Abstract
ALK oncogenic activation mechanisms were characterized in four conventional spindle-cell inflammatory myofibroblastic tumours (IMT) and five atypical IMT, each of which had ALK genomic perturbations. Constitutively activated ALK oncoproteins were purified by ALK immunoprecipitation and electrophoresis, and were characterized by mass spectrometry. The four conventional IMT had TPM3/4-ALK fusions (two cases) or DCTN1-ALK fusions (two cases), whereas two atypical spindle-cell IMT had TFG-ALK and TPM3-ALK fusion in one case each, and three epithelioid inflammatory myofibroblastic sarcomas had RANBP2-ALK fusions in two cases, and a novel RRBP1-ALK fusion in one case. The epithelioid inflammatory myofibroblastic sarcoma with RRBP1-ALK fusion had cytoplasmic ALK expression with perinuclear accentuation, different from the nuclear membranous ALK localization in epithelioid inflammatory myofibroblastic sarcomas with RANBP2-ALK fusions. Evaluation of three additional uncharacterized epithelioid inflammatory myofibroblastic sarcomas with ALK cytoplasmic/perinuclear- accentuation expression demonstrated RRBP1-ALK fusion in two cases. These studies show that atypical spindle-cell IMT can utilize the same ALK fusion mechanisms described previously in conventional IMT, whereas in clinically aggressive epithelioid inflammatory myofibroblastic sarcoma we identify a novel recurrent ALK oncogenic mechanism, resulting from fusion with the RRBP1 gene.
Keywords: inflammatory myofibroblastic tumour, epithelioid inflammatory myofibroblastic sarcoma, RRBP1, ALK, fusion gene, mass spectrometry
Introduction
Most inflammatory myofibroblastic tumours (IMT) arise in abdominopelvic locations and are composed of spindled neoplastic myofibroblasts admixed with reactive lymphoplasmacytic cells. Whereas conventional spindle-cell IMT are neoplasms of intermediate biologic potential that recur or metastasize infrequently [1-3], the epithelioid variant of IMT, known as epithelioid inflammatory myofibroblastic sarcoma (EIMS) [4,5], is clinically aggressive and has a dismal prognosis. Conventional spindle-cell IMT contain various ALK fusion oncoproteins, often involving constitutive dimerization and activation of the ALK kinase by fusion with tropomyosin (TPM3 or TPM4) coiled-coil proteins [6,7]. By contrast, EIMS typically have RANBP2-ALK fusion oncoproteins with distinctive nuclear membranous localization, due to RANBP2 roles in the nuclear pore complex [4,5]. Nonetheless, some EIMS have cytoplasmic ALK expression, implicating ALK fusion partners other than RANBP2. In this study, we used proteomic strategies to characterize novel ALK fusion oncoproteins in atypical IMT. These analyses focused on the EIMS variant, but also interrogated atypical spindled IMT with nuclear atypia or ganglion-like tumour cells, which is an IMT variant in which ALK perturbations have not been evaluated previously.
Materials and Methods
Tumour samples
IMT specimens and other ALK-positive tumours were identified from the pathology archives of the investigators’ hospitals, and the histology and immunostains for each IMT were reviewed by two soft tissue pathologists (J.L.H. and J.C.L.). The study was approved by the research ethics committees of each institution.
Immunoprecipitation and immunoblotting
Immunoprecipitations and immunoblotting were performed according to methods described previously [8]. Immunoprecipitations were performed with 1 mg of IMT protein lysate and 2μg monoclonal anti-ALK antibody (Dako, Denmark; clone ALK1). Immunoblotting was with antibodies to ALK (Invitrogen – Thermo Fisher Scientific, Carlsbad, CA; 51-3900), phospho-tyrosine (Santa Cruz Biotechnologies, Santa Cruz, CA; pY99, sc-7020), DCTN1 (Santa Cruz Biotechnologies), and RRBP1 (Novus Biologicals, Littleton, CO, USA).
Analysis of ALK fusion proteins by mass spectrometry
Aberrant-sized ALK proteins were identified by phosphotyrosine and ALK immunoblot stains, then excised from gels stained with Coomassie blue and subjected to in gel trypsin digestion, extraction, reverse-phase HPLC elution, electrospray ionization, and analysis by ion-trap mass spectrometry [8]. Peptide fragmentation patterns were matched against protein databases using SEQUEST algorithms.
RT-PCR and Sanger sequencing
Total RNA was extracted from IMT using TRIZOL LS reagent (Invitrogen, Carlsbad, CA, USA). Reverse transcription was by iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA, USA). Primer sequences for PCR and Sanger sequencing are shown in Supplementary Table 1.
Fluorescence in situ hybridization
Fluorescence in situ hybridization (FISH) analysis was performed on 4 μm paraffin sections as described previously [9]. Flanking sequences upstream of RRBP1 and downstream of ALK were detected with bacterial artificial chromosomes RP11-588F17 and RP11-373D23, respectively.
Results
Clinicopathological features
Of the 15 IMT analysed in this study (Table 1), snap-frozen materials were available for 9, which could therefore be used in the proteomic screens. Formalin-fixed paraffin-embedded (FFPE) materials were available for all IMT, and all cases were known to have ALK rearrangements, by previously-performed ALK break-apart FISH. Histological features (Table 1, Supplementary Figure S1) demonstrated three IMT subtypes: 1) conventional spindle cell low-grade IMT with lymphoplasmacytic infiltrate and ALK cytoplasmic staining (N = 4); 2) atypical spindle-cell hypercellular IMT with conspicuous ganglion-like tumour cells and nuclear atypia, along with lymphoplasmacytic infiltrate and cytoplasmic ALK expression (N = 2); and 3) EIMS (N = 9) with large round vesicular nuclei and prominent nucleoli, neutrophil-predominant inflammatory cells in a fibromyxoid stroma, and either ALK nuclear membrane staining (N = 4) or ALK cytoplasmic staining (N = 5; 4 with perinuclear accentuation).
Table 1.
The clinical, pathological, and molecular information of inflammatory myofibroblastic tumours in the current series
| Case # | Histology Type | Age | Sex | Location | ALK IHC pattern | ALK rearrangement (by FISH) |
Fusion size (by ALK IP) |
Putative partner suggested by MS |
Fusion confirmed by RT-PCR or RRBP1-ALK FISH |
Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| A1 | Atypical (epithelioid) | 42 y | M | Abdominal cavity | NM | + | 185 kDa | RANBP2 | RANBP2-ALK | Recurred at 8 months; AWD at 40 months†; |
| A2 | Atypical (spindle) | 10 y | F | Abdominal cavity | Cytoplasmic | + | 75, 85, 95 kDa | TFG | TFG-ALK | ANED at 81 months |
| A3 | Atypical (epithelioid) | 34 y | M | Liver | NM | + | 185 kDa | N.P. | RANBP2-ALK | Recurred & DOD at 5 months |
| A4 | Atypical (epithelioid) | 62 y | M | Abdominal cavity | Cytoplasmic, PN | + | 140~180 kDa | RRBP1 | RRBP1-ALK | DOD at 2 months |
| A5 | Atypical (spindle) | 14 y | M | Pelvis | Cytoplasmic | + | 80~105 kDa | N.P. | TPM3-ALK | DOD at 3 months |
| A6 | Atypical (epithelioid) | 76 y | F | Abdominal cavity | NM | + | N.P. | N.P. | RANBP2-ALK | DOD at 4 months |
| A7 | Atypical (epithelioid) | 30 y | M | Abdominal cavity | NM | + | N.P. | N.P. | N.P. | DOD at 8 months |
| A8 | Atypical (epithelioid) | 26 y | M | Abdominal cavity | Cytoplasmic, PN | + | N.P. | N.P. | RRBP1-ALK | Recurred at 7 & 16 months; AWD at 16 months, with intra-abdominal dissemination |
| A9 | Atypical (epithelioid) | 39 y | F | Abdominal cavity | Cytoplasmic, PN | + | N.P. | N.P. | RRBP1-ALK | Recurred & AWD at 10 months, with intra-abdominal and pleural dissemination |
| A10 | Atypical (epithelioid) | 7 mo | M | Abdominal cavity | Cytoplasmic, PN | + | N.P. | N.P. | RRBP1-ALK negative | DOD at 36 months |
| A11 | Atypical (epithelioid) | 16 | F | Lung | Cytoplasmic | + | N.P. | N.P. | RRBP1-ALK negative | Recurred at 1 month; AWD at 48 months† |
|
| ||||||||||
| C1 | Conventional | 23 y | F | Abdominal cavity | Cytoplasmic | + | 80~105 kDa | N.P. | TPM3-ALK | ANED at 36 months |
| C2 | Conventional | 20 y | F | Abdominal cavity | Cytoplasmic | + | 80~105 kDa | N.P. | TPM4-ALK | N.P. |
| C3 | Conventional | 31 y | F | Liver | Cytoplasmic | + | 240 kDa | DCTN1 | DCTN1-ALK | ANED at 22 months |
| C4 | Conventional | 27 y | M | Lung | Cytoplasmic | + | 240 kDa | N.P. | DCTN1-ALK | ANED at 24 months |
Notes and annotations: IP, immunoprecipitation; MS, mass spectrometry; N.P., not provided/performed (including uninformative analyses). ALK IHC (immunohistochemistry) pattern: NM, nuclear membranous; PN, perinuclear accentuation. Follow-up: ANED, alive with no evidence of disease; AWD, alive with disease; DOD, died of disease;
received crizotinib treatment.
Characterization of ALK fusion proteins
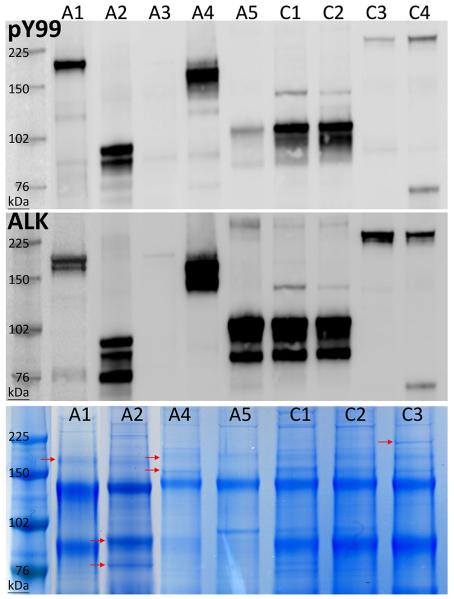
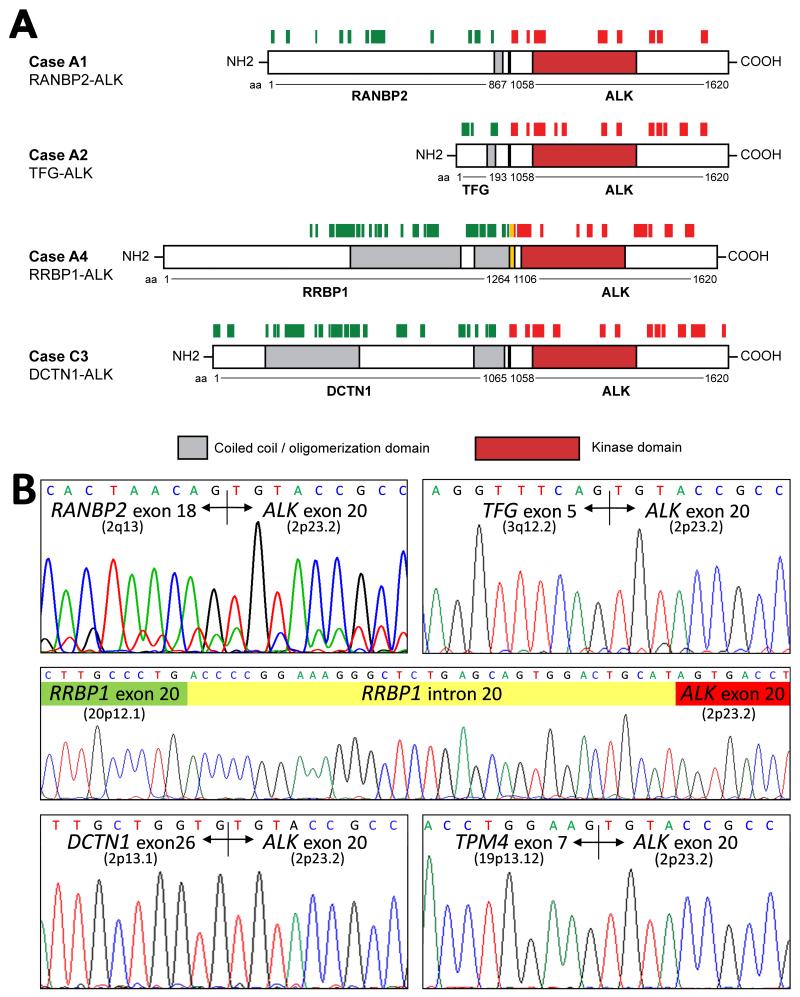
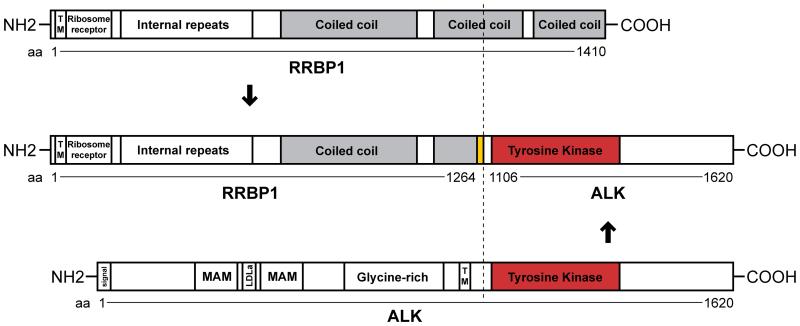
Each of the 9 snap-frozen IMT expressed tyrosine phosphorylated ALK proteins of aberrant sizes (Figure 1). Two conventional IMT (C1 and C2) and 1 atypical spindle-cell IMT (A5) expressed ALK proteins consistent in size with the TPM3/4-ALK oncoproteins reported previously by our group [10]. TPM3/4-ALK fusions were confirmed (Table 1, Figure 2) in each of these cases by RT-PCR. The remaining IMT expressed phosphorylated ALK proteins of 75-95 kDa (A2), 140-180 kDa (A4), 185 kDa (A1 and A3), and 240 kDa (C3 and C4). ALK proteins of each size (A1, A2, A4, and C3) were purified from Coomassie blue-stained gels, and characterized by mass spectrometry, revealing RANBP2-ALK, TFG-ALK, DCTN1-ALK, and RRBP1-ALK fusions in IMT A1, A2, C3 and A4, respectively (Figure 2 and Supplementary Figure S2). Each of the ALK fusion partners contributed a coiled coil domain that was retained in the ALK fusion protein (Figure 2). The protein sequences were consistent with fusions of RANBP2 exon 18, TFG exon 5, and DCTN1 exon 26 to ALK exon 20. RANBP2-ALK, TFG-ALK, and DCTN1-ALK are previously reported ALK fusions in IMT,[5,11,12] whereas RRBP1-ALK is a novel ALK fusion. RRBP1-ALK incorporates not only RRBP1 coiled-coil domains but also an RRBP1 endoplasmic reticulum transmembrane domain and a ribosome receptor domain (Figure 3).
Figure 1.
ALK immunoprecipitations were immunoblotted for phosphotyrosine (top) and ALK (middle) and were electrophoresed and stained with Coomassie blue (bottom). Coomassie blue bands indicated by arrows corresponded in size to the putative ALK fusion proteins and were excised and subjected to mass spectrometry analyses.
Figure 2.
A: Each of four aberrant ALK proteins characterized by mass spectrometry was a fusion protein in which the ALK fusion partner juxtaposed coiled coil oligomerization domains to the ALK tyrosine kinase domain. Peptides mapping to ALK and the fusion partners are indicated by red and green bars, respectively. B: The fusions were confirmed by RT-PCR and Sanger sequencing. Chromosomal cytoband locations of the ALK-fusion partner genes are as indicated in parentheses (for TPM3: 1q21.3). The RRBP1-ALK fusion incorporates an alternate splicing 33-bp intronic sequence fused to intra-exonic sequence from ALK exon 20, maintaining the open reading frame.
Figure 3.
Schematic of RRBP1-ALK domains. The dashed line indicates the breakpoints. This model is predicted using the Simple Modular Architecture Research Tool (SMART; http://smart.embl-heidelberg.de/) and the Human Protein Reference Database (http://www.hprd.org/). The ALK transmembrane domain (TM) is a cell membrane transmembrane whereas the RRBP1 TM is an organelle (most likely endoplasmic reticulum) domain.
Validation of mass spectrometry results
The N-terminal regions of DCTN1 (in IMTs C3 and C4) and RRBP1 (in IMT A4) co-precipitated and co-localized with the ALK C-terminus in these tumours, corroborating the DCTN1-ALK and novel RRBP1-ALK fusions (Supplementary Figure S3). RT-PCR demonstrated RANBP2-ALK, TFG-ALK, DCTN1-ALK, and RRBP1-ALK fusion transcripts, as predicted by mass spectrometry (Figure 2B). Likewise, TPM3-ALK or TPM4-ALK fusions were corroborated by RT-PCR in cases A5, C1, and C2 (Figure 2B, Table 1). The RRBP1-ALK fusion resulted from use of an alternate splice acceptor site within RRBP1 intron 20 (agACCCCGG….), such that the RRBP1-ALK open reading frame resulted from fusion of RRBP1 exon 20 to a 33-nucleotide sequence from RRBP1 intron 20 which was in turn fused to ALK exon 20 (Figure 2B).
RRBP1-ALK is a recurrent oncogenic mechanism in clinically aggressive EIMS
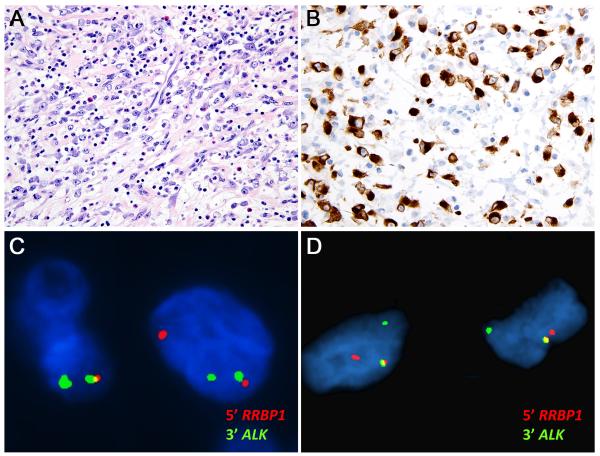
FFPE materials were studied from six additional unselected EIMS (cases different from the abovementioned IMT frozen-specimen series). Two of these (A6 and A7, Table 1) had nuclear membrane ALK expression consistent with RANBP2-ALK. RT-PCR demonstrated RANBP2-ALK in A6 and was unsuccessful in A7. The other four cases (A8, A9, A10 and A11, Table 1) had cytoplasmic ALK expression, with perinuclear accentuation in A8, A9, and A10 (Figure 4). Interphase FISH demonstrated RRBP1-ALK fusion in 34 of 51 cells (67%) from A8 and 59 of 100 cells (59%) from A9 (Figure 4). RRBP1-ALK cases A8 and A9, like A4, had fulminant clinical progression: patient A4 died of progressive EIMS 2 months after diagnosis, and patients A8 and A9 had EIMS recurrence with disseminated intra-abdominal metastases within 10 mo after resection of the primary tumour (Table 1).
Figure 4.
Cases A8 and A9 show histological features of EIMS, with mixed lymphocyte, neutrophil, and eosinophil infiltration (A), while expressing cytoplasmic ALK protein with perinuclear accentuation by immunohistochemistry (B). FISH demonstrated RRBP1-ALK fusion in both cases (C: A8 & D: A9).
RRBP1-ALK is not found in ALK-positive cancers other than EIMS
To evaluate the specificity of RRBP1-ALK, we evaluated ALK immunohistochemical expression patterns in 100 ALK-positive neoplasms including 20 lung adenocarcinomas, 25 anaplastic large-cell lymphomas, 25 epithelioid fibrous histiocytomas, and 30 conventional IMT. None of these showed the distinctive pattern of perinuclear accentuation of cytoplasmic staining exhibited by RRBP1-ALK EIMS (data not shown).
Discussion
We characterized tyrosine phosphorylated ALK oncoproteins in clinical IMT specimens (Table 1), leading to discovery of RRBP1-ALK as a novel and recurrent ALK fusion gene in clinically-aggressive EIMS. RRBP1-ALK has not been described previously in IMT, but in this study we demonstrated RRBP1-ALK in 3 of 4 EIMS in which ALK expression was cytoplasmic with perinuclear accentuation (Figure 4). The studies focused on atypical IMT, evaluating ALK fusion oncogenes in EIMS and in spindle-cell IMT with nuclear atypia and a ganglion-like component. Whereas all six IMT with RANBP2-ALK or RRBP1-ALK in this series were clinically aggressive EIMS, the TPM3-ALK and TFG-ALK fusions found in one atypical spindled IMT each have been reported previously in conventional IMT, and are therefore not specific for atypical IMT. Therefore, we hypothesize that the various ALK fusions in conventional IMT can sustain tumours that occasionally develop atypical histological features, although they remain fundamentally lower-grade and spindled entities. Acquired atypia in these IMT might result from additional genetic aberrations, as suggested by previous studies [2,13,14]. By contrast, EIMS with RANBP2-ALK or RRBP1-ALK appear to be a morphologically and clinically distinct subgroup of IMT that is of higher grade from the outset, rather than a transformation from conventional IMT. Similar to RANBP2-ALK fusions, which have been demonstrated only in EIMS and hematologic neoplasms, our studies suggest that RRBP1-ALK fusions are restricted mechanisms among human cancers. We did not find the characteristic immunohistochemical pattern of RRBP1-ALK expression in 100 ALK-positive lung adenocarcinomas, anaplastic large-cell lymphomas, epithelioid fibrous histiocytomas, and conventional IMT. Furthermore, Stransky, et al. did not find RRBP1-ALK in 6,893 human cancers from the TCGA program, using a kinase fusion-specific computational pipeline to screen RNAseq data: these cases included 102 sarcomas and included tumours in the differential diagnosis of EIMS, e.g., 33 dedifferentiated liposarcomas [15]. Similarly, Yoshihara, et al. did not find RRBP1-ALK in a partially overlapping set of 7,717 human cancers, using a different fusion caller, the Pipeline for RNA sequencing Data Analysis (PRADA) (http://54.84.12.177/PanCanFusV2/ ) [16], and neither did Giacomini, et al. find RRBP1-ALK in 974 human cancers studied by DNA Breakpoint or RNA Breakpoint analyses [17].
RRBP1 is a coiled-coil protein that functions in interactions between ribosomes and the endoplasmic reticulum, and also in microtubule binding [18]. These biologic roles likely account for the distinctive cytoplasmic and perinuclear ALK localization in EIMS with RRBP1-ALK fusions. RRBP1 overexpression has been implicated as a marker of poor prognosis in breast and colorectal cancer [19,20], but the IMT studies reported here are the first demonstration of an RRBP1 oncogenic mechanism. RRBP1-ALK retained the N-terminal RRBP1 coiled-coil domain (Figure 2 and Figure 3), and hence is one of various ALK fusion oncoproteins, such as TPM3-ALK [21], in which a coiled-coil fusion partner dictates ALK oncogenic activation.
Notably, RRBP1-ALK and RANBP2-ALK are the only recurrent oncogenic mechanisms identified, to date, in EIMS. It is intriguing that RRBP1-ALK and RANBP2-ALK have not been found in conventional low-grade spindled IMT. These findings indicate that RRBP1-ALK and RANBP2-ALK are biologically relevant only in the very high-grade epithelioid subtype of IMT, or indeed that these particular ALK fusions are directly responsible for the high proliferative status and distinctive epithelioid morphology of EIMS. Because RRBP1 and RANBP2 both interact with cell microtubule apparati through binding to KIF5B and other kinesins [22,23], it will be worthwhile to determine whether RRBP1 and RANBP2 have shared biological mechanisms in their respective ALK fusions, unique among the other various ALK fusion oncoproteins in IMT. Although mechanistic evaluations of RRBP1-ALK have not yet been performed, it is notable that forced RRBP1 overexpression has been shown to alter cell shape, possibly through microtubule interactions [18]. These observations suggest that RRBP1-ALK oncoproteins contribute to epithelioid morphology by dysregulating usual interactions between RRBP1 and microtubules. However, additional studies are needed to evaluate these hypotheses.
This study has limitations. First, although our studies and database reviews do not demonstrate evidence of RRBP1-ALK in common cancers or in IMT histological mimics, we cannot exclude that other uncommon cancer subtypes might have this novel oncogenic mechanism. Second, although the clinicopathological findings reported herein reveal an aggressive clinical course for each of three EIMS with RRBP1-ALK, additional studies are needed to determine whether this is invariably the case. Third, additional studies are needed to determine whether EIMS with RRBP1-ALK respond favourably to ALK inhibitor therapies, as do EIMS with RANBP2-ALK [24].
In conclusion, proteomic evaluations of ALK oncoproteins identified RRBP1-ALK as a novel and recurrent oncogenic mechanism in clinically aggressive EIMS. These methods were efficient in demonstrating ALK fusion identities and oncogenic functions (aberrant size and constitutive tyrosine phosphorylation) directly in the clinical specimens, rather than needing to create costly lab models which are often only approximations of the clinical entities. The novel RRBP1-ALK fusion led to cytoplasmic and perinuclear ALK expression, in contrast to the typical nuclear membranous ALK expression pattern in EIMS harbouring RANBP2-ALK fusion.
Supplementary Material
Acknowledgments
This study was supported in part by the National Cancer Institute of the National Institutes of Health under Award Numbers 1P50CA127003 and 1P50CA168512. We thank the Taplin Biological Mass Spectrometry Facility of Harvard Medical School, the sequencing core laboratory of Brigham and Women’s Hospital in Boston, MA, and the Proteomics and Protein Function Core of National Taiwan University, Taipei, Taiwan.
Footnotes
Author contributions:
J.C.L conceived and implemented the experiments and acquired and analysed the data; C.F.L, H.Y.H, M.J.Z., A.M.E, C.T.L., and W.B.O. acquired the data; J.L.H. and J.A.F. supervised the study. All authors were involved in writing the paper and had final approval of the submitted and published versions.
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Coffin CM, Watterson J, Priest JR, et al. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19:859–872. doi: 10.1097/00000478-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007;31:509–520. doi: 10.1097/01.pas.0000213393.57322.c7. [DOI] [PubMed] [Google Scholar]
- 3.Gleason BC, Hornick JL. Inflammatory myofibroblastic tumours: where are we now? J Clin Pathol. 2008;61:428–437. doi: 10.1136/jcp.2007.049387. [DOI] [PubMed] [Google Scholar]
- 4.Chen ST, Lee JC. An inflammatory myofibroblastic tumor in liver with ALK and RANBP2 gene rearrangement: combination of distinct morphologic, immunohistochemical, and genetic features. Hum Pathol. 2008;39:1854–1858. doi: 10.1016/j.humpath.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 5.Marino-Enriquez A, Wang WL, Roy A, et al. Epithelioid inflammatory myofibroblastic sarcoma: An aggressive intra-abdominal variant of inflammatory myofibroblastic tumor with nuclear membrane or perinuclear ALK. Am J Surg Pathol. 2011;35:135–144. doi: 10.1097/PAS.0b013e318200cfd5. [DOI] [PubMed] [Google Scholar]
- 6.Griffin CA, Hawkins AL, Dvorak C, et al. Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res. 1999;59:2776–2780. [PubMed] [Google Scholar]
- 7.Marino-Enriquez A, Dal Cin P. ALK as a paradigm of oncogenic promiscuity: different mechanisms of activation and different fusion partners drive tumors of different lineages. Cancer Genet. 2013 doi: 10.1016/j.cancergen.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Marino-Enriquez A, Ou WB, Weldon CB, et al. ALK rearrangement in sickle cell trait-associated renal medullary carcinoma. Genes Chromosomes Cancer. 2011;50:146–153. doi: 10.1002/gcc.20839. [DOI] [PubMed] [Google Scholar]
- 9.Lee JC, Jeng YM, Su SY, et al. Identification of a novel FN1-FGFR1 genetic fusion as a frequent event in phosphaturic mesenchymal tumour. J Pathol. 2015;235:539–545. doi: 10.1002/path.4465. [DOI] [PubMed] [Google Scholar]
- 10.Lawrence B, Perez-Atayde A, Hibbard MK, et al. TPM3-ALK and TPM4-ALK oncogenes in inflammatory myofibroblastic tumors. Am J Pathol. 2000;157:377–384. doi: 10.1016/S0002-9440(10)64550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovly CM, Gupta A, Lipson D, et al. Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov. 2014;4:889–895. doi: 10.1158/2159-8290.CD-14-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subbiah V, McMahon C, Patel S, et al. STUMP un"stumped": anti-tumor response to anaplastic lymphoma kinase (ALK) inhibitor based targeted therapy in uterine inflammatory myofibroblastic tumor with myxoid features harboring DCTN1-ALK fusion. J Hematol Oncol. 2015;8:66. doi: 10.1186/s13045-015-0160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biselli R, Ferlini C, Fattorossi A, et al. Inflammatory myofibroblastic tumor (inflammatory pseudotumor): DNA flow cytometric analysis of nine pediatric cases. Cancer. 1996;77:778–784. doi: 10.1002/(sici)1097-0142(19960215)77:4<778::aid-cncr25>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 14.Hussong JW, Brown M, Perkins SL, et al. Comparison of DNA ploidy, histologic, and immunohistochemical findings with clinical outcome in inflammatory myofibroblastic tumors. Mod Pathol. 1999;12:279–286. [PubMed] [Google Scholar]
- 15.Stransky N, Cerami E, Schalm S, et al. The landscape of kinase fusions in cancer. Nat Commun. 2014;5:4846. doi: 10.1038/ncomms5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshihara K, Wang Q, Torres-Garcia W, et al. The landscape and therapeutic relevance of cancer-associated transcript fusions. Oncogene. 2015;34:4845–4854. doi: 10.1038/onc.2014.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giacomini CP, Sun S, Varma S, et al. Breakpoint analysis of transcriptional and genomic profiles uncovers novel gene fusions spanning multiple human cancer types. PLoS Genet. 2013;9:e1003464. doi: 10.1371/journal.pgen.1003464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogawa-Goto K, Tanaka K, Ueno T, et al. p180 is involved in the interaction between the endoplasmic reticulum and microtubules through a novel microtubule-binding and bundling domain. Mol Biol Cell. 2007;18:3741–3751. doi: 10.1091/mbc.E06-12-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang X, Sun S, Zhang X, et al. Expression of ribosome-binding protein 1 correlates with shorter survival in Her-2 positive breast cancer. Cancer Sci. 2015;106:740–746. doi: 10.1111/cas.12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan Y, Cao F, Guo A, et al. Endoplasmic reticulum ribosome-binding protein 1, RRBP1, promotes progression of colorectal cancer and predicts an unfavourable prognosis. Br J Cancer. 2015;113:763–772. doi: 10.1038/bjc.2015.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amano Y, Ishikawa R, Sakatani T, et al. Oncogenic TPM3-ALK activation requires dimerization through the coiled-coil structure of TPM3. Biochem Biophys Res Commun. 2015;457:457–460. doi: 10.1016/j.bbrc.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Diefenbach RJ, Diefenbach E, Douglas MW, et al. The ribosome receptor, p180, interacts with kinesin heavy chain, KIF5B. Biochem Biophys Res Commun. 2004;319:987–992. doi: 10.1016/j.bbrc.2004.05.069. [DOI] [PubMed] [Google Scholar]
- 23.Cai Y, Singh BB, Aslanukov A, et al. The docking of kinesins, KIF5B and KIF5C, to Ran-binding protein 2 (RanBP2) is mediated via a novel RanBP2 domain. J Biol Chem. 2001;276:41594–41602. doi: 10.1074/jbc.M104514200. [DOI] [PubMed] [Google Scholar]
- 24.Butrynski JE, D'Adamo DR, Hornick JL, et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med. 2010;363:1727–1733. doi: 10.1056/NEJMoa1007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.