Abstract
Androgen loss is an important clinical concern because of its cognitive and behavioral effects. Changes in androgen levels are also suspected to contribute to neurological disease. However, the available data on the effects of androgen deprivation in areas of the brain that are central to cognition, like the hippocampus, are mixed. In this study, morphological analysis of pyramidal cells was used to investigate if structural changes could potentially contribute to the mixed cognitive effects that have been observed after androgen loss in males. Male Sprague–Dawley rats were orchidectomized or sham-operated. Two months later, their brains were Golgi-impregnated for morphological analysis. Morphological endpoints were studied in areas CA3 and CA1, with comparisons to females either intact or 2 months after ovariectomy. CA3 pyramidal neurons of orchidectomized rats exhibited marked increases in apical dendritic arborization. There were increases in mossy fiber afferent density in area CA3, as well as robust enhancements to dendritic structure in area CA3 of orchidectomized males, but not in CA1. Remarkably, apical dendritic length of CA3 pyramidal cells increased, while spine density declined. By contrast, in females overall dendritic structure was minimally affected by ovariectomy, while dendritic spine density was greatly reduced. Sex differences and subfield-specific effects of gonadal hormone deprivation on the hippocampal circuitry may help explain the different behavioral effects reported in males and females after gonadectomy, or other conditions associated with declining gonadal hormone secretion.
Keywords: Androgen, Hippocampus, Mossy fibers, Dendritic spines, Sex differences, Gonadectomy
Introduction
Changes in gonadal hormone levels affect the density of hippocampal dendritic spines (Gould et al. 1990; Woolley et al. 1990), as well as spine synapses (Leranth et al. 2003, 2004a), in rodents and non-human primates (Hao et al. 2003; Leranth et al. 2002, 2008). These structural changes are hypothesized to contribute to steroid-induced changes in mood and performance on behavioral tasks involving the hippocampus, as well as to neuropsychiatric disorders such as depression and epilepsy (Edinger and Frye 2007; Frankfurt and Luine 2015; Hajszan and MacLusky 2006; Hajszan et al. 2005, 2010; Harooni et al. 2008; Hauser and Hesdorffer 1990; Naghdi et al. 2005).
In young adult females, estradiol increases dendritic spine density in CA1 (Gould et al. 1990; Woolley et al. 1990) as well as spine synapse density in CA1, CA3 and the dentate gyrus (Hajszan et al. 2010; Leranth et al. 2002). In males, androgens induce similar effects in CA1, orchidectomy decreasing CA1 dendritic spine density (Li et al. 2012) and spine synapse density (Leranth et al. 2003, 2004b; Mendell et al. 2014; Meyer et al. 1978), while androgen replacement reverses these effects (Leranth et al. 2003; MacLusky et al. 2004). Unlike females, however, the structural changes induced by androgens in males are not associated with consistent effects on hippocampal function. In animals, androgens have been reported to have both positive and negative effects on measures of hippocampal-dependent behavior (Leonard and Winsauer 2011, for review). In men, androgen treatment has variable effects on indices of mood (Amanatkar et al. 2014; Pope et al. 2000) and cognitive function (Maki et al. 2007; Young et al. 2010), while androgen ablation therapy for treatment of prostate cancer only impairs some measures of cognitive function (particularly visuospatial function), leaving others either unaffected or enhanced (Alibhai et al. 2010; Jamadar et al. 2012; Matousek and Sherwin 2010). Although testosterone has been suggested to have proconvulsant effects via conversion to estradiol (Reddy 2004), declining androgen levels in aging men are associated with increased susceptibility to seizures (Hauser and Hesdorffer 1990).
Electrophysiological findings present a similarly confusing picture. Treatment of hippocampal slices from adult male rats with testosterone potentiates Schaffer collateral transmission to CA1 pyramidal neurons (Smith et al. 2002). However, orchidectomy does not reduce, but rather facilitates long-term potentiation (LTP) in CA1 (Harley et al. 2000). Likewise, Skucas et al. (2013) recently reported that orchidectomy increases both long-term potentiation (LTP) and brain-derived neurotrophic factor (BDNF) expression in axons from the granule cells of the dentate gyrus to area CA3 (the mossy fiber pathway), changes that are the opposite of those observed in females after ovariectomy (Kramar et al. 2009; Singh et al. 1995).
We hypothesized that these inconsistent results might reflect mixed effects of orchidectomy on the hippocampus, including both positive and negative components. To test this hypothesis, we examined the effects of orchidectomy on hippocampal structure in male rats. Our results indicate that following orchidectomy, the number of mossy fibers increases, accompanied by an extraordinary increase in the length and branching of CA3 pyramidal cell apical dendrites; yet CA3 spine density decreases, similar to results in CA1. These results contrast dramatically with the effects of ovariectomy in females in CA3, where mossy fibers and dendritic length are relatively stable, and suggest that sex differences in the impact of declining gonadal steroid levels on hippocampal function may reflect differences in the effects of these steroids on the hippocampal circuitry.
Methods and materials
Animals
Adult male and female Sprague–Dawley rats were purchased from Charles River laboratories (Kingston, NY, USA) bred in-house, weaned at 23 days and housed 2–3 per cage in plastic cages under a 12-h light/dark cycle (lights on from 0700 to 1900 hours), with food (Purina 5001, WF Fisher, Somerville, NJ, USA) and water ad libitum until used (60–70 days). Experimental protocols were consistent with the guidelines of the US National Institutes of Health and the Canadian Council on Animal Care and were approved by the relevant Institutional Animal Care and Use Committees.
Surgical procedures and tissue preparation
Rats were either gonadectomized or sham-operated (Sham) under ketamine–xylazine anesthesia (80 mg/kg ketamine hydrochloride/12 mg/kg xylazine hydrochloride) (Edwards et al. 1999a, b; Skucas et al. 2013). Intact females were monitored for reproductive cyclicity by checking vaginal cytology, and were studied after confirmation of two consecutive 4-day cycles. These animals were killed at either proestrus (when circulating estradiol is high) or metestrus (when circulating estradiol is low). Gonadectomized animals were studied 2 months after surgery. After decapitation under carbon dioxide anesthesia, brains were removed and immediately chilled in ice-cold saline. Vibratome sections (200 μm thick) were cut and processed using the Neurotechnologies FD Rapid GolgiStain™ Kit (Milatovic et al. 2010). After staining, sections were immersed in 4 % paraformaldehyde for 15 min, 14 % NH4OH for 15 min, and finally fixed in Kodak rapid fixative for 1 h. They were mounted on gelatin-chrome alum coated slides, dehydrated in ethanol, cleared in xylene and cover-slipped using Per-mount (Fisher Scientific; Pittsburgh, PA, USA). Sections used for analysis were collected between bregma −2.56 to −3.6 (Paxinos and Watson 1982).
Mossy fiber axon quantification
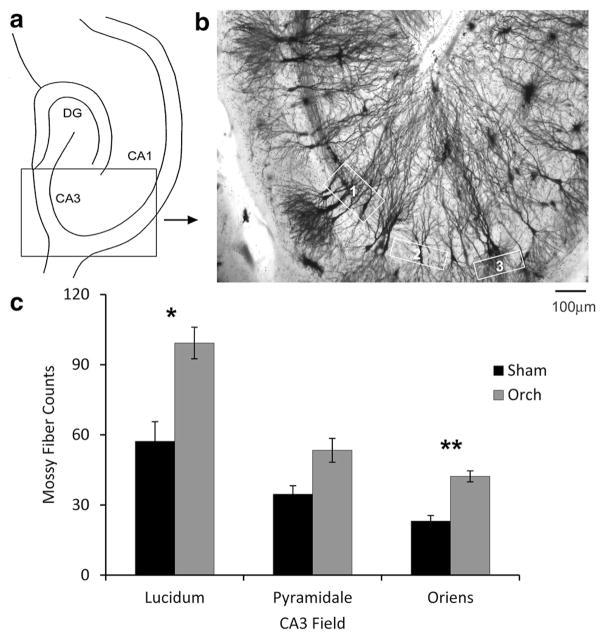
Five sections prepared from a consistent depth of the hippocampus were selected from each animal (n = 3–4 rats per group). Mossy fiber axon quantification was performed in consistent areas of the CA3 subfield in sections from different animals, hippocampal sublayers being identified as previously described (Kole et al. 2004). Mossy fiber axons were counted by moving through sections from top to bottom and counting all discernible fibers in the appropriate areas of CA3 (sampling windows are displayed in Fig. 1a). The counts across the five sections were summed for each animal. Sections were visualized and images captured using an Axio Imager D1 microscope (Carl Zeiss, Toronto, ON, Canada). Counts were verified by two independent blinded observers (A.L.M., S.A.).
Fig. 1.
a Schematic of the hippocampus, indicating the region of CA3 used in counting mossy fibers and analysis of CA3 dendrite structure. b Light micrograph of the region delineated in a from a Golgi-impregnated section, illustrating the sampling areas used for counting mossy fibers (scale bar 100 μm). The sample boxes used are outlined in white, representing the following areas: 1 stratum lucidum, 2 stratum pyramidale; and 3 stratum oriens. c Mossy fiber counts in sham-operated (Sham) and orchidectomized (ORCH) male rats. Statistical analysis: ANOVA (treatment × area of CA3), ORCH vs. Sham, F(1,18) = 17.024, p = 0.0006; interaction, F(2,18) = 1.419, p = 0.2677. ORCH male rats had significantly greater mossy fiber density in the stratum lucidum (Student’s t test, p = 0.044) and stratum oriens (Student’s t test, p = 0.0064) sampling sites than sham-operated (Sham) rats (n = 4 per group). Data represents mean ± SEM
Analysis of dendrite structure
Sholl analysis
Sholl analysis (Sholl 1953) was used to characterize the changes in overall dendrite structure for the apical and basal dendrites of representative CA1 and CA3 pyramidal neurons, as follows. Three-dimensional bright field image stacks were obtained through each section, with the images spaced 1 μm apart in the z-plane, using an Olympus BX53 microscope with an Olympus UPlanSApo 30×, 1.05 NA silicone-immersion objective (Olympus, Richmond Hill, ON, Canada; total magnification 300×). Four neurons were selected in each of CA1 and CA3 for each animal (144 neurons total; n = 3–4 rats per group). The selected neurons were traced using Neurolucida software (Version 10, MicroBrightField, Williston, VT, USA), and Sholl analysis was performed on the neuron tracings using Neurolucida Explorer (MBF Bioscience, Williston, VT, USA). To be included, neurons had to be fully contained within the section (i.e., no processes were cut-off at the edge of the section), with no breaks in dendrite branches. Given the thickness of the sections (200 μm) and the length of the apical dendrites, this meant that only apical dendrites emerging from the cell body in the same plane as the section could be studied, while dendrites projecting either upwards or downwards from this plane could not be evaluated. The data presented below should be interpreted with his limitation in mind. Values for each animal were averaged for statistical analysis.
Apical dendritic length and proximal dendritic width in CA3 neurons
Other measurements from the apical dendrites of CA3 neurons included the length of the longest identifiable apical dendrite and the diameter of the primary dendrite 10 μm from the cell body, prior to the first dendritic branch point. For this analysis, 1–3 sections were selected for each animal from the same depth of the hippocampus, in order to reduce variability in measurements along the rostro-caudal axis of the hippocampus. Image stacks were captured using an Axio Imager D1 microscope at 100× magnification, with an AxioCam MRc5 digital camera and AxioVision 4.6 software (Zeiss). The image stacks were compiled into a single two-dimensional z-projection for each stack using ImageJ processing software (version 1.38x, National Institutes of Health, Bethesda, MD, USA). Z-projection images of the stacks were merged using Adobe® Photoshop® (Version CS5, Adobe Systems Incorporated). All CA3 pyramidal neurons in each section were included for analysis, as long as they were fully contained within the section, had no breakage of primary branches, and could be traced from the soma to the tips of the distal apical dendrites. The length of the longest apical dendrite and the diameter of the primary apical dendrite were measured for every neuron that met the inclusion criteria (orchidectomized: n = 68 neurons; Sham: n = 66; Proestrus: n = 68; Metestrus: n = 32; Ovariectomized: n = 42) using ImageJ. For each neuron, the linear distance was measured from the mid-point of the soma of the pyramidal neuron to the subgranular zone at the crest of the dentate gyrus (Scharfman and Myers 2013), in order to determine whether a relationship existed between cell position in area CA3 (i.e., proximal vs. distal CA3) and the observed morphological effects.
Dendritic spine imaging and analysis
Hippocampal sections were examined under brightfield microscopy (630× total magnification) using an Axio Imager D1 microscope (Zeiss). Pyramidal neuron apical dendrite sections were chosen based on the following criteria: (1) dendrites could be continuously traced from the cell body and were fully impregnated with Golgi stain and (2) at least a 10 μm length of dendrite with well-delineated spines was visible, with the spines clearly distinct from both the dendritic shaft and (in the case of CA3) from thorny excrescences close to the cell body. Twenty-five images for each field were taken in each male and fifteen images for each field in each female, for a total of 2100 images (25 images × 6 fields × 8 males = 1200, 15 images × 6 fields × 10 females = 900; n = 3–4 rats per group). Images were analyzed using Image J by an observer (A.L.M.) blind to treatment status. Because (as described below) there were significant treatment effects on the length of the apical dendrites, we divided the dendrites into sample fields each corresponding to approximately 1/3 of the apical dendritic tree (i.e. proximal 10–30 %, medial 40–60 %, distal 70–100 %). Spine density was calculated as the number of spines per 10 μm length of dendrite. Spine lengths were measured from the distal tip of the spine to the dendrite stalk and calculated as average per dendrite segment, for each neuron (Phan et al. 2011). The number of spines was calculated by multiplying average spine density by the measured dendrite length in each sample field, to obtain an estimate of the number of spines/neuron.
Statistical analysis
Data were tested for normality and homogeneity of variance using the Komolgorov–Smirnov (K–S) test and Bartlett’s test, respectively. Where necessary (in the case of the results from Sholl analysis) data were transformed using the Box–Cox procedure (Sakia 1992). Evaluation using Bartlett’s test confirmed that this procedure was effective in removing the inhomogeneity of variance present prior to transformation. Two-way analysis of variance (ANOVA) and Student’s t tests were performed to analyze data from the mossy fiber and dendritic spine experiments. For Sholl analysis, two-way ANOVAs were performed, followed by Student’s t tests or Bonferroni post hoc tests. The dendrite lengths and proximal dendritic diameters of CA3 pyramidal neuron apical dendrites were compared by regression analysis, using two-way analysis of covariance (ANCOVA). The limits of statistical significance were set at p < 0.05.
Results
Orchidectomy increases mossy fiber axons located in CA3
Previous observations based on recordings from CA3 in response to mossy fiber stimulation suggested that orchidectomy enhances mossy fiber transmission (Skucas et al. 2013). Increased dynorphin immunoreactivity in orchidectomized rats suggested an expansion of the normal mossy fiber plexus (Skucas et al. 2013). Therefore, we first attempted to determine whether the number of detectable mossy fiber axons increased in Golgi-impregnated sections. Significantly more mossy fibers were identified in orchidectomized than in Sham males (Fig. 1). Fibers were not only in their normal location (stratum lucidum) but also expanded into stratum oriens (Fig. 1b).
Orchidectomy increases apical dendritic branching in the CA3 subfield
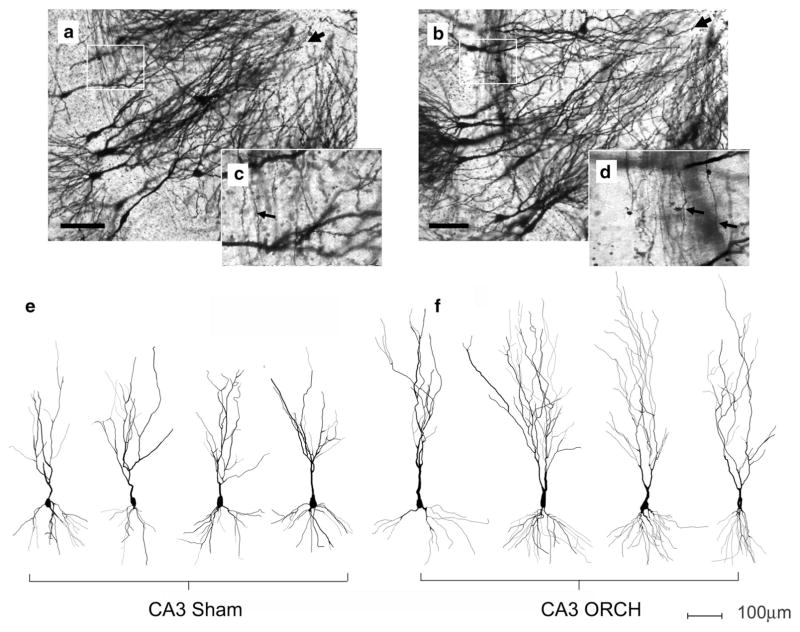
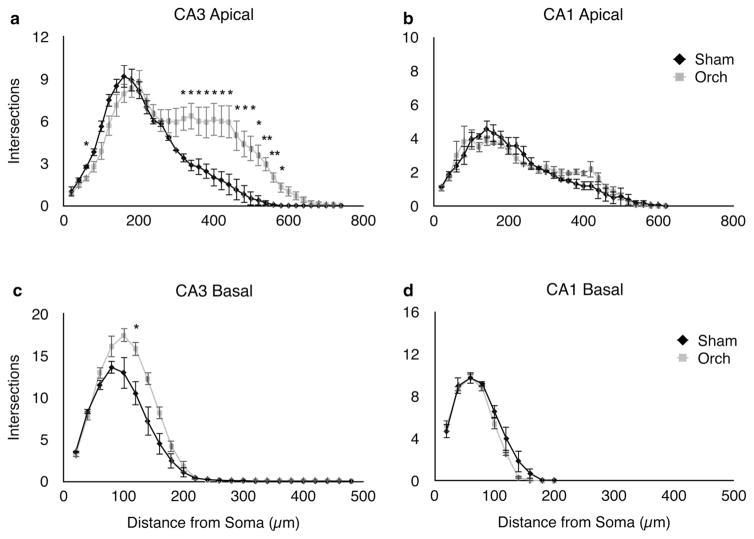
In addition to an increase in mossy fibers, there was a dramatic expansion of the apical dendrites in CA3 pyramidal neurons after orchidectomy. Figure 2 shows representative CA3 pyramidal neurons from orchidectomized males and Sham controls, illustrating the increase in arborization of apical dendrites after orchidectomy, supported by Sholl analysis (Sholl 1953) (Fig. 3). Both apical and basal dendrites were increased, but orchidectomized rats showed the most dramatic increase between 300 and 600 μm from the cell body, corresponding to the location of recurrent collateral synapses (stratum radiatum). Dendrites were also longer, increasing the dendrites located in the terminal zone of the perforant path (stratum lacunosummoleculare) (Fig. 3a). In the basal dendrites, increased branching was apparent approximately 100–150 μm from the cell body (Fig. 3c). In CA1, no changes in branching were observed in the apical or basal dendrites (Fig. 3b, d).
Fig. 2.
Effects of orchidectomy on the dendritic tree structure of pyramidal neurons in area CA3. Micrographs are shown of sections from a sham-operated (a) and an orchidectomized (b) male. Black arrow heads point to the tips of the apical dendrites. White boxes indicate the area of stratum lucidum reproduced at higher magnification in the inset panels c and d, respectively. Arrows on the inset panels point to mossy fibers coursing perpendicular to the axis of the apical dendrites. e, f Example two-dimensional representations of Neurolucida ™ tracings of Golgi-impregnated CA3 neurons from sham-operated (Sham) and orchidectomized (ORCH) animals. Horizontal scale bar on all panels 100 μm
Fig. 3.
Sholl analysis (Sholl 1953) of the apical (a, b) and basal (c, d) dendrites of neurons in areas CA3 (left hand panels) and CA1 (right hand panels). In CA3, the number of intersections recorded was significantly increased in orchidectomized (ORCH) vs. sham-operated (Sham) male rats, in both the apical (a) and basal (c) dendrites. No statistically significant differences were observed between ORCH and Sham males in the apical dendrites of CA1 (b, d). A slight reduction in the length of the CA1 basal dendrites was observed in the ORCH group. Statistical analysis: CA3 apical ANOVA (treatment × distance from soma): F(1,222) = 77.118, p <0.0001. CA3 basal ANOVA; F(1,144) = 32.467, p < 0.0001. CA1 apical ANOVA: F(1,186) = 0.421, p = 0.5174). CA1 basal ANOVA; F(1,60) = 5.167, p = 0.0266. Asterisk indicates a significant difference between ORCH and Sham animals (p < 0.05 Student’s t test). Data represents mean ± SEM (n = 4 per group)
Orchidectomy decreases dendritic spine density
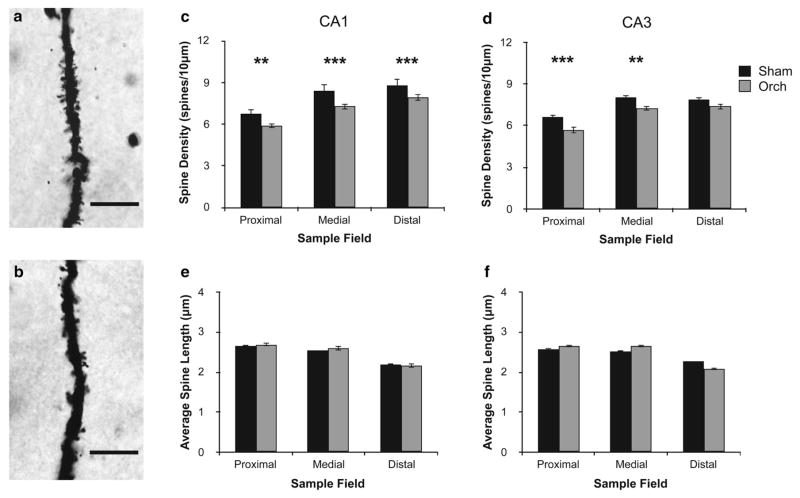
As shown in Fig. 4, orchidectomized males had slightly, but significantly fewer dendritic spines in proximal and medial apical dendrites of CA3, and in all areas of the CA1 apical dendritic tree. No effects of orchidectomy on dendritic spine length were observed (Fig. 4e, f).
Fig. 4.
Effects of orchidectomy on dendritic spines in the CA1 and CA3 regions of the rat hippocampus. Brightfield micrographs (×630) of sections of CA3 apical dendrites in the stratum radiatum from sham-operated (Sham) (a) and orchidectomized (ORCH) (b) males. Scale bars 10 μm. Spine density (c) and length (e) along the proximal, medial and distal apical dendrite segments of CA1 pyramidal neurons, from Sham and ORCH animals. d, f Corresponding data from CA3 neurons. Statistical analysis: spine density, ORCH effect ANOVA (treatment × dendritic field); F(1,36) = 6.198, p = 0.0175; interaction effect F(2,36) = 10.878, p = 0.0002. ORCH males had significantly decreased dendritic spine density in proximal (Student’s t test; p = 0.0071), medial (Student’s t test; p = 0.0001), and distal (Student’s t test; p = 0.0005) fields of CA1 pyramidal neuron apical dendrites (c), as well as proximal (Student’s t test; p = 0.0002) and medial (Student’s t test; p = 0.0083), but not distal (Student’s t test; p = 0.0881) fields of pyramidal neurons in CA3 (d). No effect of ORCH on dendritic spine length was observed (ANOVA; F(1,36) = 0.374, p = 0.5449). Data represents mean ± SEM (n = 4 per group)
Effects of reproductive state in females
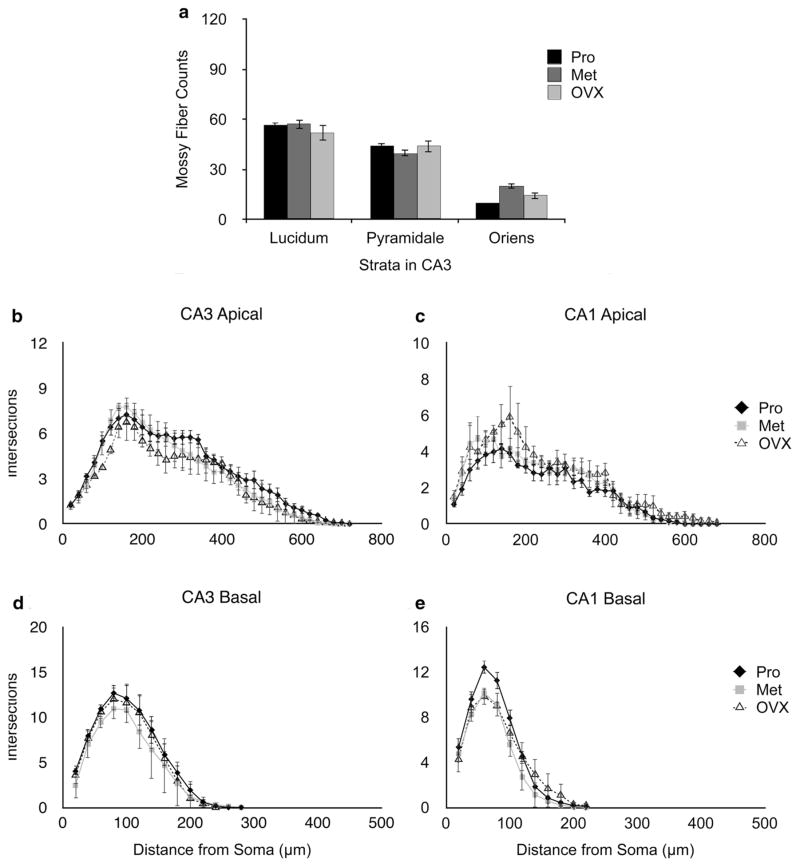
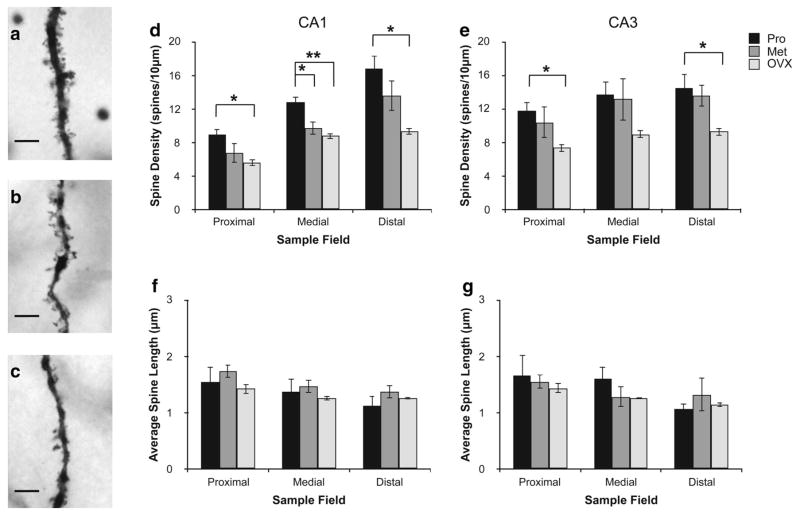
Females were examined at two stages of the estrous cycle (proestrus and metestrus, respectively, times of high and low ovarian estradiol secretion), as well as following ovariectomy. No significant differences were observed between these three groups in mossy fiber density (Fig. 5a). Effects on dendritic branching were small, compared to those in males (Fig. 5b–e). Measurements of spine density, however, revealed major intergroup differences, in both CA1 and CA3 (Fig. 6). Ovariectomized animals had significantly lower dendritic spine densities than either proestrous or metestrous females in the medial field of CA1. Dendritic spine density was reduced at metestrus compared to proestrus, in the medial field of CA1 but not CA3. No differences were observed with respect to spine length in either CA1 or CA3.
Fig. 5.
Mossy fiber counts and Sholl analysis in female rats. a Mossy fiber counts in sections from proestrus (Pro), metestrus (Met) and ovariectomized (OVX) females. Sampling sites are illustrated in Fig. 1. Statistical analysis: ANOVA (reproductive state × area of CA3); reproductive state F(2,18) = 0.299, p = 0.7454, interaction effect ANOVA; F(4,18) = 1.029, p = 0.4193. b–e Effects of stage of the cycle and ovariectomy on the dendritic tree structure of pyramidal neurons in areas CA3 (left panels) and CA1 (right panels). Sholl analysis of the apical (b) and basal (d) dendrites of neurons in area CA3. Sholl analysis of the apical (c) and basal (e) dendrites of neurons in area CA1. Small but statistically significant differences were observed between the three animal groups. Statistical analysis: CA3 apical ANOVA (reproductive state × distance from soma); F(2,252) = 11.415, p < 0.0001; branching in Pro females was significantly greater than either Met (Bonferroni post hoc test; p = 0.0095) or OVX (Bonferroni post hoc test; p = 0.0133) females. Branching in Met females was significantly greater than OVX (Bonferroni post hoc test; p < 0.0001). No statistically significant group-dependent differences were observed in the basal dendrites of CA3 (ANOVA; F(2,98) = 2.715, p = 0.0712). CA1 apical ANOVA: F(2,252) = 11.64, p < 0.0001; branching in OVX females significantly greater than both Pro (Bonferroni post hoc test; p = 0.0044) and Met (Bonferroni post hoc test; p < 0.0001) females. CA1 basal ANOVA: F(2,98) = 7.187, p = 0.0012; Pro females exhibited significantly greater dendritic branching than metestrus (Bonferroni post hoc test; p = 0.0003). Data represents mean ± SEM (n = 3–4 per group)
Fig. 6.
Effects of stage of the estrous cycle and ovariectomy on dendritic spines in the CA1 and CA3 regions of the rat hippocampus. Brightfield micrographs (×630) of sections of apical CA3 dendrites in the stratum radiatum from proestrous (Pro) (a), metestrous (Met) (b) and ovariectomized (OVX) (c) females. Scale bars 10 μm. Spine density (d) and length (f) in the proximal, medial and distal dendrite segments (see “Methods and materials” for details) of CA1 pyramidal neurons, from Pro, Met, and OVX rats. e, g Corresponding data from CA3 neurons. Statistical analysis: a significant overall treatment effect [ANOVA (reproductive state × dendritic field); F(2,42) = 23.001, p < 0.0001] and interaction effect between dendritic field and hippocampal region (ANOVA; F(2,42) = 3.502, p = 0.0405) was observed. OVX females had significantly reduced dendritic spine density compared to Pro females across all fields examined, with the exception of the medial dendrite segment of CA3. Significant differences between proestrus and metestrus animals were also observed in the medial segment (corresponding approximately to stratum radiatum) in CA1, but not CA3. Dendritic spine length did not differ significantly between the three groups (ANOVA; F(2,42) = 1.038, p = 0.3629). Data represents mean ± SEM (n = 3–4 per group)
Sex differences in CA3 dendrite length
Area CA3 varies along the proximo-distal axis (terminology of Ishizuka et al. 1995), i.e. with distance from the dentate gyrus (Ishizuka et al. 1995; Witter 2007). Therefore, the length of the apical dendrites and the diameter of the primary apical dendrite were determined for each treatment group and related to the distance of the CA3 pyramidal cell bodies from the crest of the dentate gyrus.
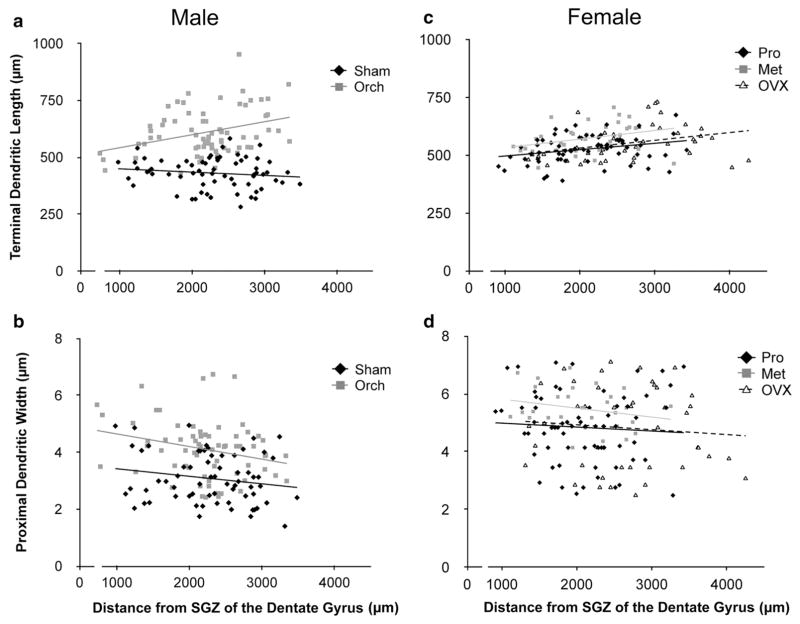
In Sham males, there was no significant change in the maximum length of dendrites along the proximo-distal axis. This pattern was dramatically altered in orchidectomized rats, with an increase in apical dendritic length being observed distal to the dentate gyrus (Fig. 7a). Regression analysis demonstrated that this change was statistically significant. The Y-intercepts of the two regression lines were indistinguishable, indicating that for neurons proximal to the dentate gyrus, maximal dendritic length was not affected by orchidectomy, possibly because the blades of the dentate gyrus limit the extent of these dendrites. However, the difference between sham and orchidectomized rats increased distally, until at the furthest distance examined (>3 mm from the hilus, corresponding approximately to area CA3a; Scharfman 1994) orchidectomized animals had dendrites approximately 60 % longer than those in Sham males (Fig. 7a).
Fig. 7.
Maximal apical dendrite length (a) and thickness of the proximal apical dendritic shaft (b) of CA3 neurons from sham-operated (Sham) and orchidectomized (ORCH) male rats plotted against distance of the cell body from the subgranular zone (SGZ) at the crest of the dentate gyrus (n = 66 neurons for Sham, n = 68 for ORCH). Comparable data for females at proestrus (Pro), metestrus (Met), and 2 months following ovariectomy (OVX) are shown in c and d (Pro n = 68 neurons, Met n = 32, OVX n = 42). Lines indicate the best fit regression lines for the data in each group. Statistical analysis: a ANCOVA comparing data for Sham vs. ORCH males: Slope F(1,130) = 9.189, p = 0.0029; Y-intercept ANCOVA: F(1,130) = 0.709, p = 0.4013. b Corresponding data for dendrite thickness: Slope ANCOVA: F(1,130) = 0.437, p = 0.5097; Y-intercept ANCOVA: F(1,130) = 4.994, p = 0.0271. c ANCOVA comparing Pro, Met and OVX females: slope ANCOVA; F(2,136) = 0.096, p = 0.9084; Y-intercept ANCOVA; F(2,136) = 0.155, p = 0.8562. d Corresponding data for dendrite thickness: slope ANCOVA; F(2,136) = 0.068, p = 0.9339; Y-intercept ANCOVA; F(2,136) = 0.507, p = 0.6036. Additional analysis of the data for dendrite length vs. distance from the hilus for males and females (a, c) revealed no significant difference in the slope of the regression lines between ORCH males and either OVX, Pro or Met females [ANCOVA F(2,202) = 0.099, p = 0.9605]
Orchidectomy also affected the diameter of the primary apical dendrites. Regression analysis revealed a slight decrease in dendrite diameter with increasing distance from the dentate gyrus in both Sham and orchidectomized animals (Fig. 7b). The Y-intercepts, but not the slopes, of the regression lines differed significantly between Sham and orchidectomized rats, indicating that orchidectomy increased the diameter of the primary apical dendrites of pyramidal neurons throughout CA3.
In contrast to males, in females there was no significant effect of gonadectomy on either maximum length of dendrites (Fig. 7c) or the diameter of the primary apical dendrites (Fig. 7d). Interestingly, data for all three groups of females were statistically indistinguishable from those in orchidectomized males, suggesting that dendrite length is reduced in males, but this sex difference is eliminated by orchidectomy.
Discussion
Structural changes in CA3 of the male after orchidectomy
Although the potential for changes in androgen levels to affect behavior and susceptibility to neuropsychiatric disorders has been known for more than 30 years, inconclusive and often apparently contradictory findings have precluded development of a clear understanding of how androgen deprivation affects the brain (Aubele and Kritzer 2012; Leranth et al. 2003, 2004b; Li et al. 2012; MacLusky et al. 2004; Mendell et al. 2014; Meyer et al. 1978). This gap in our understanding is important because declining androgen levels, which are a natural concomitant of the aging process (Yeap 2009), may predispose men to CNS disorders (Hogervorst et al. 2005; Nelson et al. 2008). A drop in androgen levels can also be precipitated abruptly by medical interventions designed to treat hormone-dependent diseases. In the present study, we demonstrate that orchidectomy induces subfield-specific effects on the hippocampal circuitry, including an increase in the length and branching of CA3 pyramidal cell apical dendrites. This may help to explain some of the previously reported electrophysiological and clinical data on the effects of declining androgen levels in men. In particular, the mixed effects on cognitive function may reflect the diverse nature of the changes induced in the hippocampal circuitry. While spine and spine synapse (Leranth et al. 2003; MacLusky et al. 2004) density decline as a result of androgen deprivation, increases in mossy fiber input and CA3 dendritic arborization may well compensate for this deficit. In particular, it seems possible that the post-orchidectomy decrease in apical spine density in CA3 may not reflect a marked overall change in the average number of spines per neuron, given that there is a concomitant expansion of the dendritic tree. The limitations of the Golgi technique preclude precise determination of the total number of spines per neuron, given the possibility that some dendrites and dendritic spines projecting perpendicular to the plane of the section may not have been counted in the analysis. However, a rough estimate of the number of spines can be obtained by multiplying the average dendritic spine densities in each apical dendritic field (Fig. 4) by the measured dendritic lengths (Fig. 3). This calculation yields estimates of the average number of dendritic spines on the apical dendrites of CA3 neurons that are almost identical in sham-operated (1684.5 ± 203.7) and orchidectomized (1686.5 ± 119.7) males, with slight increases in total dendritic spines in orchidectomized vs. sham-operated males, respectively, in the proximal (598.2 ± 71.5 vs. 501.0 ± 53.0) and distal (422.2 ± 44.4 vs. 363.8 ± 94.7) fields, compensating for a reduction in spine numbers in the medial field (666.0 ± 89.8 vs. 819.7 ± 79.3). This is consistent with the hypothesis that orchidectomy may affect the density of spines on the apical dendrites of CA3 neurons by increasing the length of the dendrites over which the spines are distributed, rather than by reducing overall spine numbers.
We have previously reported functional changes in the mossy fiber—CA3 projection after orchidectomy in adult male rats, resulting in enhanced synaptic transmission, LTP, and potentiation of recurrent collateral transmission within CA3 (Skucas et al. 2013). In addition, there was hyperexcitability after orchidectomy (Skucas et al. 2013). The anatomical correlates underlying these effects were not clear, although dynorphin immunohistochemistry suggested an expansion of the mossy fiber terminal field after orchidectomy. Since dynorphin is found in mossy fiber axons and boutons, an increase in dynorphin immunostaining was consistent with the hypothesis that that there was sprouting of the mossy fibers into the pyramidal cell layer and stratum oriens of orchidectomized animals (Skucas et al. 2013). The present results, albeit probably representing only a few of the mossy fibers in each animal because the Golgi method only impregnates a fraction of the neurons present (Das et al. 2013), are consistent with the data from this previous study. In addition, the results show increased dendritic branching in stratum radiatum of orchidectomized rats, providing an anatomical substrate for the potentiation of recurrent collateral transmission. Diameters of the primary dendritic shafts, close to the cell body, increased in orchidectomized rats. This latter observation is likely to reflect an increase in the thorny excrescences, the complex spines of pyramidal neurons which are postsynaptic to mossy fiber boutons. Thorny excrescences are clustered on the primary apical dendrites (Ishizuka et al. 1995), making it difficult to distinguish them clearly at the light microscopic level in Golgi-impregnated sections (Gonzales et al. 2001). In contrast to the expansions of axons and dendrites, dendritic spine density and spine length declined slightly or did not change after orchidectomy.
In CA1, the effects on dendrite length were very different than those in CA3. Apical dendritic length did not change significantly after orchidectomy. Spine density was reduced after orchidectomy, but these changes were small (approximately 10 %).
The data for females contrasted strikingly with those for males. In area CA3, mossy fibers were stable when comparing ovariectomized, proestrous and metestrous rats. Dendritic length and branching patterns varied little between experimental groups, consistent with prior studies (Conrad et al. 2012; McLaughlin et al. 2010). In area CA1, the results confirmed previous work (Gould et al. 1990; Woolley et al. 1990) in showing that spine density was much higher at proestrus than at metestrus, falling approximately 50 % after ovariectomy, so the differences were more pronounced in CA1 than in CA3.
Potential mechanisms mediating orchidectomy-induced hippocampal plasticity
Androgen receptors are abundant in the hippocampus (Clancy et al. 1992; DonCarlos et al. 2003; Sarkey et al. 2008; Tabori et al. 2005), and are localized to many different subcellular compartments: dendritic spines, synaptic vesicles, axons and axon terminals (Sarkey et al. 2008; Tabori et al. 2005), suggesting potentially wide-ranging effects. Previous work has suggested similarities between the effects of androgens in males and estradiol in females, testosterone inducing increases in hippocampal dendritic spine (Li et al. 2012) and spine synapse density (Leranth et al. 2003, 2004b; MacLusky et al. 2004; Meyer et al. 1978), as well as enhancing the survival of newborn neurons in the dentate gyrus (Hamson et al. 2013). The present data indicate that the effects of gonadectomy are in fact quite different in males and females. How can this be explained?
One possible contributing factor may be interactions between the direct effects of the steroids and responses mediated indirectly via BDNF. In males, orchidectomy increases BDNF protein expression in the mossy fibers, an effect that is reversed by testosterone replacement (Skucas et al. 2013). Since BDNF promotes dendritic growth and arborization (Cheung et al. 2007; Lazo et al. 2013; Takemoto-Kimura et al. 2007), while promoting mossy fiber extension (Tamura et al. 2009) the changes observed in orchidectomized males could represent a response to increased mossy fiber BDNF. Although BDNF has also been shown to enhance hippocampal dendritic spine formation (Bennett and Lagopoulos 2014) spine formation requires excitatory synaptic input (Kossel et al. 1997) and NMDA receptor activation (Hamilton et al. 2012; Kellner et al. 2014). Hippocampal NMDA receptors are up-regulated by androgen (Romeo et al. 2005). In orchidectomized animals, reduced NMDA receptor expression may thus limit the potential for new spine formation, despite the rise in BDNF. Other mechanisms could be related to the loss of testosterone metabolites after orchidectomy. Testosterone is metabolized to the androgen receptor ligand dihydrotestosterone, as well as 5α-androstane-3α17β-diol, a positive allosteric modulator of the GABAA receptor. Loss of the latter would potentially lead to increased excitability (Atwi et al. 2015; Scharfman and MacLusky 2014a). Recently it has been shown that estrogen binds to ERα in area CA1 and suppresses GABAergic inhibition via endocannabinoid release, a mechanism which is sexually differentiated (Tabatadze et al. 2015). Moreover, in addition to dynorphin, the mossy fibers contain leuenkephalin, which is estrogen regulated in females and contributes to plasticity at the mossy fiber-CA3 pyramidal cell synapse (Pierce et al. 2014). Neurophysiological data suggest that sex differences in mossy fiber plasticity are opioid receptor-dependent (Harte-Hargrove et al. 2015). Thus, multiple mechanisms capable of influencing pyramidal cell dendritic structure and function exist, as well as sex differences that could explain differences in the regulation of spine density.
Extrahippocampal afferents may also influence the structural responses of the hippocampus to orchidectomy. CA3 receives inputs from other hormone-sensitive regions of the brain. The neurotrophic effects of gonadal steroids on the hippocampus depend at least in part on afferent input (Kovacs et al. 2003; Leranth et al. 2000), from hormone-sensitive cell groups in the basal forebrain (Luine et al. 1975). A particularly important input comes from the entorhinal cortex, via the perforant pathway. Dendrites in stratum lacunosum-moleculare of CA3 receive afferents from the entorhinal cortex (Witter 2007) which produces factors that are trophic for hippocampal neurons (Kossel et al. 1997) including BDNF (Scharfman and Chao 2013, for review). Changes in the entorhinal cortex induced by orchidectomy (Scharfman and MacLusky 2014b) could thus secondarily alter the innervation of distal CA3 pyramidal neurons, contributing to the observed increases in CA3 apical dendritic length in stratum lacunosum-moleculare.
Why are the responses to gonadectomy so different in females? Females, regardless of reproductive state, may exhibit a CA3 dendritic structure similar to orchidectomized males because the normal testosterone-mediated inhibition of mossy fiber transmission observed in males (Skucas et al. 2013) is not present. Although this is a reasonable explanation for the relative stability of CA3 dendrite structure in females, as compared to males, it does not explain why gonadectomy-induced changes in spine density are so much smaller in males. Sex differences in the underlying cellular mechanisms may predominate at the spine and spine synapse level. Females exhibit rapid kinase-mediated effects of estradiol on spine formation, while males do not (Meitzen et al. 2012; Tabatadze et al. 2015). In females, estradiol induces NMDA receptors (Gazzaley et al. 1996) which, as previously mentioned, are important for spine formation (Hamilton et al. 2012; Kellner et al. 2014). Sex differences in the regulation of BDNF expression may also contribute. Although estradiol induces hippocampal BDNF expression at proestrus and estrus (Scharfman et al. 2003; Sohrabji et al. 1995), increased BDNF expression does not necessarily mean more BDNF at the presynaptic and postsynaptic sites involved in regulating dendritic architecture. Thus, in females, while estrogen induces increased hippocampal BDNF mRNA expression, this change is accompanied by decreased hippocampal levels of the mature BDNF protein (Gibbs 1999).
A caveat should, however, be interjected here because with the limitation of the methodology used in the present study (noted in the “Methods and materials” section) as well as the number of animals analyzed, the lack of significant effects on mossy fiber counts and dendrite structure observed in females must be interpreted cautiously. We cannot exclude the possibility that more complete analysis, for example using fluorescent tracer techniques to more completely delineate the complete dendritic arbor structure (Cheng et al. 2014), in larger numbers of animals, could reveal hormonally-induced differences in dendrite length in females. The effects of gonadectomy appear to be greater in males, particularly with respect to the apical dendrites of CA3 neurons; but further work will be necessary to completely delineate the nature of the sex differences.
Pathophysiological implications
Numerous studies have demonstrated sex differences in cognitive function, with females tending to excel in tasks that favor verbal memory, while males exhibit greater visuospatial ability (Halpern et al. 2007). While the neural basis of these differences remains uncertain (Miller and Halpern 2014), analogous differences exist in experimental animals and a contribution from gonadal hormones thus seems likely (Bachevalier and Hagger 1991; Zuloaga et al. 2008). Given the important role of CA3 as an autoassociative network involved in pattern recognition and pattern completion (Kesner 2007; Witter 2007), it seems possible that sex differences in hippocampal processing may involve contributions from androgen-induced sex differences in CA3.
The extent of these effects may well be relatively small, in normal men. Thus, even quite large changes in androgen levels have limited impact on cognitive processing (Maki et al. 2007; Young et al. 2010). Although concern has been expressed about possible effects of androgen deprivation therapy (ADT) on cognitive function and the incidence of neurodegenerative disease (Hogervorst et al. 2005; Nelson et al. 2008), via loss of the neurotrophic actions of testosterone (Leranth et al. 2003, 2004b; MacLusky et al. 2004; Meyer et al. 1978), ADT does not in fact seem to have marked negative cognitive sequelae: some measures (particularly visuospatial function) are impaired, while others are either unaffected or enhanced (Alibhai et al. 2010; Matousek and Sherwin 2010). This may reflect the complex nature of the hippocampal changes induced by androgen deprivation. Loss of androgen induces mossy fiber outgrowth, enhancing neurotransmission from the dentate gyrus to CA3 as well as increasing opportunities for recurrent collateral and perforant path innervation of the apical CA3 pyramidal cell dendrites; but at the same time reducing the specificity of mossy fiber innervation of the CA3 pyramidal neurons (Skucas et al. 2013). Simultaneously, there is a fall in spine and spine synapse density (Leranth et al. 2003, 2004b; MacLusky et al. 2004; Meyer et al. 1978), which may offset the changes in mossy fiber innervation and limit the potential for pyramidal cell activation.
Although the effects of androgen deprivation on hippocampal function may be limited under normal circumstances, this may not be true if the hippocampus is compromised by disease. Changes in CA3 connectivity may functionally compensate for the changes in spine synapse density induced by androgen, but they may also exacerbate pre-existing problems in the hippocampal circuitry resulting from developmental or environmental factors. Increased mossy fiber input to CA3 may represent a mixed blessing—increasing plasticity in the mossy fiber-CA3 pathway, but also raising the potential for CA3 hyperactivation and a loss of afferent specificity (Skucas et al. 2013). Low testosterone has been associated with an increased risk of seizures in men, particularly during aging (Hauser and Hesdorffer 1990), when circulating free serum testosterone levels decline (Yeap 2009). Conversely, down-regulation of the mossy fiber—CA3 connection by androgens may precipitate instability, if this pathway is already functionally impaired. Impaired connectivity in the mossy fiber system has been proposed to contribute to the development of schizophrenia (Faulkner et al. 2008; Kolomeets et al. 2007). Rapid changes in testosterone levels, which occur naturally in boys at puberty, may exacerbate this problem. Consistent with this hypothesis, schizophrenia tends to occur earlier, and is significantly more common, in young men than women, a sex difference that diminishes with age (Abel et al. 2010).
While these represent intriguing potential avenues for further investigation, an important caveat must, however, be noted: these data have been generated entirely from young adult intact and gonadectomized rats. It remains to be determined whether similar changes are observed in aging animals, or in men, after reductions in circulating androgen levels, as well as whether similar responses are observed after the kind of gradual changes in free circulating testosterone that are observed during normal aging. Further studies will be needed to determine whether the effects of androgen deprivation in aging animals are similar to those reported here.
Acknowledgments
Supported by NSERC Discovery Grants to NJM and CDCB and R01 NS 37562 from the National Institutes of Health (USA) to HES. ALM was the recipient of an Ontario Graduate Studentship during these studies.
Footnotes
Compliance with ethical standards
Conflict of interest Ari Mendell, Sarah Atwi, Craig Bailey, Dan McCloskey, Helen Scharfman and Neil MacLusky report no biomedical financial interests or potential conflicts of interest.
References
- Abel KM, Drake R, Goldstein JM. Sex differences in schizophrenia. Int Rev Psychiatry. 2010;22:417–428. doi: 10.3109/09540261.2010.515205. [DOI] [PubMed] [Google Scholar]
- Alibhai SM, Breunis H, Timilshina N, Marzouk S, Stewart D, Tannock I, Naglie G, Tomlinson G, Fleshner N, Krahn M, Warde P, Canning SD. Impact of androgen-deprivation therapy on cognitive function in men with nonmetastatic prostate cancer. J Clin Oncol. 2010;28:5030–5037. doi: 10.1200/JCO.2010.30.8742. [DOI] [PubMed] [Google Scholar]
- Amanatkar HR, Chibnall JT, Seo BW, Manepalli JN, Grossberg GT. Impact of exogenous testosterone on mood: a systematic review and meta-analysis of randomized placebo-controlled trials. Ann Clin Psychiatry. 2014;26:19–32. [PubMed] [Google Scholar]
- Atwi S, McMahon D, Scharfman HE, MacLusky NJ. Androgen modulation of hippocampal structure and function. Neuroscientist. 2015;22(1):46–60. doi: 10.1177/1073858414558065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubele T, Kritzer MF. Androgen influence on prefrontal dopamine systems in adult male rats: localization of cognate intracellular receptors in medial prefrontal projections to the ventral tegmental area and effects of gonadectomy and hormone replacement on glutamate-stimulated extracellular dopamine level. Cereb Cortex. 2012;22:1799–1812. doi: 10.1093/cercor/bhr258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J, Hagger C. Sex differences in the development of learning abilities in primates. Psychoneuroendocrinology. 1991;16:177–188. doi: 10.1016/0306-4530(91)90077-7. [DOI] [PubMed] [Google Scholar]
- Bennett MR, Lagopoulos J. Stress and trauma: BDNF control of dendritic-spine formation and regression. Prog Neurobiol. 2014;112:80–99. doi: 10.1016/j.pneurobio.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Cheng C, Trzcinski O, Doering LC. Fluorescent labeling of dendritic spines in cell cultures with the carbocyanine dye “DiI”. Front Neuroanat. 2014;8:30. doi: 10.3389/fnana.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung ZH, Chin WH, Chen Y, Ng YP, Ip NY. Cdk5 is involved in BDNF-stimulated dendritic growth in hippocampal neurons. PLoS Biol. 2007;5:e63. doi: 10.1371/journal.pbio.0050063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy AN, Bonsall RW, Michael RP. Immunohistochemical labeling of androgen receptors in the brain of rat and monkey. Life Sci. 1992;50:409–417. doi: 10.1016/0024-3205(92)90375-y. [DOI] [PubMed] [Google Scholar]
- Conrad CD, McLaughlin KJ, Huynh TN, El-Ashmawy M, Sparks M. Chronic stress and a cyclic regimen of estradiol administration separately facilitate spatial memory: relationship with hippocampal CA1 spine density and dendritic complexity. Behav Neurosci. 2012;126:142–156. doi: 10.1037/a0025770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das G, Reuhl K, Zhou R. The Golgi–Cox method. Methods Mol Biol. 2013;1018:313–321. doi: 10.1007/978-1-62703-444-9_29. [DOI] [PubMed] [Google Scholar]
- DonCarlos LL, Garcia-Ovejero D, Sarkey S, Garcia-Segura LM, Azcoitia I. Androgen receptor immunoreactivity in forebrain axons and dendrites in the rat. Endocrinology. 2003;144:3632–3638. doi: 10.1210/en.2002-0105. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Androgens’ performance-enhancing effects in the inhibitory avoidance and water maze tasks may involve actions at intracellular androgen receptors in the dorsal hippocampus. Neurobiol Learn Mem. 2007;87:201–208. doi: 10.1016/j.nlm.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Edwards HE, Burnham WM, MacLusky NJ. Testosterone and its metabolites affect afterdischarge thresholds and the development of amygdala kindled seizures. Brain Res. 1999a;838:151–157. doi: 10.1016/s0006-8993(99)01620-0. [DOI] [PubMed] [Google Scholar]
- Edwards HE, Burnham WM, Mendonca A, Bowlby DA, MacLusky NJ. Steroid hormones affect limbic afterdischarge thresholds and kindling rates in adult female rats. Brain Res. 1999b;838:136–150. doi: 10.1016/s0006-8993(99)01619-4. [DOI] [PubMed] [Google Scholar]
- Faulkner RL, Jang MH, Liu XB, Duan X, Sailor KA, Kim JY, Ge S, Jones EG, Ming GL, Song H, Cheng HJ. Development of hippocampal mossy fiber synaptic outputs by new neurons in the adult brain. Proc Natl Acad Sci USA. 2008;105:14157–14162. doi: 10.1073/pnas.0806658105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankfurt M, Luine V. The evolving role of dendritic spines and memory: interaction(s) with estradiol. Horm Behav. 2015;74:28–36. doi: 10.1016/j.yhbeh.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley AH, Weiland NG, McEwen BS, Morrison JH. Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus. J Neurosci. 1996;16:6830–6838. doi: 10.1523/JNEUROSCI.16-21-06830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB. Treatment with estrogen and progesterone affects relative levels of brain-derived neurotrophic factor mRNA and protein in different regions of the adult rat brain. Brain Res. 1999;844:20–27. doi: 10.1016/s0006-8993(99)01880-6. [DOI] [PubMed] [Google Scholar]
- Gonzales RB, DeLeon Galvan CJ, Rangel YM, Claiborne BJ. Distribution of thorny excrescences on CA3 pyramidal neurons in the rat hippocampus. J Comp Neurol. 2001;430:357–368. doi: 10.1002/1096-9861(20010212)430:3<357::aid-cne1036>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, MacLusky NJ. Neurologic links between epilepsy and depression in women: is hippocampal neuroplasticity the key? Neurology. 2006;66:S13–22. doi: 10.1212/wnl.66.66_suppl_3.s13. [DOI] [PubMed] [Google Scholar]
- Hajszan T, MacLusky NJ, Leranth C. Short-term treatment with the antidepressant fluoxetine triggers pyramidal dendritic spine synapse formation in rat hippocampus. Eur J Neurosci. 2005;21:1299–1303. doi: 10.1111/j.1460-9568.2005.03968.x. [DOI] [PubMed] [Google Scholar]
- Hajszan T, Szigeti-Buck K, Sallam NL, Bober J, Parducz A, Maclusky NJ, Leranth C, Duman RS. Effects of estradiol on learned helplessness and associated remodeling of hippocampal spine synapses in female rats. Biol Psychiatry. 2010;67:168–174. doi: 10.1016/j.biopsych.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern DF, Benbow CP, Geary DC, Gur RC, Hyde JS, Gernsbacher MA. The science of sex differences in science and mathematics. Psychol Sci Public Interest. 2007;8:1–51. doi: 10.1111/j.1529-1006.2007.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AM, Oh WC, Vega-Ramirez H, Stein IS, Hell JW, Patrick GN, Zito K. Activity-dependent growth of new dendritic spines is regulated by the proteasome. Neuron. 2012;74:1023–1030. doi: 10.1016/j.neuron.2012.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamson DK, Wainwright SR, Taylor JR, Jones BA, Watson NV, Galea LA. Androgens increase survival of adult-born neurons in the dentate gyrus by an androgen receptor-dependent mechanism in male rats. Endocrinology. 2013;154:3294–3304. doi: 10.1210/en.2013-1129. [DOI] [PubMed] [Google Scholar]
- Hao J, Janssen WG, Tang Y, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen increases the number of spinophilin-immunoreactive spines in the hippocampus of young and aged female rhesus monkeys. J Comp Neurol. 2003;465:540–550. doi: 10.1002/cne.10837. [DOI] [PubMed] [Google Scholar]
- Harley CW, Malsbury CW, Squires A, Brown RA. Testosterone decreases CA1 plasticity in vivo in gonadectomized male rats. Hippocampus. 2000;10:693–697. doi: 10.1002/1098-1063(2000)10:6<693::AID-HIPO1007>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Harooni HE, Naghdi N, Sepehri H, Rohani AH. Intra hippocampal injection of testosterone impaired acquisition, consolidation and retrieval of inhibitory avoidance learning and memory in adult male rats. Behav Brain Res. 2008;188:71–77. doi: 10.1016/j.bbr.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Harte-Hargrove LC, Varga-Wesson A, Duffy AM, Milner TA, Scharfman HE. Opioid receptor-dependent sex differences in synaptic plasticity in the hippocampal mossy fiber pathway of the adult rat. J Neurosci. 2015;35:1723–1738. doi: 10.1523/JNEUROSCI.0820-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser W, Hesdorffer D. Epilepsy: frequency, causes and consequences. Epilepsy Foundation of America; Landover: 1990. [Google Scholar]
- Hogervorst E, Bandelow S, Moffat SD. Increasing testosterone levels and effects on cognitive functions in elderly men and women: a review. Curr Drug Targets CNS Neurol Disord. 2005;4:531–540. doi: 10.2174/156800705774322049. [DOI] [PubMed] [Google Scholar]
- Ishizuka N, Cowan WM, Amaral DG. A quantitative analysis of the dendritic organization of pyramidal cells in the rat hippocampus. J Comp Neurol. 1995;362:17–45. doi: 10.1002/cne.903620103. [DOI] [PubMed] [Google Scholar]
- Jamadar RJ, Winters MJ, Maki PM. Cognitive changes associated with ADT: a review of the literature. Asian J Androl. 2012;14:232–238. doi: 10.1038/aja.2011.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner Y, Godecke N, Dierkes T, Thieme N, Zagrebelsky M, Korte M. The BDNF effects on dendritic spines of mature hippocampal neurons depend on neuronal activity. Front Synaptic Neurosci. 2014;6:5. doi: 10.3389/fnsyn.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP. Behavioral functions of the CA3 subregion of the hippocampus. Learn Mem. 2007;14:771–781. doi: 10.1101/lm.688207. [DOI] [PubMed] [Google Scholar]
- Kole MH, Costoli T, Koolhaas JM, Fuchs E. Bidirectional shift in the cornu ammonis 3 pyramidal dendritic organization following brief stress. Neuroscience. 2004;125:337–347. doi: 10.1016/j.neuroscience.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Kolomeets NS, Orlovskaya DD, Uranova NA. Decreased numerical density of CA3 hippocampal mossy fiber synapses in schizophrenia. Synapse. 2007;61:615–621. doi: 10.1002/syn.20405. [DOI] [PubMed] [Google Scholar]
- Kossel AH, Williams CV, Schweizer M, Kater SB. Afferent innervation influences the development of dendritic branches and spines via both activity-dependent and non-activity-dependent mechanisms. J Neurosci. 1997;17:6314–6324. doi: 10.1523/JNEUROSCI.17-16-06314.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs EG, MacLusky NJ, Leranth C. Effects of testosterone on hippocampal CA1 spine synaptic density in the male rat are inhibited by fimbria/fornix transection. Neuroscience. 2003;122:807–810. doi: 10.1016/j.neuroscience.2003.08.046. [DOI] [PubMed] [Google Scholar]
- Kramar EA, Chen LY, Rex CS, Gall CM, Lynch G. Estrogen’s place in the family of synaptic modulators. Mol Cell Pharmacol. 2009;1:258–262. [PMC free article] [PubMed] [Google Scholar]
- Lazo OM, Gonzalez A, Ascano M, Kuruvilla R, Couve A, Bronfman FC. BDNF regulates Rab11-mediated recycling endosome dynamics to induce dendritic branching. J Neurosci. 2013;33:6112–6122. doi: 10.1523/JNEUROSCI.4630-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard ST, Winsauer PJ. The effects of gonadal hormones on learning and memory in male mammals: a review. Curr Zool. 2011;57:543–558. [Google Scholar]
- Leranth C, Shanabrough M, Horvath TL. Hormonal regulation of hippocampal spine synapse density involves subcortical mediation. Neuroscience. 2000;101:349–356. doi: 10.1016/s0306-4522(00)00369-9. [DOI] [PubMed] [Google Scholar]
- Leranth C, Shanabrough M, Redmond DE., Jr Gonadal hormones are responsible for maintaining the integrity of spine synapses in the CA1 hippocampal subfield of female nonhuman primates. J Comp Neurol. 2002;447:34–42. doi: 10.1002/cne.10230. [DOI] [PubMed] [Google Scholar]
- Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci. 2003;23:1588–1592. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Hajszan T, MacLusky NJ. Androgens increase spine synapse density in the CA1 hippocampal subfield of ovariectomized female rats. J Neurosci. 2004a;24:495–499. doi: 10.1523/JNEUROSCI.4516-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Prange-Kiel J, Frick KM, Horvath TL. Low CA1 spine synapse density is further reduced by castration in male non-human primates. Cereb Cortex. 2004b;14:503–510. doi: 10.1093/cercor/bhh012. [DOI] [PubMed] [Google Scholar]
- Leranth C, Hajszan T, Szigeti-Buck K, Bober J, MacLusky NJ. Bisphenol A prevents the synaptogenic response to estradiol in hippocampus and prefrontal cortex of ovariectomized nonhuman primates. Proc Natl Acad Sci USA. 2008;105:14187–14191. doi: 10.1073/pnas.0806139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Masugi-Tokita M, Takanami K, Yamada S, Kawata M. Testosterone has sublayer-specific effects on dendritic spine maturation mediated by BDNF and PSD-95 in pyramidal neurons in the hippocampus CA1 area. Brain Res. 2012;1484:76–84. doi: 10.1016/j.brainres.2012.09.028. [DOI] [PubMed] [Google Scholar]
- Luine VN, Khylchevskaya RI, McEwen BS. Effect of gonadal hormones on enzyme activities in brain and pituitary of male and female rats. Brain Res. 1975;86:283–292. doi: 10.1016/0006-8993(75)90703-9. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Leranth C. Effects of dehydroepiandrosterone and flutamide on hippocampal CA1 spine synapse density in male and female rats: implications for the role of androgens in maintenance of hippocampal structure. Endocrinology. 2004;145:4154–4161. doi: 10.1210/en.2004-0477. [DOI] [PubMed] [Google Scholar]
- Maki PM, Ernst M, London ED, Mordecai KL, Perschler P, Durso SC, Brandt J, Dobs A, Resnick SM. Intramuscular testosterone treatment in elderly men: evidence of memory decline and altered brain function. J Clin Endocrinol Metab. 2007;92:4107–4114. doi: 10.1210/jc.2006-1805. [DOI] [PubMed] [Google Scholar]
- Matousek RH, Sherwin BB. A randomized controlled trial of add-back estrogen or placebo on cognition in men with prostate cancer receiving an antiandrogen and a gonadotropin-releasing hormone analog. Psychoneuroendocrinology. 2010;35:215–225. doi: 10.1016/j.psyneuen.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Wilson JO, Harman J, Wright RL, Wieczorek L, Gomez J, Korol DL, Conrad CD. Chronic 17β-estradiol or cholesterol prevents stress-induced hippocampal CA3 dendritic retraction in ovariectomized female rats: possible correspondence between CA1 spine properties and spatial acquisition. Hippocampus. 2010;20:768–786. doi: 10.1002/hipo.20678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Grove DD, Mermelstein PG. The organizational and aromatization hypotheses apply to rapid, nonclassical hormone action: neonatal masculinization eliminates rapid estradiol action in female hippocampal neurons. Endocrinology. 2012;153:4616–4621. doi: 10.1210/en.2012-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell AL, Szigeti-Buck K, MacLusky NJ, Leranth C. Orchidectomy does not significantly affect spine synapse density in the CA3 hippocampal subfield in St. Kitts vervet monkeys (Chlorocebus aethiops sabaeus) Neurosci Lett. 2014;559:189–192. doi: 10.1016/j.neulet.2013.10.061. [DOI] [PubMed] [Google Scholar]
- Meyer G, Ferres-Torres R, Mas M. The effects of puberty and castration on hippocampal dendritic spines of mice. A Golgi study. Brain Res. 1978;155:108–112. doi: 10.1016/0006-8993(78)90309-8. [DOI] [PubMed] [Google Scholar]
- Milatovic D, Montine TJ, Zaja-Milatovic S, Madison JL, Bowman AB, Aschner M. Morphometric analysis in neurodegenerative disorders. Curr Protoc Toxicol. 2010;Chapter 12(Unit 12.16) doi: 10.1002/0471140856.tx1216s43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DI, Halpern DF. The new science of cognitive sex differences. Trends Cogn Sci. 2014;18:37–45. doi: 10.1016/j.tics.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Naghdi N, Majlessi N, Bozorgmehr T. The effect of intrahippocampal injection of testosterone enanthate (an androgen receptor agonist) and anisomycin (protein synthesis inhibitor) on spatial learning and memory in adult, male rats. Behav Brain Res. 2005;156:263–268. doi: 10.1016/j.bbr.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Nelson CJ, Lee JS, Gamboa MC, Roth AJ. Cognitive effects of hormone therapy in men with prostate cancer: a review. Cancer. 2008;113:1097–1106. doi: 10.1002/cncr.23658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 1982. [DOI] [PubMed] [Google Scholar]
- Phan A, Lancaster KE, Armstrong JN, MacLusky NJ, Choleris E. Rapid effects of estrogen receptor alpha and beta selective agonists on learning and dendritic spines in female mice. Endocrinology. 2011;152:1492–1502. doi: 10.1210/en.2010-1273. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Kelter DT, McEwen BS, Waters EM, Milner TA. Hippocampal mossy fiber leuenkephalin immunoreactivity in female rats is significantly altered following both acute and chronic stress. J Chem Neuroanat. 2014;55:9–17. doi: 10.1016/j.jchemneu.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Jr, Kouri EM, Hudson JI. Effects of supraphysiologic doses of testosterone on mood and aggression in normal men: a randomized controlled trial. Arch Gen Psychiatry. 2000;57:133–140. doi: 10.1001/archpsyc.57.2.133. discussion 135–136. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Testosterone modulation of seizure susceptibility is mediated by neurosteroids 3α-androstanediol and 17β-estradiol. Neuroscience. 2004;129:195–207. doi: 10.1016/j.neuroscience.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Staub D, Jasnow AM, Karatsoreos IN, Thornton JE, McEwen BS. Dihydrotestosterone increases hippocampal N-methyl-D-aspartate binding but does not affect choline acetyl-transferase cell number in the forebrain or choline transporter levels in the CA1 region of adult male rats. Endocrinology. 2005;146:2091–2097. doi: 10.1210/en.2004-0886. [DOI] [PubMed] [Google Scholar]
- Sakia RM. The Box–Cox transformation technique: a review. Statistician. 1992;41:169–178. [Google Scholar]
- Sarkey S, Azcoitia I, Garcia-Segura LM, Garcia-Ovejero D, DonCarlos LL. Classical androgen receptors in non-classical sites in the brain. Horm Behav. 2008;53:753–764. doi: 10.1016/j.yhbeh.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. Synchronization of area CA3 hippocampal pyramidal cells and non-granule cells of the dentate gyrus in bicuculline-treated rat hippocampal slices. Neuroscience. 1994;59:245–257. doi: 10.1016/0306-4522(94)90593-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Chao MV. The entorhinal cortex and neurotrophin signaling in Alzheimer’s disease and other disorders. Cogn Neurosci. 2013;4:123–135. doi: 10.1080/17588928.2013.826184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. Differential regulation of BDNF, synaptic plasticity and sprouting in the hippocampal mossy fiber pathway of male and female rats. Neuropharmacology. 2014a;76(Pt C):696–708. doi: 10.1016/j.neuropharm.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. Sex differences in the neurobiology of epilepsy: a preclinical perspective. Neurobiol Dis. 2014b;72(Pt B):180–192. doi: 10.1016/j.nbd.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Myers CE. Hilar mossy cells of the dentate gyrus: a historical perspective. Front Neural Circuits. 2013;6:106. doi: 10.3389/fncir.2012.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J Neurosci. 2003;23:11641–11652. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Simpkins JW. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague–Dawley rats. Endocrinology. 1995;136:2320–2324. doi: 10.1210/endo.136.5.7720680. [DOI] [PubMed] [Google Scholar]
- Skucas VA, Duffy AM, Harte-Hargrove LC, Magagna-Poveda A, Radman T, Chakraborty G, Schroeder CE, MacLusky NJ, Scharfman HE. Testosterone depletion in adult male rats increases mossy fiber transmission, LTP, and sprouting in area CA3 of hippocampus. J Neurosci. 2013;33:2338–2355. doi: 10.1523/JNEUROSCI.3857-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MD, Jones LS, Wilson MA. Sex differences in hippocampal slice excitability: role of testosterone. Neuroscience. 2002;109:517–530. doi: 10.1016/s0306-4522(01)00490-0. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RCG, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci USA. 1995;92:11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatadze N, Huang G, May RM, Jain A, Woolley CS. Sex differences in molecular signaling at inhibitory synapses in the hippocampus. J Neurosci. 2015;35:11252–11265. doi: 10.1523/JNEUROSCI.1067-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabori NE, Stewart LS, Znamensky V, Romeo RD, Alves SE, McEwen BS, Milner TA. Ultrastructural evidence that androgen receptors are located at extranuclear sites in the rat hippocampal formation. Neuroscience. 2005;130:151–163. doi: 10.1016/j.neuroscience.2004.08.048. [DOI] [PubMed] [Google Scholar]
- Takemoto-Kimura S, Ageta-Ishihara N, Nonaka M, Adachi-Morishima A, Mano T, Okamura M, Fujii H, Fuse T, Hoshino M, Suzuki S, Kojima M, Mishina M, Okuno H, Bito H. Regulation of dendritogenesis via a lipid-raft-associated Ca2+/-calmodulin-dependent protein kinase CLICK-III/CaMKIgamma. Neuron. 2007;54:755–770. doi: 10.1016/j.neuron.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Tamura M, Tamura N, Ikeda T, Koyama R, Ikegaya Y, Matsuki N, Yamada MK. Influence of brain-derived neurotrophic factor on pathfinding of dentate granule cell axons, the hippocampal mossy fibers. Mol Brain. 2009;2:2. doi: 10.1186/1756-6606-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP. Intrinsic and extrinsic wiring of CA3: indications for connectional heterogeneity. Learn Mem. 2007;14:705–713. doi: 10.1101/lm.725207. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeap BB. Testosterone and ill-health in aging men. Nat Clin Pract Endocrinol Metab. 2009;5:113–121. doi: 10.1038/ncpendmet1050. [DOI] [PubMed] [Google Scholar]
- Young LA, Neiss MB, Samuels MH, Roselli CE, Janowsky JS. Cognition is not modified by large but temporary changes in sex hormones in men. J Clin Endocrinol Metab. 2010;95:280–288. doi: 10.1210/jc.2009-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga DG, Puts DA, Jordan CL, Breedlove SM. The role of androgen receptors in the masculinization of brain and behavior: what we’ve learned from the testicular feminization mutation. Horm Behav. 2008;53:613–626. doi: 10.1016/j.yhbeh.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]