Abstract
Purpose
Pain is a common but understudied quality of life concern in systemic sclerosis (SSc). This investigation sought to describe patient-reported pain during the early phase of the disease and to examine potential predictors of this over time.
Methods
A prospective cohort (N = 316) of patients with early-disease SSc from the Genetics versus ENvironment In Scleroderma Outcome Study (GENISOS) were followed for 3 years. Multilevel modeling was used to describe longitudinal changes in pain and the extent to which pain variance was explained by disease type, emotional health, perceived physical health, health worry, and social support.
Results
Patient-reported pain remained relatively stable, with slight improvement over time. More severe disease type was associated with worse initial pain, but the association was reduced to nonsignificance after accounting for the psychosocial variables. Better emotional health and perceived physical health were associated with lower initial pain. There were marginal interactive effects for perceived physical health and social support such that initial perceptions of poorer physical health, and higher social support, were predictive of greater improvements in pain over time.
Conclusions
These data suggest that emotional health, perceived physical health, and social support are more relevant to longitudinal SSc pain than disease severity and that perceived physical health and social support may impact pain trajectories. Researchers and rheumatology health professionals should consider these factors in comprehensive pain models and pain management protocols.
Keywords: Systemic sclerosis, Pain, Quality of life, Multilevel modeling
Introduction
Systemic sclerosis (SSc) is an autoimmune, connective tissue disease characterized by fibrosis of the skin and internal organs, skin thickening, and decreased organ function [1]. The disease involves multiple body systems, leading to dermatologic, vascular, pulmonary, cardiac, gastrointestinal, neurological, musculoskeletal, and renal complications [1]. SSc is more prevalent among women between ages 40 and 50 [1]. The two subtypes of SSc are distinguished primarily by the pattern and severity of skin involvement. Diffuse cutaneous SSc is more severe, with rapidly progressing fibrosis in the skin and internal organs during early disease [1, 2]. During the first 5 years, patients experience puffy fingers, tendon friction rubs, finger joint contractures, gastrointestinal problems, and visceral organ dysfunction [1]. Limited cutaneous SSc has less extensive skin fibrosis, distal to the elbows and knees [1]. Skin thickening is slower during early disease, plateauing thereafter, with less severe organ involvement [1]. Heterogeneity of symptoms and outcomes within each subtype has prompted the introduction of alternative categorizations through patterns of autoantibody expression [3, 4]. However, such approaches are in their infancy, and the majority of research and clinical efforts utilize the traditional system to approximate disease severity.
Clinical care focuses on treating disease manifestations, preserving function, and improving quality of life. Pain is a significant quality of life concern [4–10], affecting 62 % [4] to 83 % [8] of patients. Disease severity and clinical manifestations (e.g., digital ulcers) have been implicated in SSc pain [6, 9, 11–14]; however, it is not fully understood within these parameters. For example, patients with limited cutaneous disease typically report lower pain than diffuse patients, but the differences are small, and not clinically meaningful [8, 14]. Because psychosocial characteristics have been linked with pain in other populations [15, 16], it follows that several cross-sectional studies have found that emotional health [8, 17], cognitive factors [7, 18, 19], and social support [20, 21] may play a role in the prevalence and severity of SSc pain.
Little is known about whether SSc pain worsens, improves, or remains stable over time. To date, there has been one longitudinal investigation wherein 109 SSc patients with an average disease duration of 9.18 years provided data at two time-points, 8–18 months apart [22]. On average, pain did not change across observations and there were not significant differences in pain when the sample was stratified by perceived change in health status. Notably, this sample was predominantly comprised of patients who had been living with SSc for a long time, and the results may not apply to patients whose disease onset was recent.
It is important to understand pain in early SSc due to the high level of disease activity, inflammatory damage, and skin thickening during this time, and because it is a critical period of psychosocial adjustment. Knowledge about the course of early-disease pain may inform intervention opportunities to improve quality of life in the long term. Therefore, the aims of the current study were to describe pain in early-disease SSc patients over 3 years and evaluate the role of medical, psychological, and social characteristics in pain over time. Disease subtype (diffuse, limited) was used to represent disease severity. Emotional health, perceived physical health, health worry, and social support were used to represent emotional, cognitive, and social functioning. Given the deteriorating nature of SSc, it was hypothesized that pain would worsen over time. It was also hypothesized that the more severe diffuse cutaneous disease classification, poorer emotional health, poorer perceived physical health, greater health worry, and lower social support would be associated with more severe initial pain, and a poorer pain prognosis.
Method
Participants
Participants were adults with SSc (within approximately 5 years of onset, defined as the time of the first non-Raynaud’s phenomenon symptom) from the Genetics versus ENvironment In Scleroderma Outcome Study (GENISOS), a prospective cohort study of SSc morbidity and mortality. Participants lived within the geographic catchment area of a study center (University of Texas Health Science Center at Houston, University of Texas Medical Branch at Galveston, University of Texas-Health Science Center at San Antonio) and were recruited from rheumatology faculty clinics, the county hospital, and chapters of the Scleroderma Foundation [23].
Procedure
Study procedures are detailed by Reveille et al. [23]. Institutional Review Board approval was obtained at all institutions. Participants gave written informed consent prior to the baseline visit. Annual study visits took place during outpatient medical appointments or inpatient services at facilities staffed by clinician investigators. Patients received a standardized clinical exam and answered questionnaires. Enrollment in the GENISOS has been ongoing since the study’s inception in 1998; the current analysis utilizes data from the first three annual visits for each participant, starting at each individual’s own point of study enrollment.
Measures
Sample characteristics
Demographics
Sex, age, gender, income, education, and marital status data were collected.
Skin thickening
Skin thickening was measured using the modified Rodnan Skin Score (mRSS) [24]. The extent and severity of skin thickening is measured on 17 body surface areas by palpation on a 4-point scale. Scores range from 0 to 51; higher scores reflect more extensive and worse thickening.
SSc-related interstitial lung disease
Percent predicted forced vital lung capacity (%FVC) indicates the ratio of the volume of air that can be forcibly exhaled after a maximum inspiration to the same volume in age, gender, weight, height, and ethnicity matched population controls. The measurements met criteria outlined by the American Thoracic Society and were reviewed by a pulmonologist. Higher scores indicate better lung functioning.
Time-varying (level-1) outcome
Pain
The Medical Outcomes Study Short-Form Health Survey (SF-36) [25] Bodily Pain subscale describes pain intensity and impact over the past 4 weeks (α = .88). Scores (0–100) represent the percentage of the total possible score; higher scores indicate less pain severity and interference (i.e., better pain-related quality of life).
Time-varying (level-1) predictor
Disease duration
A continuous variable representing disease duration (years) was calculated by taking the difference between each study visit date and the first non-Raynaud’s phenomenon symptoms date.
Baseline (level-2) predictors
Disease type
The clinician investigators confirmed a diagnosis of diffuse or limited cutaneous SSc using the classification system established by Leroy et al. [2].
Emotional health
The SF-36 [25] Mental Health subscale was used to evaluate emotional health (α = .78). Scores (0–100) represent the percentage of the total possible score; higher scores indicate better emotional functioning and less psychological distress.
Perceived physical health
The SF-36 [25] Physical Functioning subscale was used to evaluate patient beliefs about their physical health and functioning limitations (α = .92). Scores (0–100) represent the percentage of the total possible score; higher scores indicate a perception of better physical health.
Health worry
The Illness Behavior Questionnaire (IBQ) [26] Health Worry scale for SSc [27] was used to evaluate health-related worries (α = .72). An example item is, “Do you worry a lot about your health?” Scores range from 0 to 5; higher scores indicate greater health worry and concern.
Social support
The Interpersonal Support Evaluation List (ISEL) [28] was used to measure social support (α = .88). The ISEL yields four subscales and an overall score that is derived by averaging the subscales. Scores range from 0 to 10; higher scores indicate better social support.
Analytic strategy
The hypotheses were tested using multilevel modeling (MLM), a flexible data analytic technique that handles hierarchically structured data [29]. In this study, the structure consisted of repeated measurements of pain (level-1) nested within each patient (level-2). An ordinary least squares framework was not appropriate because patient’s pain observations were related, violating the assumption of independence. Rather, MLM assumes that the level-1 observations (pain) are dependent within each cluster (patient). Fixed (regression coefficients) and random (variance components) effects are estimated simultaneously, allowing for the examination of within- and between-person variability in the same model.
MLM has several advantages in longitudinal research. First, attrition and missed appointments result in missing data. Instead of removing cases with missing observations, the full information maximum likelihood procedure (FIML) employed by MLM allows for all available data to be used by estimating a likelihood function for each person based on the variables that are present. Estimates are weighted by the amount of data each person contributes; even data from individuals with one measurement occasion can be used to stabilize mean and variance estimates. This produces unbiased parameter estimates under conditions wherein data are missing at random. Second, MLM allows for different starting times for the baseline visit and unequal spacing between visits by modeling time as a time-varying covariate on the first level of the data structure. This is relevant because patients enrolled in the GENISOS at different points after disease onset (baseline disease duration ranged from .03 to 5.95 years) and follow-up appointments ranged from 9 to 15 months between visits. Incorporating these differences, rather than operationalizing assessment waves as nominal (i.e., visit 1, 2, 3), allows for a more accurate reflection of time.
Model building
An iterative model building process was employed. Fixed and random effects were estimated for all models. Descriptive fit indices (Akaike information criteria [AIC], sample size-adjusted Bayesian information criteria [sBIC]) were evaluated, with smaller values indicating better model fit. The proportion of reduction in unexplained variance between models was examined to determine how much variance each set of predictors accounted for.
First, the null model was estimated. This unconditional model depicts each patient’s pain over time as a flat line with a slope of zero, located at each patient’s average pain. This model provides the intraclass correlation coefficient (ICC), an estimate of variance in pain between- and within-subjects that is calculated using the variance components from both levels of the data structure.
Second, an unconditional linear growth (i.e., random coefficient) model called the disease duration model was estimated by adding time as a level-1 predictor. This model examines time effects for each patient by allowing changes in each patient’s pain to be modeled with a straight line and a non-zero slope. Disease duration at each visit was selected as the metric of time; such within-person scaling operationalizes time more meaningfully in contexts where time is not a constant variable for each participant [30]. Because no measurement occasions took place at disease onset, intercepts are described as initial pain.
Three conditional linear models tested the relationship between the predictors and pain over time. Continuous variables were grand mean centered. Significant interactions were probed using the methods of Preacher, Curran, and Bauer [31]; cross-level interactions were explored by computing simple regression lines at low (−1 SD from the centered mean), mean (at the centered mean), and high (+1 SD from the centered mean) values for the relevant level-2 predictors. In all conditional models, the level-1 equation is identical to the disease duration model. The medical model was estimated by evaluating disease type1 (i.e., limited, diffuse) as a level-2 predictor of intercept and slope pain variance. Next, the psychosocial model was estimated by evaluating baseline emotional health, perceived physical health, health worry, and social support as level-2 predictors. The biopsychosocial model incorporated both the medical and psychosocial variables in a single model, specified as:
Level-1: painti = β0i + β1i(durationti) + rti
Level-2: β0i = γ00 + γ01(typei) + γ02(emotionali) + γ03 (perceivedi) + γ04(worryi) + γ05(sociali) + u0i
Level-2: β1i = γ10 + γ11(typei) + γ12(emotionali) + γ13 (perceivedi) + γ14(worryi) + γ15(sociali) + u1i
The level-1 equation states that pain at time t for patient i (painti) is a function of each patient’s mean pain (β0i), a term that reflects the estimate for each patient’s pain slope over time (β1i), and the within-persons residual term (rti). The first level-2 equation states that a patient’s pain at the intercept (β0i) is a function of the average pain for patients at baseline (γ00), the unique relationship between all predictors and pain (γ01–γ05), and the between-subjects residual term (u0i). The second level-2 equation states that each patient’s rate of change in pain over time (β1i) is a function of the average rate of change per year (γ10), the unique change attributable to each predictor (γ11–γ15), and the variance component for the slopes term (u1i).
As a final step, all conditional models were re-run with analgesic use (whether a patient reported taking acetaminophen, non-steroidal anti-inflammatory drugs, tramadol, or narcotics during the past month) as a covariate to test whether the coefficients were affected by this variable.
Missing data
The 28 patients missing a pain observation at every visit were not included. They did not differ from the remaining sample (N = 316) with regard to sex, race, marital status, education, income, disease type, disease duration, age of disease onset, death during the study, skin thickening, or analgesic use (ps > .05). They were younger (M = 43.49, SD = 12.96) than patients with ≥ 1 pain observation (M = 48.95, SD = 13.05), although the effect was small (t = 2.12, d = .22, p = .035).
Forty-nine patients died during the study (After Visit 1: 29 diffuse, 9 limited; After Visit 2: 6 diffuse, 5 limited). These patients were did not generally differ from the remaining sample on the aforementioned variables, except they were more likely to be African American or Latino, less educated, and of lower income (effect sizes = |.14– .18|, ps < .05). Baseline pain was not different for those who died versus the rest of the sample (p > .10). Pain scores for the 49 participants who died were considered “truncated by death” rather than censored or missing [32]. Therefore, the distribution of missingness was not modeled in the analyses; rather, inferences from the estimates were considered conditional on the probability of surviving (see [32, 33] for discussions of this issue).
Patterns of missing data for pain were also evaluated in relation to the aforementioned demographic and disease characteristics. The only covariate-dependent missingness pattern that emerged was for income; lower income correlated with more missing pain measurements (rs = −.12, p = .031). Heeding accepted guidelines that missing data patterns should only be accounted for when they are likely to influence results in a non-ignorable fashion [34], income was not included as a covariate because the effect was small. For completeness, all models were re-run with income, and the patterns of findings were unchanged.
Results
The sample is described in Table 1. At Visit 1, diffuse patients had greater skin thickening (t [311] = −15.06, p < .001; diffuse M = 22.16, SD = 10.80; limited M = 6.83, SD = 5.13), and group differences in interstitial lung disease approached significance (t [273] = 1.72, p = .086; diffuse M = 79.06, SD = 19.82; limited M = 82.23, SD = 20.04). Average pain scores2 were: Visit 1 (n = 302): M = 49.21, SD = 26.96; Visit 2 (n = 197): M = 55.51, SD = 26.49; Visit 3 (n = 121): M = 55.19, SD = 27.48.
Table 1.
Descriptive characteristics of sample at first study visit
| Variable | N | Percent (%) |
|---|---|---|
| Sex | ||
| Women | 266 | 84.2 |
| Men | 50 | 15.8 |
| Race/ethnicity | ||
| White | 155 | 49.1 |
| Latino | 90 | 28.5 |
| Black | 61 | 19.3 |
| Asian | 9 | 2.8 |
| American Indian | 1 | 0.3 |
| Marital status | ||
| Married/partnered | 179 | 56.6 |
| Not married/partnered | 127 | 40.2 |
| Missing | 10 | 3.2 |
| Education | ||
| Less than high school | 46 | 14.6 |
| High school/GED | 149 | 47.2 |
| Associate’s degree | 25 | 7.9 |
| Bachelor’s degree | 44 | 13.9 |
| Post-graduate | 28 | 8.9 |
| Missing | 24 | 7.6 |
| Family income | ||
| ≥$29,999 | 139 | 44.0 |
| $30,000–$49,999 | 61 | 19.3 |
| $50,000–$99,999 | 59 | 18.7 |
| ≥$100,000 | 43 | 13.6 |
| Missing | 14 | 4.4 |
| Disease type | ||
| Limited cutaneous | 131 | 41.5 |
| Diffuse cutaneous | 185 | 58.5 |
|
| ||
| M | SD | |
|
| ||
| Age (years) | 48.95 | 13.05 |
| Age at disease onset (years) | 46.47 | 13.09 |
| Disease duration (years) | 2.48 | 1.56 |
| Skin thickening (mRSS) | 15.74 | 11.66 |
| Percent predicted forced vital capacity | 80.90 | 19.99 |
| Emotional health | 67.22 | 19.32 |
| Perceived physical health | 43.70 | 28.85 |
| Health worry | 2.21 | 1.62 |
| Social support | 8.22 | 1.62 |
Parameter estimates, standard error, and fit indices are presented in Table 2. There were 620 observations for the 316 patients. The null model revealed an ICC of .612 (i.e., 61.2 % of pain variability is between-person, 38.8 % is within-person). There was significant variability (τ00 = 449.20, p < .001) around the grand mean (b = 51.42, p < .001); 95 % of patients had pain scores between 9.88 and 92.96. There was also significant variability between each patient’s observed and predicted pain (σ2 = 286.07, p < .001).
Table 2.
Parameter estimates (SE) of models predicting SSc patient pain over time
| Parameters | Model 1: intercept | Model 2: linear growth | Models 3–5: conditional models
|
||
|---|---|---|---|---|---|
| Null | Disease duration | Medical | Psychosocial | Biopsychosocial | |
| Fixed effects | |||||
| Intercept (γ00) | 51.42 (1.40)*** | 46.03 (2.26)*** | 42.50 (3.03)*** | 46.04 (1.87)*** | 48.04 (2.59)*** |
| Slope (γ10) | 1.72 (.57)** | 2.45 (.77)*** | 1.66 (.52)** | 1.55 (.71)* | |
| Disease type (γ01) | 8.82 (4.49)* | −5.10 (4.23) | |||
| Emotional health (γ02) | .32 (.10)*** | .32 (.10)*** | |||
| Perceived health (γ03) | .51 (.07)*** | .54 (.08)*** | |||
| Health worry (γ04) | −1.14 (1.34) | −1.30 (1.37) | |||
| Social support (γ05) | −.45 (1.49) | −.82 (1.56) | |||
| Type × duration (γ11) | −1.80 (1.17) | .36 (1.15) | |||
| Emotional × duration (γ12) | −.03 (.03) | −.04 (.03) | |||
| Perceived × duration (γ13) | −.04 (.02)* | −.04 (.02)† | |||
| Worry × duration (γ14) | .06 (.35) | .05 (.35) | |||
| Social × duration (γ15) | .70 (.39)† | .74 (0.41)† | |||
| Variance components | |||||
| Residual (rti) | 286.07 (30.19)*** | 281.22 (29.67)*** | 279.11 (29.20)*** | 265.14 (27.92)*** | 264.79 (27.97)*** |
| Intercept (τ00) | 449.20 (45.25)*** | 436.60 (65.25)*** | 434.44 (65.54)*** | 199.33 (45.39)*** | 192.46 (44.82)*** |
| Slope (τ11) | .61 (4.45) | .63 (4.41) | 2.12 (3.16) | 2.44 (3.19) | |
| Model summary | |||||
| AIC | 5698.87 | 5684.75 | 5684.96 | 5179.64 | 5181.06 |
| sBIC | 5702.64 | 5691.01 | 5693.74 | 5195.07 | 5198.86 |
Regression coefficient symbols in the parameters column (i.e., γ01–γ15) reflect the terms for the biopsychosocial model; Disease type was coded as 0 = diffuse, 1 = limited;
p ≤.07,
p < .05,
p < .01,
p ≤ .001
The AIC (difference of 14.12) and sBIC (difference of 11.63) values for the disease duration model were lower than the null model (proportion of reduction in unexplained variance between models was .017). Adding time (disease duration) slightly improved model fit and explained additional variance in pain. There was significant variability (τ00 = 436.60, p < .001) around the grand mean at the intercept (b = 46.03, p < .001). Pain scores increased by approximately 1.72 points annually (p = .002), suggesting that pain slightly lessened over time. There was not significant slope variance in pain growth trajectories (τ11 = .61, p = .89), indicating that there was no additional within-person variability to be accounted for. That is, time accounted for the majority of slope variance, precluding the inclusion of additional level-1 variables. This model demonstrated significant variance in each patient’s observed versus predicted pain (σ2 = 281.22, p < .001).
The medical model yielded similar AIC (difference of .21) and sBIC (difference of 2.73) values to the disease duration model (proportion of reduction in unexplained variance was .008). Model fit was similar when disease type was added to the model, and explained a small amount of additional variance in pain was explained. The intercept was significant for diffuse (b = 42.50, p < .001) and limited patients (b = 8.82, p = .049),3 indicating that limited patients reported less initial pain than diffuse patients. The slope was significant for diffuse (b = 2.45, p < .001), but not limited patients (b = −1.80, p = .12), suggesting that both diffuse and limited patients’ pain decreased at approximately the same rate. Variance estimates showed significant variability in observed versus predicted initial pain (τ00 = 434.44, p < .001) and pain over time (σ2 = 279.11, p < .001), but there was not significant slope variability in pain growth trajectories across patients (τ11 = .63, p = .89).
The psychosocial model revealed lower AIC (difference of 505.32) and sBIC (difference of 498.67) values compared to the medical model, indicating improved fit. The proportion of reduction in unexplained variance between the psychosocial model and the medical model (.05) suggested that the addition of psychosocial characteristics explained additional variance in pain. The intercept (b = 46.04, p < .001) and slope (b = 1.66, p = .001) were significant. The coefficients relating emotional health (b = .32, p = .001) and perceived physical health (b = .51, p < .001) with pain were positive, suggesting that better emotional health and perceived physical health were both associated with less initial pain. The main effects for health worry and social support were not significant (ps = .40, .76). Variance estimates showed significant intercept (τ00 = 199.33, p < .001) and residual (σ2 = 265.14, p < .001) variability, but not significant slope variability in pain growth trajectories (τ11 = 2.12, p = .50). The cross-level interactions for perceived physical health and social support were explored further. The disease duration X perceived physical health interaction approached statistical significance (b = −.04, p = .065). The intercepts for low (b = 36.18), mean (b = 46.04), and high (b = 55.89) perceived physical health were significant (ps < .001). The simple slopes for low (b = 2.37, p = .0002) and mean (b = 1.66, p = .0014) perceived physical health were significant, but the slope for high perceived physical health was not (b = .94, p = .158). This suggests that patients with average or low perceived physical health have respectively worse initial pain, but over time this improves, whereas individuals who perceive their physical health more positively have less initial pain that does not significantly change over time. The disease duration X social support interaction also approached significance (b = .75, p = .07). The intercepts for low (b = 46.77), mean (b = 46.04), and high (b = 45.31) social support were significant (ps < .001), but given that these intercepts are similar, and the main effect for pain was not significant, there was little evidence that social support was practically associated with initial pain. The simple slopes for mean (b = 1.66, p = .0014) and high (b = 2.78, p = .0095) social support were significant, although the slope for low social support was not (b = .53, p = .2397). This suggests that pain improves over time for patients with average or high social support, whereas there is no change in pain for those with low social support.
The biopsychosocial model revealed AIC (difference of 1.42) and sBIC (difference of 3.79) values were similar to the psychosocial model and that the proportion of reduction in explained variance between these two models (.001) was negligible. This suggests that the psychosocial and biopsychosocial models provide a similar fit to the data. The intercept (b = 48.04, p < .001) and slope (b = 1.55, p = .029) were significant. The coefficients for the psychosocial variables retained the patterns of the psychosocial model: better emotional health (b = .32) and perceived physical health (b = .54) were associated with less initial pain (ps ≤ .001), but health worry and social support (ps = .34, .60) were not significant. The main effect for disease type was not significant (b = −5.10, p = .23). Variance estimates were also similar to the psychosocial model (σ2 = 264.79, p < .001; τ00 = 192.46, p < .001; τ11 = 2.44, p = .44). The cross-level interactions for perceived physical health and social were probed using the procedure described above. When the disease duration X perceived physical health interaction (b = −.04, p = .065) was explored, the intercepts (low b = 37.55, mean b = 48.04, high b = 58.53; ps < .001) and slopes (low b = 2.32, p = .0002; mean b = 1.55, p = .029; high b = .77, p = .3365) retained the patterns of the psychosocial model (Fig. 1). When the disease duration X social support interaction (b = .74, p = .07) was explored, the intercepts (low b = 49.36, mean b = 48.04, high b = 46.71; ps < .001) and slopes (low b = .35, p = .7351; mean b = 1.55, p = .029; high b = 2.74, p = .0019) maintained the patterns of the psychosocial model (Fig. 2). When analgesic use was entered as a covariate, the patterns were not changed. Thus, the reported coefficients are considered the best representation of pain in this sample.
Fig. 1.
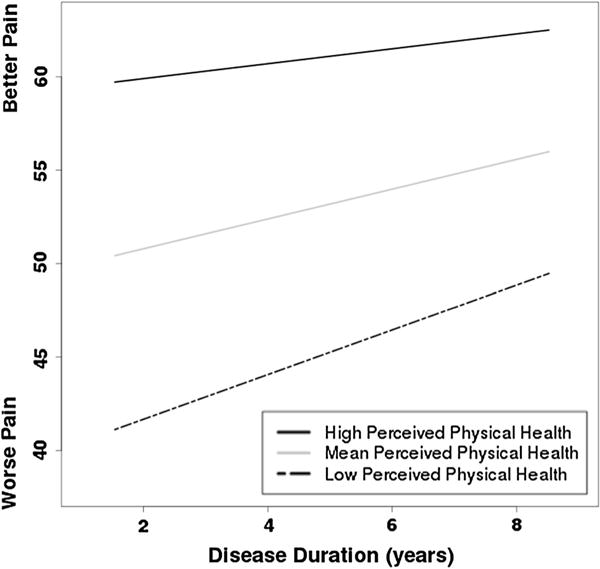
Simple slopes describing the relationship between perceived physical health (Low −1 SD from the centered mean; Mean at the centered mean; High +1 SD from the centered mean) and patient-reported pain over time
Fig. 2.
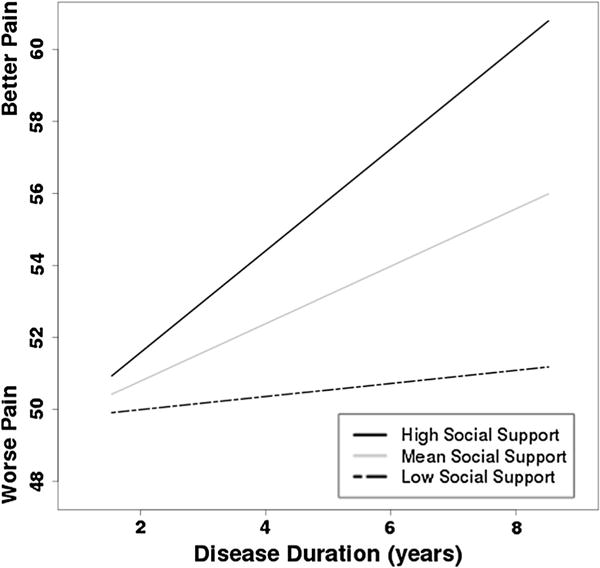
Simple slopes describing the relationship between social support (Low −1 SD from the centered mean; Mean at the centered mean; High +1 SD from the centered mean) and patient-reported pain over time
Discussion
This study described the course and correlates of patient-reported pain, a considerable but understudied quality of life concern in SSc. Contrary to hypotheses, pain changed minimally over time, and the change that did occur suggested a slight overall improvement in pain. One potential explanation for this paradoxical finding is response shift, or a change in one’s evaluation of their pain due to an adjustment of one’s internal measurement of their pain (recalibration), change in values and priorities regarding their pain (reprioritization), or changed conceptualization of what pain is (reconceptualization) [35]. Indeed, recent research using the SF-36 pain scale has demonstrated that cancer patients recalibrate their experience of pain over time as they adapt to their limitations [36] and that different types of social comparisons and post-traumatic growth may indirectly contribute to pain recalibration [37]. It follows that it is also plausible that the observed improvement in pain in the current data represents adjustment to SSc and its symptoms.
Putative predictors of patient-reported pain were tested in a series of models. The more severe diffuse subtype was associated with worse initial pain; however, pain improved at approximately the same rate for both disease types. While significant, the effect was quite small. When the psychosocial variables were evaluated (without disease severity), patterns for emotional health, perceived physical health, and social support emerged. Poorer emotional health was linked with worse initial pain, but the trajectory of pain was similar across levels of emotional functioning. Of the two cognitive variables tested, health worry was not a significant predictor of pain, but there were main and moderating effects for perceptions of physical health. Individuals whose self-perception of better physical health reported less initial pain that remained consistent, whereas individuals whose self-perception of average or poorer physical health reported respectively worse initial pain that eventually improved such that, over time, their pain became more similar to those who initially perceived their health as better. Although the main effect findings fit the study hypotheses, the trajectory of pain across different health perceptions is somewhat counterintuitive. It could be that individuals who initially perceive their physical health as poor are more likely to recalibrate their pain self-assessment as they adjust to SSc, whereas individuals who initially perceive their physical health as better do not experience this shift. Although social support was not related to initial pain, there was a trend for social support as a moderator; those with average or high levels of social support reported greater improvement in pain over time, whereas pain levels remained the same for those with lower social support. In the final model, the association between disease type and pain was reduced to nonsignificance after the psychosocial constructs were accounted for, suggesting that disease severity does not explain clinically meaningful differences in pain, as has been previously demonstrated [4, 8, 12, 38]. That is, even in the context of a disease characterized by significant tissue damage, a reductionistic medical model should not be used to conceptualize pain. Indeed, emotional health, perceived physical health, and social support are related to the initial experience of pain and whether pain remits or remains stable over time.
Clinically, these findings underscore the critical importance for rheumatology professionals to have routine discussions about pain, pain management, and psychosocial functioning with SSc patients, regardless of their diagnostic subtype or severity of disease. Over the three years of the study, patients consistently reported a significant burden of pain that was linked primarily with psychological and social characteristics. While the current findings cannot purport directionality of these effects, the clinical implication is that there are likely multiple points of intervention that could disrupt the overall cycle of pain and poor psychosocial functioning. This complex presentation has major implications for the use of cognitive-behavioral interventions that could be used alongside medical therapies. While there is no shortage of evidence that multifaceted pain interventions addressing these factors are effective in many chronic pain populations [39, 40], trials of such interventions are warranted to clarify the applicability of these approaches to SSc pain care. Given the ubiquitousness of pain, and that SSc patients often have large, interdisciplinary treatment teams, it may also be worthwhile to consider intervention delivery via clinicians who routinely interact with SSc patients (e.g., nurses, occupational and physical therapists), rather than specifically through behavioral health specialists.
This study had several limitations. First, there was substantial missing data, while the FIML procedure is able to accommodate this, lower attrition is preferable. Extensive data missingness at additional time-points in the GENISOS also precluded including these visits in the analysis; thus, it was not possible to detect the potential presence of a curvilinear pain course (see [41]). Additionally, while the sample size was large for this rare disease, power was compromised due to the heterogeneity of pain scores; a larger sample would be ideal. Obtaining sufficiently large samples with multiple time-points should be targeted in future research efforts. The cohort design also restricted the ability to determine true causality. The observed relationships are likely bidirectional, ongoing, and mutually influential; other methodologies (e.g., ecological momentary assessment [42]) might better elucidate the patterns of strength and influence, and it is possible there would be less attrition over the shorter course of such a study. Relevant cognitive styles that have been implicated in pain in rheumatologic populations (e.g., rumination, catastrophizing; [43]) were not measured as part of the GENISOS and could not be evaluated. Because the pain measure was not SSc-specific, it is possible that pain due to co-morbidities was captured. Retrospective pain reports are also subject to bias because respondents are influenced by the greatest severity and/or most recent level of pain [44]. However, given that one’s memory of pain is more influential on behavior [44], retrospective assessment may be clinically useful, even if responses did not reflect the “true” pain experienced over the previous 4 weeks. Finally, it would have been of interest to evaluate the time-varying effects of the predictor variables. Goals for future research should address these limitations by considering other designs with more frequent points of data collection, assessing other cognitive variables, exploring additional pain measurement strategies, and modeling change in the predictors over time.
In sum, pain has been understudied in SSc, despite being a hallmark of the disease. This is the first longitudinal examination of patient-reported pain in early SSc. Future research is needed to clarify several of the issues described above and translate these findings into comprehensive intervention approaches. There is a clear need to address the psychological and social concomitants of pain, in addition to medically managing SSc disease manifestations.
Acknowledgments
Funding was provided by the National Institute of Health (NIH/NIAMS) Center of Research Translation (CORT) in Scleroderma P50AR054144 (PI: Mayes); NIH-KL2RR024149 and K23AR061436 (PI: Assassi).
Footnotes
Clinical manifestations (digital ulcers, calcinosis, arthritis) were considered for inclusion in the medical model given previous research demonstrating that these correlate with pain, even after accounting for depression (Schieir et al., 2010). These variables did not have bivariate correlations with pain in the current data and thus were excluded in order to keep the model as parsimonious as possible.
Note that the SF-36 metric is such that lower scores indicate greater pain severity and interference, whereas higher scores indicate lesser pain severity and interference.
The intercept is interpreted as mean pain for those with diffuse disease (coded as 0); the slope is difference in mean pain for those diffuse versus limited cutaneous disease (coded as 1). The significant and positive slope value indicates that the mean pain score for limited patients was 8.82 points higher than the mean for diffuse patients.
References
- 1.Medsger TA. Natural history of systemic sclerosis and the assessment of disease activity, severity, functional status, and psychologic well-being. Rheumatic Disease Clinics of North America. 2003;29(2):255–273. doi: 10.1016/s0889-857x(03)00023-1. [DOI] [PubMed] [Google Scholar]
- 2.LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA, et al. Scleroderma (systemic sclerosis): Classification, subsets and pathogenesis. The Journal of Rheumatology. 1988;15(2):202–205. [PubMed] [Google Scholar]
- 3.Patterson KA, Roberts-Thomson PJ, Lester S, Tan JA, Hakendorf P, Rischmueller M, et al. Interpretation of an extended autoantibody profile in a well-characterized Australian systemic sclerosis (scleroderma) cohort using principal components analysis. Arthritis and Rheumatology. 2015;67(12):3234–3244. doi: 10.1002/art.39316. [DOI] [PubMed] [Google Scholar]
- 4.Benrud-Larson LM, Haythornthwaite JA, Heinberg LJ, Boling C, Reed J, White B, et al. The impact of pain and symptoms of depression in scleroderma. Pain. 2002;95(3):267–275. doi: 10.1016/S0304-3959(01)00409-2. [DOI] [PubMed] [Google Scholar]
- 5.Carreira PE. “Quality of pain” in systemic sclerosis. Rheumatology. 2006;45(10):1185–1186. doi: 10.1093/rheumatology/kel247. [DOI] [PubMed] [Google Scholar]
- 6.Richards H, Herrick A, Griffin K, Gwilliam P, Fortune D. Psychological adjustment to systemic sclerosis—exploring the association of disease factors, functional ability, body related attitudes and fear of negative evaluation. Psychology, Health and Medicine. 2004;9(1):29–39. [Google Scholar]
- 7.Edwards RR, Goble L, Kwan A, Kudel I, McGuire L, Heinberg L, et al. Catastrophizing, pain, and social adjustment in scleroderma: Relationships with educational level. The Clinical Journal of Pain. 2006;22(7):639–646. doi: 10.1097/01.ajp.0000210918.26159.94. [DOI] [PubMed] [Google Scholar]
- 8.Schieir O, Thombs BD, Hudson M, Boivin J-F, Steele R, Bernatsky S, et al. Prevalence, severity, and clinical correlates of pain in patients with systemic sclerosis. Arthritis Care and Research. 2010;62(3):409–417. doi: 10.1002/acr.20108. [DOI] [PubMed] [Google Scholar]
- 9.Stisi S, Sarzi-Puttini P, Benucci M, Biasi G, Bellissimo S, Talotta R, et al. Pain in systemic sclerosis. Reumatismo. 2014;66(1):44–47. doi: 10.4081/reumatismo.2014.764. [DOI] [PubMed] [Google Scholar]
- 10.Suarez-Almazor ME, Kallen MA, Roundtree AK, Mayes M. Disease and symptom burden in systemic sclerosis: A patient perspective. The Journal of Rheumatology. 2007;34(8):1718–1726. [PubMed] [Google Scholar]
- 11.Johnson SR, Glaman DD, Schentag CT, Lee P. Quality of life and functional status in systemic sclerosis compared to other rheumatic diseases. The Journal of Rheumatology. 2006;33(6):1117–1122. [PubMed] [Google Scholar]
- 12.Malcarne VL, Hansdottir I, McKinney A, Upchurch R, Greenbergs HL, Henstorf GH, et al. Medical signs and symptoms associated with disability, pain, and psychosocial adjustment in systemic sclerosis. The Journal of Rheumatology. 2007;34(2):359–367. [PubMed] [Google Scholar]
- 13.Toffolo SR, Furtado RNV, Klein A, Watanabe S, Andrade LEC, Natour J. Measurement of upper limb ulcers in patients with systemic sclerosis: Reproducibility and correlation with pain, function, and quality of life. Nursing Research. 2008;57(2):84–92. doi: 10.1097/01.NNR.0000313480.32132.db. [DOI] [PubMed] [Google Scholar]
- 14.Del Rosso A, Boldrini M, D’Agostino D, Placidi GP, Scarpato A, Pignone A, et al. Health-related quality of life in systemic sclerosis as measured by the Short Form 36: relationship with clinical and biologic markers. Arthritis and Rheumatism. 2004;51(3):475–481. doi: 10.1002/art.20389. [DOI] [PubMed] [Google Scholar]
- 15.Fava GA, Sonino N. The biopsychosocial model thirty years later. Psychotherapy and Psychosomatics. 2008;77(1):1–2. doi: 10.1159/000110052. [DOI] [PubMed] [Google Scholar]
- 16.Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychological Bulletin. 2007;133(4):581–624. doi: 10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- 17.Kwakkenbos L, van Lankveld WGJM, Vonk MC, Becker ES, van den Hoogen FHJ, van den Ende CHM. Disease-related and psychosocial factors associated with depressive symptoms in patients with systemic sclerosis, including fear of progression and appearance self-esteem. Journal of Psychosomatic Research. 2012;72(3):199–204. doi: 10.1016/j.jpsychores.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Richards HL, Herrick AL, Griffin K, Gwilliam PDH, Loukes J, Fortune DG. Systemic sclerosis: Patients’ perceptions of their condition. Arthritis and Rheumatism. 2003;49(5):689–696. doi: 10.1002/art.11385. [DOI] [PubMed] [Google Scholar]
- 19.van Lankveld W, Teunissen H, Näring G, Vonk M, van den Hoogen F. Social Support, disease-related cognitions and coping as predictors of depressed mood in systemic sclerosis. Cognitive Therapy and Research. 2007;32(3):434–447. [Google Scholar]
- 20.Merz EL, Malcarne VL, Assassi S, Nair DK, Graham TA, Yellman BP, et al. Biopsychosocial typologies of pain in a cohort of patients with systemic sclerosis. Arthritis Care and Research. 2014;66(4):567–574. doi: 10.1002/acr.22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savelkoul M, Post MW, de Witte LP, van den Borne HB. Social support, coping and subjective well-being in patients with rheumatic diseases. Patient Education and Counseling. 2000;39(2–3):205–218. doi: 10.1016/s0738-3991(99)00033-6. [DOI] [PubMed] [Google Scholar]
- 22.Sekhon S, Pope J, Baron M. The minimally important difference in clinical practice for patient-centered outcomes including health assessment questionnaire, fatigue, pain, sleep, global visual analog scale, and SF-36 in scleroderma. The Journal of Rheumatology. 2010;37(3):591–598. doi: 10.3899/jrheum.090375. [DOI] [PubMed] [Google Scholar]
- 23.Reveille JD, Fischbach M, McNearney T, Friedman AW, Aguilar MB, Lisse J, et al. Systemic sclerosis in 3 US ethnic groups: A comparison of clinical, sociodemographic, serologic, and immunogenetic determinants. Seminars in Arthritis and Rheumatism. 2001;30(5):332–346. doi: 10.1053/sarh.2001.20268. [DOI] [PubMed] [Google Scholar]
- 24.Kahaleh MB, Sultany GL, Smith EA, Huffstutter JE, Loadholt CB, LeRoy EC. A modified scleroderma skin scoring method. Clinical and Experimental Rheumatology. 1986;4(4):367–369. [PubMed] [Google Scholar]
- 25.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Medical Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 26.Pilowsky I, Spence ND. Patterns of illness behaviour in patients with intractable pain. Journal of Psychosomatic Research. 1975;19(4):279–287. doi: 10.1016/0022-3999(75)90026-4. [DOI] [PubMed] [Google Scholar]
- 27.Merz EL, Malcarne VL, Roesch SC, Sharif R, Harper BE, Draeger HT, et al. Measuring illness behavior in patients with systemic sclerosis. Arthritis Care and Research. 2013;65(4):585–593. doi: 10.1002/acr.21874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen S, Mermelstein R, Kamarck T, Hoberman HM. Measuring the functional components of social support. In: Sarason IG, Sarason BR, editors. Social support: Theory, research and applications. Dordrecht: Springer, Netherlands; 1985. pp. 73–94. [Google Scholar]
- 29.Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2nd. Thousand Oaks: Sage; 2002. [Google Scholar]
- 30.Mehta PD, West SG. Putting the individual back into individual growth curves. Psychological Methods. 2000;5(1):23–43. doi: 10.1037/1082-989x.5.1.23. [DOI] [PubMed] [Google Scholar]
- 31.Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31(4):437–448. [Google Scholar]
- 32.Zhang JL, Rubin DB. Estimation of causal effects via principal stratification when some outcomes are truncated by “death”. Journal of Educational and Behavioral Statistics. 2003;28(4):353–368. [Google Scholar]
- 33.Kurland BF, Johnson LL, Egleston BL, Diehr PH. Longitudinal data with follow-up truncated by death: Match the analysis method to research aims. Statistical Science. 2009;24(2):211. doi: 10.1214/09-STS293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hedeker D, Gibbons RD. Application of random-effects pattern-mixture models for missing data in longitudinal studies. Psychological Methods. 1997;2(1):64–78. [Google Scholar]
- 35.Schwartz CE, Andresen EM, Nosek MA, Krahn GL. Response shift theory: Important implications for measuring quality of life in people with disability. Archives of Physical Medicine and Rehabilitation. 2007;88(4):529–536. doi: 10.1016/j.apmr.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 36.Verdam MGE, Oort FJ, Sprangers MAG. Using structural equation modeling to detect response shifts and true change in discrete variables: An application to the items of the SF-36. Quality of Life Research. 2016;25(6):1361–1383. doi: 10.1007/s11136-015-1195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Visser MRM, Oort FJ, Van Lanschot JJB, Van Der Velden J, Kloek JJ, Gouma DJ, et al. The role of recalibration response shift in explaining bodily pain in cancer patients undergoing invasive surgery: An empirical investigation of the Sprangers and Schwartz model. Psycho-Oncology. 2013;22(3):515–522. doi: 10.1002/pon.2114. [DOI] [PubMed] [Google Scholar]
- 38.Georges C, Chassany O, Toledano C, Mouthon L, Tiev K, Meyer O, et al. Impact of pain in health related quality of life of patients with systemic sclerosis. Rheumatology. 2006;45(10):1298–1302. doi: 10.1093/rheumatology/kel189. [DOI] [PubMed] [Google Scholar]
- 39.Ehde DM, Dillworth TM, Turner JA. Cognitive-behavioral therapy for individuals with chronic pain: Efficacy, innovations, and directions for research. American Psychologist. 2014;69(3):22–29. doi: 10.1037/a0035747. [DOI] [PubMed] [Google Scholar]
- 40.McCracken LM, Vowles KE. Acceptance and commitment therapy and mindfulness for chronic pain: model, process, and progress. American Psychologist. 2014;69(2):178–187. doi: 10.1037/a0035623. [DOI] [PubMed] [Google Scholar]
- 41.Sterba SK. Fitting nonlinear latent growth curve models with individually varying time points. Structural Equation Modeling. 2014;21:630–647. [Google Scholar]
- 42.Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annual Review of Clinical Psychology. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- 43.Edwards RR, Cahalan C, Calahan C, Mensing G, Smith M, Haythornthwaite JA. Pain, catastrophizing, and depression in the rheumatic diseases. Nature Reviews Rheumatology. 2011;7(4):216–224. doi: 10.1038/nrrheum.2011.2. [DOI] [PubMed] [Google Scholar]
- 44.Redelmeier DA, Katz J, Kahneman D. Memories of colonoscopy: A randomized trial. Pain. 2003;104(1–2):187–194. doi: 10.1016/s0304-3959(03)00003-4. [DOI] [PubMed] [Google Scholar]


