Abstract
Objectives
Electronic health record (EHR) systems have been infrequently used to support HIV service delivery models to optimise HIV prevention and treatment cascades. We have studied the implementation, uptake and use of an EHR among Thai men who have sex with men (MSM) and transgender (TG) women.
Methods
Participants, e-counselled via the Adam's Love ( www.adamslove.org) support platforms, after having completed risk behaviour questionnaires and being assessed for their HIV risk by online counsellors, were enrolled based on their preference into one of three EHR-supported arms: (1) private clinic-based HIV testing and counselling (HTC); (2) online pretest counselling and private clinic-based HIV testing (hybrid); and (3) online supervised HIV self-testing and counselling (eHTC).
Results
Between December 2015 and May 2016, of a total of 489 MSM and TG women were introduced to the study, 186 (38%) enrolled into the study, with 89, 72 and 25 participants joining the HTC, hybrid and eHTC arms, respectively. Seeking sex online was reported by 83.9%. HIV prevalence was highest (16%) in the eHTC arm, and participants in this arm were more likely to be younger (median age 25 vs 29 vs 27 years; P=0.01), bisexual (16% vs 9.7% vs 5.6%; P=0.005), with an unknown history of HIV or first-time HIV testers (48% vs 25% vs 19.1%; P=0.01) or had tested >1 year ago (15.8% vs 4.8% vs 3.4%, P=0.04), compared with those in the hybrid and HTC arms. Around half (48.3%) of them revisited the EHR at least once to access laboratory results, read post-test summaries and make an appointment for another HIV test. The participants in the eHTC arm had reduced odds of revisiting the EHR twice or more as compared with participants in the HTC [odds ratio (OR) 0.14, 95% confidence interval (CI) 0.03–0.67, P=0.01] and hybrid arms (OR 0.10, 95% CI 0.02–0.44, P=0.003). Overall the EHR satisfaction was high at 4.4 (SD 0.68) on a Likert scale of 5.
Conclusions
Young and high-risk MSM and some TG women engaged successfully with the Adam's Love EHR system, showing its potential to support innovative service delivery models and target hard-to-reach and vulnerable populations.
Keywords: Electronic health record, health informatics, HIV testing, HIV prevention and treatment cascade, men who have sex with men, transgender women, Thailand
Introduction
In Thailand, men who have sex with men (MSM) and transgender (TG) women account for the largest number of new HIV infections. In Bangkok, one in three MSM is infected with HIV [1], and incidence rates, especially among young MSM aged 15–21 years, are alarmingly high (12.2 per 100 person-years) [2]. Thailand aims to end the AIDS epidemic by 2030. Mathematical modelling suggests that the only way to achieve this goal is to increase HIV testing to cover 90% of key populations [3] and to treat all HIV cases with antiretroviral therapy (ART) regardless of the CD4 count.
A major priority in strategic planning is to test more individuals and treat all of them on the recruit–test–treat–retain cascade. However, the HIV testing rate, the first critical step to ending the epidemic in particular among MSM populations, remains very low at 29% [4]. Only 7% of those reached through conventional face-to-face outreach activities have been tested for HIV [5], and in most conventional HIV testing programmes less than 50% who come for HIV testing are first-time testers [6].
Among Thai HIV-positive individuals in the National AIDS Program, only 73% have started ART and almost half of them had CD4 counts below 100 cells/mm3 at treatment initiation [7]. The overall retention rate in the programme is 78%, with a higher rate (87%) among those who are on ART than in those who are not (31%) [7].
A number of structural, societal and individual factors are barriers in terms of optimising the HIV prevention and treatment cascade among MSM and TG women. These include traditional low rate of outreach models [5,8], sexual identity and HIV-related stigmatisation [9], conventional HIV testing facilities with long waiting times, ineffective linkage to HIV care models, lack of adherence and retention support strategies [10,11], fragmented healthcare systems [12], perceived discrimination from providers, poor patient–provider communication, lack of guidance and follow-up, and logistic barriers [13–15]. Innovative interventions and service delivery models that help optimise this cascade by overcoming these barriers are a priority.
Electronic health record (EHR) systems are one example of an emerging healthcare technology that helps to reduce the fragmentation of care. They empower clients to engage in their own care, communicate with providers in virtual non-judgemental settings, schedule appointments and access medical health records by providing clients instant personal health information (PHI). Such models have become an important part of healthcare delivery, especially in the west [16].
However, the potential of EHR in optimising the HIV prevention and care cascade, especially among MSM and TG women, remains unknown. Past use has been limited, focusing primarily on removing the need for local clinic infrastructure, enhancing data sharing, providing a centralised source of data for epidemiological research [17] and reducing gaps, specifically in the HIV treatment continuum [18]. Costs, fear of and actual data breaches, as well as securing client confidentiality [19–22], remain key challenges to EHR adoption and effective use, especially among populations with stigmatising health conditions [23–25].
Adam's Love ( www.adamslove.org), a leading technology-based HIV outreach and testing initiative in Asia for MSM and TG individuals, was launched in 2011 by The Thai Red Cross AIDS Research Centre (TRCARC) and has engaged more than 3 million website visitors since its launch [26–28]. It has demonstrated success in engaging hard-to-reach Thai and Asian MSM and TG groups in e-counselling, assessing risks in real time and effectively linking them online to relevant clinical services using its novel online-to-offline model [26,28–34]. These vulnerable groups with high Internet and technology literacy could further benefit from innovative models such as the EHR.
We have developed and launched the Adam's Love EHR system in an effort to facilitate a completely online HIV prevention and treatment cascade. Because data on EHR implementation and impact on MSM and TG women's health are scarce, this study hopes to describe the implementation of a system that supports different types of HIV service delivery models and analyse participant characteristics according to their choice of EHR-supported service delivery model. We also have examined the factors associated with EHR uptake and utilisation among various MSM and TG subgroups and its impact on their health-seeking behaviours for six months following implementation, as well as the costs involved in the design and implementation of the system, to provide recommendations for future enhancements.
Methods
The myhealth.adamslove.org, a web browser-based EHR system, a recent addition to Adam's Love, was launched by the TRCARC in December 2015 [35] as part of the Online Test and Treat Study approved by the institutional review board of the Faculty of Medicine, Chulalongkorn University in Bangkok, Thailand, an amfAR GMT Initiative implementation science research project.
Between December 2015 and May 2016, the e-counselling support delivered via Adam's Love integrated e-platforms, including Adam's Love website ( www.adamslove.org), social media networks and instant messaging applications helped engage MSM and TG individuals at risk. They completed risk behaviour questionnaires and were assessed for their risk by Adam's Love online counsellors and staff who introduced them to the Adam's Love clinic-based or home-based HIV testing and counselling service options, and enrolled them into one of three study arms of their choice: (1) Participants in the HIV testing and counselling (HTC) arm received HIV testing and counselling at the MSM- and TG-friendly Adam's Love private and by-appointment clinic and were given access to the EHR system post HIV testing; (2) The participants in the hybrid arm received online pretest counselling, scheduled appointments via EHR and visited Adam's Love clinic to perform HIV testing; and (3) Participants in the online supervised HIV self-testing and counselling (eHTC) arm completed all of the above online via Adam's Love EHR, including scheduling for real-time home-based HIV testing and guidance, pretest counselling, online supervised finger-prick HIV self-testing and online referral to HIV treatment sites for those diagnosed as HIV positive (Box 1).
Box 1. Description of the three electronic health record (EHR)-supported HIV service delivery models.
| HIV testing and counselling (HTC) arm | Hybrid arm | Online supervised HIV self-testing and counselling (eHTC) arm | |
|---|---|---|---|
| Scheduling an appointment | Use e-counselling support platform to schedule HTC at a private Adam's Love clinic | Use EHR to schedule online pretest counselling
Use EHR to schedule HIV testing at an Adam's Love clinic |
Use EHR to schedule online pretest counselling
Use EHR to schedule the delivery of an HIV self-testing kit Use EHR to schedule online supervised HIV testing |
| Pretest counselling | At Adam's Love clinic | Online pretest counselling via live video | Online pretest counselling via live video |
| HIV testing | At Adam's Love clinic | At Adam's Love clinic | Online supervised HIV self-testing with real-time guidance by counsellor |
| Post-test counselling | At Adam's Love clinic, along with registration into the EHR system | At Adam's Love clinic | Online post-test counselling |
All participants received e-counselling via Adam's Love online support platforms (such as web message boards, social networks, instant communication applications), complete risk behaviour questionnaires and were assessed for their risk by online counsellors prior to being introduced to the study and choosing their preferred arm.
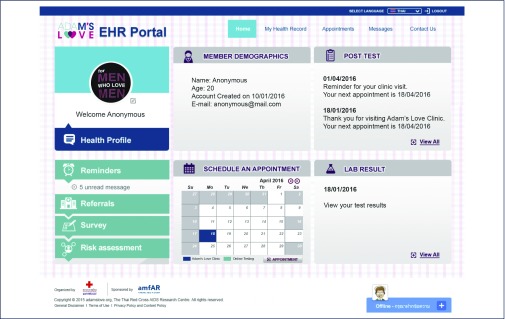
Registered participants in all three arms received access to all EHR features and functionalities illustrated in Figure 1.
The Adam's Love EHR model allowed a real-time data collection for study and monitoring purposes. Data elements can be categorised into the following items: (1) sociodemographic data: age, sexual identity, education, income, sexual and drug use behaviours, social network use characteristics, HIV and sexually transmitted infection (STI) testing history were self-reported in the questionnaires completed by participants; (2) EHR utilisation and activity tracking of participants in the HTC, hybrid and eHTC arms: appointment schedule and actual testing, time stamps, login frequency, functions accessed, EHR satisfaction, EHR system errors, troubleshooting and maintenance, and recommended engagement strategies needed were gathered using EHR data metrics and participant-completed questionnaires; and (3) clinical and laboratory data: HIV test results were entered manually in the EHR system by clinic counsellors and nurses.
Statistical analysis was conducted with Stata 14 (StataCorp LP, College Station, TX, USA). Sociodemographic and subject behavioural characteristics of participants in each arm were compared using a Kruskal–Wallis test for continuous covariates and chi-square or Fisher's exact tests for categorical covariates. Thereafter multiple logistic regression was used to assess which participant characteristics were associated with revisiting the EHR twice or more. Covariates in univariate analysis where the P for one of the categories vs the reference group was <0.1 were adjusted for in a multivariate model.
Development of Adam's Love EHR
The Adam's Love EHR was developed using an IBM web server application with steadfast security that included intuitively designed displays, aligned with cognitive workflows and decision making, which offered participants multiple user-friendly functions and features. The system was shaped and conceptualised by the study team, including HIV prevention and technology leaders, clinicians, biostatisticians and public health professionals, with the aim of facilitating an optimal online HIV prevention and treatment cascade as illustrated above (Figure 1).
Figure 1.
Adam's Love Electronic Health Record (EHR) features and functions
Key features of the system
-
–Online consent, knowledge assessment and registration
- ability to conduct online consent by electronic signature, test eligibility and participant understanding prior to joining the study; collect personal sociodemographic details during registration process and send signed consent form copies to the EHR database, project team and participant's email; issue EHR personal identification number; and conduct multiple surveys and self-risk assessment.
-
–Appointment scheduling/rescheduling and cancellation
- e-calendar with the ability to display available date and time slots for services at Adam's Love private clinic sites; enable participants to create, reschedule, or cancel appointments for pretest e-counselling, home-based HIV testing with live online guidance or Adam's Love clinic-based offline HIV testing.
-
–Synchronous e-counselling
- enable participants to communicate electronically and securely with counsellors through web conferencing, online meetings, screen sharing, live chats, and receive real-time HIV testing guidance and e-counselling.
-
–Post-test summary, laboratory results and health records:
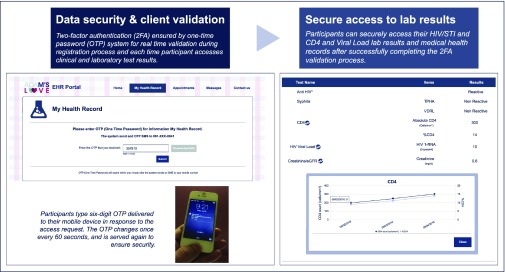
- enable participants to check their post-test summaries; access past HIV/STI test and other laboratory results such as CD4 count, HIV-RNA level, history of ART medication and other PHI data collected and entered manually by Adam's Love clinic counsellors and nurses in the EHR system; and view auto-generated graphs showing CD4 counts and HIV-RNA levels over time (Figure 2).
-
–Reminders and messages
- automated appointment reminders and personalised messaging capabilities to engage and retain participants in care.
-
–ART hospital referrals
- ability to facilitate the early linkage to care at one of the five collaborative hospitals for ART initiation, enable online registration with the linked HIV treatment facility, share participants’ HIV results and related health records with linked ART hospital nurses and provide a registration code online allowing a fast-track access to HIV treatment providers.
-
–Data storage and security
- The EHR system designed and built using IBM WebSphere Portal and DB2 database server included a range of steadfast security features to prevent unauthorised intrusion and help ensure secure data storage and back-up, participant access and sensitive HIV/STI data protection (Figure 2).
Figure 2.
Adam's Love Electronic Health Record (EHR) advanced data security system and one-time password (OTP) verification.
Advanced security features included an email verification process and a two-factor authentication (2FA) ensured by an OTP for real-time validation during the registration process and each time participants accessed clinical and laboratory test results
EHR usability training was conducted for participants, moderators, counsellors and ART hospital staff who were assigned various levels of access and authorisation permissions based on their administrative roles.
Results
Between December 2015 and May 2016, a total of 489 e-counselled MSM and TG women were assessed for their risk and introduced to the study arms by online counsellors, of whom 186 (38%) enrolled into the Adam's Love EHR system. Of the enrolled participants, 89, 72 and 25 chose to join the HTC, hybrid and eHTC arms, respectively, with an age range of 17–50 years (Table 1).
Table 1.
Sociodemographics of participants in the HTC, hybrid and eHTC arms
| Characteristics | HTC | Hybrid | eHTC | P-value |
|---|---|---|---|---|
| N=89 | N=72 | N=25 | ||
| N (%) | N (%) | N (%) | ||
| Age, year | 0.01 | |||
| Median (IQR) | 27(23–32) | 29(25–35) | 25(23–28) | |
| [Min–Max] | [17–50] | [19–49] | [20–43] | |
| Sexual orientation | 0.005a | |||
| Gay | 79(88.8) | 53(73.6) | 14(56.0) | |
| Bisexual | 5(5.6) | 7(9.7) | 4(16.0) | |
| Male | 3(3.4) | 10(13.9) | 4(16.0) | |
| Transgender | 2(2.2) | 2(2.8) | 3(12.0) | |
| Education level | 0.995 | |||
| Less than bachelor's degree | 17(19.1) | 14(19.4) | 5(20.0) | |
| Bachelor's degree or higher | 72(80.9) | 58(80.6) | 20(80.0) | |
| Monthly income | 0.82 | |||
| ≤500 USD | 24(27.0) | 17(23.6) | 6(24.0) | |
| 501–1000 USD | 42(47.2) | 31(43.1) | 10(40.0) | |
| ≥1001 USD | 23(25.8) | 24(33.3) | 9(36.0) | |
| HIV test result | ||||
| Negative | 87(97.8) | 71(98.6) | 21(84.0) | 0.01a |
| Positive | 2(2.2) | 1(1.4) | 4(16.0) | |
| Current residence | 0.59 | |||
| Bangkok | 72(80.9) | 55(76.4) | 18(72.0) | |
| Others | 17(19.1) | 17(23.6) | 7(28.0) | |
| Time spent on social media per day | <0.001 | |||
| Less than 4 hours | 27(30.3) | 21(29.2) | 6(24.0) | |
| 4–8 hours | 13(14.6) | 30(41.7) | 13(52.0) | |
| 8–24 hours | 49(55.1) | 21(29.2) | 6(24.0) | |
| Seek sexual partners online | 0.53 | |||
| No | 17(19.1) | 9(12.5) | 4(16.0) | |
| Yes | 72(80.9) | 63(87.5) | 21(84.0) | |
| Online/social media channels to seek sex | N=72 | N=63 | N=21 | |
| 37(51.4) | 26(41.3) | 8(38.1) | 0.38 | |
| Grindr | 17(23.6) | 53(84.1) | 12(57.1) | <0.001 |
| Hornet | 45(62.5) | 55(87.3) | 12(57.1) | 0.002 |
| Jack'd | 37(51.4) | 55(87.3) | 15(71.4) | <0.001 |
| Camfrog | 15(20.8) | 6(9.5) | 9(42.9) | 0.003 |
| 10(13.9) | 0(0.0) | 0(0.0) | 0.002a | |
| 8(11.1) | 2(3.2) | 1(4.8) | 0.18a | |
| Others(BeeTalk, GROWLr, PlanetRomeo) | 3(4.2) | 1(1.6) | 0(0.0) | 0.79a |
| Ever tested for HIV | 0.01a | |||
| Did not answer | 0(0.0) | 10(13.9) | 6(24.0) | |
| Yes | 72(80.9) | 54(75.0) | 13(52.0) | |
| No | 17(19.1) | 8(11.1) | 6(24.0) | |
| Previous HIV test | N=89 | N=62 | N=19 | 0.04a |
| Never tested | 17(19.1) | 8(12.9) | 6(31.6) | |
| Less than 6 months | 57(64.0) | 34(54.8) | 7(36.8) | |
| 6 months–1 year | 12(13.5) | 17(27.4) | 3(15.8) | |
| >1 year | 3(3.4) | 3(4.8) | 3(15.8) | |
| Lifetime sexual relationship with | ||||
| Male | 89(100.0) | 72(100.0) | 25(100.0) | N/A |
| Female | 16(18.0) | 13(18.1) | 5(20.0) | 0.934a |
| Transgender | 2(2.2) | 0(0.0) | 2(8.0) | 0.07a |
| Condom use in the past 6 months | <0.001 | |||
| Always | 54(60.7) | 17(23.9) | 7(28.0) | |
| Sometimes | 23(25.8) | 50(70.4) | 16(64.0) | |
| Never | 9(10.1) | 3(4.2) | 2(8.0) | |
| No anal sex in the past 6 months | 3(3.4) | 1(1.4) | 0(0.0) | |
| Substance use in the past 6 months(including alcohol) | 0.40 | |||
| No | 53(59.6) | 50(69.4) | 17(68.0) | |
| Yes | 36(40.4) | 22(30.6) | 8(32.0) | |
| Substance used in the past 6 months | N=36 | N=22 | N=8 | |
| Alcohol | 20(55.6) | 12(54.5) | 6(75.0) | 0.64a |
| Poppers | 17(47.2) | 12(54.5) | 2(25.0) | 0.42a |
| Methamphetamine | 4(11.1) | 3(13.6) | 0(0.0) | 0.86a |
| Ketamine | 0(0.0) | 1(4.5) | 0(0.0) | 0.46a |
| Viagra | 3(8.3) | 4(18.2) | 1(12.5) | 0.50a |
| Cannabis | 1(2.8) | 1(4.5) | 0(0.0) | 0.81a |
| Reasons for using substance | N=31 | N=21 | N=7 | |
| Sexual arousal | 10(32.3) | 9(42.9) | 3(42.9) | 0.69a |
| Forget sadness | 0(0.0) | 3(14.3) | 0(0.0) | 0.08a |
| Try/friends recommend | 4(12.9) | 5(23.8) | 0(0.0) | 0.40a |
| For fun | 15(48.4) | 12(57.1) | 5(71.4) | 0.53a |
| Dare to do things | 0(0.0) | 1(4.8) | 0(0.0) | 0.48a |
| Prolong sex | 2(6.5) | 0(0.0) | 0(0.0) | 0.62a |
| Ever had an STI | 0.02a | |||
| Never | 52(58.4) | 51(70.8) | 20(80.0) | |
| Yes | 24(27.0) | 8(11.1) | 5(20.0) | |
| I'm not sure | 13(14.6) | 13(18.1) | 0(0.0) | |
| Had group sex in the past 6 months | 0.10 | |||
| No | 84(94.4) | 61(84.7) | 21(84.0) | |
| Yes | 5(5.6) | 11(15.3) | 4(16.0) |
eHTC: online supervised HIV self-testing and counselling; HTC: private clinic-based HIV testing and counselling; IQR: interquartile range; STI: sexually transmitted infection.
Fisher's exact test.
The majority (>80%) of participants in the three arms had obtained bachelor degrees or higher, more than a quarter had a monthly salary above 1000 USD, most (>72%) lived in Bangkok, a majority (>69.7%) spent more than 4 hours using social media and most (83.9%) had sought sex online. Almost one-third (>30%) had used drugs in the past 6 months, mostly alcohol (>54%), poppers (>25%) and Viagra (>8%), with popper use highest among participants in the hybrid arm (54.5%). Those in the HTC arm as compared with those in the hybrid and eHTC arms were more likely to self-identify as gay (88.8%, P=0.005), spent more than 8 hours per day on social media (55.1% vs 29.2% vs 24%, P<0.001), consistently used condoms in the past 6 months (60.7% vs 23.9% vs 28%, P<0.001) and had been previously diagnosed with an STI compared with those in the other two arms (27% vs 11.1% vs 20%, P=0.02), respectively.
The participants in the eHTC arm were more likely to be younger, with a median age of 25 vs 29 for the hybrid and 27 years for the HTC arm (P=0.01), bisexual (16% vs 9.7% vs 5.6%, P=0.005), have never tested for HIV or refused to answer (48% vs 25% vs 19.1%; P=0.01) or tested more than 1 year ago (15.8% vs 4.8% vs 3.4%, P=0.04). The participants in the eHTC and hybrid arms tended to have had more group sex (16% vs 15.3% vs 5.6%; P=0.10) than did those in the HTC arm, although this difference was not significant. The participants in the eHTC arm had never used methamphetamine, although some in the HTC (11.1%) and hybrid (13.6%) arms reported having used it.
Platforms used for seeking sex varied significantly among the three arms, with Camfrog (video chat program) having the highest use among participants in the eHTC arm (P=0.003); Jack'd (P<0.001), Hornet (P=0.002) and Grindr (P<0.001) dating applications had the highest use among participants in the hybrid arm; and Twitter (social networking application) was highest among participants in the HTC arm (P=0.002).
HIV prevalence was highest among participants in the eHTC arm (16% vs 1.4% vs 2.2%, P=0.01). However, none of the study's five collaborative treatment sites for online referral was covered by the national health insurance plan, which is based on the national identification number and address in Thailand of the HIV-positive participant. These individuals were successfully linked to care through conventional offline referral methods rather than the online model via the EHR's electronic referral system.
The median (interquartile range) duration of study follow-up was 2.3 (1.8–5.9) months and is consistent across arms. Almost half of all participants in the HTC (48.3%), hybrid (62.5%) and eHTC (48%) arms revisited the EHR at least once after baseline registration. The median revisit time was 4 vs 4 vs 6 days, respectively. The second revisit rate was least among participants in the eHTC arm (8%), whereas more than a quarter of the participants in the HTC (27%) and hybrid (26.4%) arms revisited three or more times. Participants in the HTC, hybrid and eHTC arms revisited to check messages sent by the study team (90.7% vs 82.2% vs 41.7%, P=0.002), access laboratory results (79.1 vs 71.1% vs 33.3%, P=0.01), read post-test counselling summaries (53.5% vs 55.6% vs 33.3%) and make their next clinic appointment to receive an HIV test (51.2% vs 66.7% vs 0%, P<0.001) (Table 2).
Table 2.
EHR utilisation among participants in the HTC, hybrid and eHTC arms
| EHR utilisation | HTC (n=89) | Hybrid (n=72) | eHTC (n=25) | P-value |
|---|---|---|---|---|
| Revisit after baseline | ||||
| No | 46(51.7) | 27(37.5) | 13(52.0) | 0.17 |
| Yes | 43(48.3) | 45(62.5) | 12(48.0) | |
| Number of EHR revisits after baseline(times) | 0.001a | |||
| 0 | 46(51.7) | 27(37.5) | 13(52.0) | |
| 1 | 9(10.1) | 11(15.3) | 10(40.0) | |
| 2 | 10(11.2) | 15(20.8) | 2(8.0) | |
| ≥3 | 24(27.0) | 19(26.4) | 0(0.0) | |
| Duration between baseline and first revisit(days) | N=43 | N=45 | N=12 | 0.627 |
| Median(IQR) | 4(1–8) | 4(2–11) | 6(2–12) | |
| [Min–Max] | [1–72] | [1–71] | [1–27] | |
| EHR activities at revisit | N=43 | N=45 | N=12 | |
| Check messages | 39(90.7) | 37(82.2) | 5(41.7) | 0.002a |
| Check lab results | 34(79.1) | 32(71.1) | 4(33.3) | 0.01a |
| View post-test summary | 23(53.5) | 25(55.6) | 4(33.3) | 0.41a |
| Appointment making | 22(51.2) | 30(66.7) | 0(0.0) | <0.001 |
| Complete survey | 5(11.6) | 10(22.2) | 7(58.3) | 0.004a |
EHR: electronic health record; eHTC: online supervised HIV self-testing and counselling; HTC: private clinic-based HIV testing and counselling; IQR: interquartile range.
Fisher's exact test.
In a multivariate model adjusting for sexual identity, three factors were independently associated with more than two EHR revisits. Participants in the eHTC arm had reduced odds of revisiting compared with participants in the HTC [odds ratio (OR) 0.14, 95% confidence interval (CI) 0.03–0.67, P=0.01] and hybrid arms (OR 0.10, 95% CI 0.02–0.44, P=0.003). Those having an income of ≤500 USD (OR 4.21, 95% CI 1.21–14.71, P=0.02) or between 501 and 1000 USD (OR 3.38, 95% CI 1.04–11.03, P=0.04) vs ≥1500 USD per month, and those having group sex in the past 6 months (OR 3.71, 95% CI 1.16–11.81, P=0.03), had increased odds for revisiting the EHR twice or more. None of the participants who were diagnosed as HIV positive ever revisited on two or more occasions (Table 3).
Table 3.
Univariate and multivariate analysis of factors associated with revisiting the EHR at least twice
| Covariate | Did not revisit or revisit once | Revisit (≥2 times) | Unadjusted OR (95%CI) | P | Adjusted OR (95%CI) | P |
|---|---|---|---|---|---|---|
| Arm | 0.001 | 0.002 | ||||
| HTC | 55(61.8) | 34(38.2) | Reference | Reference | ||
| Hybrid | 38(52.8) | 34(47.2) | 1.45(0.77–2.72) | 0.25 | 1.38(0.70–2.72) | 0.35 |
| eHTC | 23(92.0) | 2(8.0) | 0.14(0.03–0.63) | 0.01 | 0.14(0.03–0.67) | 0.01 |
| Sexual orientation | 0.33 | 0.44 | ||||
| Gay | 87(59.6) | 59(40.4) | Reference | |||
| Bisexual | 13(81.2) | 3(18.8) | 0.34(0.09–1.25) | 0.68 | 0.34(0.09–1.38) | 0.13 |
| Male | 11(64.7) | 6(35.3) | 0.80(0.28–2.29) | 0.54 | 1.08(0.32–3.60) | 0.90 |
| Transgender | 5(71.4) | 2(28.6) | 0.59(0.11–3.14) | 0.10 | 0.87(0.13–5.56) | 0.89 |
| Education level | 0.58 | |||||
| Less than bachelor's degree | 21(58.3) | 15(41.7) | 1.69(0.64–4.44) | |||
| Bachelor's degree or higher | 95(63.3) | 55(36.7) | Reference | |||
| Monthly income | 0.19 | 0.11 | ||||
| ≤500 USD | 26(55.3) | 21(44.7) | 3.23(1.04–10.06) | 0.04 | 4.21(1.21–14.71) | 0.02 |
| 501–1000 USD | 50(60.2) | 33(39.8) | 2.64(0.90–7.73) | 0.08 | 3.38(1.04–11.03) | 0.04 |
| 1001–1500 USD | 20(64.5) | 11(35.5) | 2.20(0.65–7.49) | 0.21 | 3.00(0.78–11.59) | 0.11 |
| ≥1500 USD | 20(80.0) | 5(20.0) | Reference | Reference | ||
| Anti-HIV result | 0.05a | |||||
| Negative | 109(60.9) | 70(39.1) | Reference | |||
| Positive | 7(100.0) | 0(0.0) | 0.16(0–1.12) | |||
| Time using social media per day | 0.57 | |||||
| Less than 4 hours | 35(64.8) | 19(35.2) | Reference | |||
| 4–8 hours | 37(66.1) | 19(33.9) | 0.95(0.43–2.08) | 0.89 | ||
| 8–24 hours | 44(57.9) | 32(42.1) | 1.34(0.64–2.75) | 0.43 | ||
| Condom use in the past 6 months | 0.86 | |||||
| Always/no anal sex in the past 6 months | 52(63.4) | 30(36.6) | Reference | |||
| Sometimes/never | 64(62.1) | 39(37.9) | 1.06(0.58–1.93) | |||
| Used substance in the past 6 months(including alcohol) | 0.50 | |||||
| No | 77(64.2) | 43(35.8) | Reference | |||
| Yes | 39(59.1) | 27(40.9) | 1.24(0.67–2.30) | |||
| Had group sex in the past 6 months | 0.10 | 0.03 | ||||
| No | 107(64.5) | 59(35.5) | Reference | Reference | ||
| Yes | 9(45.0) | 11(55.0) | 2.22(0.87–5.65) | 3.71(1.16–11.81) | ||
| Ever had an STI | 0.76 | |||||
| No | 75(61.0) | 48(39.0) | Reference | |||
| Yes | 25(67.6) | 12(32.4) | 0.75(0.34–1.63) | 0.47 | ||
| I'm not sure | 16(61.5) | 10(38.5) | 0.98(0.41–2.33) | 0.96 |
EHR, electronic health record; eHTC, online supervised HIV self-testing and counselling; HTC, private clinic-based HIV testing and counselling.
OR is the median unbiased estimate calculated using exact logistic regression.
The majority of participants (n=110) who accessed the EHR system also completed the satisfaction survey. Overall satisfaction was reported to be high, with a mean rating of 4.4 (SD=0.68) on a Likert scale of 5. Participants also favourably rated each existing EHR feature, including design and comprehensive interface (4.34, SD=0.78), online consent and understanding (4.58, SD=0.57), ease of registration process (4.51, SD=0.63), online HIV/STI data security (4.64, SD=0.53), ease of accessing laboratory results and post-test counselling summaries (4.37, SD=0.70) and the video chat quality for online HIV self-testing guidance (4.71, SD=0.47). Future and most desirable EHR enhancement features were real-time e-counselling by doctors, a sexual health forum, educational videos, a reward and incentive structure for achieving milestones, a personalised memo/diary to maintain health notes and chat rooms to connect with the community.
In total there were 24 incidents (12.9% user incident reports) reported during the 6-month period, including interoperability issues, survey completion errors, e-calendar scheduling issues, booking viewing made at e-calendar and some other interface issues. There were also initial technical difficulties with the one-time password (OTP) system due to password delivery interruptions. These were addressed by troubleshooting directly with local mobile network operators and white listing internet protocol numbers. Other technical issues included inconsistent microphone or webcam function and disruptions in live video chat due to slow internet connections.
Discussion
Our study demonstrates the feasibility of creating an innovative EHR system tailored for MSM and TG women. Three different types of service delivery models helped reach diverse and at-risk subgroups of mostly MSM, varying by age, time spent per day using social media, substance use, online sex-seeking behaviours, condom use, and HIV/STI testing history and prevalence. Of a total of 186 MSM and TG participants enrolled in the study, nearly one-third had used substances, most had sought sex online and a quarter of them were first-time testers. Almost half (48.3%) revisited the EHR at least once to access laboratory results, read post-test summaries and make an appointment for their next HIV test. The overall satisfaction with the system was reported to be high by all participants. Young, bisexual and high-risk MSM and TG women with high psychotropic drug use, had more group sex and who never or less frequently tested for HIV were more likely to prefer the eHTC or hybrid arm. The HIV prevalence was highest (16%) in the eHTC arm. The EHR-supported innovative online and offline service delivery models ensured secure and safe access to highly sensitive HIV/STI data storage and access over the Internet. The platform has, therefore, a high potential to store and enable access to cascade data for both HIV prevention and treatment programmes.
Several studies have demonstrated that many participants would review and interact with their medical record on an ongoing basis if the record was made available to them [36–39].
Usage and revisits were highest among participants in the hybrid arm who used this system for checking laboratory results, checking post-test counselling summaries and making appointments for their next clinic visit, representing, therefore, possibly the most feasible, acceptable and engaging model for high-risk Thai MSM and TG groups who have high Internet and technology utilisation, and those who use multiple applications to seek sex online and those who engage in group sex.
Interestingly in our multivariate model analysis, those with a lower monthly income who would be less likely to afford private healthcare were more likely to revisit the EHR on two or more occasions. It is possible that they felt more engaged and empowered by our innovative model than they did in the traditional healthcare systems, where they might feel hesitant in communicating with providers and in seeking support due to multiple barriers, including differences in social status and self-stigmatisation perceptions towards the use of these services [40–42]. Provision of virtual and non-judgemental medical services and easy and free access to PHI via EHR for vulnerable groups with lower socio-economic status could help reduce health disparities.
The participants in the eHTC arm had the least number of EHR revisits, with those diagnosed as HIV positive especially hard to engage online. This may be due to a lack of in-person counselling post HIV testing and an insufficient interaction/relationship building with the offline counsellors. We were unable to refer and link online via the EHR those who were diagnosed positive in the eHTC arm. They were linked via the offline referral system to treatment sites covered by their healthcare coverage plans, which might also be a factor for reduced engagement and EHR utilisation.
Evidence of the impact EHRs have on clinical outcomes remains mixed in diabetes [43] and HIV care. These systems have shown a potential to improve quality of care [44] and communication between patients and providers [45] as well as ART adherence [46]. In our study the EHR integrated with innovative service delivery models helped improve client engagement in terms of their health and reduce some of the known barriers in conventional HIV prevention and care models.
The absence of meaningful end-user engagement has repeatedly been highlighted as a key factor contributing to ‘failed’ EHR implementations [47]. Our participants highlighted the importance of integrating user-driven engagement strategies to enhance the involvement in their health and increase interactions with providers to improve health outcomes.
We are aware of several limitations in our study. Participants in the eHTC arm diagnosed as HIV positive had to be linked to care by offline referral systems as the collaborative treatment sites that supported the online referral and linkage to care were not covered by the participant's national healthcare plan. A wider collaboration with other treatment sites in Bangkok to facilitate online linkage to care facilities is our next focus. We also believe that we reached only few TG women, and more efforts are needed to engage this high-risk group in our type of intervention.
The Adam's Love EHR system performed with no unauthorised data breaches, intrusions detected or reported. A combination of security features in its implementation offered layers of protection to the client data and usage. The OTP verification system (two-factor authentication) was acceptable and added further credibility. Contrary to previous EHR studies [25], our quantitative data revealed that Adam's Love EHR system was perceived extremely secure by MSM and TG participants who were highly satisfied with it. This system was extremely robust with 12.9% user incident reports in a six-month period, most being of medium priority and which could be resolved by bug-fixing. The EHR model is scalable and replicable in settings for those who are not reached and for those vulnerable groups with high Internet use. With the capacity to support 1000 users, the investment for our initial set-up was 130,000 USD and the annual post-implementation maintenance cost was 21,100 USD.
In conclusion, our study demonstrated the feasibility of creating a secure and completely online HIV prevention and care cascade using the Adam's Love EHR system. It underlines the significance of implementing such innovative interventions and service delivery models to reach high-risk MSM and some TG women who remain hard to reach or would shy away from conventional HIV prevention interventions.
Acknowledgements
Adam's Love Electronic Health Record (EHR) system was supported by the amfAR GMT Initiative Grant No. 109072-57-HGMM. The authors are extremely grateful to the amfAR – Foundation for AIDS Research and thank all research participants and study staff for their significant contribution.
Competing interests
None.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily reflect the views or policies of amfAR or the US Army and the US Department of Defense.
Authors' contributions
All authors contributed to the outline of the paper. Tarandeep Anand assumed primary responsibility for writing and revising the manuscript. Nittaya Phanuphak, Chattiya Nitpolprasert, Stephen J Kerr and Tanakorn Apornpong substantially contributed to the preparation of this paper. All authors edited and reviewed the manuscript and gave their final approval for submission to the journal.
References
- 1. Griensven F, Varangrat A, Wimonsate W et al. Trends in HIV prevalence, estimated HIV incidence, and risk behavior among men who have sex with men in Bangkok, Thailand, 2003–2007. J Acquir Immune Defic Syndr 2010; 53: 234– 239. [DOI] [PubMed] [Google Scholar]
- 2. Ananworanich J, Chitwarakorn A, Wimonsate W et al. HIV and syphilis infection among men who have sex with men – Bangkok, 2005–2011. MMWR Morb Mortal Wkly Rep 2013; 62: 558. [PMC free article] [PubMed] [Google Scholar]
- 3. UNAIDS Smart investments. 2013. Available at: www.unaids.org/sites/default/files/media_asset/20131130_smart-investments_en_1.pdf ( accessed December 2016).
- 4. National AIDS Committee Thailand AIDS Response Progress Report 2012 reporting period: 2010–2011. 2012. Available at: www.aidsdatahub.org/sites/default/files/documents/UNGASS_2012_Thailand_Narrative_Report.pdf.
- 5. Wolf RC. Thailand global fund round 8 external evaluation: men who have sex with men (MSM). 2012. Available at: www.researchgate.net/publication/260293958_Thailand_Global_Fund_Round_8_External_Evaluation_MSM_Thailand_Global_Fund_Round_8_External_Evaluation_Men_Who_Have_Sex_with_Men_%28MSM%29_Thailand_Global_Fund_Round_8_External_Evaluation_MSM_ACKNOWLEDGEMENTS ( accessed December 2016).
- 6. Phanuphak N, Keeratikongsakul J, Barisri N et al. High uptake of HIV testing, HIV prevalence and incidence among MSM clients of the Thai Red Cross mobile clinic to night entertainment venues in Bangkok AIDS 2014. Melbourne, Australia, July 2014. Abstract MOPE369. [Google Scholar]
- 7. National AIDS Management Center, Department of Disease Control MoPH . 2015 Thailand AIDS Response Progress Report. Bangkok, Thailand, 2015. [Google Scholar]
- 8. Lee SW, Deiss RG, Segura ER et al. A cross-sectional study of low HIV testing frequency and high-risk behaviour among men who have sex with men and transgender women in Lima, Peru. BMC Public Health 2015; 15: 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wei C, Yan H, Yang C et al. Accessing HIV testing and treatment among men who have sex with men in China: a qualitative study. AIDS Care 2014; 26: 372– 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nachega JB, Uthman OA, Rio C et al. Addressing the Achilles' heel in the HIV care continuum for the success of a test-and-treat strategy to achieve an AIDS-free generation. Clin Infect Dis 2014; 59 ( suppl 1): S21– S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meehan SA, Leon N, Naidoo P et al. Availability and acceptability of HIV counselling and testing services. A qualitative study comparing clients' experiences of accessing HIV testing at public sector primary health care facilities or non-governmental mobile services in Cape Town, South Africa. BMC Public Health 2015; 15: 845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Doshi RK, Malebranche D, Bowleg L et al. Health care and HIV testing experiences among Black men in the South: implications for ‘Seek, Test, Treat, and Retain’ HIV prevention strategies. AIDS Patient Care STDS 2013; 27: 123– 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meanley S, Gale A, Harmell C et al. The role of provider interactions on comprehensive sexual healthcare among young men who have sex with men. AIDS Educ Prev 2015; 27: 15– 26. [DOI] [PubMed] [Google Scholar]
- 14. Churcher S. Stigma related to HIV and AIDS as a barrier to accessing health care in Thailand: a review of recent literature. WHO South East Asia J Public Health 2013; 2: 12. [DOI] [PubMed] [Google Scholar]
- 15. Liu Y, Osborn CY, Qian HZ et al. Barriers and facilitators of linkage to and engagement in HIV care among HIV-positive men who have sex with men in China: a qualitative study. AIDS Patient Care STDS 2016; 30: 70– 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lam JG, Lee BS, Chen PP. The effect of electronic health records adoption on patient visit volume at an academic ophthalmology department. BMC Health Serv Res 2016; 16: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tierney WM, Rotich JK, Hannan TJ et al. The AMPATH medical record system: creating, implementing, and sustaining an electronic medical record system to support HIV/AIDS care in western Kenya. Stud Health Technol Inform 2007; 129: 372– 376. [PubMed] [Google Scholar]
- 18. Haskew J, Ro G, Turner K et al. Implementation of a cloud-based electronic medical record to reduce gaps in the HIV treatment continuum in rural Kenya. PLoS ONE 2015; 10: e0135361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Appari A, Johnson ME. Information security and privacy in healthcare: current state of research. Int J Internet Enterp Manag 2010; 6: 279– 314. [Google Scholar]
- 20. Hersh W. Health care information technology: progress and barriers. JAMA 2004; 292: 2273– 2274. [DOI] [PubMed] [Google Scholar]
- 21. Litvin CB. In the dark – the case for electronic health records. N Engl J Med 2007; 356: 2454– 2455. [DOI] [PubMed] [Google Scholar]
- 22. Swartz N. Electronic medical records' risks feared. Inf Manag 2005; 39: 9. [Google Scholar]
- 23. Salomon RM, Blackford JU, Rosenbloom ST et al. Openness of patients' reporting with use of electronic records: psychiatric clinicians' views. J Am Med Inform Assoc 2010; 17: 54– 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malebranche DJ, Peterson JL, Fullilove RE et al. Race and sexual identity: perceptions about medical culture and healthcare among Black men who have sex with men. J Natl Med Assoc 2004; 96: 97– 107. [PMC free article] [PubMed] [Google Scholar]
- 25. Stablein T, Lorenzo J, Nissenbaum H et al. Gay males and electronic health records: privacy perceptions, age, and negotiating stigma. Available at: http://sharps.org/wp-content/uploads/STABLEIN-ESS-Annual-Meeting.pdf ( accessed December 2016).
- 26. Zablotska I, Grulich AE, Phanuphak N et al. PrEP implementation in the Asia-Pacific region: opportunities, implementation and barriers. J Int AIDS Soc 2016; 19: 21119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Frellick M. Apps and Social Media Can Reach High-Risk HIV Populations. Medscape, WebMD, LLC, 2016. Available at: www.medscape.com/viewarticle/871137 ( accessed 23 November 2016). [Google Scholar]
- 28. Nitpolprasert C, Anand T, Ananworanich J et al. ‘Adam's Love: for men who love men’, an online communication campaign through an edutainment website to promote HIV testing among men who have sex with men (MSM) in Thailand In: AIDS 2012. Washington, DC, USA, July 2012. Abstract THPE250. [Google Scholar]
- 29. Anand T, Nitpolprasert C, Trachunthong D et al. A novel online-to-offline (O2O) model for PrEP and HIV testing scale up In: AIDS 2016. Durban, South Africa: July 2016. Abstract LBPE035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anand T, Nitpolprasert C, Ananworanich J et al. Characteristics of HIV risks among Thai young men who have sex with men and transgender youth using an eCounseling platform In: AIDS 2016. Durban, South Africa, July 2016. Abstract TUPEC229. [Google Scholar]
- 31. Anand T, Nitpolprasert C, Sohn AH et al. TemanTeman.org, an integrated public-private sector communication strategy to scale up early and routine HIV testing among most-at-risk populations (MARPs) in Indonesia In: AIDS 2014. Melbourne, Australia, July 2014. Abstract TUPE174. [Google Scholar]
- 32. Anand T, Ananworanich J, Parwati Merati T et al. A culturally sensitive online communication campaign to reach hidden men who have sex with men for HIV/STI prevention and testing in Indonesia: TemanTeman.org In: IAS 2013. Kuala Lumpur, Malaysia, June–July 2013. Abstract WEPE590. [Google Scholar]
- 33. Anand T, Nitpolprasert C, Ananworanich J et al. Innovative strategies using communications technologies to engage gay men and other men who have sex with men into early HIV testing and treatment in Thailand. J Virus Erad 2015; 1: 111– 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Anand T, Nitpolprasert C, Ananworanich J et al. How effective are innovative strategies that use communication technology in scaling up HIV testing and engaging MSM in HIV awareness? A case study from Thailand In: IAS 2015. Vancouver, Canada, July 2015. Abstract MOPEC428. [Google Scholar]
- 35. Anand T, Nitpolprasert C, Phanuphak P et al. Creating an online HIV prevention and treatment cascade using the Adam's love electronic health record system In: AIDS 2016. Durban, South Africa, July 2016. Abstract THPEE506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hassol A, Walker JM, Kidder D et al. Patient experiences and attitudes about access to a patient electronic health care record and linked web messaging. J Am Med Inform Assoc 2004; 11: 505– 513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pyper C, Amery J, Watson M et al. Patients' experiences when accessing their on-line electronic patient records in primary care. Br J Gen Pract 2004; 54: 38– 43. [PMC free article] [PubMed] [Google Scholar]
- 38. Wiljer D, Bogomilsky S, Catton P et al. Getting results for hematology patients through access to the electronic health record. Can Oncol Nurs J 2006; 16: 154– 156. [DOI] [PubMed] [Google Scholar]
- 39. Pyper C, Amery J, Watson M et al. Patients' access to their online electronic health records. J Telemed Telecare 2002; 8 ( suppl 2): 103– 105. [DOI] [PubMed] [Google Scholar]
- 40. Perry MJ. Gender, race and economic perspectives on the social epidemiology of HIV infection: implications for prevention. J Prim Prev 1998; 19: 97– 104. [Google Scholar]
- 41. Smith MW, Kreutzer RA, Goldman L et al. How economic demand influences access to medical care for rural Hispanic children. Med Care 1996; 34: 1135– 1148. [DOI] [PubMed] [Google Scholar]
- 42. Jirojwong S, Manderson L. Physical health and preventive health behaviors among Thai women in Brisbane, Australia. Health Care Women Int 2002; 23: 197– 206. [DOI] [PubMed] [Google Scholar]
- 43. Graetz I, Huang J, Brand R et al. The impact of electronic health records and teamwork on diabetes care quality. Am J Manag Care 2015; 21: 878– 884. [PMC free article] [PubMed] [Google Scholar]
- 44. Appari A, Johnson EM, Anthony D. Meaningful use of electronic health record systems and process quality of care: evidence from a panel data analysis of U.S. acute-care hospitals. Health Serv Res 2013; 48: 354– 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Delbanco T, Walker J, Bell SK et al. Inviting patients to read their doctors' notes: a quasi-experimental study and a look ahead. Ann Intern Med 2012; 157: 461– 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Saberi P, Catz SL, Leyden WA et al. Antiretroviral therapy adherence and use of an electronic shared medical record among people living with HIV. AIDS Behav 2015; 19 ( suppl 2): 177– 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cresswell K, Morrison Z, Crowe S et al. Anything but engaged: user involvement in the context of a national electronic health record implementation. Inform Prim Care 2011; 19: 191– 206. [DOI] [PubMed] [Google Scholar]