Abstract
Introduction
Thailand has been heralded as a global leader in HIV prevention and treatment, and its experience with the HIV/AIDS epidemic holds valuable lessons for public health. This paper documents Thailand's response to its HIV epidemic from the late 1980s until today, and analyses its epidemiological impact (incidence and mortality). We discuss the association between the trajectory of HIV incidence and mortality rates over time, and the programmatic investments, policies and interventions that were implemented in the last three decades.
Methods
This is a review paper that draws on published literature, unpublished sources and routine behavioural and serological surveillance data since 1989. It is informed by the modelling of epidemiological impacts using the AIDS Epidemic Model. The AIDS Epidemic Model and Spectrum were used to assess the impact on incidence and mortality. Apart from epidemiological data, National AIDS Spending Assessment and programme data were also used to assess financial investments.
Results
Thailand is well on its way to meeting the 90-90-90 targets, the goal that by 2020, 90% of people living with HIV know their HIV status, 90% of people with diagnosed HIV infection receive sustained antiretroviral therapy, and 90% of people receiving antiretroviral therapy (ART) are virally suppressed. In Thailand, 89% of people living with HIV know their status, 72% receive ART and 82% have viral load testing – 99% of whom are suppressed. The public health response to HIV in Thailand has averted 5.7 million infections since 1991. If Thailand had not responded in 1991 to the HIV epidemic, and had there been no prevention and ART provision, the country would have experienced an estimated 158,000–225,000 deaths in the 2001–2006 period. This figure would have risen to 231,000–268,924 in the 2007–2014 period. A total of 196,000 deaths were averted between 2001 and 2014. If ART scale-up had not occurred in 2001, Thailand would have experienced between 50,000 and 55,000 deaths per year in the period 2001–2006, and 31,000–46,000 annual deaths between 2007 and 2014. The main impact in terms of deaths averted is seen from 2004 onwards, reflecting treatment scale up.
Conclusions
Thailand's AIDS response has prevented needless morbidity and mortality due to the HIV epidemic. In the context of Thailand's ageing population, it is faced with the twin challenges of maintaining life-long quality services among HIV patients and sustaining behaviour change to maintain primary prevention gains. Keeping the focus of the policy makers and health administrators on ‘Ending the HIV epidemic’ will require consistent advocacy, and evidence-based, innovative and efficient approaches.
Keywords: HIV/AIDS, Thailand, incidence, impact, interventions, ART, universal health, health governance
Introduction
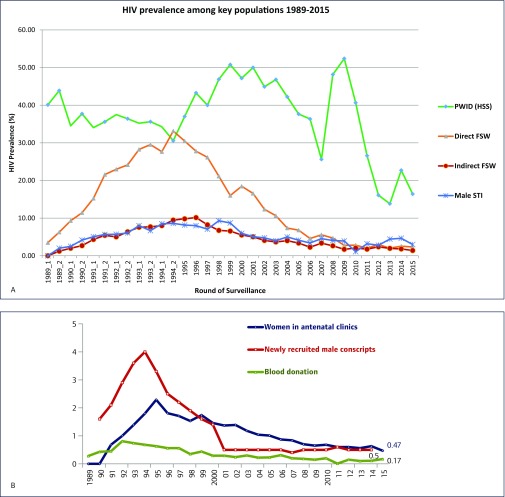
Thailand's first case of HIV was reported in 1984. The epidemic has evolved and changed strikingly over the last three decades. The early phase of the epidemic was mostly that of HIV-1, subtype B, which rapidly escalated among people who inject drugs (PWID) in 1988 [1,2]. The virus then quickly spread to populations of female sex workers (FSWs), with increasing documentation of subtype E [3]. The epidemic spread rapidly in the early 1990s, driven by infections among sex workers and their clients [4–6]. The prevalence among direct FSWs was much higher, peaking in the mid-1990s, and declining rapidly after that (Figure 1a). There were clear geographical differences in the prevalence of HIV. The upper-northern provinces accounted for a disproportionate number of HIV case reports [7]. By 1993, some 600,000–800,000 people were estimated to be living with HIV [11]. At the same time, the prevalence of HIV in the general population – as measured by women attending antenatal clinics, newly recruited male conscripts as well as blood donors – also showed an increase, peaking in the early 1990s, and then declining slowly (Figure 1b).
Figure 1.
(A) HIV prevalence among key populations from 1989 to 2015. The decline in the prevalence of HIV among PWID is probably linked to sampling issues. The sample size for PWID was inadequate and limited to fewer than 10 sites after 2009. Source: Bureau of Epidemiology, Ministry of Public Health Thailand, Sentinel Surveillance Survey data 1989–2013. (B) HIV prevalence in the general population in Thailand, 1989–2015. Source: Bureau of Epidemiology, Thai Ministry of Public Health, Sentinel Surveillance Survey data 1989–2015
Data based on AIDS case surveillance between 1984 and 1998 showed that the most frequently reported opportunistic infections were tuberculosis (19%), Pneumocystis carinii pneumonia (19%), cryptococcosis (17%), candidiasis of oesophagus, trachea or lung (5%) and recurrent bacterial pneumonia (4%) [2]. Cross-sectional survey data of hospital admissions between 1993 and 1996 also indicated that the most common AIDS-defining conditions were cryptococcosis, tuberculosis and HIV-wasting syndrome; PWID were more likely to have tuberculosis or suffer from HIV-wasting syndrome [8].
As HIV prevalence began to decline among FSWs and their clients in the mid-1990s, data from serial prospective cohorts among young Thai military conscripts also showed simultaneous declines in the incidence of both HIV and sexually transmitted infections, suggesting successful interventions and changes in transmission patterns [7]. The early 2000s saw marked changes in the transmission routes in Thailand, with sharp increases in the estimated HIV incidence among young men who have sex with men (MSM) – from 4.1% to 7.7% between 2003 and 2007 [9], with a median of 9.2% as a national estimate in 2014. Prevalence among MSM in Thailand has remained high (Integrated Biological Behavioral Surveillance Round, 2014). HIV incidence among MSM is especially high among those living in large urban areas and international tourist destinations for example, Bangkok, Chiang Mai, Phuket and Pattaya. In a large clinic-based study of MSM coming forward for testing at the Silom Community Clinic in Bangkok, an incidence of 12.2 per 100 person years was found among 15–21 year old men, this is almost twice as high as among all ages, which was 6.3 per 100 person years [10].
The HIV epidemic up to 2015 is mature and abating rapidly. According to the AIDS Epidemic Model, Global AIDS Report for Thailand 2015, in 2014 there were an estimated 445,504 people living with HIV in Thailand, including 175,716 women and 6875 children. The estimated HIV prevalence among adults was 0.83%. There were an estimated 7816 new infections in 2014, including 121 in newborns. A quarter of adult infections (1944) occurred in women, of them 221 in FSW, and the remaining 1723 in other groups of women, particularly discordant couples and partners of members of key populations.
The transmission of HIV from parents to children has been successfully controlled. According to programme data from the Department of Health, the parent-to-child transmission (PTCT) rate was 1.9% in 2015. AIDS-related deaths have been steadily falling since 2001, with a sharp drop observed from 2006 following the scaling up of ART. The National AIDS Management Centre estimates that there were 20,492 deaths among people living with HIV/AIDS in Thailand in 2014 (modelling estimates from the AIDS Epidemic Model). However, programme data from the National Health Security Office, based on an analysis of records from the Ministry of Interior's Civil Vital Registration System, suggests that there may be fewer than 16,000 AIDS-related deaths.
Thailand has been heralded as a global leader in HIV prevention and treatment, and its experience with the AIDS epidemic holds valuable lessons for public health. This paper documents Thailand's response to its HIV epidemic from the late 1980s until today, and analyses its scope and epidemiological impact (incidence and mortality). We discuss the association between the trajectory of HIV incidence and mortality rates over time, and the programmatic investments, policies and interventions that were implemented in the last three decades. In doing so, we document and describe not just the public health interventions, but also consider issues of governance, universal health coverage as well as structural and policy constraints that influence public health outcomes.
Methods
This review draws on published literature and unpublished sources and routine behavioural and serological surveillance data since 1989. It is informed by the modelling of epidemiological impacts using the AIDS Epidemic Model (AEM). Electronic data sources include Medline, PubMed, the Social Sciences Citation Index, Social Sciences Index and Abstracts, and the International Bibliography of the Social Sciences. Key peer-reviewed journals published between 1984 and 2015 were searched. Serological and behavioural data collected by the Bureau of Epidemiology (BOE), covering the period since the establishment of the HIV sero-surveillance system (1989) and the behaviour sentinel surveillance programme (1995) were also reviewed. These data provide information on key affected populations (KAPs) and the general population. Finally, AEM models to assess impact on incidence and mortality in conjunction with vital registration data were also analysed.
Apart from epidemiological data, we used information from National AIDS Spending Assessment (NASA) and programme data from the Ministry of Public Health (MOPH) and National Health Security Office (NHSO) to assess financial investment and track monetary flows to specific interventions.
Results
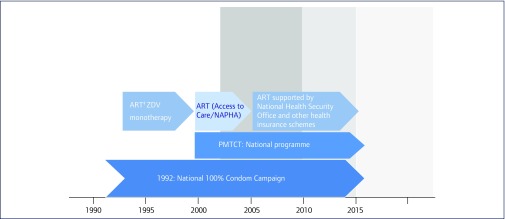
We distinguish our findings over two phases. First, we present the outcomes and impact of Thailand's early prevention interventions (1990–2000). Second, we present and discuss the impact of the country's prevention of mother-to-child transmission (PMTCT) programme, the scaling up of treatment with antiretroviral drugs (2000–2015; Figure 2).
Figure 2.
Timeline of HIV interventions and investments in Thailand, 1990–2015. Prevention of mother-to-child transmission (PMTCT), National access for people living with HIV/AIDS (NAPHA) to ART, which was a highly active antiretroviral treatment (HAART) regimen funded by the Thai government and the Global Fund between 2002 and 2005. The ART programme started in 1992 with ZDV monotherapy and later continuing with dual therapy
Phase 1
The public health responses started within the Division of Venereal Diseases under the Communicable Disease Control Department and the Division of Epidemiology of the Office of Permanent Secretary Office after the first AIDS case report from a tertiary hospital in Bangkok in 1984. This led to HIV being classified as a reportable disease and the development of the surveillance system, which resulted in the case-based reporting system in 1984. The National AIDS Programme was launched in 1987 with the establishment of the Center of AIDS Prevention and Control, which subsequently became the Division of AIDS under the Department of Communicable Disease Control of the Ministry of Public Health. By 1989, a surveillance system had been established across Thailand and an accurate assessment of high-risk groups and behavioural patterns provided strategic information for evaluations and resource allocation [2]. By 1992, the HIV/AIDS programme was being co-managed by the Ministry of Public Health and the Office of the Prime Minister – controlling the epidemic had become a priority national agenda.
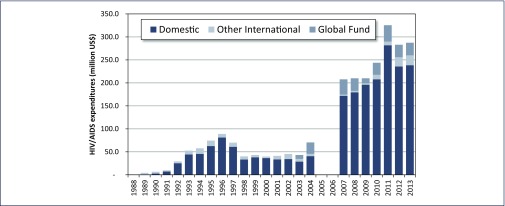
Concomitantly, the Thai Government stepped up its investment in HIV control, from just US$180,000 in 1988, to US$44.33 million in 1993. By 1996, the government allocated US$81.96 million to its response to control the spread of HIV [11].
A remarkable aspect of the Thai national response to HIV has been the government's strong financial ownership of the programme, even when Thailand was classified as a ‘lower-middle income’ country. With the exception of 1989, Thai domestic resources have accounted for the vast majority of funding for the AIDS response. In addition, the early prevention efforts and treatment scale-up were funded through the national budget (Figure 3). Despite the financial collapse during the Asian financial crisis in the late 1990s, Thailand sustained a lowered, but substantial investment in the AIDS response. This financial commitment reflects the Thai government's strong commitment to control HIV. Funding from the Global Fund for HIV, TB and Malaria (GFATM) first became available in 2003, and has accounted for between 10 and 15% of the money spent in Thailand's response since then, with most funds being used for treatment and prevention in young people in the first 5 years and later on a focus in prevention among KAPs (Figure 3).
Figure 3.
Thailand's national HIV/AIDS expenditures by source of funding, 1988–2013. Source: National AIDS Spending Assessment conducted from 2007 to 2013, reported to UNAIDS, National AIDS Account data 1988–2004
By the end of 1991, Thailand's well-documented 100% Condom Use Programme had been initiated following the Ratcharaburi model [12–14]. Condom use increased dramatically in sex-work settings from 14% to 94% between 1989 and 1993 [15]. A 79% decrease in sexually transmitted infection (STIs) rates among men was attributed to the 100% Condom Use Programme [16]. Other studies among male conscripts during the period 1991–1993 (n=4086) also showed that HIV incidence declined from 2.48 per 100 person years between 1991 and 1993 to 0.55 between 1993 and 1995 [17]. STI rates in the 1991 cohort declined even more sharply: from 17 per 100 person years to 1.8 per 100 person years in the 1993 cohort [17].
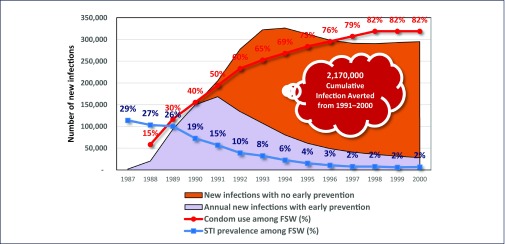
The AEM shows that the impact of early prevention in Thailand averted 2,170,000 infections (Figure 4). The annual number of new infections fell dramatically after 1992, from 168,485 in 1991 to 28,241 in 2000 (Thai Working Group on HIV Estimation and Projection, 2015). Modelling using the AEM suggests that by 2013, the total number of averted infections since 1991 had risen to 5.7 million (Thai Working group on HIV Estimation and Projection 2015). If Thailand had not responded in 1991 to the HIV epidemic, and had there been no prevention and ART provision, the country would have experienced an estimated 158,000–225,000 deaths in the period 2001–2006. This figure would have risen to 231,000–268,924 in the period 2007–2014 (Thai Working Group on HIV Estimation and Projection, 2015).
Figure 4.
Impact of early prevention on new infections in Thailand (1991–2000), and the potential costs of inaction. FSW: female sex worker; STI: sexually transmitted infection. Source: Thai Working Group on HIV estimation and projection, 2015
Phase 2
In 2000, Thailand initiated its nationwide PMTCT programme [18]. It provided voluntary and free testing for all pregnant women, provision of free ART to pregnant women and newborn infants, and free formula feeding for infants for the first 12 months [19]. The effectiveness of Thailand's PMTCT programme has been rigorously assessed [20,21]. In the period 2001–2003, the transmission risk among those completing a short course of zidovudine (ZDV)-only regimen declined from 18.9–24.2% to 6.8% (CI 5.2–8.9%). Among those who received ZDV along with nevirapine (NVP), the transmission was 3.9% (CI 2.2–6.6%) [19]. By 2005, 89.8% of HIV-positive pregnant women were receiving ART to reduce MTCT. By 2009, this share had risen to 94.7% [22]. In 2010, a triple ART regimen began to be used for PMTCT, when the Thailand National Health Security Office, supported by cost–benefit analysis data, advised the use of HAART over ZDV + single-dose-NVP in HIV-positive women [23,24]. In 2015, 95.8% of Thai and non-Thai HIV-positive pregnant women received drugs to reduce MTCT. Some 76% of infants born to HIV-positive mothers received virological testing within two months of birth, and only 2.1% of infants born to HIV-positive mothers were infected [25,26]. Unpublished estimates using Spectrum by the National AIDS Management Centre indicate that between 2000 and 2014, the PMTCT programme prevented a total of 15,760 infants from being infected and 7440 deaths. In 2016 Thailand was officially certified by the World Health Organization as having eliminated mother-to-child transmission of HIV and congenital syphilis.
HIV treatment with antiretroviral drugs was first started in 1992 with ZDV monotherapy, and later, dual therapy. At the end of 1995, approximately 4200 people were being treated [27]. In 2000 the concept of providing ART free of charge took concrete shape under the Access to Care (ATC) programme, drawing on the principles of equal access to HAART and quality of services for all. In 2002, two critical events facilitated the massive scale-up of ART in Thailand. First, the Government Pharmaceutical Organisation (GPO) began producing GPO-VIR (a fixed-dose generic combination of stavudine, lamivudine and NVP). Second, more funding was made available: the government doubled the budget for ART due to the exclusion of ART from universal health coverage and in 2004 Thailand received supplemental support from Round 1 of the GFATM for the ART programme. The ATC programme was renamed the National Access to Antiretrovirals Programme for People living with HIV/AIDS (NAPHA), and massively scaled up ART – treating 58,133 PLHIV, with a total budget of ฿800 million (approximately US$23 million) [27].The roll-out of ARVs was made a priority not just for adults, but also for children, with 7543 children put on ART between 2000 and 2007 [28]. An assessment of treatment outcomes for ART among adults in Thailand (2000–2007) showed that outcomes remained good, with much improved survival rates, despite the rapid scale-up of ART [29]. By 2010, more than 150,000 patients were receiving ART [30] with doctors using a treatment initiation criterion of CD4 cell count <350cells/mm3 [30]. In 2014, based on new evidence, new guidance recommended ART initiation irrespective of an individuals’ CD4 cell count [31].
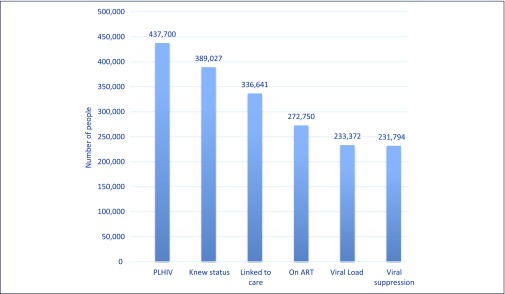
At the end of 2015, Thailand was well on its way to reach the 90-90-90 targets. Of the 437,700 estimated PLHIV in 2015, 389,027 (89%) had been diagnosed with the virus (these figures exclude HIV tests in the private sector), and 336,541 were in care (National ADS Programme Database, National Health Security Office, 2015). Of those in care, 288,231 were on ART and 231,794 were virally suppressed (NAP Database 2015). The main areas of loss from the care cascade (defined by more than a 10% difference between any two points in the cascade) were between those in HIV care and those commencing ART, and those on ART and those who were virally suppressed (see Figure 5).
Figure 5.
Testing and treatment cascade, Thailand 2015. This data excludes tests and treatment in the private sector. An estimated additional 15,481 people are on ART in the private sector, which is not routinely reported, bringing the total number on ART to 288,231 (estimates based on data from the Government Pharmaceutical Organisation). Source: National AIDS Programme Database, National Health Security Office, 2015
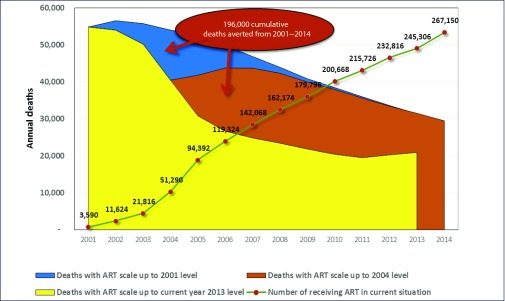
The impact of the large-scale provision of ART in Thailand between 2001 and 2014 was assessed using the AEM. A total of 196,000 deaths were averted between 2001 and 2014 (Figure 6). If ART scale-up had not occurred in 2001, Thailand would have experienced between 50,000 and 55,000 deaths per year in the period 2001–2006, and between 31,000 and 46,000 annual deaths in the period 2007–2014 (Thai Working Group on HIV Estimation and Projection, 2015). The vast majority of the impact in terms of deaths averted is seen from 2004 onwards, reflecting treatment scale up (Figure 6).
Figure 6.
The impact of ART scale up on deaths due to AIDS in Thailand, 2001–2014. Source: Thai Working Group on HIV estimation and projection, 2015
The impact of the response to HIV in Thailand is also reflected in the burden of disease analysis. In 2004, HIV was the top cause of death in men (26,400 deaths), and the second most common cause in women (11,000 deaths) [32]. However, between 1999 and 2004, the burden of death and disease attributable to HIV/AIDS fell from 32.3 to 21.1 disability-adjusted life years per 1000 men, and to a lesser extent in women (from 12.2 to 9.3) [32]. In 2015, HIV/AIDS was the sixth most common cause of death surpassed only by coronary heart disease, stroke, road traffic accidents and other causes [33].
Discussion
This paper takes stock of the HIV/AIDS response in Thailand, and looks at more than 30 years of prevention, care and treatment efforts in the country. We have reviewed and synthesised published evidence, programme data and the results of modelling exercises to gauge the impact that these efforts have had. We argue that while the evidence and our analysis does not allow us to attribute direct causality, there are strong temporal associations between these efforts and the impact on HIV incidence and AIDS-related mortality. We have assessed the impact of the programme specifically in terms of declines in incidence and mortality. It is important, however, to acknowledge that some key governance, financial and policy inputs into the national AIDS control efforts have strongly influenced those outcomes. The role of Thailand's well-developed health infrastructure, the government's strong political commitment and the stewardship from the Prime Minister's office have been well described elsewhere [11]. We highlight three key issues that have been critical in ensuring that interventions in Thailand could be implemented early, at scale and in a sustained fashion: health governance, reform and partnership with civil society.
Health governance and reform
Thailand has gone through a major reform of governance and its health service system with the Decentralization Act in November 1999 and the introduction of universal health coverage in 2002. Government reform involved the devolution of authority for some operations from the central government to the provincial and local administrations. There were changes to the structure of the Ministry of Public Health, and in the management of the AIDS budget as part of this decentralisation. Following enactment of the official ministerial proclamation in 2002, the Ministry of Public Health implemented structural reforms at central and regional levels. In particular, at the central level, the role of the National AIDS Committee shifted from policy and budget support for implementation and development to co-ordination, monitoring and technical support. A portion of the prevention budget and much of the task of implementation was decentralised to local administrative organisations. Other related line ministries made budget requests for HIV prevention activities through their own agencies. Apart from antiretroviral treatment, which has been centrally managed, the budget for HIV clinical services for opportunistic infections was integrated into the national health insurance scheme, and allocated to health service outlets in the form of per capita lump sum payments [34].
The national AIDS response is integrated into numerous and diverse programmes of participating agencies and line ministries. Up until 2005, these ministries prepared AIDS budgets in collaboration with the Ministry of Public Health. However, starting in 2005, no specific AIDS budget was defined. It became the responsibility of each ministry to allocate a budget line for HIV control. The budget for health of the population was allocated as a lump sum based on per capita needs, including AIDS. This approach promoted a multi-sectoral response and removed the constraints of a centralised budget. For example, under the arrangement, local administrative organisations were made responsible for paying a monthly allowance to PLHIV. Provinces were also expected to prioritise and budget for health issues at the local level. This made financing directly available at the local level (rather than indirectly through a centralised funding mechanism). While this decentralisation has led to some positive changes, the risk that there may be varying capacities and awareness across provinces regarding continued investment and engagement with HIV has remained a challenge [35].
With the ultimate goal of equal rights to the access of quality health services for all – as stipulated under Section 52 of the 1997 Constitution – the government also implemented the Universal Health Security Scheme namely the ‘30 Baht scheme’ (in addition to the Social Security Scheme and the Civil Servant Medical Benefit Scheme). This package entitled all Thai citizens to free medical services and health promotion and prevention. At the introduction of the scheme, antiretroviral treatment was excluded from the service package, but included in 2006. The National Health Security Office-supported ART programme is highly cost effective at less than US$1 per day per patient, and was supported by the government's bold policies in initiating generic production, cost negotiation and compulsory licensing of ARV drugs, specifically for efavirenz and lopinavir/ritonavir [36,37].
Partnership
The Ministry of Public Health had begun to engage with civil society partners in Thailand on the issue of HIV prevention and treatment since the early 1990s. A key partner is the Thai NGO Coalition on AIDS (TNCA), a network of 168 Thai NGOs, which aims to improve the quality of life of PLHIV. It is notable that the Bureau of AIDS, TB and STIs (BATS) not only worked in close partnership with TNCA from the beginning of the epidemic, but also provided it with an annual budget of ฿65–90 million to support their activities. TNCA was seen, along with the Thai Network of People Living with HIV/AIDS (TNP+), as an equal partner in the AIDS response, with a dedicated line of funding from the NHSO.
Apart from working closely with the government, Thai civil society has successfully held governments accountable, and championed the cause of equal access. For example, on 30 November 2001, 1200 PLHIVs from all parts of the country demonstrated in front of parliament and met with Minister of Public Health. The minister agreed, in principle, to their demands, and doubled the budget for ART and also committed the government to include ARVs in the universal health scheme. The working committee, which consisted of representatives from TNP+, NGO/AIDS and the government, was set up to prepare for implementation of the scheme. At that time, there were fewer than 4000 individuals receiving ART. Arguably, civil society action has been fundamental in shaping government policy, an illustration that a well-informed and motivated civil society, which is able to negotiate and partner with government agencies, can be highly beneficial to the AIDS response.
Conclusions
We conclude by noting that, despite the outstanding successes of Thailand's AIDS response, the programme is faced by a multitude of challenges. A key dilemma is how to position HIV in the era of the sustainable development goals and move towards ‘Ending AIDS’. HIV is a chronic disease, and in the context of Thailand's ageing population, it poses the twin challenges of maintaining life-long quality services for HIV patients and sustaining behavioural change to maintain primary prevention gains. Keeping the focus of policymakers and health administrators on ‘Ending the HIV epidemic’ requires consistent advocacy, evidence-based cost effectiveness and innovative approaches to addressing shortages of human resources.
Stigma and discrimination in healthcare settings is still a major obstacle to a more effective response to HIV. Observed behaviours towards KAPs among health staff in two Thai provinces indicate disturbing levels of discrimination [38]. Thailand has also struggled with ongoing policy and legislative barriers that have an impact on access and quality of services. Despite recent progress in reducing barriers to access (for example, no further requirement for parental consent for HIV testing in young people; a pilot harm reduction policy in 19 provinces; and health insurance for healthcare for migrants) Thailand has a rocky road to travel before ending AIDS [24]. Some regulations, such as those that only allow a ‘medical technologist’ under the responsibility of a physician to provide HIV test results, do not promote community-based testing.
To support Thailand in achieving the ambitious ‘Fast Track’ End AIDS and move towards the attainment of the Sustainable Development Goals, the Ministry of Public Health and other partners are working towards establishing policies and systems to increase funding flows to community partners at national and regional levels, including the accreditation of community services, and continuing ‘test and start ART’ for all HIV-infected people. The Ministry of Public Health is also implementing a programme to reduce system-wide stigma and discrimination in healthcare settings, and address human rights concerns.
In order to ‘End AIDS’, Thailand will need to focus on areas that enhance the ability of the programme staff, service providers, health insurance agencies, civil society partners and PLHIV to work in a co-ordinated manner, and develop the capacity of the health and community system to move beyond a ‘control’ agenda to an ‘Ending AIDS’ agenda.
Acknowledgements
We would like to acknowledge Dr Nima Asgari from WHO Thailand for reviewing early drafts of this paper. We would also like to acknowledge Tom F Joehnk for editorial support.
Declaration of conflict of interest
The authors do not have any conflict of interest to declare.
Disclaimer
MS is a staff member of the World Health Organization. The authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the World Health Organization.
References
- 1. Weniger BG, Limpakarnjanarat K, Ungchusak K et al. The epidemiology of HIV infection and AIDS in Thailand, AIDS 1991; 5 Suppl 2: S71– 85. [DOI] [PubMed] [Google Scholar]
- 2. Saengwonloey O, Jiraphongsa C, Foy H.. Thailand report: HIV/AIDS surveillance 1998. J Acquir Immune DeficSyndr 2003; 32 Suppl 1: S63– 67. [DOI] [PubMed] [Google Scholar]
- 3. Ou CY, Takebe Y, Weniger BG et al. Independent introduction of two major HIV-1 genotypes into distinct high-risk populations in Thailand. Lancet 1993; 341: 1171– 1174. [DOI] [PubMed] [Google Scholar]
- 4. Siraprapasiri T, Thanprasertsuk S, Rodklay A et al. Risk factors for HIV among prostitutes in Chiangmai, Thailand. AIDS 1991; 5: 579– 582. [PubMed] [Google Scholar]
- 5. Sawanpanyalert P, Ungchusak K, Thanprasertsuk S, Akarasewi P.. HIV seroconversion rates among female sex workers in Chiang Mai, Thailand: a multi-cross-sectional study. AIDS 1994; 8: 825– 829. [DOI] [PubMed] [Google Scholar]
- 6. Brown T, Sittitrai W, Vanichseni S, Thisyakorn U.. The recent epidemiology of HIV and AIDS in Thailand. AIDS 1994; 8 Suppl 2: S131– 141. [PubMed] [Google Scholar]
- 7. Celentano DD, Bond KC, Lyles CM et al. Preventive intervention to reduce sexually transmitted infections: a field trial in the Royal Thai Army. Arch Intern Med 2000; 160: 535– 540. [DOI] [PubMed] [Google Scholar]
- 8. Tansuphasawadikul S, Amornkul PN, Tanchanpong C et al. Clinical presentation of hospitalized adult patients with HIV infection and AIDS in Bangkok, Thailand. J Acquir Immune DeficSyndr 1999; 21: 326– 332. [DOI] [PubMed] [Google Scholar]
- 9. Griensven F, Varangrat A, Wimonsate W et al. Trends in HIV prevalence, estimated HIV incidence, and risk behavior among men who have sex with men in Bangkok, Thailand, 2003–2007. J Acquir Immune DeficSyndr 2010; 53: 234– 239. [DOI] [PubMed] [Google Scholar]
- 10. Ananworanich J, Chitwarakorn A, Wimonsate W et al. HIV and syphilis infection among men who have sex with men – Bangkok, Thailand, 2005–2011. MMWR Morb Mortal Wkly Rep 2013; 62: 518– 520. [PMC free article] [PubMed] [Google Scholar]
- 11. Phoolcharoen W, Ungchusak K, Sittitrai W, Brown T.. Thailand: lessons from a strong national response to HIV. AIDS 1998; 12 Suppl B: S123– 135. [PubMed] [Google Scholar]
- 12. Brown T, Sittitrai W.. Estimates of recent HIV infection levels in Thailand. Research Report No. 9. Program on AIDS, Thai Red Cross Society, Bangkok; 1993.
- 13. Punpanich W, Ungchusak K, Detels R.. Thailand's response to the HIV epidemic: yesterday, today, and tomorrow. AIDS Educ Prev 2004; 16 Suppl A: 119– 136. [DOI] [PubMed] [Google Scholar]
- 14. Hanenberg RS, Rojanapithayakorn W, Kunasol P et al. Impact of Thailand's HIV control programme as indicated by the decline of sexually transmitted diseases. Lancet 1994; 344: 243– 245. [DOI] [PubMed] [Google Scholar]
- 15. Visrutaratna S, Lindan CP, Sirhorachai A, Mandel JS. ‘ Superstar’ and ‘model brothel’: developing and evaluating a condom promotion program for sex establishments in Chiang Mai, Thailand. AIDS 1995; 9 Suppl 1: S69– 75. [PubMed] [Google Scholar]
- 16. Rojanapithayakorn W, Hanenberg RS.. The 100% condom programme in Thailand. AIDS 1996; 10: 1– 7. [DOI] [PubMed] [Google Scholar]
- 17. Celentano DD, Nelson KE, Lyles CM et al. Decreasing incidence of HIV and sexually transmitted diseases in young Thai men: evidence for success of the HIV/AIDS control and prevention program. AIDS 1998; 12: F29– 36. [DOI] [PubMed] [Google Scholar]
- 18. Kanshana S, Simonds RJ.. National programme for preventing mother to child HIV transmission in Thailand: successful implementation and lessons learned. AIDS 2002; 16: 953– 959. [DOI] [PubMed] [Google Scholar]
- 19. Phanuphak N, Chokephaibulkit K, Boonsuk S et al. Evolution of interventions to prevent mother-to-child transmission of hiv: perspective from Thailand. Siriraj Med J 2011; 63: 20– 24. [Google Scholar]
- 20. Plipat T, Naiwatanakul T, Rattanasuporn N et al. Reduction in mother-to-child transmission of HIV in Thailand, 2001–2003: results from population-based surveillance in six provinces. AIDS 2007; 21: 145– 151. [DOI] [PubMed] [Google Scholar]
- 21. Teeraratkul A, Simonds RJ, Asavapiriyanont S et al. Evaluating programs to prevent mother-to-child HIV transmission in two large Bangkok hospitals, 1999–2001. J Acquir Immune Defic Syndr 2005; 38: 208– 212. [DOI] [PubMed] [Google Scholar]
- 22. National AIDS Prevention and Alleviation Committee, Thailand ; 2010. UNGASS Country Progress Report. Available at: http://data.unaids.org/pub/Report/2010/thailand_2010_country_progress_report_en.pdf ( accessed November 2016).
- 23. Health Intervention and Technology Assessment Program (HITAP) Cost benefit and feasibility study of HAART for prevention of mother-to-child HIV transmission in Thailand. Presented at Steering Group Committee of HIV/AIDS System Development. Bangkok, 2009, cited in Phanuphak et al [24].
- 24. Phanuphak N, Lolekha R, Chokephaibulkit K et al. for the Thai National HIV Guidelines Working Group . Thai national guidelines for the prevention of mother-to-child transmission of HIV: March 2010. Asian Biomed 2010; 4: 529– 540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. National AIDS Committee, Thailand Thailand Ending AIDS. AIDS Response Progress Report, 2015. Available at: www.unaids.org/sites/default/files/country/documents/THA_narrative_report_2015.pdf ( accessed November 2016).
- 26. Ministry of Public Health Thailand Validation of elimination of Mother-To-Child-Transmission of HIV and Syphilis in Thailand 2013–2015 Report. Department of Health report to WHO Global Validation Committee for Elimination of mother to child transmission of HIV and congenital syphilis May 2016.
- 27. Chasombat S, Lertpiriyasuwat C, Thanprasertsuk S et al. The National Access to Antiretroviral Program for PHA (NAPHA) in Thailand. Southeast Asian J Trop Med Public Health 2006; 37: 704– 715. [PubMed] [Google Scholar]
- 28. McConnell MS, Chasombat S, Siangphoe U et al. National program scale-up and patient outcomes in a pediatric antiretroviral treatment program, Thailand, 2000–2007. J Acquir Immune Defic Syndr 2010; 54: 423– 429. [DOI] [PubMed] [Google Scholar]
- 29. Chasombat S, McConnell MS, Siangphoe U et al. National expansion of antiretroviral treatment in Thailand, 2000–2007: program scale-up and patient outcomes. J Acquir Immune Defic Syndr 2009; 50: 506– 512. [DOI] [PubMed] [Google Scholar]
- 30. Sungkanuparpha S, Techasathit W, Utaipiboon C et al. for the Adults and Adolescents Committee of the Thai National HIV Guidelines Working Group . Thai national guidelines for antiretroviral therapy in HIV-1 infected adults and adolescents 2010. Asian Biomed 2010; 4: 515– 528. [Google Scholar]
- 31. Manosuthi W, Ongwandee S, Bhakeecheep S et al. Guidelines for antiretroviral therapy in HIV-1 infected adults and adolescents 2014, Thailand. AIDS Res Ther 2015; 12: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bundhamcharoen K, Bundhamcharoen K, Odton P et al. Burden of disease in Thailand: changes in health gap between 1999 and 2004. BMC Public Health 2011; 11: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. World Health Organization Thailand: WHO statistical profile. Available at: http://www.who.int/gho/countries/tha.pdf?ua=1 ( accessed November 2016).
- 34. Mahidol University, Asean Institute for Health Development Evaluation of the National AIDS Response in Thailand. Available at: https://www.mottmac.com/download/file/127/7038/2011_evaluation_of_the_national_aids_response_in_thailand.pdf ( accessed November 2016).
- 35. Ministry of Public Health Thailand and the World Health Organisation, SEARO External Review of the Health Sector Response to HIV/AIDS in Thailand 2005. Available at: http://apps.searo.who.int/PDS_DOCS/B0181.pdf ( accessed November 2016).
- 36. Cohen J. Thailand's do-it-yourself therapy. Science 2003; 301: 1662. [DOI] [PubMed] [Google Scholar]
- 37. Rungpry S, Kelly EJ.. Compulsory Licensing Developments in Thailand. Asia law IP Review. July 2008. Available at: www.tilleke.com/sites/default/files/compulsory_licensing_developments_TH.pdf ( accessed November 2016).
- 38. International Health Policy Programme, Ministry of Public Health, Thailand 2014. Report of a pilot: Developing Tools and Methods to measure HIV related stigma and discrimination in Health Care Settings in Thailand. Available at: http://ihppthaigov.net/DB/publication/attachresearch/349/chapter1.pdf ( accessed November 2016).