bishydroxycoumarin-like rodenticide. J Am Med Assoc. 1952;148:935–937. doi: 10.1001/jama.1952.62930110003013a. [DOI] [PubMed] [Google Scholar]
- 83.Pollock BE. Clinical experience with warfarin (coumadin) sodium, a new anticoagulant. J Am Med Assoc. 1955;159:1094–1097. doi: 10.1001/jama.1955.02960280016004. [DOI] [PubMed] [Google Scholar]
- 84.Jarvik LF. Congenital malformations attributed to sleeping pill (Thalidomide) Eugen Q. 1962;9:95–97. doi: 10.1080/19485565.1962.9987510. [DOI] [PubMed] [Google Scholar]
- 85.Palumbo A, et al. Thalidomide for treatment of multiple myeloma: 10 years later. Blood. 2008;111:3968–3977. doi: 10.1182/blood-2007-10-117457. [DOI] [PubMed] [Google Scholar]
- 86.Perkins RG. A study of munitions intoxications in France. Public Health Rep. 1919;34:2335–2374. [Google Scholar]
- 87.Cutting WC, Mehrtens HG, Tainter ML. Actions and Uses of Dinitrophenol. Promising Metabolic Applications. JAMA. 1933;101:193. [Google Scholar]
- 88.Tainter ML, Cutting WC, Stockton AB. Use of Dinitrophenol in Nutritional Disorders: A Critical Survey of Clinical Results. Am J Public Health Nations Health. 1934;24:1045–1053. doi: 10.2105/ajph.24.10.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Horner WD. A Study of Dinitrophenol and Its Relation to Cataract Formation. Trans Am Ophthalmol Soc. 1941;39:405–437. [PMC free article] [PubMed] [Google Scholar]
- 90.Petróczi A, Ocampo JA, Shah I, Jenkinson C, New R, James RA, Taylor G, Naughton DP. Russian roulette with unlicensed fat-burner drug 2,4-dinitrophenol (DNP): evidence from a multidisciplinary study of the internet, bodybuilding supplements and DNP users. Subst Abuse Treat Prev Policy. 2015;10:39. doi: 10.1186/s13011-015-0034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goldgof M, Xiao C, Chanturiya T, Jou W, Gavrilova O, Reitman ML. The chemical uncoupler 2,4-dinitrophenol (DNP) protects against diet-induced obesity and improves energy homeostasis in mice at thermoneutrality. J Biol Chem. 2014;289:19341–19350. doi: 10.1074/jbc.M114.568204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rolfe DF, Brand MD. Contribution of mitochondrial proton leak to skeletal muscle respiration and to standard metabolic rate. Am J Physiol. 1996;271:C1380–1389. doi: 10.1152/ajpcell.1996.271.4.C1380. [DOI] [PubMed] [Google Scholar]
- 93.Nicholls DG, Bernson VS, Heaton GM. The identification of the component in the inner membrane of brown adipose tissue mitochondria responsible for regulating energy dissipation. Experientia Supplementum. 1978;32:89–93. doi: 10.1007/978-3-0348-5559-4_9. [DOI] [PubMed] [Google Scholar]
- 94.Perry RJ, Zhang D, Zhang XM, Boyer JL, Shulman GI. Controlled-release mitochondrial protonophore reverses diabetes and steatohepatitis in rats. Science. 2015;347:1253–1256. doi: 10.1126/science.aaa0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Perry RJ, Kim T, Zhang XM, Lee HY, Pesta D, Popov VB, Zhang D, Rahimi Y, Jurczak MJ, Cline GW, Spiegel DA, Shulman GI. Reversal of hypertriglyceridemia, fatty liver disease, and insulin resistance by a liver-targetedmitochondrial uncoupler. Cell Metab. 2013;18:740–748. doi: 10.1016/j.cmet.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Caldeira da Silva CC, Cerqueira FM, Barbosa LF, Medeiros MH, Kowaltowski AJ. Mild mitochondrial uncoupling in mice affects energy metabolism, redox balance and longevity. Aging Cell. 2008;7:552–560. doi: 10.1111/j.1474-9726.2008.00407.x. [DOI] [PubMed] [Google Scholar]
- 97.Sebollela A, Freitas-Corrêa L, Oliveira FF, Mendes CT, Wasilewska-Sampaio AP, Camacho-Pereira J, Galina A, Brentani H, Passetti F, De Felice FG, Dias-Neto E, Ferreira ST. Expression profile of rat hippocampal neurons treated with the neuroprotective compound 2,4-dinitrophenol: up-regulation of cAMP signaling genes. Neurotox Res. 2010;18:112–123. doi: 10.1007/s12640-009-9133-y. [DOI] [PubMed] [Google Scholar]
- 98.De Felice FG, Ferreira ST. Novel neuroprotective, neuritogenic and anti-amyloidogenic properties of 2,4-dinitrophenol: the gentle face of Janus. IUBMB Life. 2006;58:185–191. doi: 10.1080/15216540600702198. [DOI] [PubMed] [Google Scholar]
- 99.Duan W, Guo Z, Mattson MP. Brain-derived neurotrophic factor mediates an excitoprotective effect of dietary restriction in mice. J Neurochem. 2001;76:619–626. doi: 10.1046/j.1471-4159.2001.00071.x. [DOI] [PubMed] [Google Scholar]
- 100.Morrison RS, Wenzel HJ, Kinoshita Y, Robbins CA, Donehower LA, Schwartzkroin PA. Loss of the p53 tumor suppressor gene protects neurons from kainate-induced cell death. J Neurosci. 1996;16:1337–1345. doi: 10.1523/JNEUROSCI.16-04-01337.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kashiwaya Y, Bergman C, Lee JH, Wan R, King MT, Mughal MR, Okun E, Clarke K, Mattson MP, Veech RL. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer;s disease. Neurobiol Aging. 2013;34:1530–1539. doi: 10.1016/j.neurobiolaging.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]