Abstract
Background
Inflammation and coagulation biomarkers are independent predictors of morbidity and mortality in HIV-infected patients. The impact of country of residence on these biomarkers is unknown and was investigated in persons at similar stages of HIV infection.
Methods
Cryopreserved plasma specimens were analysed from 267 ART-naive patients with CD4 cell counts <100 cells/μl from Mexico (n=124) and South Africa (n=143). Biomarkers were compared and dimension reduction analyses were performed to highlight biosignatures according to nationality, gender and tuberculosis co-infection.
Results
Mexican patients were significantly different from South Africans with regard to age, gender, CD4 cell count, haemoglobin, presence of AIDS-defining illness and prevalence of active tuberculosis. After adjusting for baseline characteristics, patients from Mexico had higher levels of IFN-γ, IL-8, and CXCL-10 whereas patients from South Africa had higher levels of fibrinogen, LTB4, P-selectin, protein S, and sCD40 ligand. The effect of country on the profile of biomarker expression was stronger than gender differences and tuberculosis co-infection.
Conclusion
Inflammation and coagulation biomarkers vary significantly by country. Further studies are needed to evaluate how these differences may contribute to HIV pathogenesis and prognosis in diverse populations and how they can be accounted for in studies using biomarkers as surrogate end points.
Keywords: HIV, nationality, inflammation, biomarkers
Introduction
Biomarkers of inflammation and coagulation have emerged as independent predictors of morbidity and mortality in HIV-infected patients in several studies [1–6]. In particular IL-6 and D-dimer [3,7–9], CRP [8] and sCD14 [5] have been strongly associated with all-cause mortality and cardiovascular mortality [10]. IL-6, CRP and D-dimers have also been linked with cardiovascular disease [1]. Most of these studies included large numbers of patients from several countries, but predominantly from North America and Europe.
Many studies focused on cardiovascular disease have shown that the inflammatory profile may vary by geographic location or ethnicity [11–18]. Nationality- or ethnicity-driven variation of biomarker concentrations in the blood have been explored in the context of infectious diseases such as tuberculosis [12]. Nevertheless, the potential effects of country of residence on the inflammatory profile of HIV-infected persons remain largely unknown. Recognising a possible association may have important implications for the potential use of biomarkers as surrogate markers or clinical trial endpoints.
In the present study, we examined the potential effect of nationality on circulating biomarkers by studying HIV-infected, ART naive patients participating in a randomised clinical trial in two different countries (CADIRIS trial, NCT00988780), a trial in which biomarkers predictive of IRIS were recently analysed [19]. Our aim was to evaluate whether patients living with HIV in different regions (country of residence), but at similar stages of infection, have significant differences in plasma levels of biomarkers of inflammation and coagulation.
Methods
Study outline
The CADIRIS trial (CCR5 Antagonism to Decrease the Incidence of the Immune Reconstitution Inflammatory Syndrome in HIV-infected Patients, NCT00988780) was a double-blinded trial that randomly allocated 276 ART-naive patients with AIDS (CD4 cell count <100 cells/μL) to receive maraviroc or placebo in combination with a standard antiretroviral regimen (efavirenz, tenofovir and emtricitabine) to assess whether maraviroc could decrease the incidence of IRIS [20,21]. Patients were screened from 2009 to 2012 at clinical sites in Mexico and South Africa. The primary endpoint was IRIS diagnosis by week 24 of ART initiation. All patients provided written informed consent. A full description of the clinical trial and outcomes has been previously published [21]. This study was sponsored by the Instituo Nacional de Cicencias Medicas y Nutricion Salvador Zubiran and approved by the Ministry of Health and Federal Commission for Sanitary Risks Protection of Mexico, and the Medicines Control Council and Human Research Ethics Committee of South Africa. We evaluated levels of biomarkers based upon nationality, gender, and TB status of the CADIRIS population in 267 available cryopreserved plasma specimens from the pre-ART timepoint.
Biomarker measurements
All plasma specimens were obtained at the time of enrolment, prior to ART initiation, and were stored at −80°C. Biomarkers and coagulation markers were measured in plasma. Specimens were collected in EDTA or citrate (for protein C, S, von Willebrand factor and vitamin D measurement). Levels of IFN-γ, IL-10, IL-12p70, IL-1β, IL-6, TNF-α, CRP, SAA, P-selectin, CXCL10 and IL-17 were measured by electrochemiluminescence using a multiplex plate (MESO scale discovery). Levels of sCD14, sCD40L, sCD163, vWF, fibrinogen, protein C and S were measured using enzyme-linked immunosorbent assays (ELISAs) according to the manufacturers’ instructions (R&D Systems, AdipoBioscience, Zymutest). Levels of 25-hydroxyvitamin D were also measured using ELISA (ALPCO). D-dimer was measured using an enzyme-linked fluorescent assay (Biomeriuex) and hyaluronic acid (HA) was measured using an enzyme coagulation kit (Corgenix).
Statistical methods
Baseline characteristics were summarised with frequencies or medians and interquartile ranges (IQR) as appropriate. Demographic data were analysed using Wilcoxon rank-sum test and Fisher's test. Data were logarithmically transformed, and comparisons of biomarker distributions by country, gender and TB status were performed using the Student's t-test. Confounding factors were chosen based upon significant differences in baseline characteristics and adjusted for using a multivariate generalised linear model. To compensate for multiple comparisons, we defined statistical significance as a P-value ≤0.01.
Dimension reduction analyses
A principal component analysis (PCA) model, utilising a combination of plasma concentrations of several biomarkers that exhibited statistical differences assessed in univariate analyses between patients from Mexico and South Africa, was employed to test whether individuals from these different countries could be distinguished. A similar approach has been successfully used previously [22–24]. Unsupervised two-way hierarchical cluster analyses (Ward's method) with bootstrap, were used to test whether males and females from the different countries exhibiting differential levels of biomarkers in plasma could be grouped separately. The same analysis was performed to assess the overall biomarker profile between patients with TB versus those without TB co-infection. This tested whether gender differences or differences based on TB would influence the potential differences observed in the analysis by country.
The inferential networks (host interactome) were generated from Spearman correlation matrices containing values of each biomarker measured in the plasma samples and blood parameters. In this analysis, each mediator is selected as a target, and the software program searches among the other mediators for those that are correlated, with the target calculating a correlation matrix using Spearman rank tests. As a result, the features related to the selected target are linked. The links shown in the networks represent highly statistically significant Spearman rank correlations (P<0.001). To compensate for differences in the number of individuals in the distinct study groups (e.g. male vs female or TB vs no TB), we employed the bootstrap method (100X). Only correlations remaining with P<0.001 in at least 40 out of 100 times were considered.
Sparse canonical correlation analysis (CCA) modelling was employed to assess whether combinations of circulating biomarkers can discriminate between patients from Mexico or South Africa. The CCA model was chosen because many variables were studied. This model is able to perform dimensionality reduction for two co-dependent data sets (biomarker profile and baseline characteristics profile) simultaneously so that the discrimination of the clinical endpoints represents a combination of variables that are maximally correlated. Thus, trends of correlations between parameters in different clinical groups rather than their respective distribution within each group are the key components driving the discrimination outcome. In our CCA model, simplified and adapted from previously reported investigations of biomarkers for TB diagnosis [25,26], linear regression graphs represent coefficients from different combinations of plasma factors and baseline characteristics. In the biomarker profile dataset, we included values of variables that exhibited significant differences (P<0.05) in the multivariate analysis (CXCL10, fibrinogen, hyaluronic acid, IFN-γ, IL-8, LTB4, P-selectin, protein S, vitamin D and sCD40L; see Appendix 1 for details). The diagnostic class prediction values obtained were calculated using receiver operator characteristics curve analysis.
Results
Baseline characteristics
The 267 participants who had baseline pre-ART samples available had similar baseline demographics in the maraviroc and placebo arms and the incidence of IRIS did not differ in the two randomisation arms [21]. One hundred and twenty-four patients were enrolled in Mexico (five clinical sites) and 143 patients were enrolled in South Africa (one clinical site). The Mexican sites were three HIV tertiary care clinics in university-affiliated hospitals, one sexually transmitted diseases clinic and one HIV clinic, and the South African site was an HIV-referral clinic based at a tertiary care university-affiliated hospital in Johannesburg.
When comparing baseline demographics between the two nations, we found that patients from the Mexican cohort were younger compared to the South African cohort (35 vs 38 years, respectively, P=0.006; Table 1) and included a lower proportion of women (12% vs 54% female, respectively, P<0.0001). Patients from South Africa were predominantly black (95.8%) with a small proportion of patients who were Asian (1.4%) and mixed race (2.8%). Most patients from Mexico were of mixed race (88.71%), while the remaining patients were white (11.29%)
Table 1.
Baseline characteristics of study participants based upon country of origin
| Mexico (n=124) | South Africa (n=143) | P-valuea | |
|---|---|---|---|
| Age (years) | 35[29–41] | 38[33–44] | 0.006 |
| Gender (% female) | 12 | 54 | <0.001* |
| CD4 cell count (cells/μL) | 31[15–53] | 37[21–64] | 0.030 |
| CD8 cell count (cells/μL) | 478[361–786] | 473[326–680] | 0.320 |
| Plasma HIV RNA (log10 copies/mL) | 5.4[5.1–5.7] | 5.4[5–5.8] | 0.560 |
| Haemoglobin (g/dL) | 12.4[11–14] | 12[10.8–13] | 0.030 |
| AIDS-defining conditions | 79%(98) | 43%(61) | <0.001 |
| HIV-associated wasting | 52%(64) | 5%(7) | <0.001 |
| Tuberculosis | 17%(21) | 28%(40) | 0.040 |
Data are reported as median [IQR] or as percentage
Significance was determined using Wilcoxon rank-sum test or Fisher's test*
Patients in the Mexican cohort also had a lower median CD4 cell count than did patients from South Africa (31 vs 37 cells/μL, P=0.034) and more AIDS-defining illnesses (79 vs 43% of patients, respectively, P≤0.001). Baseline HIV viral load and CD8 T cell count did not differ significantly by country. The patients in the South African cohort had lower haemoglobin levels than did the Mexican cohort (12 vs 12.4 g/dL, P=0.030).
The most common AIDS-defining illnesses in the CADIRIS trial at enrolment were HIV-associated wasting and tuberculosis [21]. The Mexican cohort had a higher prevalence of HIV-associated wasting than did the South African cohort (52% vs 5%, respectively, P≤0.0001), whereas the South African cohort had a higher prevalence of active TB infection (28% vs 17% of patients, P=0.04; Table 1). At enrolment, 89% of patients with active TB infection were on therapy, while 100% of patients in the Mexican cohort on therapy, and 83% of patients in the South African cohort on therapy (P=0.08). The remaining opportunistic infections included CMV disease, Kaposi's sarcoma, oesophageal candidiasis, cryptococcosis, histoplasmosis, cryptosporidiosis, Pneumocystis jirovecii pneumonia, progressive multifocal leukoencephalopathy and isoporiasis [21].
Biomarkers by country
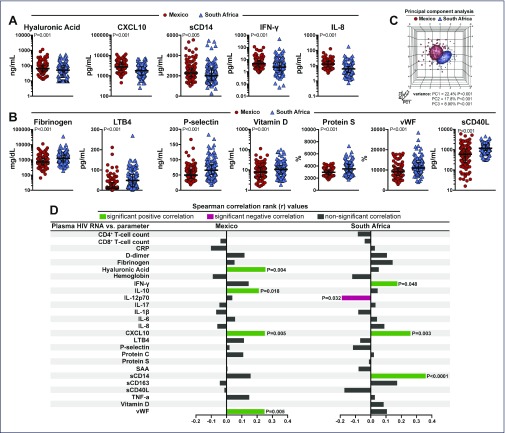
Median levels of the baseline cytokines and biomarkers from each country were compared using univariate analysis with a Student's t-test. As shown in Figure 1, patients from Mexico had significantly higher levels of hyaluronic acid, IFN-γ, IL-8, CXCL10 and sCD14 than did patients from South Africa, whereas patients from South Africa had higher levels of vitamin D, fibrinogen, LTB4, P-selectin, protein S, and sCD40 ligand (Figure 1A–C and Appendix 1). To explore the hypothesis that HIV viral clades (Mexican vs South African) could have different effects on plasma markers, we performed correlation analyses between viraemia and all biomarkers assessed separately in Mexicans and South Africans (Figure 1D). We found that in Mexico, HIV viraemia was robustly positively correlated with hyaluronic acid, IL-10, CXCL10 and vWF (Figure 1D). In South Africa, HIV viraemia remained positively correlated with CXCL10; however, additional positive correlations became significant such as those with sCD14 and IFN-γ (Figure 1D). Moreover, viraemia was negatively correlated with circulating levels of IL-12p70 in South Africa but not in Mexico. These findings indicate that viral clades could influence distinct aspects of the host immune responses in different countries.
Figure 1.
Differences in expression profile of plasma biomarkers between Mexico and South Africa. (A, B) Plasma concentrations of several biomarkers were compared between patients from Mexico (n=124) or South Africa (n=143) with univariate analysis using a generalised linear model. (C) Principal component analysis was employed using data from the biomarkers displayed in (A) and (B). Normal ellipsoids represent area estimated to contain 50% of the population of a group. (D) Spearman correlations between HIV viremia and several biomarkers are shown for patients from Mexico or South Africa. P-values of significant correlations are shown
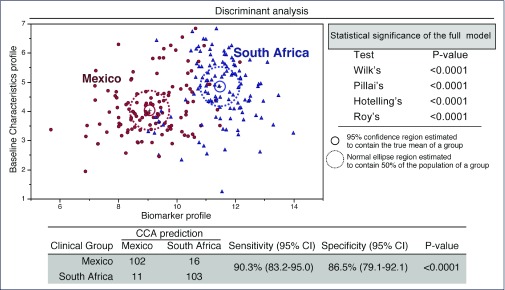
In order to further assess the relationship of country with biomarker levels, sparse canonical correlation analysis (sCCA)was performed to demonstrate the distribution of biomarkers between the two countries as shown in Figure 2. This model had 90.3 % sensitivity and 86.5 % specificity to accurately predict the country based upon the biomarker profile. When these values were adjusted for significant differences in baseline characteristics, IFN-γ, IL-8 and CXCL10 remained significantly elevated in the Mexican cohort, and vitamin D, fibrinogen, LTB4, P-selectin, protein S, and sCD40 ligand remained significantly elevated in the South African cohort (Appendix 1). With adjustments for multiple comparisons, IL-8 and CXCL10 remained significantly elevated in the Mexican cohort, and fibrinogen, LTB4, P-selectin, protein S and sCD40 ligand remained elevated in the South African cohort (Appendix 1).
Figure 2.
Discrimination of country of origin using combination of plasma biomarkers. In an exploratory approach, a sparse canonical correlation analysis (sCCA) was employed to test whether patients from Mexico or South Africa could be distinguished. The sCCA performs dimensionality reduction for two co-dependent data sets (biomarker profile and baseline characteristics profile) so that the trends of correlations between parameters from both countries represent a combination of variables that are maximally correlated. In the biomarker profile dataset, values of variables that were exhibited significant differences (P<0.05) in the multivariate analysis (CXCL10, fibrinogen, IFN-γ, IL-8, LTB4, P-selectin, protein S, vitamin D and sCD40L; see Appendix 1 for details). The overall performance of the model in discriminating participants from Mexico versus South Africa is shown
Biomarkers by gender
Based upon findings of significant differences among biomarkers by gender in the cardiovascular literature [13,14,27–29] and the significant skew of gender between the two countries, we decided to investigate the biomarker profile by gender in our CADIRIS cohort.
Baseline characteristics are demonstrated in Appendix 2. In addition to the significant difference of gender proportions by country, haemoglobin levels were found to be significantly different by gender with lower haemoglobin in females (11.45 vs 12.8 g/dL, P<0.001). Age, CD4 cell count, CD8 cell count, viral load, and TB status did not significantly differ by gender. Biomarkers did not significantly differ by oral contraceptive use in female patients.
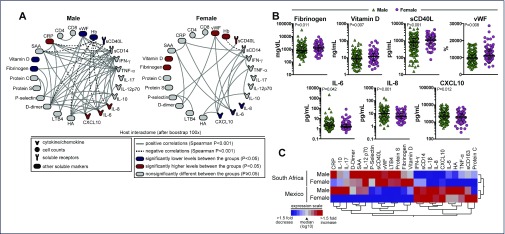
Female patients had significantly higher median levels of fibrinogen, vitamin D, sCD40 ligand and vWF, whereas male patients had significantly higher median values of IL-6, IL-8 and CXCL10 (Figure 3B,C; Appendix 2). When these biomarkers were adjusted for country of origin, there was no significant difference between genders (Appendix 2).
Figure 3.
Gender differences in expression of plasma biomarkers. (A) Network analysis shows significant correlations (P<0.001, after bootstrap of 100X) between several biomarkers assessed by Spearman correlation matrices in male (n=175) and female (n=92) patients. Network densities were compared between the groups using the permutation test. (B) Plasma concentrations of selected biomarkers were compared between male and female patients with univariate analysis using a generalised linear model. (C) A hierarchical clustering analysis (Ward's method) of the plasma expression profile of several biomarkers was employed to test whether male and female patients from either Mexico or South Africa could be clustered separately. The colours represent the level of expression relative to the median
Because differences in biomarkers by gender have been reported in the literature and yet we found none in our study, we decided to perform a network analysis to investigate whether the relationships of the biomarkers, rather than their respective concentrations values per se, could be different by gender. As shown in Figure 3A, we found positive correlations between inflammatory markers including D-dimer, IL-6, TNF-α and IFN-γ in addition to negative correlation with haemoglobin, which can be suggestive of a tightly coordinated inflammatory response in men. Interestingly this observation is not seen in the female cohort, which may indicate that inflammatory responses may vary by gender. Moreover, hierarchical cluster analysis of the overall biomarker expression profile in plasma underlined that male and female patients from either Mexico or South Africa exhibit a distinct biosignature (Figure 3C), further highlighting the role of nationality in driving the differences detected in the biomarker values.
Biomarkers by TB status
We then investigated whether TB, the most prevalent opportunistic infection in this cohort, which was also significantly more prevalent in South Africa, had an important effect on the biomarker profile.
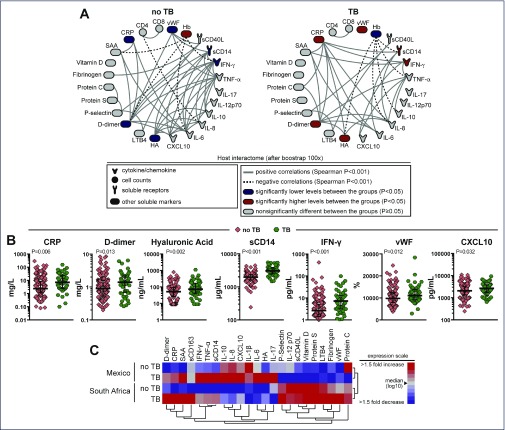
Patients infected with tuberculosis had higher median levels of CRP, D-dimer, hyaluronic acid, IFN-γ, CXCL10, TNF-α and sCD14 than did patients without tuberculosis as shown in Figure 4B (see also Appendix 3).
Figure 4.
Differential expression of plasma biomarkers in tuberculosis. (A) Network analysis shows significant correlations (P<0.001, after bootstrap of 100X) between several biomarkers assessed by Spearman correlation matrices in patients with no TB (n=206) or with a TB diagnosis (n=61). Network densities were compared between the groups using the permutation test. (B) Plasma concentrations of selected biomarkers were compared between patients with (green) or without TB (pink) with univariate analysis using a generalised linear model. (C) A hierarchical clustering analysis (Ward's method) of the plasma expression profile of several biomarkers was employed to test whether patients with or without TB and from either Mexico or South Africa could be clustered separately. The colours represent the level of expression relative to the median
When these biomarkers were adjusted for country of origin, haemoglobin and viral load, IFN-γ, CRP, hyaluronic acid, sCD14 and sCD40 ligand remained significantly higher in patients with tuberculosis (Appendix 3). After adjustments for multiple comparisons, IFN-γ and sCD14 remained significantly higher in patients with TB (Appendix 3).
Network analysis was performed, see Figure 4A. We observed that in patients without TB, inflammatory markers such as D-dimer, IL-6, TNF-α and IFN-γ were tightly correlated. In addition, significant negative correlations involving haemoglobin levels were observed in both groups of patients. A new hierarchical cluster analysis of the overall biomarker expression profile in plasma demonstrated that patients with or without TB from either Mexico or South Africa exhibit a distinct biosignature (Figure 4C). These findings suggest nationality as the central driver of certain observed differences in pro-inflammatory biomarker expression in plasma of HIV patients.
Discussion
In this study, we evaluated the effects of country of residence on the inflammatory and coagulation profile in HIV infection based upon nationality prior to ART initiation. To our knowledge this is one of the first studies to show a significant role of country of residence on levels of certain relevant biomarkers during HIV infection, which may have important pathogenesis implications and affect use of biomarkers as clinical trial endpoints.
In our study, which included patients from Mexico and South Africa at similar stages of HIV infection, we demonstrated a unique biomarker profile by country of origin during HIV infection prior to ART initiation. Although both cohorts had elevations of markers associated with immune activation, the South African cohort appeared to have higher baseline levels of coagulation markers (fibrinogen and protein S), platelet activation (sCD40 ligand) and leukocyte adhesion (LTB4 and P-selectin).
Elevated levels of P-selectin, fibrinogen, and hyaluronic acid have been associated with morbidity and mortality in HIV [4,6,30–32]. Boulware et al. has demonstrated that higher levels of hyaluronic acid levels pre-ART identified patients at higher risk of AIDS or death following ART initiation, and Musselwhite et al. found that elevated levels of P-selectin and hyaluronic acid were associated with increased risk of venous thromboembolism [4,30]. In addition, Tien et al. demonstrated that fibrinogen was associated with an increased risk of mortality in HIV-patients [6].
In studies including HIV-seronegative patients, there have been conflicting reports regarding fibrinogen levels in patients of different ethnicities [15,33,34], particularly when comparing Caucasians with black individuals. These studies were performed in different countries so it is possible that the country of origin plays an important role in addition to race.
P-selectin, protein S and fibrinogen have been evaluated in different ethnic groups in HIV-seronegative patients [15,35]. Miller et al. demonstrated significantly lower levels of P-selectin in people of African origin compared to Caucasians in the United Kingdom [15]. Dunne et al. investigated protein S levels in healthy individuals by ethnicity, and found that black Africans had significantly lower protein S levels than black Caribbeans, and Caucasians in the United Kingdom [35].
The Mexican cohort had significantly elevated levels of chemokines, specifically CXCL10, which has been shown to be involved with the Type 1 helper cell (TH1) response as well as lymphocyte recruitment, and IL-8, a chemokine produced by myeloid cells to promote chemotaxis and phagocytosis. During acute HIV infection, increased CXCL10levels have been associated with low CD4 counts later in the chronic phase31 [31] and elevated levels of IL-8 have been associated with mortality during an opportunistic infection [36] and IRIS [37].
In the context of the current literature on biomarkers and nationality, we found that levels of CXCL10 have been compared in studies; however, no studies compared South Africans and Mexicans. Sekikawa et al. found that Japanese individuals had higher levels of CXCL10 than did Caucasians and Americans of Japanese origin [38].
We further investigated biomarkers based on gender and TB status, as the gender distribution and TB status differed between the two countries. We did not see a significant difference when looking at biomarkers by gender in HIV infection prior to ART initiation, in contrast to observations in cardiovascular disease [13,14,39]; however, our network analysis is suggestive of distinguishable relationships among these inflammatory markers by gender.
When we investigated biomarkers by TB status, we found that sCD14 and IFN-γ were significantly higher in patients with TB. Our network analysis also demonstrated less coordination of biomarkers during TB infection, which is suggestive of a perturbation of the inflammatory environment with TB. During HIV infection, sCD14 has been associated with morbidity and mortality [4,32]. Other studies have shown an elevation of sCD14 [40] during TB infection, and increased IFN-γ production is a known feature of TB infection.
The finding of a distinguishable biomarker profile by country is intriguing. What could be contributing to these differences? There may be many factors driving the difference amongst biomarkers between these two countries. Foremost, there could be underlying genetic differences by country, specifically genetic differences in the biomarker, its receptor, or the determinants of expression, distribution or elimination. For example, P-selectin [41,42] and IL-8 [43,44] are known to have polymorphisms conferring disease risk that vary among different ethnic populations. The difference by geographic location suggests a role for viral clades influencing biomarker status. We explored the notion that HIV viral clades could have an impact on the plasma markers, and we found distinct biomarkers correlated with viraemia by country. However, these biomarkers were different when compared to biomarkers associated with country, thus making the influence of viral clade on the country's biomarker profile unclear.
Different countries may have unique endemic infectious organisms that could influence immunological responses and biomarker levels. Additionally, the microbiome could affect biomarker status, as diet and flora can vary by location. Finally, underlying behavioural and psychosocial factors may play a role as well.
Our study had several strengths including comparable populations by HIV disease stage and large numbers. The data could be generalisable to settings where patients with HIV receive routine clinical care, as the patients were accrued in clinical centres in two different regions of the world with different epidemics and distributions of opportunistic infections. Limitations include the lack of an uninfected control group to compare biomarkers by country of residence. Additionally, despite randomisation we did have baseline differences between countries requiring adjustment. We also had no data on BMI, race, smoking status or cardiovascular or other comorbidities, which could be potential confounding factors of our findings.
Our data suggest that inflammatory and coagulation biomarkers may vary significantly by region and that country-specific data may be needed in studies using biomarkers as predictors or clinical trial endpoints. Further questions remain, including whether these elevated biomarkers constitute an individual's normal range or reflect differences in HIV pathogenesis and adverse cardiovascular outcomes. In this light, if these baseline biomarkers reflect a persistent state of inflammation, this could contribute to an abnormal immune reconstitution following ART. In this regard, elevated plasma levels of inflammatory and coagulation indices both before and after ART-mediated suppression of viraemia are similarly predictive of non-AIDS morbidities and mortality after ART [45]. Overall these findings suggest that the country of residence could play a role in HIV pathogenesis, and further studies are needed to evaluate how these differences may contribute to HIV treatment, response and prognosis in diverse populations.
Acknowledgements
We thank Dr Kiyoshi Fukutani (FIOCRUZ, Brazil) for assistance with statistical analysis. We also thank all the CADIRIS participants and the study team members at domestic and international study sites. We thank Pfizer for the unrestricted research grant provided to the CADIRIS study team to support conduct of the clinical trial from which the clinical specimens were obtained.
Appendix 1. Baseline biomarkers from CADIRIS study based upon country of origin
| Mexico (n=124) | South Africa (n=143) | Univariate P-value* | Multivariate P-value† | |
|---|---|---|---|---|
| CRP (mg/L) | 3.36[1.59–9.14] | 2.93[1.17–11.13] | 0.482 | 0.600 |
| D-dimer (mg/L) | 0.87[0.51–1.58] | 1.10[0.58–1.61] | 0.200 | 0.140 |
| Fibrinogen (mg/dL) | 723[448–1300] | 1229[715–1945] | <0.001 | <0.001 |
| HA (ng/mL) | 61.5[37.6–98.1] | 48.1[16.9–92.3] | 0.001 | 0.098 |
| IFN-γ (pg/mL) | 4.09[2.20–7.41] | 2.19[1.17–5.58] | <0.001 | 0.034 |
| IL-10 (pg/mL) | 12.2[8.24–18.3] | 10.4[7.63–15.7] | 0.186 | 0.877 |
| IL-12p70 (pg/mL) | 1.06[0.42–2.72] | 1.30[0.85–3.28] | 0.602 | 0.450 |
| IL-17 (pg/mL) | 0.33[0.16–0.57] | 0.31[0.18–0.59] | 0.762 | 0.923 |
| IL-1β (pg/mL) | 0.28[0.00–0.81] | 0.20[0.10–0.51] | <0.001 | 0.002 |
| IL-6 (pg/mL) | 2.25[1.54–3.57] | 1.56[1.12–2.78] | 0.080 | 0.445 |
| IL-8 (pg/mL) | 11.8[7.77–18.1] | 5.79[3.40–8.31] | <0.001 | <0.001 |
| CXCL10 (pg/mL) | 2575[439–1780] | 1691[999–2693] | <0.001 | <0.001 |
| LTB4 (pg/mL) | 10.2[10.2–37.2] | 47.8[16.5–76.6] | <0.001 | <0.001 |
| P-Selectin (ng/mL) | 48.5[37.2–64.7] | 64.7[50.1–82.6] | <0.001 | <0.001 |
| Protein C (%) | 3797[3267–4323] | 3768[3112–4378] | 0.993 | 0.348 |
| Protein S (%) | 3550[3037–3960 | 4376[3658,5430] | <0.001 | <0.001 |
| SAA (mg/L) | 5.29[2.03–12.79] | 5.54[1.52–17.19] | 0.742 | 0.632 |
| TNF-α (pg/mL) | 17.3[13.5–25.4] | 15.5[12.0–22.1] | 0.611 | 0.811 |
| Vitamin D (ng/mL) | 7.72[4.44–13.4] | 10.4[6.90–17.1] | 0.001 | 0.026 |
| sCD14 (μg/mL) | 2221[1895–2686] | 1957[1530–2615] | 0.005 | 0.123 |
| sCD163 (ng/mL) | 705[471–957] | 629[419–903] | 0.521 | 0.882 |
| sCD40 L(pg/mL) | 586[227–1096] | 1157[870–1716] | <0.001 | <0.001 |
| vWF (%) | 9162[5861–10,880] | 10,990[9259–13,770] | <0.001 | <0.001 |
Data are reported as median[IQR] or as percentage.
Univariate analysis was determined with a generalised linear model.
Multivariate analysis was determined using a generalised linear model and was adjusted for age, CD4 cell count, haemoglobin, gender, and AIDS-defining conditions.
Appendix 2. Baseline biomarkers from CADIRIS study based upon gender
| Females | Males | |||||
|---|---|---|---|---|---|---|
| Mexico | South Africa | Mexico | South Africa | Univariate | Multivariate | |
| (n=15) | (n=77) | (n=109) | (n=66) | P-value* | P-value† | |
| Agea (years) | 38.0 | 38.0 | 35.0 | 38.0 | 0.249 | |
| [30.0–44.0] | [31.0–44.5] | [29.0–40.5] | [34.0–43.2] | |||
| CD4 cell counta (cells/μL) | 15.0 | 37.0 | 32.0 | 36.5 | 0.752 | |
| [3.00–36.0] | [21.5–62.5] | [16.0–53.5] | [21.0–66.3] | |||
| CD8 cell counta (cells/μL) | 475 | 465 | 480 | 476 | 0.337 | |
| [351–725] | [337–673] | [362–824] | [304–718] | |||
| Plasma HIV RNAa (log10 c/mL) | 5.44 | 5.32 | 5.38 | 5.41 | 0.221 | |
| [5.23–5.65] | [4.90–5.69] | [5.04–5.72] | [5.00–5.82] | |||
| HgBa (g/dL) | 11.7 | 11.4 | 12.9 | 12.8 | <0.001 | |
| [10.8–12.5] | [10.5–12.6] | [11.05–14.1] | [11.4–13.7] | |||
| TBb (%) | 19.6 (18) | 24.6 (43) | 0.443 | |||
| Countryb (%) | 16.3 Mexico | 62.3 Mexico | <0.001 | |||
| CRP (mg/L) | 1.83 | 2.30 | 3.57 | 3.67 | 0.086 | 0.117 |
| [0.70–2.38] | [0.88, 10.5] | [1.81–10.3] | [1.37–12.3] | |||
| D-dimer (mg/L) | 0.71 | 0.99 | 0.95 | 1.11 | 0.488 | 0.903 |
| [0.45–1.23] | [0.60–1.78] | [0.52–1.59] | [0.53–1.52] | |||
| Fibrinogen (mg/dL) | 670 | 1288 | 726 | 1009 | 0.011 | 0.533 |
| [403–1004] | [781–2288] | [463–1318] | [581–1730] | |||
| HA (ng/mL) | 55.3 | 46.1 | 63.4 | 48.1 | 0.122 | 0.952 |
| [34.1–85.5] | [20.4–88.5] | [40.7–101] | [9.00, 104] | |||
| IFN-γ (pg/mL) | 3.93 | 2.64 | 4.16 | 1.74 | 0.119 | 1.000 |
| [2.83–8.11] | [1.38–4.71] | [2.14–7.31] | [0.98–8.78] | |||
| IL-10 (pg/mL) | 8.97 | 10.0 | 12.3 | 11.0 | 0.203 | 0.453 |
| [5.43–18.0] | [7.52–16.3] | [8.42–18.6] | [7.97–15.2] | |||
| IL-12p70 (pg/mL) | 0.49 | 1.31 | 1.17 | 1.29 | 0.382 | 0.476 |
| [0.16–1.34] | [0.76–3.27] | [0.52–2.74] | [0.90–3.28] | |||
| IL-17 (pg/mL) | 0.26 | 0.31 | 0.34 | 0.30 | 0.211 | 0.211 |
| [0.05–0.52] | [0.15–0.57] | [0.17–0.57] | [0.21–0.64] | |||
| IL-1β (pg/mL) | 0.28 | 0.20 | 0.28 | 0.21 | 0.065 | 0.607 |
| [0.09–0.44] | [0.10–0.45] | [0.00–0.82] | [0.08–0.52] | |||
| IL-6 (pg/mL) | 2.01 | 1.52 | 2.40 | 1.68 | 0.042 | 0.165 |
| [1.36– 2.25] | [1.13–2.67] | [1.57–3.69] | [1.12–2.86] | |||
| IL-8 (pg/mL) | 10.8 | 5.83 | 11.8 | 5.76 | <0.001 | 0.416 |
| [5.65–15.0] | [3.69–833] | [8.32–18.5] | [3.32–8.23] | |||
| CXCL10 (pg/mL) | 2791 | 1710 | 2522 | 1565 | 0.012 | 0.755 |
| [1954–4130] | [999–2748] | [1703–4207] | [1001–2426] | |||
| LTB4 (pg/mL) | 10.2 | 44.5 | 10.2 | 54.8 | 0.153 | 0.087 |
| [10.2–53.4] | [14.7–67.6] | [10.2–34.5] | [30.1–83.2] | |||
| P-Selectin (ng/mL) | 47.4 | 56.8 | 48.7 | 75.8 | 0.631 | 0.079 |
| [34.9–61.2] | [48.1–77.7] | [37.2–65.8] | [51.1–88.2] | |||
| Protein C (%) | 3984 | 3739 | 3747 | 3792 | 0.332 | 0.281 |
| [3523–4512] | [3208–4426] | [3224–4322] | [3029–4349] | |||
| Protein S (%) | 3560 | 4286 | 3550 | 4474 | 0.132 | 0.128 |
| [3040–3682] | [3467–5334] | [3034–4018] | [3806–5634] | |||
| SAA (mg/L) | 2.35 | 5.47 | 5.60 | 6.38 | 0.122 | 0.117 |
| [0.67–7.92] | [1.21–17.3] | [2.32–15.6] | [1.82–15.8] | |||
| TNF-α (pg/mL) | 16.9 | 15.3 | 17.4 | 16.14 | 0.109 | 0.125 |
| [12.1–19.7] | [10.8–22.4] | [13.5–26.3] | [13.0– 2.0] | |||
| Vitamin D (ng/mL) | 6.35 | 13.9 | 8.07 | 9.18 | 0.007 | 0.160 |
| [4.31–9.59] | [7.95–19.7] | [4.45–13.7] | [5.83–14.6] | |||
| sCD14 (μg/mL) | 2263 | 2034 | 2214 | 1904 | 0.461 | 0.533 |
| [1978–2625] | [1542–2604] | [1877–2713] | [1500–2626] | |||
| sCD163 (ng/mL) | 735 | 607 | 700 | 670 | 0.358 | 0.482 |
| [511–982] | [380, 895] | [462–953] | [424–956] | |||
| sCD40 L(pg/mL) | 481 | 1165 | 601 | 1148 | <0.001 | 0.774 |
| [214–983] | [902–1749] | [257–1140] | [817–1695] | |||
| vWF (%) | 9181 | 11,139 | 9143 | 10,792 | 0.002 | 0.339 |
| [7442–10,564] | [9065–14,653] | [7090–10,944] | [9576–13,077] | |||
Data are reported as median [IQR] or as percentage.
Significance of baseline characteristics was obtained using Wilcoxon analysisa or Fisher's T testb.
Univariate analysis was determined with generalised linear model to compare biomarkers by gender.
Multivariate analysis was determined with generalised linear model to compare biomarkers by gender and was adjusted for country.
Appendix 3. Baseline biomarkers from CADIRIS study based upon TB status
| Tuberculosis | No Tuberculosis | |||||||
|---|---|---|---|---|---|---|---|---|
| Mexico | South Africa | Mexico | South Africa | Univariate | Multivariate | |||
| (n=21) | (n=40) | (n=103) | (n=103) | P-value* | P-value† | |||
| Agea | 35.0 | 37.5 | 35.0 | 38.0 | 0.600 | |||
| (years) | [26.5–38.5] | [34.0–42.0] | [29.0–42.0] | [32.0–45.0] | ||||
| CD4 cell counta | 31.0 | 32.0 | 30.0 | 40.0 | 0.760 | |||
| (cells/μL) | [21.5–60.5] | [20.3–64.0] | [14.0, 52.0] | [22.0–66.0] | ||||
| CD8 cell counta | 423 | 414 | 526 | 478 | 0.091 | |||
| (cells/μL) | [286–538] | [290–707] | [378–823] | [341–680] | ||||
| Plasma HIV RNAa | 5.23 | 5.64 | 5.40 | 5.19 | 0.014 | |||
| (log10copies/mL) | [5.08–5.68] | [5.22–5.94] | [5.05–5.72] | [4.92–5.63] | ||||
| HgBa | 11.3 | 11.3 | 12.7 | 12.1 | <0.001 | |||
| (g/dL) | [9.75–12.9] | [10.2–13.1] | [11.2–14.1] | [11.1–13.0] | ||||
| Genderb (%) | 30 Female | 36 Female | 0.443 | |||||
| Countryb (%) | 34.4 Mexico | 50 Mexico | 0.0404 | |||||
| CRP (mg/L) | 6.00 | 7.37 | 2.89 | 2.21 | 0.006 | 0.026 | ||
| [2.50–28.0] | [2.09–19.5] | [1.37–8.16] | [1.12–9.20] | |||||
| D-dimer (mg/L) | 1.26 | 1.48 | 0.83 | 0.96 | 0.013 | 0.223 | ||
| [0.66–2.15] | [0.77–2.18] | [0.50–1.55] | [0.54–1.56] | |||||
| Fibrinogen (mg/dL) | 575 | 1449 | 743 | 1150 | 0.190 | 0.511 | ||
| [477–1667] | [757–2324] | [446–1291] | [707–1876] | |||||
| HA (ng/mL) | 120 | 60.2 | 54.3 | 46.1 | 0.002 | 0.017 | ||
| [65.9–223] | [20.8–145] | [34.1–87.8] | [10.5–91.4] | |||||
| IFN-γ (pg/mL) | 8.11 | 5.64 | 3.22 | 1.74 | <0.001 | <0.001 | ||
| [4.05–17.6] | [2.59–11.5] | [2.06–6.23] | [0.91–3.80] | |||||
| IL-10 (pg/mL) | 12.6 | 11.0 | 12.0 | 10.3 | 0.846 | 0.672 | ||
| [7.07–15.9] | [8.11–16.7] | [8.27–18.4] | [7.39–14.6] | |||||
| IL-12p70 (pg/mL) | 0.80 | 1.23 | 1.10 | 1.30 | 0.300 | 0.408 | ||
| [0.32–2.34] | [0.90–3.27] | [0.41–2.74] | [0.77–3.37] | |||||
| IL-17 (pg/mL) | 0.34 | 0.29 | 0.31 | 0.31 | 0.610 | 0.410 | ||
| [0.16–0.54] | [0.16–0.38] | [0.15–0.59] | [0.19–0.64] | |||||
| IL-1β (pg/mL) | 0.26 | 0.20 | 0.28 | 0.21 | 0.168 | 0.179 | ||
| [0–1.18] | [0.08–0.42] | [0–0.72] | [0.10–0.55] | |||||
| IL-6 (pg/mL) | 3.54 | 1.65 | 2.22 | 1.47 | 0.247 | 0.752 | ||
| [1.60–5.73] | [1.19–3.20] | [1.40–3.23] | [1.11–2.68] | |||||
| IL-8 (pg/mL) | 13.08 | 7.08 | 11.6 | 5.44 | 0.987 | 0.977 | ||
| [9.04–17.8] | [3.35–9.65] | [7.37–18.5] | [3.50–8.27] | |||||
| CXCL10 (pg/mL) | 2917 | 2156 | 2480 | 1515 | 0.032 | 0.066 | ||
| [2068–4739] | [1527–3462] | [1703–4152] | [947–2292] | |||||
| LTB4 (pg/mL) | 13.9 | 52.2 | 10.2 | 45.2 | 0.591 | 0.584 | ||
| [10.2–44.6] | [10.2–78.1] | [8.1–40.0] | [18.0–76.6] | |||||
| P-Selectin (ng/mL) | 34.9 | 68.9 | 51.4 | 62.2 | 0.120 | 0.053 | ||
| [27.7–47.6] | [42.2–82.8] | [40.3–66.4] | [50.4–82.6] | |||||
| Protein C (%) | 3494 | 3803 | 3849 | 3768 | 0.195 | 0.256 | ||
| [3080–4058] | [3147–4482] | [3323–4412] | [3088–4312] | |||||
| Protein S (%) | 3420 | 4421 | 3561 | 4329 | 0.424 | 0.910 | ||
| [2764–4015] | [3683–5744] | [3045–3948] | [3574–5299] | |||||
| SAA (mg/L) | 10.6 | 10.1 | 4.70 | 5.35 | 0.825 | 0.858 | ||
| [2.27–57.3] | [0.86–21.8] | [1.88–10.3] | [1.52–14.1] | |||||
| TNF-α (pg/mL) | 22.0 | 19.7 | 16.7 | 15.2 | 0.115 | 0.465 | ||
| [14.6–29.0] | [14.3–26.0] | [13.3–23.8] | [11.3–20.7] | |||||
| Vitamin D (ng/mL) | 7.55 | 10.4 | 7.96 | 10.6 | 0.534 | 0.818 | ||
| [5.00–12.7] | [6.10–19.9] | [4.28–13.7] | [7.29–16.8] | |||||
| sCD14 (μg/mL) | 3452 | 2906 | 2096 | 1800 | <0.001 | <0.001 | ||
| [2576–3882] | [2247–3305] | [1784–2407] | [1424–2174] | |||||
| sCD163 (ng/mL) | 713 | 840 | 704 | 607 | 0.451 | 0.526 | ||
| [360–953] | [425–1133] | [480–980] | [381–851] | |||||
| sCD40 L(pg/mL) | 429 | 1124 | 604 | 1229 | 0.256 | 0.030 | ||
| [109–772] | [609–1695] | [263–1141] | [906–1744] | |||||
| vWF (%) | 9575 | 12,491 | 9057 | 10,668 | 0.012 | 0.022 | ||
| [8169–11,324] | [10,112–14,793] | [7023–10,790] | [8776–13,317] | |||||
Data are reported as median [IQR] or as percentage.
Significance of baseline characteristics was obtained using Wilcoxon analysisa or Fishers T testb.
Univariate analysis was determined with generalised linear model to compare biomarkers by TB status.
Multivariate analysis was determined with generalised linear model to compare biomarkers by TB status and was adjusted for country, haemoglobin and viral load.
Funding
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases of the National Institutes of Health and the National Cancer Institute through Contract No HHSN261200800001E.
Conflict of interest
MML reports grants from Pfizer to support the conduct of this study. JGH reports grants from BMS, Pfizer, MSD, and Stendhal outside the submitted work. IS, MM, BBA, RD, WG, LWM, BZ and AR have nothing to disclose.
This work was been presented at the 2015 International AIDS Society Conference in Vancouver.
References
- 1. Duprez DA, Neuhaus J, Kuller LH et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One 2012; 7: e44454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Justice AC, Freiberg MS, Tracy R et al. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin Infect Dis 2012; 54: 984– 994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kuller LH, Tracy R, Belloso W et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008; 5: e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Musselwhite LW, Sheikh V, Norton TD et al. Markers of endothelial dysfunction, coagulation and tissue fibrosis independently predict venous thromboembolism in HIV. AIDS 2011; 25: 787– 795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sandler NG, Koh C, Roque A et al. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology 2011; 141: 1220– 1230, 1230 e1221–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tien PC, Choi AI, Zolopa AR et al. Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort. J Acquir Immune Defic Syndr 2010; 55: 316– 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hamlyn E, Fidler S, Stohr W et al. Interleukin-6 and D-dimer levels at seroconversion as predictors of HIV-1 disease progression. AIDS 2014; 28: 869– 874. [DOI] [PubMed] [Google Scholar]
- 8. Ledwaba L, Tavel JA, Khabo P et al. Pre-ART levels of inflammation and coagulation markers are strong predictors of death in a South African cohort with advanced HIV disease. PLoS One 2012; 7: e24243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McDonald B, Moyo S, Gabaitiri L et al. Persistently elevated serum interleukin-6 predicts mortality among adults receiving combination antiretroviral therapy in Botswana: results from a clinical trial. AIDS Res Hum Retroviruses 2013; 29: 993– 999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nordell AD, McKenna M, Borges AH et al. Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation. J Am Heart Assoc 2014; 3: e000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Albert MA. Inflammatory biomarkers, race/ethnicity and cardiovascular disease. Nutr Rev 2007; 65: S234– 238. [DOI] [PubMed] [Google Scholar]
- 12. Coussens AK, Wilkinson RJ, Nikolayevskyy V et al. Ethnic variation in inflammatory profile in tuberculosis. PLoS Pathog 2013; 9: e1003468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferguson JF, Patel PN, Shah RY et al. Race and gender variation in response to evoked inflammation. J Transl Med 2013; 11: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khera A, McGuire DK, Murphy SA et al. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol 2005; 46: 464– 469. [DOI] [PubMed] [Google Scholar]
- 15. Miller MA, Cappuccio FP. Ethnicity and inflammatory pathways – implications for vascular disease, vascular risk and therapeutic intervention. Curr Med Chem 2007; 14: 1409– 1425. [DOI] [PubMed] [Google Scholar]
- 16. Nazmi A, Victora CG. Socioeconomic and racial/ethnic differentials of C-reactive protein levels: a systematic review of population-based studies. BMC Public Health 2007; 7: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paalani M, Lee JW, Haddad E, Tonstad S. Determinants of inflammatory markers in a bi-ethnic population. Ethn Dis 2011; 21: 142– 149. [PMC free article] [PubMed] [Google Scholar]
- 18. Stowe RP, Peek MK, Cutchin MP, Goodwin JS. Plasma cytokine levels in a population-based study: relation to age and ethnicity. J Gerontol A Biol Sci Med Sci 2010; 65: 429– 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Musselwhite LW, Andrade BB, Ellenberg SS et al. Vitamin D, D-dimer, Interferon gamma, and sCD14 Levels are Independently Associated with Immune Reconstitution Inflammatory Syndrome: A Prospective, International Study. EBioMedicine 2016; 4: 115– 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sierra-Madero JG, Ellenberg S, Rassool MS et al. A randomized, double-blind, placebo-controlled clinical trial of a chemokine receptor 5 (CCR5) Antagonist to decrease the occurrence of immune reconstitution inflammatory syndrome in HIV-infection: the CADIRIS study. Lancet HIV 2014; 1: e60– 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sierra-Madero JG, Ellenberg SS, Rassool MS et al. Effect of the CCR5 antagonist maraviroc on the occurrence of immune reconstitution inflammatory syndrome in HIV (CADIRIS): a double-blind, randomised, placebo-controlled trial. Lancet HIV 2014; 1: e60– 67. [DOI] [PubMed] [Google Scholar]
- 22. Andrade BB, Pavan Kumar N, Amaral EP et al. Heme oxygenase-1 regulation of matrix metalloproteinase-1 expression underlies distinct disease profiles in tuberculosis. J Immunol 2015; 195: 2763– 2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Andrade BB, Pavan Kumar N, Sridhar R et al. Heightened plasma levels of heme oxygenase-1 and tissue inhibitor of metalloproteinase-4 as well as elevated peripheral neutrophil counts are associated with TB-diabetes comorbidity. Chest 2014; 145: 1244– 1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mesquita ED, Gil-Santana L, Ramalho D et al. Associations between systemic inflammation, mycobacterial loads in sputum and radiological improvement after treatment initiation in pulmonary TB patients from Brazil: a prospective cohort study. BMC Infect Dis 2016; 16: 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mayer-Barber KD, Andrade BB, Oland SD et al. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature 2014; 511: 99– 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rousu J, Agranoff DD, Sodeinde O et al. Biomarker discovery by sparse canonical correlation analysis of complex clinical phenotypes of tuberculosis and malaria. PLoS Comput Biol 2013; 9: e1003018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Albert MA, Ridker PM. C-reactive protein as a risk predictor: do race/ethnicity and gender make a difference? Circulation 2006; 114: e67– 74. [DOI] [PubMed] [Google Scholar]
- 28. Morimoto Y, Conroy SM, Ollberding NJ et al. Ethnic differences in serum adipokine and C-reactive protein levels: the multiethnic cohort. Int J Obes (Lond) 2014; 38: 1416– 1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Patel DA, Srinivasan SR, Xu JH et al. Distribution and metabolic syndrome correlates of plasma C-reactive protein in biracial (black-white) younger adults: the Bogalusa Heart Study. Metabolism 2006; 55: 699– 705. [DOI] [PubMed] [Google Scholar]
- 30. Boulware DR, Hullsiek KH, Puronen CE et al. Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis 2011; 203: 1637– 1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Graham SM, Mwilu R, Liles WC. Clinical utility of biomarkers of endothelial activation and coagulation for prognosis in HIV infection: a systematic review. Virulence 2013; 4: 564– 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leeansyah E, Malone DF, Anthony DD, Sandberg JK. Soluble biomarkers of HIV transmission, disease progression and comorbidities. Curr Opin HIV AIDS 2013; 8: 117– 124. [DOI] [PubMed] [Google Scholar]
- 33. Cook DG, Cappuccio FP, Atkinson RW et al. Ethnic differences in fibrinogen levels: the role of environmental factors and the beta-fibrinogen gene. Am J Epidemiol 2001; 153: 799– 806. [DOI] [PubMed] [Google Scholar]
- 34. Lutsey PL, Cushman M, Steffen LM et al. Plasma hemostatic factors and endothelial markers in four racial/ethnic groups: the MESA study. J Thromb Haemost 2006; 4: 2629– 2635. [DOI] [PubMed] [Google Scholar]
- 35. Jerrard-Dunne P, Evans A, McGovern R et al. Ethnic differences in markers of thrombophilia: implications for the investigation of ischemic stroke in multiethnic populations: the South London Ethnicity and Stroke Study. Stroke 2003; 34: 1821– 1826. [DOI] [PubMed] [Google Scholar]
- 36. Grant PM, Komarow L, Sanchez A et al. Clinical and immunologic predictors of death after an acute opportunistic infection: results from ACTG A5164. HIV Clin Trials 2014; 15: 133– 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haridas V, Pean P, Jasenosky LD et al. TB-IRIS, T-cell activation, and remodeling of the T-cell compartment in highly immunosuppressed HIV-infected patients with TB. AIDS 2015; 29: 263– 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sekikawa A, Kadowaki T, Curb JD et al. Circulating levels of 8 cytokines and marine n-3 fatty acids and indices of obesity in Japanese, white, and Japanese American middle-aged men. J Interferon Cytokine Res 2010; 30: 541– 548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weiner SD, Ahmed HN, Jin Z et al. Systemic inflammation and brachial artery endothelial function in the Multi-Ethnic Study of Atherosclerosis (MESA). Heart 2014; 100: 862– 866. [DOI] [PubMed] [Google Scholar]
- 40. Lawn SD, Labeta MO, Arias M et al. Elevated serum concentrations of soluble CD14 in HIV- and HIV+ patients with tuberculosis in Africa: prolonged elevation during anti-tuberculosis treatment. Clin Exp Immunol 2000; 120: 483– 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Reiner AP, Carlson CS, Thyagarajan B et al. Soluble P-selectin, SELP polymorphisms, and atherosclerotic risk in European-American and African-African young adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Arterioscler Thromb Vasc Biol 2008; 28: 1549– 1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Volcik KA, Ballantyne CM, Coresh J et al. Specific P-selectin and P-selectin glycoprotein ligand-1 genotypes/haplotypes are associated with risk of incident CHD and ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis 2007; 195: e76– 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gao P, Zhao H, You J et al. Association between interleukin-8 -251A/T polymorphism and risk of lung cancer: a meta-analysis. Cancer Invest 2014; 32: 518– 525. [DOI] [PubMed] [Google Scholar]
- 44. Wang XB, Li YS, Li J et al. Interleukin-8 -251A/T gene polymorphism and lung cancer susceptibility: a meta-analysis. J Cell Mol Med 2015; 19: 1218– 1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tenorio AR, Zheng Y, Bosch RJ et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014; 210: 1248– 1259. [DOI] [PMC free article] [PubMed] [Google Scholar]