Abstract
Background
Plasma HIV-RNA viral load (VL) of HIV-infected persons is an important prognostic factor in HIV management. We determined the VL among antiretroviral therapy (ART)-naive patients to identify the association between patients’ demographic, clinical and laboratory characteristics with VL.
Method
A cross-sectional study of 224 ART-naive HIV-1-infected patients (≥15 years of age) accessing care at the Jos University Teaching Hospital AIDS Prevention Initiative in Nigeria ART treatment centre, from October 2010 to April 2011. A log-linear model was used to determine if VL was related to demographic and clinical variables.
Results
The patients had a median (interquartile range) age of 34 (28–41) years with females in the majority (59%). Females compared to males and pulmonary tuberculosis (PTB) co-infected compared to not co-infected patients had a significantly higher VL (14.9 loge versus 11.5 loge, P=0.003 and 11.31 loge versus 11.89 loge, P=0.047, respectively). VL tended to decrease with increasing CD4+ cell count levels in females, but remained relatively unchanged in males across all values of CD4+ cell counts. The difference (β) in the mean change in VL between males and females was loge 0.64 copies/mL, P=0.005.
Conclusion
In ART-naive HIV-1-infected patients in our setting, females had significantly higher VL and lower CD4+ cell count, at the same VL threshold, compared to males, and hence were more likely to be at a higher risk of rapid progression to AIDS. Therefore, gender-based strategies for early identification and engaging females into care are required in this setting to mitigate against rapid progression to AIDS.
Keywords: HIV, viral load, CD4+ cell count, sex difference, ART naive
Introduction
Plasma HIV-1 RNA viral load (VL) levels of HIV-infected persons is a strong predictor of: sexual and perinatal transmission of HIV; rapidity of achieving viral suppression with antiretroviral therapy (ART) [1–3]; and the probability of progression to AIDS or death [4]. Pre-ART HIV-RNA VL levels can be exploited in the strategic timing of ART, particularly in the settings of limited resources [5]. An African study that modelled the relationship of VL and heterosexual transmission risk in discordant couples concluded that 90% of new HIV infections could be eliminated by treating only people with HIV VL >10,000 copies/mL [6].
Several factors are associated with VL in untreated patients: female sex [7,8], and a number of host factors such as HLA-B*5701, HLA-B*27, CCR5 delta-32 heterozygosity and allelic variation in HLA-C and KIR [9–11] have been shown to be associated with lower VL, while older age is associated with higher VL [12]. Other factors associated with VL include race, with some studies reporting lower VL in black compared with white groups [13,14].
The association between VL and CD4+ cell count, a marker of immune status, is well described. A study reported that an average of 1 log10 increase in viral load is associated with a 55 cell/mm3 CD4+ cell count decrease [15]. But Rodríguez et al. evaluated the association between VL on rate of CD4+ decline in untreated patients in two US cohorts and concluded that presenting VL predicts the rate of CD4+ cell decline only minimally [16]. It is likely that a mixture of factors drives CD4+ cell losses in HIV infection. It is therefore important to understand an individual's risk for disease progression as this may have profound implications for therapeutic management decisions.
In view of the prognostic importance of VL and possible demographic differences in VL in ART-naive HIV-1 patients, it is important to conduct locally relevant studies to identify the risk factors for high viral loads. Such information could guide programmatic policies, especially with respect to ART initiation. The purpose of this study was to determine the HIV-1 VL levels among ART-naive patients and to identify the association between patients’ demographic, clinical and laboratory characteristics with VL.
Methods
This was a cross-sectional study in which 224 patients (≥15 years of age) accessing care at the Jos University Teaching Hospital (JUTH) AIDS Prevention Initiative in Nigeria (APIN) ART treatment centre from October 2010 to April 2011 were consecutively recruited. Patients were eligible for ART based on the Nigerian National Adult ART guidelines at time of enrolment [17], which closely followed the World Health Organization (WHO) guidelines at the time of patient enrolment [18]. Patients who were on ART prior to enrolment were excluded from the study. The study used data already captured in the electronic database as a part of ongoing HIV/AIDS care, treatment and support programmes. Data regarding: demographics (age, sex, marital status, occupation, residence, education level, mode of HIV transmission, spouse HIV status, spouse ART status); clinical; World Health Organization (WHO) staging; pulmonary tuberculosis (PTB), oropharyngeal candidiasis and Kaposi's sarcoma (KS), and laboratory parameters; hepatitis B virus (HBV) status; HIV-1 RNA viral load and CD4+ cell count were extracted. For HIV-1 serodiagnosis, collected whole blood samples and plasma were tested using rapid HIV tests: Uni-Gold (Trinity Biotech Plc, Bray, Ireland) and Determine HIV-1/2 test (Determine Alere Medical, Japan) with Statpak (Chembio Diagnostic Systems, New York, USA) as the tiebreaker assay for resolving discordant results. T-lymphocyte count (CD4+) was measured by Partec flow Cytometry (Munster, Germany). Hepatitis B surface antigen was determined using enzyme immunoassay (EIA) (Monolisa HBsAg Ultra3, Bio-Rad, Hercules, CA, USA). The plasma portion of the sample was separated after centrifugation and stored at -80°C for HIV-1 RNA viral load testing. The HIV-1 RNA viral load was measured using the Roche Cobas Amplicor HIV-1 Monitor (version 1.5, Roche Diagnostics GmbH, Mannheim, Germany). PTB was diagnosed from clinical symptoms (including chronic cough ≥2 weeks and weight loss), chest radiographs and a positive sputum smear microscopy using Ziehl–Neelsen (Z-N) staining for Mycobacterium tuberculosis (Mtb) acid-fast bacilli (AFB). Ethical clearance for the study was obtained from the JUTH institutional review board and the enrolled patients provided written informed consent. The use of secondary data was approved by the Harvard School of Public Health and JUTH APIN management.
Data analysis
To determine whether VL was related to demographic and clinical variables, we used a log-linear model in which we included demographic and clinical variables likely to correlate with natural log (loge) transformed VL. We excluded individuals for whom there was missing information for any of the explanatory variables considered.
A model in which all variables were added including two-way interactions and gender, with all other explanatory variables was built. Using a backward stepwise procedure, the least significant of the variables was removed and the model tested again. This procedure was repeated until further removal of a variable did not improve the Akaike Information Criterion (AIC). The final statistical model accounted for 67% variability in the data (adjusted R Square=0.67). The R statistical software (Statistical Computing, Vienna, Austria) was used for the data analyses and P values of <0.05 were considered statistically significant.
Results
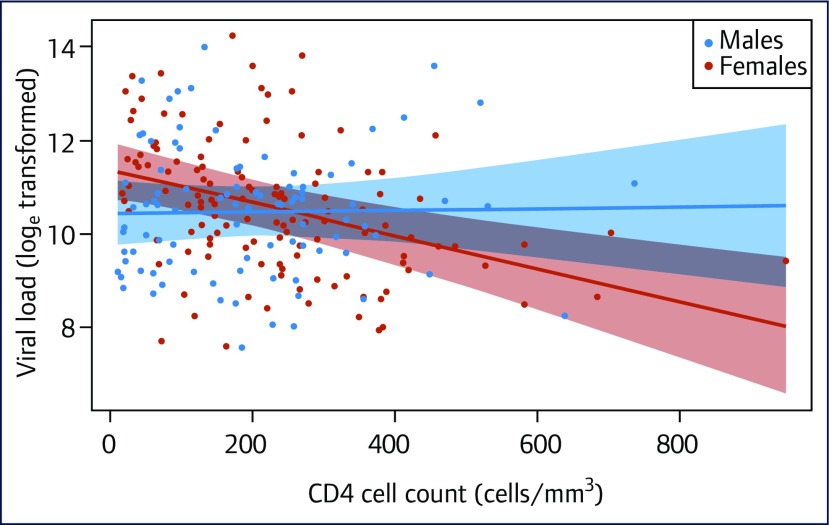
The characteristics of the study participants, summarised in Table 1, show that the participants had a median age of 34 years (interquartile range [IQR] 28–41) with females in the majority (59%). Heterosexual transmission was the predominant mode of HIV transmission (98%). Approximately 10% of the participants had no formal education, while the proportion of those with secondary education (36%) was higher in comparison to other levels of education. Most of the participants were married with a little above half reporting their partner HIV status as negative. Other infections such as HBV, candidiasis, and Kaposi's sarcoma were uncommon with each having a prevalence of less than 10%, while the prevalence of PTB was above 10%. Over half of the participants were at WHO clinical stage 3 or 4. When the clinical and demographic characteristics of females and males were compared (Table 1), females had a lower median age than men (32 versus 36 years; P<0.001), while a higher proportion of males than females had HIV-positive partners (55% men compared to 41% females; P=0.041). There was no significant difference in other demographic and clinical characteristics between females and males. A summary of the mean (log transformed) viral load for the different categories is presented in Table 2. The results showed females compared to males and PTB negative compared to PTB positive patients had a significantly higher VL (14.9 loge versus 11.5 loge, P=0.003 and 11.31 loge versus 11.89 loge, P=0.047, respectively). The difference (β) in the mean change in VL between males and females was loge0.64 copies/mL (Table 2). The change in VL between males and females is illustrated in Figure 1. Viral load tended to decrease with increasing CD4+ cell counts in females, but remained relatively unchanged in males across all values of CD4+ cell counts (Figure 1).
Table 1.
Clinical and demographic information collected from study subjects stratified by gender
| Variable | Subgroup | N (%) | Female N (%) | Male (%) | P* |
|---|---|---|---|---|---|
| Age (years) | Median (IQR) | 34(28–41) | 32(27–38) | 36(32–43) | <0.001 |
| Mode of HIV transmission | Heterosexual | 219(97.7) | 129(97.7) | 90(97.8) | 0.955 |
| Transfusion | 5(2.2) | 3(2.3) | 2(2.2) | ||
| Educational Level | Illiterate | 23(10.2) | 13(9.9) | 10(10.9) | 0.982 |
| Primary | 49(21.8) | 30(22.7) | 19(20.7) | ||
| Secondary | 81(36.1) | 47(35.6) | 34(37) | ||
| Tertiary | 71(31.7) | 42(31.8) | 29(31.5) | ||
| Marital status | Divorced | 3(1.3) | 2(1.5) | 1(1.1) | 0.118 |
| Married | 148(66.0) | 79(59.9) | 69(75) | ||
| Separated | 12(5.3) | 9(6.8) | 3(3.3) | ||
| Single | 45(20.0) | 29(22) | 16(17.4) | ||
| Widowed | 16(7.1) | 13(9.9) | 3(3.3) | ||
| Partner HIV status | Negative | 119(53.1) | 78(59.1) | 41(44.6) | 0.041 |
| Positive | 105(46.8) | 54(40.9) | 51(55.4) | ||
| Spouse ART | Negative | 202(90.1) | 117(88.6) | 85(92.4) | 0.494 |
| Positive | 22(9.8) | 15(11.4) | 7(7.6) | ||
| HBV | Negative | 208(92.8) | 124(93.9) | 84(91.3) | 0.599 |
| Positive | 16(7.1) | 8(6.1) | 8(8.7) | ||
| PTB | Negative | 198(88.3) | 120(90.9) | 78(84.8) | 0.204 |
| Positive | 26(11.6) | 12(9.1) | 14(15.2) | ||
| Candidiasis | Negative | 148(66.0) | 90(68.2) | 58(63) | 0.474 |
| Positive | 76(33.9) | 42(31.8) | 34(37) | ||
| Kaposi's sarcoma | No | 216(96.4) | 127(96.2) | 89(96.7) | 0.985 |
| Yes | 8(3.5) | 5(3.8) | 3(3.3) | ||
| WHO staging | 1 | 41(18.3) | 25(18.9) | 16(17.4) | 0.16 |
| 2 | 54(24.1) | 37(28) | 17(18.5) | ||
| 3 | 74(33.0) | 44(33.3) | 30(32.6) | ||
| 4 | 55(24.5) | 26(19.7) | 29(31.5) | ||
| CD4+ cell count(cells/mm3) | Median(IQR) | 193.0(92.7–284.0) | 202(122.3–299.0) | 178(69–269.8) | 0.075 |
P values are based on chi-squared contingency table test, Fisher's exact test for groups with less than five counts, and Mann–Whitney U-tests for age and CD4+ cell count.
N: number of subjects; IQR: interquartile range; ART: antiretroviral therapy; HBV: hepatitis B virus; PTB: pulmonary tuberculosis; WHO: World Health Organization.
Table 2.
Relationship between viral load and clinical and demographic parameters
| Parameter | Loge transformed mean viral load (CI) | β (CI)† | P |
|---|---|---|---|
| Sex | |||
| Female | 14.9(11.71–16.94) | 3.38(1.13–5.64) | 0.003 |
| Male* | 11.5(8.14–13.718) | 0 | |
| Mode of transmission | |||
| Heterosexual | 11.54(11.67–16.99) | −0.12(−1.39–1.15) | 0.858 |
| Transfusion | 11.65(11.37–17.52) | 0 | |
| Education level | |||
| Illiterate | 11.42(11.71–16.94) | −0.13(−0.83–0.57) | 0.719 |
| Primary | 11.73(12.07–17.21) | 0.18(−0.37–0.73) | 0.516 |
| Secondary | 11.69(12.04–17.15) | 0.14(−0.33–0.61) | 0.561 |
| Tertiary* | 11.55(11.97–16.96) | 0 | |
| Marital status | |||
| Divorced | 12.18(11.71–16.94) | 0.87(−0.9–2.65) | 0.333 |
| Married | 11.15(11.2–15.39) | −0.15(−0.91–0.59) | 0.686 |
| Separated | 11.87(11.71–16.29) | 0.56(−0.54–1.67) | 0.316 |
| Single | 11.46(11.53–15.69) | 0.16(−0.73–1.04) | 0.728 |
| widowed* | 11.31(11.29–15.62) | 0 | |
| Partner HIV status | |||
| Negative | 11.52(11.71–16.94) | −0.16(−0.61–0.29) | 0.476 |
| Positive* | 11.68(11.89–17.08) | 0 | |
| Spouse on ART | |||
| No | 11.7(11.71;16.94) | 0.21(−0.49–0.91) | 0.564 |
| Yes* | 11.49(11.46;16.75) | 0 | |
| HBV | |||
| Negative | 11.34(11.71–16.94) | −0.52(−1.26–0.22) | 0.169 |
| Positive* | 11.86(12.13–17.56) | 0 | |
| PTB | |||
| Negative | 11.31(11.71–16.94) | −0.98(−1.24–0.08) | 0.047 |
| Positive* | 11.89(12.17–17.65) | 0 | |
| Candidiasis | |||
| Negative | 11.61(11.71–16.94) | 0.027(−0.44–0.49) | 0.911 |
| Positive* | 11.58(11.68–16.93) | 0 | |
| Kaposi's sarcoma | |||
| No | 11.6(11.67–16.94) | 0.06(−0.99–1.12) | 0.910 |
| Yes* | 11.6(11.38–17.1) | 0 | |
| WHO staging | |||
| 1 | 11.3(11.67–16.94) | −0.31(−1.09–0.47) | 0.432 |
| 2 | 11.6(12.05–17.21) | 0.01(−0.69–0.72) | 0.977 |
| 3 | 11.8(12.23–17.36) | 0.18(−0.42–0.77) | 0.555 |
| 4* | 11.627(12.14–17.1) | 0 | |
| Log CD4 cell count | −0.04(−0.39–0.31) | 0.008 | |
| Age | 0.01(−0.02–0.03) | 0.636 | |
| Sex log CD4 cell count* | 0.005 | ||
| Females log CD4 cell count* | −0.64(−1.07–0.19) | ||
| Males log CD4 cell count* | 0 | ||
Reference group for factor variables.
β: difference of means for group variables and the slope for continuous variables.
ART: antiretroviral therapy; HBV: hepatitis B virus; PTB: pulmonary tuberculosis; WHO: World Health Organization.
Figure 1.
Change in loge transformed viral load in relation to CD4+ cell counts in males and females with the shaded bands representing 95% confidence intervals
Discussion
Our result showed that VL in ART-naive HIV-1-infected patients was significantly influenced by sex, PTB status and CD4+ cell count. Females and patients with PTB had a significantly higher VL, while viral load decreased with increasing CD4+ cell count; however, this decrease was more significant in females compared to males.
HIV RNA levels were significantly higher at lower CD4+ cell counts in females than males in this study, whereas earlier studies [8,19–21] found HIV VL to be lower in females compared to males at similar stages of HIV infection. However, the trend towards higher VL in females in our study was reversed at higher CD4+ cell count levels above 300 cell/mm3, with females having lower HIV VL. The finding of our study is not surprising as most patients present late (with low CD4 cell counts) for treatment at the study site [22]. Gender-based strategies with a focus on women are needed in our setting to promote early identification and integration of women into care as this can potentially reduce HIV-associated mortality in the population.
Interestingly, whereas VL levels tend to be lower in females with higher CD4+ cell counts, it remained relatively constant at different CD4+ cell count levels in males, which suggests a higher rate of CD4+ cell decline at the same level of viraemia and a faster progression to AIDS in ART-naive females compared to males in our setting. It is not surprising that more females than males present for treatment at more advanced stage of HIV disease in our setting [22]. Consistent with the finding of this study, some studies showed that at the same level of viraemia, progression to AIDS is faster in women than in men [19,23]. The mechanism underpinning sex differences in HIV disease progression is unclear. A comprehensive immunological assessment that included the analysis of CD4+ and CD8+ T cell activation of 980 ART-naive HIV-1-infected subjects (514 men and 109 women) followed at US sites, showed a substantial sex difference in the response of plasmacytoid dendritic cells (pDCs) to HIV-1. Plasmacytoid dendritic cells derived from women produce markedly more interferon-alpha (IFN-alpha) in response to HIV-1-encoded Toll-like receptor 7 (TLR7) ligands than pDCs derived from men, resulting in stronger secondary activation of CD8(+) T cells. The sex difference in TLR-mediated activation of pDCs may account for higher immune activation in women compared to men at a given HIV-1 viral load and provide a mechanism by which the same level of viral replication might result in faster HIV-1 disease progression in women compared to men [23]. In line with the WHO HIV treatment approach [24], which recommends ART in all adults living with HIV regardless of WHO clinical stage and CD4 cell count, early identification of HIV-infected females and initiation of treatment will improve treatment prognosis and programmatic success. Unlike previous studies where older patients tended to have higher VL levels [12], this study did not find any significant association between age and HIV VL in ART-naive patients.
Consistent with other studies [25,26], we found a significantly higher VL in HIV/PTB co-infected patients compared to those infected with HIV only. Available evidence suggests that the increase in HIV viraemia that occurs in association with tuberculosis may result from upregulation of CXCR4 and CCR5 on CD4+ T cells, thereby causing acceleration of HIV infection [27]. Other cellular mechanisms involve induction by Mtb of HIV-1 replication in HIV-infected cell lines, including in mononuclear cells from HIV-1 infected subjects [28]. When mononuclear cells from subjects with tuberculosis (TB) are activated, they become more susceptible to a productive infection by HIV-1 [29,30]. Additionally, the cytokine profile of the tuberculous microenvironment is conducive for HIV-1 replication [31]. Considering the effect of TB on viral load, and the high burden of TB in Nigeria [32], TB chemoprophylaxis in HIV-1-infected patients in our setting might be beneficial and should be explored.
The cross-sectional design of this study has some inherent limitations that should be considered in interpreting the results of the study. HIV RNA was measured at a single point in time and hence we are unable to describe the trend in disease progression. Furthermore, the interval between seroconversion was not considered in evaluating the association between the VL. However, using the population baseline VL we were able to describe the association between several demographic and clinical characteristics and VL in ART-naive patients.
Conclusion
In ART-naive HIV-1-infected patients in our setting, females compared to males had significantly higher VL and lower CD4+ cell counts at the same VL threshold. This data suggests that more females than males were presenting late for treatment in our setting and hence were more likely to be at a higher risk of rapid progression to AIDS. Therefore, gender-based strategies for early identification and integration of females into care are required in this setting to mitigate against rapid progression to AIDS. Further studies in African settings are required to characterise the pattern and magnitude of viral load that may be unique to males and females in this setting.
References
- 1. Garcia PM, Kalish LA, Pitt J et al. . Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N Engl J Med 1999; 341: 394– 402. [DOI] [PubMed] [Google Scholar]
- 2. Gray RH, Wawer MJ, Brookmeyer R et al. . Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet 2001; 357: 1149– 1153. [DOI] [PubMed] [Google Scholar]
- 3. Lingappa JR, Hughes JP, Wang RS et al. . Partners in prevention HSV/HIV transmission study team. estimating the impact of plasma HIV-1 RNA reductions on heterosexual HIV-1 transmission risk. PloS One 2010; 5: e12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Egger M, May M, Chêne G et al. . Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: A collaborative analysis of prospective studies. Lancet 2002; 360: 119– 129. [DOI] [PubMed] [Google Scholar]
- 5. Korenromp EL, Williams BG, Schmid GP, Dye C. Clinical prognostic value of RNA viral load and CD4 cell counts during untreated HIV-1 Infection. A quantitative review. PLoS One 2009; 4: e5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lingappa JR, Hughes JP, Wang RS et al. . Estimating the impact of plasma HIV-1 RNA reductions on heterosexual HIV-1 transmission risk. PLoS One 2010; 5: e12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Farzadegan H, Hoover DR, Astemborski J et al. . Sex differences in HIV-1 viral load and progression to AIDS. Lancet 1998; 352: 1510– 1514. [DOI] [PubMed] [Google Scholar]
- 8. Sterling TR, Vlahov D, Astemborski J et al. . Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N Engl J Med 2001; 344: 720– 725. [DOI] [PubMed] [Google Scholar]
- 9. Martin MP, Qi Y, Gao X et al. . Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat Genet 2007; 39: 733– 740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McLaren PJ, Coulonges C, Ripke S et al. . Association study of common genetic variants and HIV-1 acquisition in 6,300 infected cases and 7200 controls. PLoS Pathog 2013; 9: e1003515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Migueles SA, Sabbaghian MS, Shupert WL et al. . HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci U S A 2000; 97: 2709– 2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Touloumi G, Pantazis N, Babiker AG et al. . Differences in HIV RNA levels before the initiation of antiretroviral therapy among 1864 individuals with known HIV-1 seroconversion dates. AIDS 2004; 18: 1697– 1705. [DOI] [PubMed] [Google Scholar]
- 13. Anastos K, Gange SJ, Lau B et al. . Association of race and gender with HIV-1 RNA levels and immunologic progression. J Acquir Immune Defic Syndr 2000; 24: 218– 226. [DOI] [PubMed] [Google Scholar]
- 14. Smith PR, Sarner L, Murphy M et al. . Ethnicity and discordance in plasma HIV-1 RNA viral load and CD4+ lymphocyte count in a cohort of HIV-1-infected individuals. J Clin Virol 2003; 26: 101– 117. [DOI] [PubMed] [Google Scholar]
- 15. Lima VD, Fink V, Yip B et al. . Association between HIV-1 RNA level and CD4 cell count among untreated HIV-infected individuals. Am J Public Health 2009; 1: S193– S196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rodríguez B, Sethi AK, Cheruvu VK et al. . Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. JAMA 2006; 296: 1498. [DOI] [PubMed] [Google Scholar]
- 17. Federal Ministry of Health (FMoH) Nigeria ( 2010). National Guidelines for HIV and AIDS Treatment and Care in Adolescents and Adults. Available at: www.who.int/hiv/pub/guidelines/nigeria_art.pdf ( accessed August 2015).
- 18. World Health Organization Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. 2006. World Health Organization, Geneva, 1– 134. [PubMed] [Google Scholar]
- 19. Addo MM, Altfeld M. Sex-based differences in HIV type 1 pathogenesis. J Infect Dis 2014; 209: S86– S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Evans JS, Nims T, Cooley J et al. . Serum levels of virus burden in early-stage human immunodeficiency virus type 1 disease in women. J Infect Dis 1997; 175: 795– 800. [DOI] [PubMed] [Google Scholar]
- 21. Gandhi M, Bacchetti P, Miotti P et al. . Does patient sex affect human immunodeficiency virus levels? Clin Infect Dis 2002; 35: 313– 322. [DOI] [PubMed] [Google Scholar]
- 22. Agaba PA, Meloni ST, Sule HM et al. . Patients who present late to HIV care and associated risk factors in Nigeria. HIV Med 2014; 15: 396– 405. [DOI] [PubMed] [Google Scholar]
- 23. Meier A, Chang JJ, Chan E et al. . Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med 2009; 15: 955– 959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Health Organization Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2nd edn 2016. Available at: http://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf ( accessed December 2016). [PubMed]
- 25. Goletti D, Weissman D, Jackson RW et al. . Effect of Mycobacterium tuberculosis on HIV replication. Role of immune activation. J Immunol 1996; 157: 1271– 1278. [PubMed] [Google Scholar]
- 26. Toossi Z, Mayanja-Kizza H, Hirsch CS et al. . Impact of tuberculosis (TB) on HIV-1 activity in dually infected patients. Clin Exp Immunol 2001; 123: 233– 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Juffermans NP, Speelman P, Verbon A et al. . Patients with active tuberculosis have increased expression of HIV coreceptors CXCR4 and CCR5 on CD4(+) T cells. Clin Infect Dis 2001; 32: 650– 652. [DOI] [PubMed] [Google Scholar]
- 28. Lederman MM, Georges DL, Kusner DJ et al. . Mycobacterium tuberculosis and its purified protein derivative activate expression of the human immunodeficiency virus. J Acquir Immune Deficy Syndr 1994; 7: 727– 733. [PubMed] [Google Scholar]
- 29. Toossi Z, Xia L, Wu M, Salvekar A. Transcriptional activation of HIV by Mycobacterium tuberculosis in human monocytes. Clin Exp Immuno 1999; 117: 324– 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vanham G, Edmonds K, Qing L et al. . Generalized immune activation in pulmonary tuberculosis: co-activation with HIV infection. Clin Exp Immun 1996; 103: 30– 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Diedrich CR, and Flynn JL. HIV-1/Mycobacterium tuberculosis coinfection immunology: how does HIV-1 exacerbate tuberculosis? Infect Immun 2011; 79: 1407– 1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. World Health Organization| Regional Office for Africa AIDS, TB and Malaria. 2015. Available at: www.afro.who.int/en/nigeria/country-programmes/aids-tb-and-malaria.html ( accessed December 2016).