Abstract
PURPOSE
Little research examines whether adiposity or post-diagnosis weight changes influence CVD among breast cancer patients for whom effects may differ due to treatment and recovery.
METHODS
We studied Stage I–III breast cancer survivors 18–<80 years, without pre-existing CVD, diagnosed from 1997–2013 at Kaiser Permanente. Women reported weight at diagnosis and weight and waist circumference (WC) around 24 months post-diagnosis. Using Cox models for time to incident coronary artery disease, heart failure, valve abnormality, arrhythmia, stroke, or CVD death, we examined at-diagnosis body mass index (BMI, n=3,109) and post-diagnosis WC (n=1,898) and weight change (n=1,903, stable, ±5–<10-lbs or ±>=10-lbs).
RESULTS
Mean (SD) age was 57 (11) years and BMI was 28 (6) kg-m2. Post-diagnosis, 25% of women gained and 14% lost ≥10-lbs; mean (SD) WC was 90 (15) cm. Over a median of 8.28 years, 915 women developed CVD. BMI 25–30-kg/m2 (versus BMI<25-kg/m2) was not associated with CVD while BMI≥35-kg/m2 increased risk by 33% (HR:1.33; 95%CI:1.08–1.65), independent of lifestyle and tumor/treatment factors. The increased risk at BMI≥35-kg/m2 attenuated with adjustment for pre-existing CVD risk factors to HR:1.20; 95%CI:0.97–1.50. By contrast, even moderate elevations in WC increased risk of CVD, independent of pre-existing risk factors (HR:1.93; 95%CI:1.31–2.84 comparing ≥100-cm versus ≤80-cm). Post-diagnosis weight change had no association with CVD.
CONCLUSION
Extreme adiposity and any elevation in WC increased risk of CVD among breast cancer survivors; however, changes in weight in the early post-diagnosis period were not associated with CVD. Survivors with high WC and existing CVD risk factors should be monitored.
Keywords: Cardiovascular Diseases, Breast Cancer, Survivors, Body Mass Index, Waist Circumference, Body Weight Changes, Breast Neoplasms
INTRODUCTION
Cardiovascular disease (CVD) is one of the leading causes of death among early-stage breast cancer patients,[1] and some studies suggest survivors may be at increased risk relative to women without a breast cancer history.[2,3] Potential reasons for excess risk include direct effects of treatment (e.g., radiation-induced cardiovascular injury and cardio-toxic effects of systemic therapies) and indirect effects (e.g, de-conditioning). CVD and cancer share common risk factors.[4] Thus, rates of CVD among survivors are likely to increase with improvements in cancer-specific survival and increasing cancer incidence in the aging population, threatening future gains in quality of life and overall survival.
The increasing burden of CVD after breast cancer gives urgent clinical importance to identifying interventions to reduce CVD among survivors. As obesity and elevated waist circumference (WC) increase CVD risk in adults without cancer history,[5] lifestyle modification, including weight loss, has been suggested for overweight breast cancer survivors.[6] Yet, prior research suggests that large changes in bodyweight after cancer diagnosis – gains, but also losses - are associated with reduced survival[7–12] and higher rates of CVD mortality[11] among breast cancer survivors. This raises the possibility that the relationship of adiposity and weight change to CVD among breast cancer survivors could differ from that in women without a cancer history due to treatment effects and the demands of the cancer process. Understanding whether adiposity and weight change at and after diagnosis influence survivors’ CVD risk will inform treatment decisions and targeted lifestyle interventions to improve overall survival and management of co-morbidities.
To date, studies among breast cancer survivors have examined at-diagnosis body mass index (BMI) and broad outcomes such as all-cause mortality without detailed assessment of CVD morbidity or death due to CVD. Additionally, whether weight change after diagnosis influences CVD among early-stage patients has not been examined. This study of early-stage breast cancer patients addresses this gap by examining whether BMI and WC and/or changes in weight after diagnosis predict incident CVD. Importantly, we examine individual CVD endpoints including heart failure (HF) and coronary artery disease (CAD) and consider detailed adjustment for cancer treatments, lifestyle behaviors, and pre-existing CVD risk factors.
METHODS
Population and setting
Participants were drawn from a well-characterized population of Kaiser Permanente Northern California (KPNC) health plan members enrolled in two prospective cohorts with similar characteristics: LACE [19]) and Pathways [20]. The 2,135 LACE participants were diagnosed with breast cancer (AJCC stage I-IIIA) from 1997–2000 and were 18–79 years old. Women enrolled within 39-months (mean [standard deviation, SD] months from diagnosis to enrollment was 21.96 [6.49]), had completed adjuvant therapy if received, and had no prior cancer history within 5 years. Pathways enrolled 4,505 women diagnosed with AJCC Stage I–IV breast cancer from 2006–2013 with no invasive cancer history, and were at least 21 years old. Women enrolled within 2-months (mean [SD] months from diagnosis to enrollment was 1.89 [0.69]). Participants provided informed consent under human subjects’ protocols approved by the institutional review boards at KPNC.
Weight, waist circumference and other covariates
Demographic and cancer risk factors were collected at enrollment via mailed questionnaire or in-person interview, and included age at breast cancer diagnosis, race/ethnicity, education, menopausal status, smoking, moderate-vigorous physical activity (metabolic equivalent (MET)-hours/week), and dietary intake assessed via food frequency questionnaire.
At enrollment, LACE women reported their current weight and height and recalled their weight 12 months before diagnosis. Pathways women reported weight and height at similar time points by completing an enrollment questionnaire and 24-month follow-up questionnaire (mean [SD] months from diagnosis to follow-up questionnaire was 26.08 [2.05]).
From the first reported weights and heights, we computed “at-diagnosis” BMI according to WHO guidelines in kilograms divided by meters squared (kg/m2): normal-weight (18.5–<25-kg/m2), overweight (25–<30- kg/m2), Class I obesity, (30–<35-kg/m2), and Class II/III obesity (≥35-kg/m2). We further computed “post-diagnosis weight change” by subtracting the first (around diagnosis) reported weight from the second (around 24 months post-diagnosis) reported weight. Post-diagnosis weight change was categorized: stable (±5-pounds of diagnosis weight), small gains or losses (>5–<10-pounds), and large gains or losses (≥10-pounds). In sensitivity analysis we considered relative changes (i.e., ±5%, and gains and losses of 5–<10% or of ≥10%).
We also examined WC, which was available at the 24 month post-diagnosis assessments. Women were mailed tape measures and recorded WC in centimeters (cm) one inch above the navel according to a standardized protocol.
Of the 3,619 women who met inclusion criteria for this analysis (Stage I–III, <80 years old, and a KPNC member at diagnosis), we excluded 428 women with CVD events prior to enrollment; 63 women who were underweight BMI (<18.5-kg/m2) at diagnosis; and 19 lacking chemotherapy information, making our analytic sample for at-diagnosis BMI n=3,109. For post-diagnosis weight change, we excluded an additional 1,206 women missing a second weight measurement, leaving n=1,903. For WC, we excluded 5 additional women without waist measurements, leaving n=1,898. Women excluded due to missing data were similar in age, stage, and BMI to those included, but less likely to be non-Hispanic white (64% versus 72%).
Cardiovascular Disease End Points
The primary endpoint was the first occurrence of any newly diagnosed CVD event: coronary artery disease (CAD, nonfatal myocardial infarction or death from coronary causes), heart failure (HF), valve abnormality, arrhythmia, stroke, or CVD death, occurring after the exposure assessment (study enrollment for at-diagnosis BMI or 24-month follow-up for WC and weight change). CVD was continually updated in real time in the Electronic Medical Record (EMR) for all health plan members. All CVD events were ascertained using standardized International Classification of Diseases codes for CVD conditions and CVD death (Supplemental Table 2). Cause of death was from death certificates supplemented with medical records.
Statistical Analysis
Women were followed from the enrollment through July 2015 until first occurrence of CVD or CVD death unless censored due to death from other causes or dis-enrolling from the health plan. Cox proportional hazards regression was used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between at-diagnosis BMI and post-diagnosis WC and changes in weight and incidence of CVD or CVD death, adjusted for covariates. CAD and HF were treated as individual endpoints in secondary analyses. Covariates were ascertained from enrollment questionnaires, medical chart review, the EMR and tumor registry abstraction, and were: age (<60, <70, and 70 years), race/ethnicity (non-Hispanic white, black, Hispanic/Latino, Asian/Pacific Islander or other), menopausal status, smoking status (current, former or never), tumor stage (I, II or III), adjuvant therapy (chemotherapy [no chemotherapy, doxorubicin or non–doxorubicin-containing regimens], and/or radiation), and the presence of CVD risk factors at diagnosis (hypertension, diabetes, hyperlipidemia, and/or peripheral vascular disease). The time scale in regression models and for calculation of unadjusted Kaplan-Meier survivor functions was time since the weight or waist assessment date (e.g., diagnosis for at-diagnosis BMI, and the date of 24-month follow-up questionnaire return for the post-diagnosis weight change or WC exposures) allowing for delayed entry into the cohort (with study entry ranging from 0 to 3 years post-diagnosis).
Due to similar results when stratifying by study (LACE or Pathways) and lack of a significant interaction with the exposures of interest, we combined the two for analysis. Further, study was not associated with CVD and adjustment for study did not alter results; this covariate was omitted from multivariable models.
Additionally, we examined whether associations differed between subgroups defined by cancer treatment (receipt of chemotherapy and/or radiotherapy), and, for post-diagnosis weight change, by at-diagnosis BMI category. In sensitivity analyses, we accounted for competing risks using the %PSHREG macro to fit the proportional sub-distribution hazards model (Fine and Gray, 1999).[13–16] We considered omitting CVD events in the first year of follow-up, and including additional treatment (endocrine and HER2–directed therapy) and dietary (total energy intake [kilocalories/day] and fruit/vegetable intake [servings/day]) covariates in multivariable models. Results were similar; as such, only data from the primary analyses are presented.
Statistical analyses were conducted using SAS Version 9.3 (SAS Institute, Cary, NC). A significance probability <0.05 was considered statistically significant. Proportional hazards were assessed through product terms between the exposures and logged follow-up time; no violations were detected. All statistical inferences were two-sided.
RESULTS
As shown in Table 1, mean (SD) age at diagnosis was 57 (11) years, BMI was 28 (6) kg/m2, WC was 90 (15) cm. One quarter (25%) of women gained ≥10-lbs and 14% lost ≥10-lbs. Half of women had stage I cancer, and half had pre-existing CVD risk factors at diagnosis including diabetes, hyperlipidemia, hypertension or peripheral vascular disease. Compared to leaner breast cancer survivors, obese women were less likely to be physically active and more likely to have pre-existing CVD risk factors at diagnosis and to be black or Hispanic/Latina. Normal-weight survivors were more likely to be pre-menopausal and to gain weight following diagnosis.
Table 1.
Characteristics of Early Stage Breast Cancer Survivors Enrolled in the LACE-Pathways Cohort by Body Mass Index at Diagnosis (n=3,109)
| Overall, n=3,109 | Normal weight, n=1286 | Overweight, n=939 | Class I obesity, n=510 | Class II obesity, n=374 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean, % | SD | N | Mean, % | SD | N | Mean, % | SD | N | Mean, % | SD | N | Mean, % | SD | |
|
|
|||||||||||||||
| Age, years | 3109 | 56.86 | 10.69 | 1286 | 55.78 | 11.20 | 939 | 57.71 | 10.6 | 510 | 58.19 | 10.43 | 374 | 56.62 | 8.93 |
| BMI, kg/m2 | 3109 | 27.54 | 6.13 | 1286 | 22.39 | 1.66 | 939 | 27.24 | 1.40 | 510 | 32.00 | 1.41 | 374 | 39.94 | 4.75 |
| Waist circumference, cm | 1898 | 89.61 | 14.78 | 833 | 79.22 | 8.93 | 598 | 91.64 | 9.28 | 279 | 100.11 | 9.79 | 188 | 113.57 | 14.32 |
| Weight change, lbs | 1903 | 2.60 | 15.06 | 834 | 4.82 | 10.62 | 601 | 3.01 | 14.05 | 280 | 0.71 | 16.66 | 188 | −5.76 | 25.65 |
| Race/Ethnicity, % | |||||||||||||||
| Non-Hispanic White | 2228 | 72 | 919 | 72 | 690 | 73 | 362 | 71 | 255 | 68 | |||||
| Black | 201 | 6 | 41 | 3 | 55 | 6 | 57 | 11 | 48 | 13 | |||||
| Hispanic | 306 | 10 | 100 | 8 | 91 | 10 | 61 | 12 | 54 | 14 | |||||
| Asian/Pacific | 304 | 10 | 194 | 15 | 83 | 9 | 22 | 4 | 5 | 1 | |||||
| Other | 70 | 2 | 30 | 2 | 20 | 2 | 8 | 2 | 12 | 3 | |||||
| Stage, % | |||||||||||||||
| I | 1526 | 49 | 648 | 50 | 477 | 51 | 237 | 46 | 164 | 44 | |||||
| II | 1363 | 44 | 574 | 45 | 398 | 42 | 216 | 42 | 175 | 47 | |||||
| III | 220 | 7 | 64 | 5 | 64 | 7 | 57 | 11 | 35 | 9 | |||||
| Chemotherapy, % | |||||||||||||||
| None | 1351 | 43 | 551 | 43 | 420 | 45 | 233 | 46 | 147 | 39 | |||||
| Non-doxorubicin | 394 | 13 | 155 | 12 | 118 | 13 | 64 | 13 | 57 | 15 | |||||
| Doxorubicin | 1364 | 44 | 580 | 45 | 401 | 43 | 213 | 42 | 170 | 45 | |||||
| Radiation | 1664 | 54 | 689 | 54 | 497 | 53 | 277 | 54 | 201 | 54 | |||||
| Hormone receptor, % | |||||||||||||||
| ER+ | 2558 | 82 | 1064 | 83 | 774 | 82 | 427 | 84 | 293 | 78 | |||||
| PR+ | 2094 | 67 | 871 | 68 | 625 | 67 | 346 | 68 | 252 | 67 | |||||
| HER2+ | 477 | 15 | 211 | 16 | 146 | 16 | 60 | 12 | 60 | 16 | |||||
| Pre-menopausal, % | 1010 | 32 | 483 | 38 | 267 | 28 | 147 | 29 | 113 | 30 | |||||
| Prior CVD risk, %a | 1555 | 50 | 475 | 31 | 475 | 51 | 330 | 65 | 275 | 74 | |||||
| Physically active, %b | 1518 | 49 | 712 | 55 | 477 | 51 | 220 | 43 | 109 | 29 | |||||
| Smoking status, % | |||||||||||||||
| Never smoker | 1625 | ||||||||||||||
| Former smoker | 1268 | 52 | 689 | 54 | 477 | 51 | 261 | 51 | 198 | 53 | |||||
| Current smoker | 216 | 41 | 506 | 39 | 403 | 43 | 208 | 41 | 151 | 40 | |||||
| Pre-menopausal, % | 1010 | 7 | 59 | 7 | 41 | 8 | 25 | 7 | 91 | 3 | |||||
CVD risk factor at diagnosis (Hypertension, Hyperlipidemia, Diabetes Mellitus)
Met physical activity guidelines for Americans: >9 Metabolic Equivalent Task (MET)-hours/week
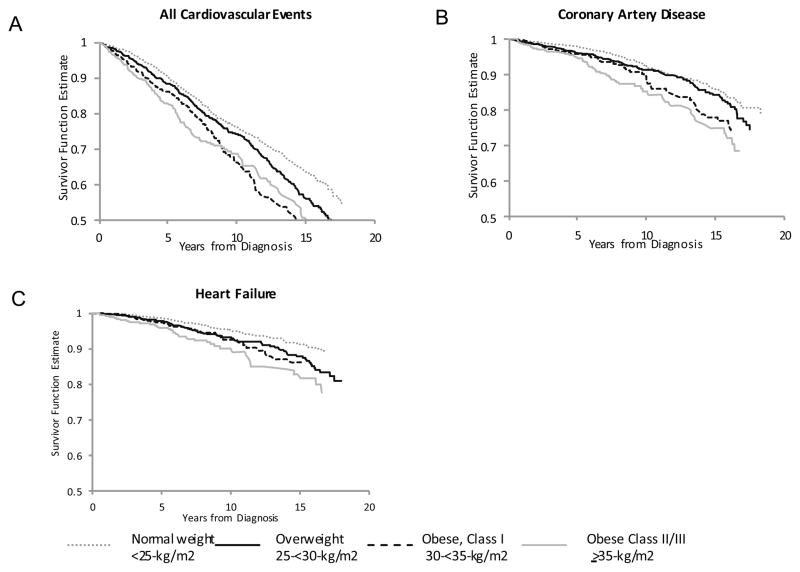
Over a median follow-up of 8.28 years, we observed 915 incident CVD events, including 336 cases of HF and 222 of CAD. Figure 1A shows the unadjusted survivorship function for CVD-free survival by BMI at diagnosis: women who were normal-weight at diagnosis survived the longest without developing CVD, whereas CVD-free survival was shortest among obese women (all log-rank p-values<0.05). Similarly, women with WC≥80 cm were more likely to develop CVD than those with WC<80 cm (all log-rank p-values<0.05, Supplemental Figure 1A). Similar patterns were observed for CAD and HF.
FIGURE 1.
Unadjusted Kaplan-Meier curves of CVD-Free Survival by Body Mass Index at Diagnosis
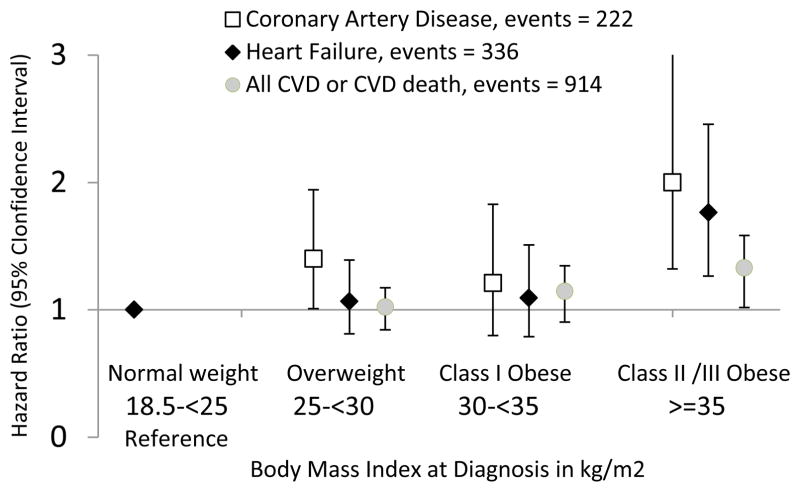
Table 2 shows the association of at-diagnosis BMI and subsequent CVD. In models adjusted for race/ethnicity, age and menopausal status, increasing BMI was associated with risk of CVD in a dose-response fashion: women with Class I obesity experienced a 23% increased risk (HR: 1.23; 95%CI: 1.01–1.48) and women with Class II/III obesity experienced a 44% increased risk (HR: 1.44; 95%CI: 1.17–1.78). After adjustment for lifestyle, tumor and treatment factors, these risks attenuated substantially, and after adjustment for pre-existing CVD risk factors at diagnosis only the trend remained statistically significant: the HR per 5-kg/m2 was 1.07; 95%CI: 1.01–1.14. Results were similar across CVD endpoints, though slightly stronger for CAD than HF (Figure 2; Supplemental Table 1). There was little evidence that associations differed by receipt of chemotherapy (Supplemental Table 3).
Table 2.
Body Mass Index at Diagnosis and Incident CVD Event or Death n=3,109; events= 915
| Overweight 25–<30
kg/m2 N=939 |
Class I Obese 30–<35
kg/m2 N=510 |
Class II/III Obese ≥35
kg/m2 N=374 |
Normal Weight 18.5–<25
kg/m2 N=1286 |
Per 5
kg/m2 N=3,109 |
|
|---|---|---|---|---|---|
|
|
|||||
| Mean (SD) BMI | 27.24 (1.40) | 32.00 (1.41) | 39.94 (4.75) | 22.39 (1.66) | 27.54 (6.13) |
|
|
|||||
| Hazard Ratio for CVD (95% Confidence Interval) | |||||
| CVD Event or Death a | 283 | 166 | 130 | 336 | 915 |
|
|
|||||
| Age & race-adjusted b | 1.05 (0.89, 1.23) | 1.23 (1.01, 1.48) | 1.44 (1.17, 1.78) | Ref. | 1.31 (1.07, 1.19) |
| +lifestyle c | 1.02 (0.87, 1.20) | 1.16 (0.95, 1.40) | 1.36 (1.10, 1.68) | Ref. | 1.11 (1.05, 1.17) |
| +tumor & treatment d | 1.02 (0.87, 1.20) | 1.14 (0.94, 1.38) | 1.33 (1.08, 1.65) | Ref. | 1.10 (1.04, 1.17) |
| +pre-existing CVD risk factors e | 0.99 (0.84, 1.16) | 1.05 (0.86, 1.27) | 1.20 (0.97, 1.50) | Ref. | 1.07 (1.01, 1.14) |
CVD events are composite outcome of both incident CVD events (post-diagnosis heart failure, coronary artery disease, valve abnormality, arrhythmia or stroke) or death in which CVD was the primary cause
Adjusted for age in categories (=<60, <70, or >=70 at diagnosis), menopausal status and race/ethnicity (white, black, Asian/Pacific Islander, Hispanic)
Additionally adjusted for smoking status at diagnosis (never, current or former), and physical activity (MET hours/week of recreational activity at enrollment).
Additionally adjusted for tumor stage, hormone receptor status (ER+, PR+, HER2+) and treatment (receipt of and type of chemotherapy, e.g., Doxorubicin or other, and/or radiation) characteristics
Additionally adjusted for any CVD risk factor at diagnosis (Hypertension, Hyperlipidemia, Diabetes Mellitus)
FIGURE 2.
Body Mass Index at Diagnosis and Subtype of CVD
Adjusted for age in categories (=<60, <70, or >=70 at diagnosis), menopausal status, race/ethnicity (white, black, Asian/Pacific Islander, Hispanic), smoking status at diagnosis (never, current or former), physical activity (MET hours/week of recreational activity at enrollment), tumor stage, hormone receptor status (ER+, PR+, HER2+) and treatment (receipt of and type of chemotherapy, e.g., Doxorubicin or other, and/or radiation).
Table 3 shows that post-diagnosis WC was associated with subsequent CVD among the subset of n=1,898 women with available data (median follow-up time was 6.90 years, with 596 CVD events). Even after adjustment for pre-existing CVD risk factors, women with extreme abdominal obesity experienced an almost 2-fold increase in CVD risk (the HR comparing WC ≥100cm to <80 cm was 1.93; 95%CI: 1.31–2.84). Results were similar for individual CVD endpoints (Supplemental Table 1).
Table 3.
Post-Diagnosis Waist Circumference and Incident CVD Event or Death n=1,898; events= 596
| 80–<90
cm N=480 |
90–<100
cm N=463 |
≥100 cm N=417 |
<80 cm N=535 |
Per 5 cm N=1,898 |
|
|---|---|---|---|---|---|
|
|
|||||
| Mean (SD) Waist circumference | 84.64 (2.71) | 94.61 (2.86) | 110.66 (10.60) | 73.38 (4.67) | 89.61 (14.78) |
|
|
|||||
| Hazard Ratio for CVD (95% Confidence Interval) | |||||
| CVD Event or Death a | 154 | 162 | 156 | 124 | 596 |
|
|
|||||
| Age & race-adjusted b | 1.31 (1.02, 1.68) | 1.34 (1.04, 1.73) | 1.63 (1.26, 2.11) | Ref. | 1.06 (1.03, 1.09) |
| +lifestyle and BMI c | 1.52 (1.15, 2.00) | 1.67 (1.20, 2.32) | 2.01 (1.37, 2.94) | Ref. | 1.09 (1.05, 1.14) |
| +tumor & treatment d | 1.50 (1.14, 1.97) | 1.63 (1.17, 2.26) | 2.02 (1.37, 2.97) | Ref. | 1.09 (1.04, 1.14) |
| +pre-existing CVD risk factors e | 1.48 (1.12, 1.95) | 1.60 (1.15, 2.22) | 1.93 (1.31, 2.84) | Ref. | 1.08 (1.03, 1.13) |
CVD events are composite outcome of both incident CVD events (post-diagnosis heart failure, coronary artery disease, valve abnormality, arrhythmia or stroke) or death in which CVD was the primary cause
Adjusted for age in categories (=<60, <70, or >=70 at diagnosis), menopausal status and race/ethnicity (white, black, Asian/Pacific Islander, Hispanic)
Additionally adjusted for body mass index at time of waist measurement, smoking status at diagnosis (never, current or former), and physical activity (MET hours/week of recreational activity at enrollment).
Additionally adjusted for tumor stage, hormone receptor status (ER+, PR+, HER2+) and treatment (receipt of and type of chemotherapy, e.g., Doxorubicin or other, and/or radiation) characteristics
Additionally adjusted for any CVD risk factor at diagnosis (Hypertension, Hyperlipidemia, Diabetes Mellitus)
Table 4 shows associations of post-diagnosis weight change with subsequent CVD. In this subset of n=1,903 women with weight measurements around diagnosis and 24 months post-diagnosis, median follow-up time after the weight change period was 6.89 years, with 599 CVD events. Most women maintained body weight (±5-lbs: 38%) or gained weight (39%); 23% of women lost ≥5 lbs following diagnosis. Women who gained weight following diagnosis were younger and less likely to have reached menopause or to have pre-existing CVD risk factors at diagnosis (data not shown). Post-diagnosis weight change was not associated with subsequent CVD. In models adjusted for age, race/ethnicity and menopausal status, there was the suggestion of a U-shaped relationship between large gains or losses in bodyweight and CVD (compared to stable weight, the HR for ≥10-lb losses was 1.20; 95%CI: 0.93–1.56 and the HR for ≥10-lb gains was 1.13; 95%CI: 0.90–1.43). These HRs became attenuated substantially after adjustment for lifestyle, tumor and treatment factors. There was no evidence that at-diagnosis BMI modified the association of post-diagnosis weight change and subsequent CVD: results were similar across BMI subgroups (Supplemental Table 4).
Table 4.
Post-Diagnosis Weight Change and Incident CVD Event or Death N=1,903; events = 599
| >10 lb loss n=265 |
5–<10 lb
loss n=170 |
5–<10 lb
gain n=260 |
≥10 lb n=484 |
gain +/− 5 lb
stable n=724 |
|
|---|---|---|---|---|---|
|
|
|||||
| CVD Event or Death a | 92 | 59 | 68 | 151 | 229 |
| Mean (SD) Weight Change | −20.24 (14.68) | −7.01 (1.37) | 6.81 (1.40) | 19.77 (11.46) | 0.23 (2.58) |
| Hazard Ratio for CVD (95% Confidence Interval) | |||||
|
|
|||||
| Age & race-adjusted b | 1.20 (0.93, 1.56) | 1.11 (0.82, 1.49) | 0.92 (0.69, 1.23) | 1.13 (0.90, 1.43) | Ref. |
| + lifestyle c | 1.20 (0.93, 1.56) | 1.09 (0.81, 1.46) | 0.93 (0.70, 1.25) | 1.05 (0.84, 1.33) | Ref. |
| +tumor & treatment d | 1.17 (0.90, 1.52) | 1.08 (0.81, 1.46) | 0.93 (0.69, 1.24) | 1.01 (0.80, 1.28) | Ref. |
| +CVD risk factors e | 1.13 (0.87, 1.48) | 1.04 (0.78, 1.41) | 0.93 (0.70, 1.25) | 1.01 (0.79, 1.28) | Ref. |
CVD events are composite outcome of both incident CVD events (post-diagnosis heart failure, coronary artery disease, valve abnormality, arrhythmia or stroke) or death in which CVD was the primary cause
Adjusted for age in categories (=<60, <70, or >=70 at diagnosis), menopausal status, race/ethnicity (white, black, Asian/Pacific Islander, Hispanic) and body mass index at diagnosis.
Additionally adjusted for smoking status at diagnosis (never, current or former), and physical activity (MET hours/week of recreational activity at enrollment).
Additionally adjusted for adjusted for tumor stage, hormone receptor status (ER+, PR+, HER2+) and treatment (chemotherapy and/or radiation) characteristics
Additionally adjusted for any CVD risk factor at diagnosis (Hypertension, Hyperlipidemia, Diabetes Mellitus)
When post-diagnosis weight changes were modeled as a percentage, results were similar. Analyses accounting for competing risks due to patients dying of other causes (e.g., breast cancer) before developing CVD yielded similar estimates for all exposures.
DISCUSSION
In this prospective study of n=3,109 early-stage breast cancers survivors, nearly 30% of patients had an incident CVD event following diagnosis. Examining at-diagnosis BMI, an increased risk of CVD emerged with Class II/III obesity (BMI ≥35-kg/m2), and was largely explained by pre-existing CVD risk factors. However, even moderate elevations in WC strongly increased risk of CVD independent of pre-existing risk factors, suggesting central adiposity is a more salient measure of CVD risk among breast cancer survivors. We found little indication that weight changes during the early post-diagnosis period influenced CVD risk. While we did not have information on the intentionality of weight loss, our results do not support a CVD benefit of weight loss in the early post-diagnosis period, regardless of at-diagnosis BMI.
Our study is the first to examine adiposity, weight change and risk of incident CVD including CAD and HF among breast cancer survivors controlling for lifestyle, cancer-related and preexisting CVD risk factors, making few studies directly comparable. Results for at-diagnosis BMI are broadly consistent with prior studies of all-cause and CVD mortality: for example, Nichols found that breast cancer survivors with BMI≥30 kg/m2 had a 65% increased risk of CVD mortality compared to normal-weight survivors.[11] In our study, which assessed CVD incidence/morbidity in addition to mortality, the risk of CVD did not become statistically significant until BMI≥35-kg/m2, and even in this group the risk attenuated substantially after adjustment for pre-existing CVD risk factors. A potential explanation is that the cardio-toxic influence of cancer treatment and accompanying declines in physical activity exacerbate/potentiate underlying CVD in breast cancer survivors. However, we observed similarly adverse results regardless of whether chemotherapy was received or included doxorubicin, and no evidence of an interaction between at-diagnosis BMI and treatment type. Thus, the ill effects of Class II/III obesity are not restricted to patients receiving chemotherapy.
In contrast to emergence of increased risk only at a BMI≥35-kg/m2, even moderate elevations in WC were associated with subsequent CVD independent of pre-existing CVD risk factors. While to our knowledge no prior research has examined WC and incident CVD among survivors, this is consistent with the Carolina Breast Cancer Study in which higher waist:hip ratio, but not BMI, was associated with all-cause mortality.[17] Since BMI does not describe fat distribution, and adipose tissue depots differ in their associations with disease, it is unsurprising that WC would show a more graded and consistent association. For CVD, the most relevant exposure may be visceral adiposity, for which WC is a proxy.
In contrast to our finding that weight gain in the early post-diagnosis period had no relationship with CVD, Nichols et al found that each 5-kg of weight gain in the later post-diagnosis period (5–7 years post-diagnosis) was associated with a 19% increase in CVD mortality. While the outcome of CVD mortality is not directly comparable to the composite endpoint of incidence and mortality examined in our study, the different findings may also be due to exposure timing: we examined weight change from diagnosis to 2-years post-diagnosis, during the period when lifestyle recommendations would be made in oncology practice or integrated into survivorship care plans. By contrast, Nichols et al. examined weight changes from diagnosis to 5–7 years post-diagnosis, when many survivors would be in the primary care setting. The later post-diagnosis period may be the most appropriate time for intensive lifestyle intervention to prevent CVD. Consistent with this, in a prior study, we found no association of weight gain in the early post-diagnosis period and subsequent survival.[12] However, an adverse association of weight gain emerged later in the post-diagnosis period among women who were overweight at-diagnosis BMI 25–<30 kg/m2.[12]
Importantly, BMI and weight change do not capture body composition and WC is an imperfect proxy for visceral adiposity. Changes in muscle and fat mass during the post-diagnosis period may ocur even with stable weight, and these changes may have metabolic consequences that influence future risk of CVD. Changes in visceral adiposity may matter most for CVD risk, and these are not adequately described by non-specific measures like weight change, underscoring the need for body composition assessment and/or interventions that target body composition. For example, physical activity may favorably influence body composition; among overweight adults without cancer these interventions increase muscularity while reducing visceral adiposity.[18] A prior study in our cohorts found greater physical activity was associated with lower CVD risk in a dose-response fashion, independent of BMI at diagnosis.[19] Physical activity interventions among breast cancer survivors could be a promising area for CVD prevention: to date no controlled study has had sufficient follow-up or sample size to demonstrate effects on CVD morbidity/mortality, though many have demonstrated safety/efficacy for treatment-related side effects, quality of life, resting heart rate and blood pressure.[20–22]
Strengths and Limitations
This is the first study of early-stage breast cancer survivors to examine associations of adiposity and weight change with incident CVD including detailed information on CVD morbidities and CVD subtypes. Another novel feature is the multiple measures of adiposity (BMI and WC) and inclusion of post-diagnosis weight changes. However, analyses of WC and post-diagnosis weight change excluded participants for whom these exposures were unavailable and excluded CVD events prior to the 24-month follow-up evaluation, resulting in lower power to detect associations for these exposures than for BMI. An additional limitation is that we could not distinguish whether weight changes were intentional: though it is possible that intentional weight loss would reduce CVD in a randomized controlled trial,[23] it is striking that there was no indication of an increased risk of CVD even with large amounts of weight gain in the early post-diagnosis period. Though weights were self-reported, they had excellent correlation with measured weights among the n=1,139 women for whom both were available, both overall (r=0.97) and within subgroups (e.g., BMI category).
CONCLUSION
There is a high burden of CVD among breast cancer survivors; nearly 30% of our early-stage cohort had an incident CVD event following diagnosis. Similar to adults without cancer, greater abdominal adiposity increases risk of CVD among breast cancer survivors. However, weight changes in the early post-diagnosis period have no association with subsequent CVD. Breast cancer survivors with Class II/III obesity, elevated WC and pre-existing CVD risk factors at diagnosis should be monitored closely in primary care and cardio-oncology practices.
Supplementary Material
Acknowledgments
Funding acknowledgement: This study was funded by the National Cancer Institute, grant numbers R01 CA105274 (PI: Kushi LH), U01 CA195565 (PI: Kushi LH) and R01 CA129059 (B.J. Caan, PI)
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Participants provided informed consent under human subjects’ protocols approved by the institutional review boards at KPNC.
References
- 1.Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13(3):R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradshaw PT, Stevens J, Khankari N, Teitelbaum SL, Neugut AI, Gammon MD. Cardiovascular Disease Mortality Among Breast Cancer Survivors. Epidemiology. 2016;27(1):6–13. doi: 10.1097/ede.0000000000000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hooning MJ, Aleman BM, van Rosmalen AJ, Kuenen MA, Klijn JG, van Leeuwen FE. Cause-specific mortality in long-term survivors of breast cancer: a 25-year follow-up study. International Journal of Radiation Oncology* Biology* Physics. 2006;64(4):1081–1091. doi: 10.1016/j.ijrobp.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 4.Foraker RE, Abdel-Rasoul M, Kuller LH, Jackson RD, Van Horn L, Seguin RA, Safford MM, Wallace RB, Kucharska-Newton AM, Robinson JG, Martin LW, Agha G, Hou L, Allen NB, Tindle HA. Cardiovascular Health and Incident Cardiovascular Disease and Cancer: The Women’s Health Initiative. Am J Prev Med. 2016;50(2):236–240. doi: 10.1016/j.amepre.2015.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss an update of the 1997 American Heart Association Scientific statement on obesity and heart disease from the obesity committee of the council on nutrition, physical activity, and metabolism. Circulation. 2006;113(6):898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 6.Demark-Wahnefried W, Campbell KL, Hayes SC. Weight management and its role in breast cancer rehabilitation. Cancer. 2012;118(S8):2277–2287. doi: 10.1002/cncr.27466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caan BJ, Kwan ML, Hartzell G, Castillo A, Slattery ML, Sternfeld B, Weltzien E. Pre-diagnosis body mass index, post-diagnosis weight change, and prognosis among women with early stage breast cancer. Cancer Causes Control. 2008;19(10):1319–1328. doi: 10.1007/s10552-008-9203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caan BJ, Kwan ML, Shu XO, Pierce JP, Patterson RE, Nechuta SJ, Poole EM, Kroenke CH, Weltzien EK, Flatt SW, Quesenberry CP, Jr, Holmes MD, Chen WY. Weight change and survival after breast cancer in the after breast cancer pooling project. Cancer Epidemiol Biomarkers Prev. 2012;21(8):1260–1271. doi: 10.1158/1055-9965.epi-12-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawkes AL, Lynch BM, Owen N, Aitken JF. Lifestyle factors associated concurrently and prospectively with co-morbid cardiovascular disease in a population-based cohort of colorectal cancer survivors. European Journal of Cancer. 2011;47(2):267–276. doi: 10.1016/j.ejca.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol. 2005;23(7):1370–1378. doi: 10.1200/jco.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 11.Nichols HB, Trentham-Dietz A, Egan KM, Titus-Ernstoff L, Holmes MD, Bersch AJ, Holick CN, Hampton JM, Stampfer MJ, Willett WC, Newcomb PA. Body mass index before and after breast cancer diagnosis: associations with all-cause, breast cancer, and cardiovascular disease mortality. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1403–1409. doi: 10.1158/1055-9965.epi-08-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cespedes Feliciano EM, Kroenke CH, Bradshaw PT, Chen WY, Prado CM, Castillo A, Weltzein E, BJC . Cancer Epidemiology, Biomarkers and Prevention. 2016. Post-diagnosis Weight Change and Survival Following a Diagnosis of Early Stage Breast Cancer. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American statistical association. 1999;94(446):496–509. [Google Scholar]
- 14.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. The Annals of statistics. 1988:1141–1154. [Google Scholar]
- 15.Kohl M, Heinze G. PSHREG: A SAS macro for proportional and nonproportional substribution hazards regression with competing risk data. Vienna, Austria: 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin G, So Y, Johnston G. Analyzing survival data with competing risks using SAS® software. SAS Global Forum; Citeseer: 2012. [Google Scholar]
- 17.Sun X, Nichols HB, Robinson W, Sherman ME, Olshan AF, Troester MA. Post-diagnosis adiposity and survival among breast cancer patients: influence of breast cancer subtype. Cancer Causes & Control. 2015;26(12):1803–1811. doi: 10.1007/s10552-015-0673-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slentz CA, Aiken LB, Houmard JA, Bales CW, Johnson JL, Tanner CJ, Duscha BD, Kraus WE. Inactivity, exercise, and visceral fat. STRRIDE: a randomized, controlled study of exercise intensity and amount. Journal of Applied Physiology. 2005;99(4):1613–1618. doi: 10.1152/japplphysiol.00124.2005. [DOI] [PubMed] [Google Scholar]
- 19.Jones LW, Habel LA, Weltzien E, Castillo A, Gupta D, Kroenke CH, Kwan ML, Quesenberry CP, Scott J, Sternfeld B. Exercise and Risk of Cardiovascular Events in Women With Nonmetastatic Breast Cancer. Journal of Clinical Oncology. 2016 doi: 10.1200/JCO.2015.65.6603. JCO656603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott JM, Adams SC, Koelwyn GJ, Jones LW. Cardiovascular Late-Effects and Exercise Treatment in Breast Cancer: Current Evidence and Future Directions. Canadian Journal of Cardiology. 2016 doi: 10.1016/j.cjca.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Speck RM, Courneya KS, Mâsse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Journal of Cancer Survivorship. 2010;4(2):87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 22.Sturgeon KM, Ky B, Libonati JR, Schmitz KH. The effects of exercise on cardiovascular outcomes before, during, and after treatment for breast cancer. Breast cancer research and treatment. 2014;143(2):219–226. doi: 10.1007/s10549-013-2808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NCIC Clinical Trials Group. Alliance for Clinical Trials in Oncology. MAC20 (ALLIANCE A011401) Randomized Phase III Trial Evaluating the Role of Weight Loss In Adjuvant Treatment of Overweight and Obese Women with Early Breast Cancer. 2015 doi: 10.1038/s41523-017-0040-8. https://www.ctg.queensu.ca/public/all-disease-sites. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.