Abstract
Background: Though iodine deficiency in pregnancy is a matter of public-health concern, a functional measure of iodine status is lacking. The thyroid-specific protein thyroglobulin (Tg), which reflects thyroid size, has shown promise as a functional measure in studies of children and adults, but data in pregnancy are sparse. In a cohort of mildly to moderately iodine-deficient pregnant women, this study aimed to explore whether serum Tg is a sensitive functional biomarker of iodine status and to examine longitudinal change in Tg with gestational age.
Method: A total of 230 pregnant women were recruited at an antenatal clinic at 12 weeks of gestation to the Selenium in PRegnancy INTervention study, in Oxford, United Kingdom. Repeated measures of urinary iodine-to-creatinine ratio, serum thyrotropin (TSH), and Tg at 12, 20, and 35 weeks of gestation were made. Women were dichotomized by their iodine-to-creatinine ratio (<150 or ≥150 μg/g) to group them broadly as iodine deficient or iodine sufficient. Women with thyroid antibodies were excluded; data and samples were available for 191 women.
Results: Median Tg concentrations were 21, 19, and 23 μg/L in the first, second, and third trimesters, respectively. In a linear mixed model, controlling for confounders, Tg was higher in the <150 μg/g group than it was in the ≥150 μg/g group (p < 0.001) but there was no difference in TSH (p = 0.27). Gestational week modified the effect of iodine status on TSH (p = 0.01) and Tg (p = 0.012); Tg did not increase with gestational week in the ≥150 μg/g group, but it did in the <150 μg/g group, and TSH increased more steeply in the <150 μg/g group.
Conclusions: Low iodine status (<150 μg/g) in pregnancy is associated with higher serum Tg, suggesting that the thyroid is hyperstimulated by iodine deficiency, which causes it to enlarge. Tg is a more sensitive biomarker of iodine status in pregnancy than is TSH.
Keywords: : iodine, thyroglobulin, pregnancy, thyroid, nutrition
Introduction
An adequate dietary supply of iodine is essential for the production of the thyroid hormones thyroxine (T4) and triiodothyronine (T3). Hence, the recent recognition that the iodine status of teenage schoolgirls (1), women of child-bearing age (2,3), and pregnant women (4–6) in the United Kingdom appears to be suboptimal has raised concern. The finding of an association between the mild-to-moderate degree of iodine deficiency found in British pregnant women and lower IQ and reading scores in their eight- to nine-year-old children (7) has increased that concern.
Iodine status is commonly assessed by measurement of urinary iodine concentration (UIC) from a spot-urine sample (8). However, UIC is not a functional biomarker of iodine status; it only reflects recent intake (past 24–48 h) and may therefore not be representative of usual intake in an individual. Although the thyroid hormones (T3 and T4) and thyrotropin (TSH) are functional measures, they are not sensitive markers of iodine status, as values can remain in the normal reference range in individuals with suboptimal iodine intake because of tight homeostatic regulation (9). By contrast, the thyroid-specific protein thyroglobulin (Tg) shows promise as a functional biomarker of iodine status that better reflects long-term iodine intake (weeks or months) (8,9). Serum Tg concentration is considered to reflect thyroid volume in both iodine-deficient and iodine-excessive settings (10). In iodine deficiency, high Tg concentration results from TSH stimulation of the thyroid, leading to thyroid enlargement (9). Antibodies to Tg (TgAb) can interfere with Tg measurement, resulting in either a higher Tg concentration when measured by radioimmunometric assay (RIA) or a lower concentration when measured by an immunometric assay (11). TgAb therefore need to be measured concurrently so that those individuals who are TgAb-positive can be excluded from analysis.
While studies have explored the relationship between iodine status and serum Tg in both adults and children (10,12), there is little exploration of this relationship in pregnancy (9), especially with a repeated-measure study design. Furthermore, there are no Tg data from pregnant women in the United Kingdom—a region of mild-to-moderate iodine deficiency. This study therefore aimed to investigate the relationship between iodine status (as measured by the iodine-to-creatinine ratio) and serum Tg concentration in a cohort of British pregnant women to test whether Tg might be a useful functional biomarker of low iodine status and to understand the change in Tg concentration during pregnancy under conditions of mild-to-moderate iodine deficiency.
The hypotheses of this study were that iodine status would be negatively associated with serum Tg and that the association would be stronger than with serum TSH. Furthermore, it was hypothesized that the profile of TSH and Tg throughout pregnancy would differ between those classified as iodine deficient and iodine sufficient. A lag effect was anticipated such that TSH would increase more steeply in iodine-deficient women as pregnancy advanced and stores of iodine became depleted.
Materials and Methods
This study used samples and data collected as part of the Selenium in Pregnancy INTervention (SPRINT) study, a double-blind placebo-controlled randomized trial (ISRCTN37927591) that investigated the effect of selenium supplementation on markers of risk of pre-eclampsia. Two hundred and thirty primiparous women (sample size calculated to detect differences in biological markers of pre-eclampsia) were recruited when attending for an ultrasound scan at 12–14 weeks of gestation at the John Radcliffe Hospital (Oxford, United Kingdom) between July 2009 and June 2011. Relevant exclusion criteria were current smoking, being on thyroid medication, and taking a selenium-containing supplement. As most prenatal supplements contain both iodine and selenium, this exclusion criterion meant that very few women were taking an iodine-containing supplement (13). One woman was recruited in error, as she was taking levothyroxine and was therefore excluded. Full details of the SPRINT study have already been reported (14).
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Milton Keynes Research Ethics Committee (REC ref no. 08/H0603/46). A non-substantial amendment for additional laboratory measurements in stored samples was approved by NRES Committee South Central Berkshire (July 27, 2011). Written informed consent was obtained from all subjects.
Procedures
Blood and urine samples were collected at approximately 12, 20, and 35 weeks of gestation. TSH, free T4 (fT4), and thyroid peroxidase antibodies (TPOAb) were measured in serum samples using a Modular Analytics E170 analyzer (Roche Diagnostics, Mannheim, Germany) at the Department of Clinical Chemistry, Máxima Medical Center (Veldhoven, The Netherlands). Tg and TgAb were measured using an electrochemiluminescence immunoassay on a Cobas e601 analyzer (Roche Diagnostics) at the Máxima Medical Center. The serum Tg assay was calibrated against the Certified Reference Material for human Tg (CRM-457). TPOAb concentrations >35 IU/mL and TgAb >115 KIU/L (manufacturer's cutoff) were considered as positive for TPOAb and TgAb, respectively. Women were defined as thyroid-antibody positive if they were positive for TPOAb and/or TgAb. The use of trimester- and method-specific reference ranges from an iodine-sufficient and thyroid-antibody negative population are recommended for thyroid function tests in pregnancy (15). However, no method-specific (i.e., Roche) reference ranges exist in the literature (16). Trimester-specific reference ranges for TSH and fT4 derived from women without thyroid autoimmunity from an iodine-sufficient region were therefore used (17). The reference ranges and the definitions of overt hypothyroidism, hyperthyroidism, subclinical hypothyroidism, and isolated hypothyroxinemia are reported in our previous study that had the same participants as the current study (18). For assessment of Tg, a cutoff of 40 μg/L was used to indicate high Tg concentration based on previous research in adults and children (9).
Measurement of urinary iodine and creatinine concentration was carried out at the Trace Element Unit, Southampton General Hospital, as previously described (13). Briefly, iodine was measured using a dynamic reaction cell inductively coupled plasma mass spectrometry (ICP-MS), and certified reference materials were used to ascertain the accuracy of the method. Creatinine was measured using the UniCel DxC Synchron Clinical System Analyzer by the Jaffe rate method.
The iodine-to-creatinine ratio was used in preference to the UIC, as SPRINT women were requested to attend the hospital with a full bladder for their ultrasound scan, and as a result, some urine samples were very dilute; use of the UIC would have overstated iodine deficiency in the cohort (13).
Two hundred and nineteen women (95.6% of the cohort) completed a Food Frequency Questionnaire (FFQ) at approximately 12 weeks of gestation (13), and data on milk intake from the FFQ were used to explore the association with serum Tg concentration. Our previous study in this cohort found an association between milk intake and the urinary iodine-to-creatinine ratio (13).
Statistical analysis
As the selenium intervention had no effect on urinary iodine-to-creatinine ratio (13), TSH, or fT4 in antibody-negative women in the trial (18), data were pooled to analyze the women as one group, regardless of intervention.
There is a known effect of autoimmune thyroid disease on TSH (19), and TgAbs can interfere with the interpretation of Tg analysis (9). Therefore, women who were positive for TPOAb and/or TgAb at 12 weeks of gestation (n = 34; 14.9%) and those with overt thyroid disease (one woman [0.4%] had overt hyperthyroidism at 12 weeks of gestation) were excluded in order to describe more accurately the relationship between iodine status and thyroid function. Two additional women with a urinary iodine-to-creatinine ratio >700 μg/g who were clear outliers (i.e., values suggestive of excessive iodine intake) were excluded, and one woman with transient subclinical hyperthyroidism at 12 weeks was also excluded, as inclusion prevented convergence of the linear mixed models. This left 191 women for statistical analysis.
Women were dichotomized on the basis of maternal urinary iodine-to-creatinine ratio as ≥150 μg/g (i.e., sufficient) or <150 μg/g (i.e., deficient), as in our previous study (7). The groups were further divided by iodine-to-creatinine ratio as <100, 100–149, 150–249, and ≥250 μg/g, based on methodology in a Belgian study (20).
Normally distributed data (fT4) are reported as mean (standard deviation [SD]). Non–normally distributed data (UIC, iodine-to-creatinine ratio, TSH, and Tg) were transformed using the natural logarithm. TSH and Tg values are reported as geometric means and confidence intervals [CI] by back-transformation into the original units. The study compared (log) Tg concentration between antibody-positive and antibody-negative women, and between categories of milk consumption using t-tests and one-way analysis of variance, respectively. The chi-square test was used for comparison of categories of milk intake and percentage of women with Tg >40 μg/L.
A linear mixed model was used to maximize the utilizable data, as some subjects had missing data. The predictors of TSH and Tg were explored by building models that included variables that are known to affect the concentration of both (20). These variables were iodine-to-creatinine ratio (<150 vs. ≥150 μg/g), gestational week, maternal age (years), smoking status (ex-smoker vs. non-smoker), body mass index (BMI) at 12 weeks of gestation (kg/m2), and ethnicity (Caucasian or other). Season (summer or winter) was also included as a time-varying confounder to account for the underlying change in season with progress of gestation, as has been done previously (13). The model included random effects, with random coefficients at the subject level (i.e., intercept and gestational week). A model was constructed with an interaction term between the iodine variable and gestational week, which allowed us to explore our hypothesis that the effect of gestation would differ according to the iodine status of the individual. Interactions were tested between the iodine variable and other confounders, but these were not statistically significant. The standardized residuals at levels 1 (within-subject) and 2 (between-subjects) were visually assessed for normality. The multivariable-adjusted geometric mean ratios of TSH and Tg were estimated.
fT4 concentration was not modeled because the model would not converge if the same linear mixed model was applied as for the Tg and TSH variables (gestational week could not be included as a random effect). It was therefore not possible to evaluate the predictors of fT4 in the same way as TSH and Tg, as the models were not comparable. Furthermore, there are known problems with fT4 measurement in pregnancy that suggest that the measure may not be reliable (21).
Statistical analysis was conducted using IBM SPSS Statistics for Windows v21.0 (IBM Corp., Armonk, NY). Statistical significance was set at 5%.
Results
At week 12 of gestation, 25 (11%) women were TPOAb-positive, 22 (9.9%) were TgAb-positive, of whom nine (3.9%) had isolated TgAb positivity (i.e., not TPOAb-positive). Geometric mean Tg concentration was lower in the 34 (14.9%) women who were positive for TgAb and/or TPOAb in the first (16 vs. 21; p = 0.17), second (13 vs. 18; p = 0.12), and third trimesters (13 vs. 21; p = 0.02). These women were excluded from further analysis.
The demographic data for the whole cohort of 230 women have been reported previously (14). In these 191 women, the mean (SD) age was 30.6 (4.2) years, the mean BMI was 24.4 (4.1) kg/m2, and 92.1% were Caucasian. The group was classified as mildly-to-moderately iodine deficient in all trimesters, as previously reported (13) (Table 1). TSH increased and fT4 concentration decreased over the course of pregnancy (Table 1).
Table 1.
Thyroid Function Parameters and Urinary Iodine Concentrations in Pregnant Women Negative for TPOAb and/or TgAb Without Overt Thyroid Disease
| First trimester | n | Second trimester | n | Third trimester | n | |
|---|---|---|---|---|---|---|
| Gestational week of samplea,b | 12 (9, 14) | 190 | 20 (17, 23) | 185 | 35 (30, 36) | 178 |
| Iodine concentration (μg/L)a,b | 39.4 (23.3, 84.4) | 190 | 55.4 (32.1, 103) | 186 | 73.2 (44.7, 126.5) | 177 |
| Iodine/creatinine ratio (μg/g)a,b | 104 (66, 172) | 190 | 119 (82, 186) | 186 | 127 (86, 184) | 177 |
| Tg (μg/L)a | 21 (13, 33) | 185 | 19 (12, 30) | 183 | 23 (13, 33) | 179 |
| TSH (mU/L)a | 1.3 (0.1, 4.2) | 190 | 1.8 (0.4, 4.0) | 185 | 2.0 (0.4, 5.4) | 179 |
| fT4 (pmol/L)a | 15.1 (1.8) | 190 | 12.6 (1.4) | 185 | 11.2 (1.4) | 179 |
Values are expressed as median (25th, 75th percentile) for iodine concentration, iodine-to-creatinine ratio, and Tg; fT4 values are expressed as mean (SD); TSH and gestational week are expressed as median (min, max).
Values differ from reference 13, as women with thyroid dysfunction are excluded here.
TPOAb, thyroid peroxidase antibodies; TgAb, thyroglobulin antibodies; Tg, thyroglobulin; TSH, thyrotropin; fT4, free thyroxine.
The median Tg concentration was 21 μg/L in the first trimester (n = 185), 19 μg/L in the second trimester (n = 183), and 23 μg/L in the third trimester (n = 179; Table 1). In the first, second, and third trimesters, 29 (15.7%), 16 (8.7%), and 33 (18.4%) women, respectively, had a high Tg, defined as a concentration >40 μg/L according to the reference range suggested in studies of schoolchildren and adults (9).
A linear mixed model was constructed to explore the effect of iodine status and gestational week on both TSH and Tg while controlling for season, maternal age, smoking status, BMI, and ethnicity. The interaction term between the iodine variable (<150 and ≥150 μg/g) and gestational week was significant in both the TSH (p = 0.010) and Tg (p = 0.012) models. To interpret the effect of the iodine-status group on Tg and TSH concentration, estimated marginal means were reported while holding the continuous interacting variable, gestational week, at the mean. Tg was significantly (p < 0.001) higher in the <150 μg/g group compared with the ≥150 μg/g group (estimated marginal mean 18 vs. 16 μg/L; p < 0.001). By contrast, there was no significant difference in TSH concentration between the <150 and ≥150 μg/g groups at the mean gestational week (estimated marginal mean TSH 1.49 vs. 1.55 mIU/L; p = 0.27).
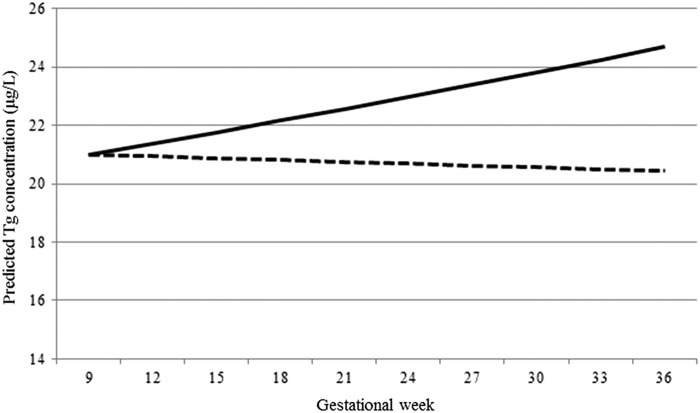
The interaction between gestational week and the dichotomized iodine variable meant that there was no significant increase in Tg with advancing pregnancy in the ≥150 μg/g group, whereas there was an increase in Tg in the <150 μg/g group (Fig. 1 and Table 2). Tg was higher in the <150 μg/g group than it was in the ≥150 μg/g at each time point of gestation, but the difference between the groups was greater in the later stages of pregnancy (Table 3). TSH increased with advancing gestation in both iodine-status groups, but the increase was greater in the <150 μg/g group than it was in the 150 μg/g group (Table 2).
FIG. 1.
Change in thyroglobulin (Tg) throughout gestation according to iodine-status group. Predicted values for Tg based on median at baseline and geometric mean ratio for a one-week increase in the <150 μg/g group (solid line) and the ≥150 μg/g group (dashed line). Results are from a linear mixed model (on log-transformed data), controlling for the effects of season (winter/summer), body mass index (<25 vs. ≥25 kg/m2), smoking status (never vs. ex-smoker), ethnicity (Caucasian vs. other), and maternal age. The interaction between iodine status and gestational week was significant (p = 0.012).
Table 2.
Linear Mixed Model Exploring the Interaction Between Iodine Status and Week of Gestation on TSH and Tg Concentrations
| TSH | Tg | ||||
|---|---|---|---|---|---|
| Urinary iodine-to-creatinine ratio group | Geometric mean ratio [CI]a | p-Value for interactionb | Geometric mean ratio [CI]a | p-Value for interactionb | |
| One week increase in gestation | <150 μg/g | 1.020 [1.016–1.023] | 0.010 | 1.006 [1.003–1.008] | 0.012 |
| ≥150 μg/g | 1.011 [1.001–1.021] | 0.999 [0.992–1.007] | |||
Exponential of β from linear mixed model, controlling for the effects of season (winter/summer), body mass index (<25 vs. ≥25 kg/m2), smoking status (never vs. ex-smoker), ethnicity (Caucasian vs. other), and maternal age.
p-Value for the difference in slope (<150 vs. ≥150 μg/g).
CI, confidence interval.
Table 3.
Tg Concentration in the First, Second, and Third Trimesters of Pregnancy According to Iodine Group
| Tg concentration (μg/L)a | ||||
|---|---|---|---|---|
| 12 weeks | 20 weeks | 35 weeks | ||
| Urinary iodine-to-creatinine ratio group | <150 μg/g | 17 [14–21] | 18 [15–22] | 20 [16–24] |
| ≥150 μg/g | 16 [13–20] | 16 [13–20] | 16 [13–20] | |
| p-Valueb | 0.056 | <0.001 | <0.001 | |
Data are estimated marginal mean [CI]. Estimated marginal means are calculated from the linear mixed-model that controlled for the effects of season (winter/summer), body mass index (<25 vs. ≥25 kg/m2), smoking status (never vs. ex-smoker), ethnicity (Caucasian vs. other), and maternal age. It also included an interaction term between iodine group and gestational week. The estimated marginal means were computed for these time points to reflect the study design.
p-Value comparing the Tg concentration in the <150 and ≥150 μg/g iodine group at each time point.
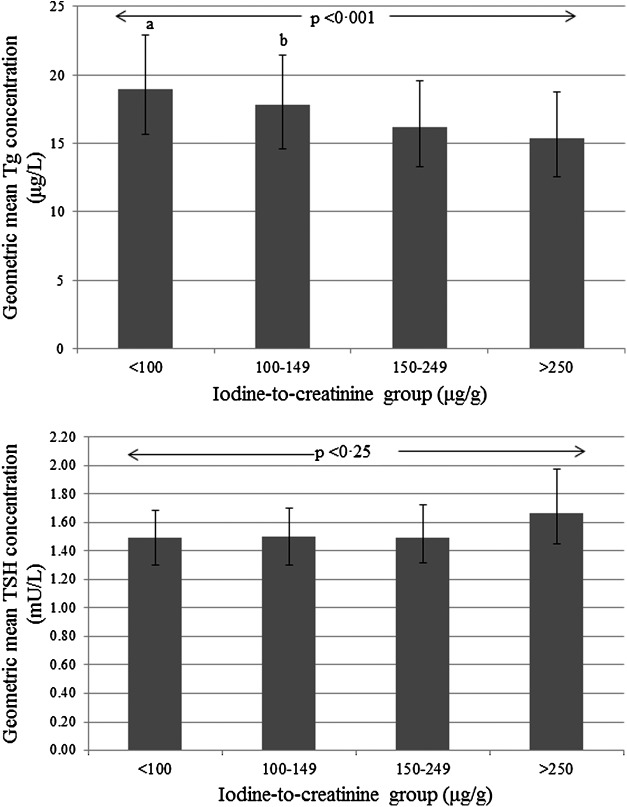
When the four iodine-status groups (<100, 100–149, 150–249, and ≥250 μg/g) were explored, there was no significant interaction between the iodine variable and gestational week on either TSH (p = 0.07) or Tg (p = 0.054) concentration (likely because of reduced power with a smaller sample size in each group). Therefore, only main effects are reported. While there was no difference in TSH concentration between the four groups (p = 0.25), Tg concentrations differed significantly (p < 0.001; Fig. 2). Women in the <100 μg/g and 100–149 μg/g groups had significantly higher Tg concentrations than those in the 150–249 and the ≥250 μg/g groups (Fig. 2). Against the reference group of 150–249 μg/g, the geometric mean ratio of the Tg concentration was 17% higher in the <100 μg/g group (1.17, [CI 1.10–1.25]) and 10% higher in the 100–149 μg/g group (1.10 [CI 1.03–1.17]). There was no significant difference between the >250 μg/g group and the 150–249 μg/g group (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/thy).
FIG. 2.
Adjusted geometric mean TSH and Tg concentration by iodine status (as iodine-to-creatinine ratio, μg/g). The error bars are the confidence intervals of the geometric mean. Results are computed by back transformation of estimated marginal means from a linear mixed model (on log-transformed data), controlling for the effects of gestational week, season (winter/summer), body mass index (<25 vs. ≥25 kg/m2), smoking status (never vs. ex-smoker), ethnicity (Caucasian vs. other), and maternal age. aSignificantly higher than the 150–249 μg/g group (p < 0.001) and the ≥250 μg/g group (p < 0.001). bSignificantly higher than the 150–249 μg/g group (p = 0.005) and the ≥250 μg/g group (p = 0.002).
The study explored whether dietary intake of milk was associated with Tg concentration at 12 weeks of gestation (i.e., when the FFQ was completed). An inverse relationship was found between Tg concentration and milk intake, such that median Tg was lowest in the top milk-intake group (i.e., 24, 21, and 18 μg/L in the <140 mL, 140–280 mL, and >280 mL/day groups, respectively; p = 0.13). The difference between those in the <140 mL group and those in the >280 mL group was significant at the 90% level (p = 0.06). Milk consumption was significantly inversely associated with the percentage of women with high Tg (p = 0.02), with 28.3%, 12.2%, and 8.9% of women in the <140 mL, 140–280 mL, and >280 mL groups having a Tg concentration >40 μg/L. There was no relationship between milk intake and TSH (data not shown).
Discussion
In line with the hypothesis, a negative association was found between iodine status (as measured by the iodine-to-creatinine ratio) and serum Tg concentration. Tg was higher in the group with an iodine-to-creatinine ratio <150 μg/g than it was in the group with a ratio >150 μg/g. Furthermore, when the <150 μg/g and the ≥150 μg/g groups were subdivided, there was a trend for increasing Tg concentration across the four iodine-status groups, with the highest Tg concentration being in those with an iodine-to-creatinine ratio <100 μg/g. These results suggest that Tg may be used as a functional marker of iodine deficiency in a mildly-to-moderately iodine deficient population, and is a notably better marker than TSH.
The results also demonstrate that the effect of advancing gestation on TSH and Tg differs between iodine-sufficient and iodine-deficient women, as was hypothesized. The study shows that in the <150 μg/g group, the increase in TSH during pregnancy was greater than it was in the ≥150 μg/g group. Furthermore, Tg concentration increased throughout pregnancy in the <150 μg/g group, whereas there was no significant change in the ≥150 μg/g group. The difference in Tg concentration between the iodine groups was greatest in later pregnancy. Taken together, these results suggest that even mild-to-moderate iodine deficiency during pregnancy stresses the thyroid. It should be remembered here that thyroid-antibody positive women were excluded from the analysis, which means that the Tg concentration was not influenced by autoimmune destruction of thyroid cells.
The increase in TSH throughout gestation in both iodine-status groups reflects the well-known physiological change (15). In the first trimester, TSH is suppressed as a result of the transient effect of human chorionic gonadotropin (hCG). TSH then increases in the second and third trimesters (15). However, the finding of a greater increase in TSH in the <150 μg/g group may reflect hyperstimulation of the thyroid under conditions of iodine deficiency. The increase in Tg concentration in the iodine-deficient women suggests that thyroid volume is increasing as pregnancy advances. Initially (first trimester), this is likely to be a result of hyperstimulation of the thyroid by hCG, but the later increase probably reflects thyroidal adaptation to the low dietary iodine supply. It has previously been suggested that an increase in thyroid size in pregnancy may be a risk factor for impaired supply of thyroid hormones to the fetus and that the increased size may not completely regress after pregnancy, leading to later thyroid dysfunction in the mother (22).
Randomized controlled trials (RCTs) in pregnant women from regions of mild-to-moderate iodine deficiency have found that iodine supplementation can prevent the increase in thyroid size and Tg concentration seen in the control groups with advancing pregnancy (23–25). The present results support the findings from those RCTs, as no increase in Tg was found in the iodine-sufficient group (i.e., those with an iodine-to-creatinine ratio ≥150 μg/g).
Tg has shown promise as an iodine-status marker, and has been used successfully, along with UIC, in schoolchildren from different countries (12) and in adults in China (26). In a recent RCT, Tg was shown to be responsive to improved iodine status in adults following 24 weeks of iodine supplementation (27). However, the potential for Tg to be a longer-term biomarker of iodine status in pregnant women has seldom been explored, and this is the first such study in British women.
A strength of this study is that it used repeated measures for both Tg and urinary iodine excretion in pregnant women. Although two other studies also had repeated measures, they either did not report the relationship between urinary iodine status and Tg (28), or they only used simple cross-sectional analysis at each trimester (29). Therefore, to the authors' knowledge, this is the first study to use a repeated-measures analysis to relate iodine status to Tg concentration and to explore the change in Tg concentration during pregnancy in relation to urinary iodine excretion. Other studies in pregnancy have measured iodine status and Tg at one time point and have not found an association with either UIC (30,31) (possibly as a result of small sample sizes) or with 24 h urine excretion (32). However, two studies found a significant negative association between UIC and Tg that was stronger than the association between UIC and TSH (20,29). These studies support the present findings. A recent review of Tg found the median Tg to be ≥13 μg/L in the majority of studies of pregnant women from iodine-deficient areas (27), as was the case in our study of mildly to moderately deficient women where the median Tg concentration was 21, 19, and 23 μg/L in trimesters one, two, and three, respectively. There is evidence, both from schoolchildren (12) and pregnant women (33), that the relationship between UIC and Tg is U-shaped. However, there was no evidence of non-linearity in this study, probably because most women were deficient, and it was therefore not possible to explore the effects of iodine excess.
Low intake of milk, the major source of iodine in the United Kingdom, was found to be associated with a higher Tg concentration, suggesting that low iodine intake, either pre-pregnancy or early in pregnancy (the FFQ reflected intake over the previous 12 months), results in increased thyroid size. This finding is similar to that from a study in Denmark that found an association between a low iodine index (based on milk and fish intake) and higher Tg concentration in adults (males and females) (34). Furthermore, Danish pregnant mothers who took an iodine-containing supplement had significantly lower Tg levels at term (35).
The significant interaction found between iodine status and gestational week suggests that simple cross-sectional analysis may not reveal the relationship between iodine and TSH because there may be a lag between inadequate iodine intake and evidence of thyroid dysfunction. Indeed, when women were dichotomized (<150 and ≥150 μg/g) according to their iodine-to-creatinine ratio at 12 weeks, although there was no difference in TSH concentration, which was also measured at 12 weeks, TSH was higher at 20 (p = 0.008) and 35 (p = 0.06) weeks of gestation in the women who were in the <150 μg/g group at 12 weeks. This suggests a lag effect of low iodine status on serum TSH, and might explain why previous studies in pregnant women in iodine-deficient regions that have only examined relationships cross-sectionally have not found significant associations between iodine status and TSH (6,30,36–40).
This study has a number of limitations. First, the women were recruited as part of a trial, and therefore may be of higher socioeconomic and educational status than the general population. It has previously been shown that maternal education is positively associated with iodine-to-creatinine ratio in pregnant women in this and in another cohort (7,13). Second, the study was conducted in a region of mild-to-moderate iodine deficiency, reducing the ability to explore the relationship between iodine and Tg across the full range of iodine status. Third, women with thyroid antibodies were excluded, and therefore the findings relate to “healthy” pregnant women without overt (or subclinical) thyroid dysfunction. Fourth, thyroid volume was not assessed by ultrasound, hence it was not possible to correlate higher Tg concentration with thyroid size.
In conclusion, Tg shows promise as a long-term marker of iodine status in pregnant women. A method for measuring Tg in dried blood spots (DBS) has recently been developed in samples from pregnant women that may be useful in future studies (41). However, the inter-assay variability (even if calibrated to CRM-457) makes the determination of a general clinical cutoff for Tg concentration challenging. Tg measurement is invasive and an additional expense, but this study suggests that urinary iodine excretion and Tg may be complementary measures of iodine status and may give a better picture of status than either measure alone. Future studies should therefore consider measurement of Tg concentration (concurrently with TgAb, as 10% of women in this study were TgAb-positive, which tended to decrease Tg) in addition to urinary iodine-to-creatinine ratio when assessing the iodine status of pregnant women.
Supplementary Material
Acknowledgments
We are extremely grateful to all the women who participated in the SPRINT study, the Research Team, and The Women's Centre, John Radcliffe Hospital, Oxford, where the women were recruited. We are grateful to the following: Dr. Huib Vader of Máxima Medical Center, Veldhoven, for assistance with the analysis of TSH, fT4, and TPOAb; Mr. Alessandro Leidi at the Statistical Services Centre, Reading, for help with the linear mixed model analysis; and Dr. Christine Sieniawska of the Trace Element Unit, Southampton University Hospital NHS Trust, for urine-sample analysis.
A Medical Research Council (MRC) Population Health Scientist Fellowship (MR/K02132X/1) supported S.C.B. The SPRINT study was financially supported by the Wellcome Trust (grant no. 083918/Z/07/Z). A PhD studentship (for S.C.B., 2009–2012) funded by Wassen International and the Waterloo Foundation covered the costs the urinary iodine and creatinine measurements and the Tg and TgAb measurements.
Author Disclosure Statement
V.J.M.P., M.A.C.B., and M.P.R. have nothing to declare. V.L.F.-O. was employed by Danone Nutricia Early Life Nutrition. S.C.B. has received lecture fees from The Dairy Council.
References
- 1.Vanderpump MP, Lazarus JH, Smyth PP, Laurberg P, Holder RL, Boelaert K, Franklyn JA. 2011. Iodine status of UK schoolgirls: a cross-sectional survey. Lancet 377:2007–2012 [DOI] [PubMed] [Google Scholar]
- 2.Bath SC, Sleeth ML, McKenna M, Walter A, Taylor A, Rayman MP. 2014. Iodine intake and status of UK women of childbearing age recruited at the University of Surrey in the winter. Br J Nutr 112:1715–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Combet E, Lean ME. 2014. Validation of a short food frequency questionnaire specific for iodine in UK females of childbearing age. J Hum Nutr Diet 27:599–605 [DOI] [PubMed] [Google Scholar]
- 4.Bath SC, Walter A, Taylor A, Wright J, Rayman MP. 2014. Iodine deficiency in pregnant women living in the South East of the UK: the influence of diet and nutritional supplements on iodine status. Br J Nutr 111:1622–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kibirige MS, Hutchison S, Owen CJ, Delves HT. 2004. Prevalence of maternal dietary iodine insufficiency in the north east of England: implications for the fetus. Arch Dis Child Fetal Neonatal Ed 89:F436–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearce EN, Lazarus JH, Smyth PP, He X, Dall'amico D, Parkes AB, Burns R, Smith DF, Maina A, Bestwick JP, Jooman M, Leung AM, Braverman LE. 2010. Perchlorate and thiocyanate exposure and thyroid function in first-trimester pregnant women. J Clin Endocrinol Metab 95:3207–3215 [DOI] [PubMed] [Google Scholar]
- 7.Bath SC, Steer CD, Golding J, Emmett P, Rayman MP. 2013. Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet 382:331–337 [DOI] [PubMed] [Google Scholar]
- 8.Zimmermann MB, Andersson M. 2012. Assessment of iodine nutrition in populations: past, present, and future. Nutr Rev 70:553–570 [DOI] [PubMed] [Google Scholar]
- 9.Ma ZF, Skeaff SA. 2014. Thyroglobulin as a biomarker of iodine deficiency: a review. Thyroid 24:1195–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knudsen N, Bulow I, Jorgensen T, Perrild H, Ovesen L, Laurberg P. 2001. Serum Tg—a sensitive marker of thyroid abnormalities and iodine deficiency in epidemiological studies. J Clin Endocrinol Metab 86:3599–3603 [DOI] [PubMed] [Google Scholar]
- 11.Netzel BC, Grebe SK, Carranza Leon BG, Castro MR, Clark PM, Hoofnagle AN, Spencer CA, Turcu AF, Algeciras-Schimnich A. 2015. Thyroglobulin (Tg) testing revisited: Tg assays, TgAb assays, and correlation of results with clinical outcomes. J Clin Endocrinol Metab 100:E1074–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmermann MB, Aeberli I, Andersson M, Assey V, Yorg JA, Jooste P, Jukic T, Kartono D, Kusic Z, Pretell E, San Luis TO, Jr., Untoro J, Timmer A. 2013. Thyroglobulin is a sensitive measure of both deficient and excess iodine intakes in children and indicates no adverse effects on thyroid function in the UIC range of 100–299 μg/L: a UNICEF/ICCIDD study group report. J Clin Endocrinol Metab 98:1271–1280 [DOI] [PubMed] [Google Scholar]
- 13.Bath SC, Furmidge-Owen VL, Redman CW, Rayman MP. 2015. Gestational changes in iodine status in a cohort study of pregnant women from the United Kingdom: season as an effect modifier. Am J Clin Nutr 101:1180–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rayman MP, Searle E, Kelly L, Johnson S, Bodman-Smith K, Bath SC, Mao J, Redman CW. 2014. Effect of selenium on markers of risk of pre-eclampsia in UK pregnant women: randomized, controlled pilot trial. Br J Nutr 112:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, Nixon A, Pearce EN, Soldin OP, Sullivan S, Wiersinga W. 2011. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 21:1081–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medici M, Korevaar TI, Visser WE, Visser TJ, Peeters RP. 2015. Thyroid function in pregnancy: what is normal? Clin Chem 61:704–713 [DOI] [PubMed] [Google Scholar]
- 17.Stricker R, Echenard M, Eberhart R, Chevailler MC, Perez V, Quinn FA, Stricker R. 2007. Evaluation of maternal thyroid function during pregnancy: the importance of using gestational age-specific reference intervals. Eur J Endocrinol 157:509–514 [DOI] [PubMed] [Google Scholar]
- 18.Mao J, Pop VJ, Bath SC, Vader HL, Redman CW, Rayman MP. 2016. Effect of low-dose selenium on thyroid autoimmunity and thyroid function in UK pregnant women with mild-to-moderate iodine deficiency. Eur J Nutr 55:55–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearce EN, Oken E, Gillman MW, Lee SL, Magnani B, Platek D, Braverman LE. 2008. Association of first-trimester thyroid function test values with thyroperoxidase antibody status, smoking, and multivitamin use. Endocr Pract 14:33–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreno-Reyes R, Glinoer D, Van Oyen H, Vandevijvere S. 2013. High prevalence of thyroid disorders in pregnant women in a mildly iodine-deficient country: a population-based study. J Clin Endocrinol Metab 98:3694–3701 [DOI] [PubMed] [Google Scholar]
- 21.Lee RH, Spencer CA, Mestman JH, Miller EA, Petrovic I, Braverman LE, Goodwin TM. 2009. Free T4 immunoassays are flawed during pregnancy. Am J Obstet Gynecol 200:260.e1–6 [DOI] [PubMed] [Google Scholar]
- 22.Glinoer D. 2007. The importance of iodine nutrition during pregnancy. Public Health Nutr 10:1542–1546 [DOI] [PubMed] [Google Scholar]
- 23.Brucker-Davis F, Panaia-Ferrari P, Gal J, Fenichel P, Hieronimus S. 2013. Iodine Supplementation throughout pregnancy does not prevent the drop in fT4 in the second and third trimesters in women with normal initial thyroid function. Eur Thyroid J 2:187–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glinoer D, De Nayer P, Delange F, Lemone M, Toppet V, Spehl M, Grun JP, Kinthaert J, Lejeune B. 1995 A randomized trial for the treatment of mild iodine deficiency during pregnancy: maternal and neonatal effects. J Clin Endocrinol Metab 80:258–269 [DOI] [PubMed] [Google Scholar]
- 25.Pedersen KM, Laurberg P, Iversen E, Knudsen PR, Gregersen HE, Rasmussen OS, Larsen KR, Eriksen GM, Johannesen PL. 1993. Amelioration of some pregnancy-associated variations in thyroid function by iodine supplementation. J Clin Endocrinol Metab 77:1078–1083 [DOI] [PubMed] [Google Scholar]
- 26.Chong W, Shi X, Shan Z, Teng X, Teng D, Guan H, Li Y, Jin Y, Yu X, Fan C, Yang F, Dai H, Yu Y, Li J, Chen Y, Zhao D, Hu F, Mao J, Gu X, Yang R, Tong Y, Wang W, Gao T, Li C, Teng W. 2015. Tg in adults as a sensitive biomarker of iodine status: a 5-year follow up population study in different levels of iodine intake regions. PLoS One 10:e0135553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma ZF, Venn BJ, Manning PJ, Cameron CM, Skeaff SA. 2016. Iodine supplementation of mildly iodine-deficient adults lowers thyroglobulin: a randomized controlled trial. J Clin Endocrinol Metab 101:1737–1744 [DOI] [PubMed] [Google Scholar]
- 28.Costeira MJ, Oliveira P, Ares S, Roque S, de Escobar GM, Palha JA. 2010. Parameters of thyroid function throughout and after pregnancy in an iodine-deficient population. Thyroid 20:995–1001 [DOI] [PubMed] [Google Scholar]
- 29.Eltom A, Elnagar B, Elbagir M, Gebre-Medhin M. 2000. Thyroglobulin in serum as an indicator of iodine status during pregnancy. Scand J Clin Lab Invest 60:1–7 [DOI] [PubMed] [Google Scholar]
- 30.Raverot V, Bournaud C, Sassolas G, Orgiazzi JJ, Claustrat F, Gaucherand P, Mellier G, Claustrat B, Borson-Chazot F, Zimmermann M. 2012. Pregnant French women in the Lyon area are iodine deficient and have elevated serum thyroglobulin concentrations. Thyroid 22:522–528 [DOI] [PubMed] [Google Scholar]
- 31.Brough L, Jin Y, Shukri NH, Wharemate ZR, Weber JL, Coad J. 2015. Iodine intake and status during pregnancy and lactation before and after government initiatives to improve iodine status, in Palmerston North, New Zealand: a pilot study. Matern Child Nutr 11:646–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C, Peng S, Zhang X, Xie X, Wang D, Mao J, Teng X, Shan Z, Teng W. 2016. The urine iodine to creatinine as an optimal index of iodine during pregnancy in an iodine adequate area in China. J Clin Endocrinol Metab 101:1290–1298 [DOI] [PubMed] [Google Scholar]
- 33.Shi X, Han C, Li C, Mao J, Wang W, Xie X, Xu B, Meng T, Du J, Zhang S, Gao Z, Zhang X, Fan C, Shan Z, Teng W. 2015. Optimal and safe upper limits of iodine intake for early pregnancy in iodine-sufficient regions: a cross-sectional study of 7,190 pregnant women in China. J Clin Endocrinol Metab 100:1630–1638 [DOI] [PubMed] [Google Scholar]
- 34.Abalovich M, Amino N, Barbour LA, Cobin RH, De Groot LJ, Glinoer D, Mandel SJ, Stagnaro-Green A. 2007. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 92:S1–47 [DOI] [PubMed] [Google Scholar]
- 35.Andersen SL, Nohr SB, Wu CS, Olsen J, Pedersen KM, Laurberg P. 2013. Thyroglobulin in smoking mothers and their newborns at delivery suggests autoregulation of placental iodide transport overcoming thiocyanate inhibition. Eur J Endocrinol 168:723–731 [DOI] [PubMed] [Google Scholar]
- 36.Luton D, Alberti C, Vuillard E, Ducarme G, Oury JF, Guibourdenche J. 2011. Iodine deficiency in northern Paris area: impact on fetal thyroid mensuration. PLoS One 6:e14707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez-Vega J, del Rey FE, Farinas-Seijas H, de Escobar GM. 2008. Inadequate iodine nutrition of pregnant women from Extremadura (Spain). Eur J Endocrinol 159:439–445 [DOI] [PubMed] [Google Scholar]
- 38.Pettigrew-Porter A, Skeaff S, Gray A, Thomson C, Croxson M. 2011. Are pregnant women in New Zealand iodine deficient? A cross-sectional survey. Aust N Z J Obstet Gynaecol 51:464–467 [DOI] [PubMed] [Google Scholar]
- 39.Aguayo A, Grau G, Vela A, Aniel-Quiroga A, Espada M, Martul P, Castano L, Rica Ib. 2013. Urinary iodine and thyroid function in a population of healthy pregnant women in the North of Spain. J Trace Elem Med Biol 27:302–306 [DOI] [PubMed] [Google Scholar]
- 40.Brucker-Davis F, Ferrari P, Gal J, Berthier F, Fenichel P, Hieronimus S. 2013. Iodine status has no impact on thyroid function in early healthy pregnancy. J Thyroid Res 2:187–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stinca S, Andersson M, Erhardt J, Zimmermann M. 2015. Development and validation of a new low-cost enzyme-linked immunoassay for serum and dried blood spot thyroglobulin. Thyroid 25:1297–1305 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.