Abstract
One of the major pathophysiological features of primary hypertension is an inappropriate activation of the sympathetic nervous system, which is mediated by excessive synthesis and secretion of catecholamine into the blood. Tyrosine hydroxylase (TH), a rate-limiting enzyme in the synthesis of catecholamine, has been highlighted because genetic variations of TH could alter the activity of the sympathetic nervous system activity and subsequently contribute to the pathogenesis of hypertension. Here, we discuss the role of TH as a regulator of sympathetic activity and review several studies that investigated the relationship between genetic variations of TH and hypertension.
Keywords: Tyrosine hydroxylase, Hypertension, Single nucleotide polymorphism
Introduction
Hypertension remains a major contributing factor to cardio-cerebrovascular disease, a leading cause of death in developed countries. The etiologies of hypertension are highly complex and multifactorial: genetic and environmental factors interact to initiate and progress hypertensive diseases. Hereditability of hypertension was first reported in the early 1900s1). Since then, several observational studies have reported that hypertension is much more common in individuals who had hypertensive parents or in identical twins2,3,4). These findings led clinicians and geneticists to investigate genetic variations of genes involved in the pathogenesis of hypertension. With the improvement in tools and techniques for genetic analysis over the past few decades, novel genetic variations are now being recognized increasingly as contributing factors for hypertension. The estimated genetic contribution to the development of hypertension ranges from 30 to 60%5).
Tyrosine hydroxylase (TH) is a key enzyme that converts L-tyrosine to catecholamine in the adrenal medulla, as well as in noradrenergic and dopaminergic neurons. Sympathetic overactivity is one of the characteristic features in hypertension6); therefore, TH has received much attention as a master regulator of catecholamine synthesis. Moreover, several studies reported that certain variations of the TH gene were also associated with the risk of psychiatric disorders, raising the possibility that genetic variations of TH could influence the pathogenesis of hypertension. In this review, we will briefly discuss the importance of sympathetic overactivity and how TH is involved in the pathogenesis of hypertension. Thereafter, we will review several studies that demonstrated the association between the genetic variations of TH and hypertension.
The Sympathetic Nervous System and Its Roles in Hypertension
One of the most widely investigated and accepted theory in the pathogenesis of hypertension is deregulation of the autonomic nervous system, especially increased activity of the sympathetic nervous system(SNS)7). Several studies have demonstrated that the basal activity of the SNS is higher in patients with primary hypertension compared with that in normotensive controls6,8,9). Moreover, sympathetic overactivity has been reported in other, more specialized forms of hypertension, such as isolated systolic hypertension, hypertension of the young, and elderly, hypertension without dipping phenomenon, obesity-related hypertension, hypertension induced by renal impairment, and pregnancy-induced hypertension10,11,12,13,14,15,16,17). These data suggested that excessive activity of the SNS is a universal phenomenon, irrespective of clinical situations that provoke hypertension. However, in contrast to our deep understanding of the importance of sympathetic overactivity in hypertension, the mechanism of how this dysregulated sympathetic activity occurs is not completely understood, despite intensive research. Possible mechanisms for this phenomenon include concurrent activation of the reninangiotensin-aldosterone system, dysfunctions of cardiopulmonary and baro-reflex activities, chemoreceptor activation, and the effects of insulin7). To date, sympathetic overactivity could not be explained by one theory, suggesting that complex interactions are involved in the regulation of the SNS. Further research to determine the mechanisms underlying pathological elevations of sympathetic nerve activity are needed to develop more targeted therapeutic strategies in this field.
Tyrosine Hydroxylase as a Master Regulator of Catecholamine Synthesis
TH is a rate-limiting enzyme for the synthesis of both catecholamines, epinephrine and norepinephrine. TH catalyzes the conversion of L-tyrosine to dihydroxyphenylalanine, which is subsequently converted to dopamine, epinephrine, and norepinephrine. Systemic effects of the SNS are mostly mediated by circulating catecholamines. In response to activation of the SNS, chromaffin cells in the adrenal medulla and sympathetic ganglia synthesize and secret catecholamines into the blood, resulting in increased cardiac output and systemic vascular resistance.
Human TH is encoded by the single-copy TH gene, which is located on chromosome 11p15.5 and has 13 exons. This enzyme is present mainly in the central nervous system and adrenal medulla, and its activity is regulated tightly by multiple levels of control, such as modulation of gene expression through alternative splicing of pre-mRNA, post-transcriptional modification of mRNA, and phosphorylation of TH18,19,20). Traditionally, TH was investigated in the field of neuropsychiatry, because both dopamine and norepinephrine are relevant neurotransmitters synthesized by TH in brain cells. Several studies demonstrated that genetic mutations of TH were important etiologies of nervous system diseases, such as bipolar disorders, schizophrenia, and Parkinson disease21,22,23). These studies raised the possibility that genetic mutations of TH could be involved in the pathogenesis of hypertension, because numerous reports suggested that adrenergic hormones play an important role in the development of hypertension. Moreover, normotensive individuals whose parents were diagnosed with hypertension showed elevated plasma levels of catecholamines, indicating that sympathetic overactivity could be one of the reasons why primary hypertension exhibited a heritable trait24). Taken together, these findings suggested that genetic variations of TH might be one of the determinant factors for overall sympathetic activity, as well as the development of hypertension.
Genetic Variations of Tyrosine Hydroxylase and Hypertension
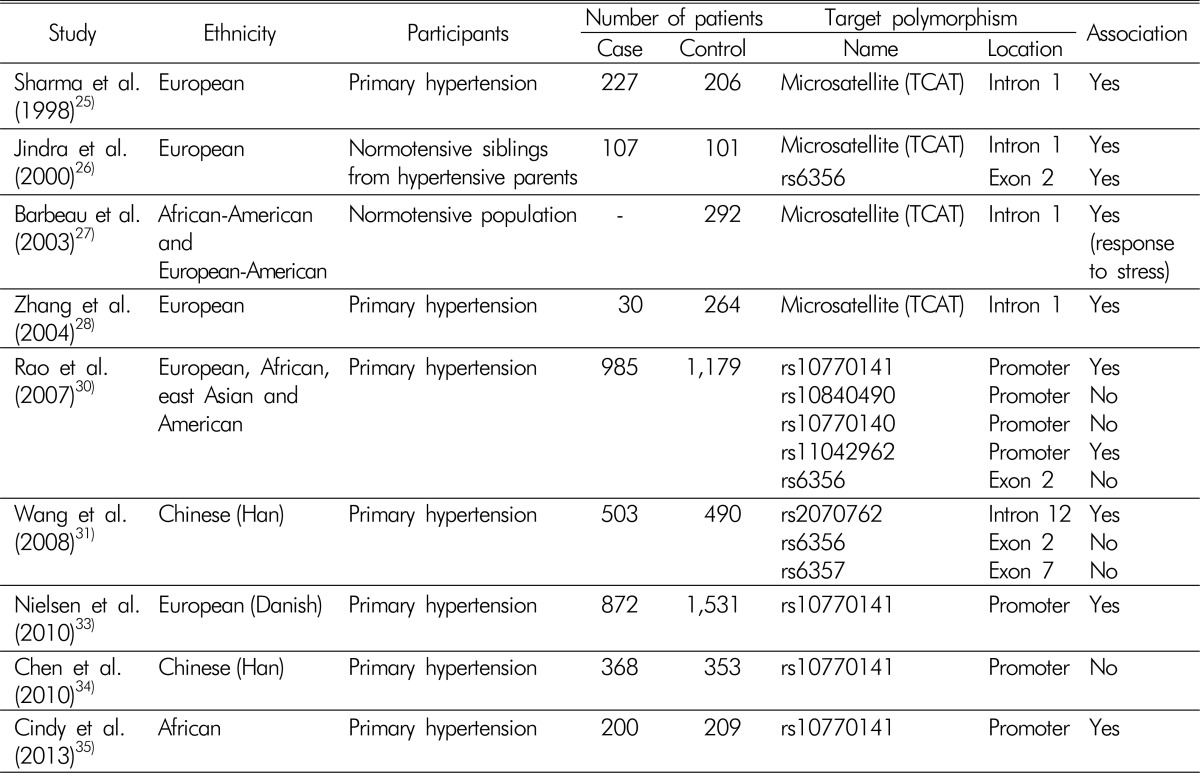
Association studies that investigated genetic variations of TH and hypertension are summarized in Table 1. The relationship between TH and hypertension was first reported in 1998. Sharma et al.25) investigated whether a tetranucleotide (TCAT) microsatellite repeat marker, located in the first intron of TH gene, was associated with hypertension. They analyzed 227 patients with primary hypertension and 206 age and gender-matched control subjects. Five different microsatellite alleles were revealed and designated as A through E, according to the number of short tandem repeats. They found that patients with hypertension were more likely to have allele E, and normotensive controls showed high proportions of allele D. Levels of plasma norepinephrine were higher in those with allele E, while they was lower in those with allele D, although those results did not reach statistical significance. The relationships among variations of the TCAT microsatellite, catecholamine secretion, and hypertension were replicated in other studies, which showed that genetic differences in TH could affect the development of hypertension through the regulation of catecholamine synthesis26,27,28). One of the major limitations of these studies was that they could not determine how an intronic polymorphism could result in the observed phenotypic changes. Nonetheless, those studies highlighted the importance of TH in the pathogenesis of hypertension.
Table 1. Association studies between genetic variations of tyrosine hydroxylase and hypertension.
Within the TH gene, single nucleotide polymorphism (SNP) rs6356 has been the most examined SNP in association studies, because it results in the substitution Val81Met, which is located in the regulatory domain of the tetrameric enzyme and seems to have an inhibitory effect on enzyme function29). However, rs6356 is located in the regulatory domain of TH, a region that is not as functionally critical as the catalytic domain, where all disease-causing mutations are clustered. The benign change caused by rs6356 is consistent with its high frequency in the general population. Indeed, another study showed no significant association of rs6356 with clinical manifestations30,31). Later, Rao et al.30) performed intensive investigations to assess the genetic variations of TH and their effects on hypertension. They analyzed the sequences of TH gene from 80 ethnically diverse individuals, and discovered 49 SNPs. Fourteen SNPs were found frequently (defined by a minor allele frequency >5%) in this population: four in the promoter region, two in coding regions, four in the untranslated regions, and four in introns. Functional studies revealed that SNPs within coding regions did not alter the phenotypes significantly, as assessed by catecholamine secretion and blood pressure response to cold stress. By contrast, the C-824T polymorphism (rs10770141), located in the promoter region and affecting binding sites of transcription factors MEF2, FOXD1, and SRY32), increased urinary norepinephrine excretion significantly and caused an increase in blood pressure in response to cold stress. These associations correlated with the minor allele (T). Thereafter, the effects of the C-824T polymorphism on the SNS were validated in two independent case-control studies, both of which comprised nearly 1,000 patients with hypertension. Encouraged by these findings, Nielsen et al.33) performed an additional study to confirm the relationship between the C824T polymorphism and hypertension with a different ethnic group. Analyses of 2,656 Danish subjects demonstrated that those with T/T allele were associated with higher prevalence of hypertension, reinforcing the contribution of SNP C-824T to the development of hypertension. Interestingly, the C-824T polymorphism showed high diversity across different ethnicities; Asians were among the least affected by the trait in the analyzed populations (16.7% for Asians versus 60.3% for Africans)30). A subsequent study demonstrated that the C-824T polymorphism was not associated with the incidence of hypertension in a Chinese population34), which contrasted with another study that showed a positive association between these two variables35). Therefore, functional variations of the promoter region of TH gene could explain the racial differences in sympathetic overactivity and the development of hypertension.
Another study to identify relevant SNPs in the TH gene was conducted in China31). Laiyuan et al. analyzed the presence of SNPs in the TH gene from 503 patients with hypertension and 490 age, gender, and area-matched normotensive controls. They searched HapMap data (http://www.hapmap.org/) and selected four candidate TH SNPs; the promoter region was excluded from the analysis. Among the four candidate SNPs, the minor allele of rs2070762, which is located within intron 12 of TH gene, was present at a higher frequency in the patients with hypertension compared with the controls. Further cell experiments demonstrated that the allelic change from the major to the minor allele of rs2070762 enhanced the transcriptional activity of a heterologous promoter in SH-SY5Y cells, suggesting the functional significance of the rs2070762 intronic SNP. However, an important limitation was that the levels of catecholamines in clinical samples were not reported in this study.
Therapeutic implications
Metyrosine, a potent inhibitor of TH, has been used to treat hypertension. However, the nonspecific nature of the TH inhibition induced by metyrosine resulted in several side effects, especially severe extrapyramidal neurological symptoms caused by dopamine depletion. Currently, metyrosine is contraindicated in patients with primary hypertension and the only clinical indication is preoperative management of pheochromocytoma to reduce hemodynamic instability during surgery and perioperative cardiovascular complications36). Theoretically, patients with hypertension caused by genetic variations of TH or sympathetic over-reactivity could be managed more effectively by the introduction of TH inhibitors. Selective inhibition of adrenal TH, without affecting brain cells, might be helpful for the clinical application of TH inhibitors to patients with primary hypertension.
Perspectives and Conclusions
Over the past two decades, TH has received significant research attention because of the identification of sympathetic hyperactivity as an important pathophysiological mechanism in the development of hypertension. The high prevalence of hypertension worldwide led physicians to discover several genomic variations of TH that are associated with the pathogenesis of hypertension. However, to date, these novel findings have not resulted in specific therapeutic interventions.
Nevertheless, revealing the genetic diversity of the TH gene will increase our understanding of the complex roles of sympathetic overactivity in the pathogenesis of hypertension, and will lead to more targeted interventions to manage patients with hypertension in the future.
Acknowledgements
All the authors declare no conflicts of interest.
References
- 1.Luft FC. Hypertension as a complex genetic trait. Semin Nephrol. 2002;22:115–126. doi: 10.1053/snep.2002.30211. [DOI] [PubMed] [Google Scholar]
- 2.Croft JB, Foster TA, Parker FC, Cresanta JL, Hunter SM, Webber LS, Srinivasan SR, Berenson GS. Transitions of cardiovascular risk from adolescence to young adulthood--the Bogalusa Heart Study: I. Effects of alterations in lifestyle. J Chronic Dis. 1986;39:81–90. doi: 10.1016/0021-9681(86)90064-0. [DOI] [PubMed] [Google Scholar]
- 3.Hunt SC, Hasstedt SJ, Kuida H, Stults BM, Hopkins PN, Williams RR. Genetic heritability and common environmental components of resting and stressed blood pressures, lipids, and body mass index in Utah pedigrees and twins. Am J Epidemiol. 1989;129:625–638. doi: 10.1093/oxfordjournals.aje.a115175. [DOI] [PubMed] [Google Scholar]
- 4.Williams RR, Hasstedt SJ, Hunt SC, Wu LL, Hopkins PN, Berry TD, Stults BM, Barlow GK, Kuida H. Genetic traits related to hypertension and electrolyte metabolism. Hypertension. 1991;17:I69–I73. doi: 10.1161/01.hyp.17.1_suppl.i69. [DOI] [PubMed] [Google Scholar]
- 5.Harrap SB. Hypertension: genes versus environment. Lancet. 1994;344:169–171. doi: 10.1016/s0140-6736(94)92762-6. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein DS. Plasma catecholamines and essential hypertension. An analytical review. Hypertension. 1983;5:86–99. doi: 10.1161/01.hyp.5.1.86. [DOI] [PubMed] [Google Scholar]
- 7.Mancia G, Grassi G. The autonomic nervous system and hypertension. Circ Res. 2014;114:1804–1814. doi: 10.1161/CIRCRESAHA.114.302524. [DOI] [PubMed] [Google Scholar]
- 8.Mancia G. Bjorn Folkow Award Lecture. The sympathetic nervous system in hypertension. J Hypertens. 1997;15:1553–1565. doi: 10.1097/00004872-199715120-00056. [DOI] [PubMed] [Google Scholar]
- 9.Grassi G. Role of the sympathetic nervous system in human hypertension. J Hypertens. 1998;16:1979–1987. doi: 10.1097/00004872-199816121-00019. [DOI] [PubMed] [Google Scholar]
- 10.Esler M, Lambert G, Jennings G. Regional norepinephrine turnover in human hypertension. Clin Exp Hypertens A. 1989;11(Suppl 1):75–89. doi: 10.3109/10641968909045414. [DOI] [PubMed] [Google Scholar]
- 11.Julius S, Krause L, Schork NJ, Mejia AD, Jones KA, van de Ven C, Johnson EH, Sekkarie MA, Kjeldsen SE, Petrin J, et al. Hyperkinetic borderline hypertension in Tecumseh, Michigan. J Hypertens. 1991;9:77–84. doi: 10.1097/00004872-199101000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Converse RL, Jr, Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F, Victor RG. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327:1912–1918. doi: 10.1056/NEJM199212313272704. [DOI] [PubMed] [Google Scholar]
- 13.Schobel HP, Fischer T, Heuszer K, Geiger H, Schmieder RE. Preeclampsia -- a state of sympathetic overactivity. N Engl J Med. 1996;335:1480–1485. doi: 10.1056/NEJM199611143352002. [DOI] [PubMed] [Google Scholar]
- 14.Grassi G, Seravalle G, Bertinieri G, Turri C, Dell'Oro R, Stella ML, Mancia G. Sympathetic and reflex alterations in systo-diastolic and systolic hypertension of the elderly. J Hypertens. 2000;18:587–593. doi: 10.1097/00004872-200018050-00012. [DOI] [PubMed] [Google Scholar]
- 15.Grassi G, Seravalle G, Dell'Oro R, Turri C, Bolla GB, Mancia G. Adrenergic and reflex abnormalities in obesity-related hypertension. Hypertension. 2000;36:538–542. doi: 10.1161/01.hyp.36.4.538. [DOI] [PubMed] [Google Scholar]
- 16.Grassi G, Quarti-Trevano F, Dell'oro R, Mancia G. Essential hypertension and the sympathetic nervous system. Neurol Sci. 2008;29(Suppl 1):S33–S36. doi: 10.1007/s10072-008-0882-9. [DOI] [PubMed] [Google Scholar]
- 17.Grassi G, Quarti-Trevano F, Seravalle G, Arenare F, Volpe M, Furiani S, Dell'Oro R, Mancia G. Early sympathetic activation in the initial clinical stages of chronic renal failure. Hypertension. 2011;57:846–851. doi: 10.1161/HYPERTENSIONAHA.110.164780. [DOI] [PubMed] [Google Scholar]
- 18.Grima B, Lamouroux A, Boni C, Julien JF, Javoy-Agid F, Mallet J. A single human gene encoding multiple tyrosine hydroxylases with different predicted functional characteristics. Nature. 1987;326:707–711. doi: 10.1038/326707a0. [DOI] [PubMed] [Google Scholar]
- 19.Nagatsu T. Tyrosine hydroxylase: human isoforms, structure and regulation in physiology and pathology. Essays Biochem. 1995;30:15–35. [PubMed] [Google Scholar]
- 20.Tank AW, Xu L, Chen X, Radcliffe P, Sterling CR. Post-transcriptional regulation of tyrosine hydroxylase expression in adrenal medulla and brain. Ann N Y Acad Sci. 2008;1148:238–248. doi: 10.1196/annals.1410.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kunugi H, Kawada Y, Hattori M, Ueki A, Otsuka M, Nanko S. Association study of structural mutations of the tyrosine hydroxylase gene with schizophrenia and Parkinson's disease. Am J Med Genet. 1998;81:131–133. [PubMed] [Google Scholar]
- 22.Souery D, Lipp O, Rivelli SK, Massat I, Serretti A, Cavallini C, Ackenheil M, Adolfsson R, Aschauer H, Blackwood D, Dam H, Dikeos D, Fuchshuber S, Heiden M, Jakovljevic M, Kaneva R, Kessing L, Lerer B, Lonnqvist J, Mellerup T, Milanova V, Muir W, Nylander PO, Oruc L, Mendlewicz J, et al. Tyrosine hydroxylase polymorphism and phenotypic heterogeneity in bipolar affective disorder: a multicenter association study. Am J Med Genet. 1999;88:527–532. doi: 10.1002/(sici)1096-8628(19991015)88:5<527::aid-ajmg17>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Chao HM, Richardson MA. Aromatic amino acid hydroxylase genes and schizophrenia. Am J Med Genet. 2002;114:626–630. doi: 10.1002/ajmg.10606. [DOI] [PubMed] [Google Scholar]
- 24.Bianchetti MG, Beretta-Piccoli C, Weidmann P, Ferrier C. Blood pressure control in normotensive members of hypertensive families. Kidney Int. 1986;29:882–888. doi: 10.1038/ki.1986.81. [DOI] [PubMed] [Google Scholar]
- 25.Sharma P, Hingorani A, Jia H, Ashby M, Hopper R, Clayton D, Brown MJ. Positive association of tyrosine hydroxylase microsatellite marker to essential hypertension. Hypertension. 1998;32:676–682. doi: 10.1161/01.hyp.32.4.676. [DOI] [PubMed] [Google Scholar]
- 26.Jindra A, Jachymova M, Horky K, Peleska J, Umnerova V, Bultas J, Savlikova J. Association analysis of two tyrosine hydroxylase gene polymorphisms in normotensive offspring from hypertensive families. Blood Press. 2000;9:250–254. doi: 10.1080/080370500448623. [DOI] [PubMed] [Google Scholar]
- 27.Barbeau P, Litaker MS, Jackson RW, Treiber FA. A tyrosine hydroxylase microsatellite and hemodynamic response to stress in a multi-ethnic sample of youth. Ethn Dis. 2003;13:186–192. [PubMed] [Google Scholar]
- 28.Zhang L, Rao F, Wessel J, Kennedy BP, Rana BK, Taupenot L, Lillie EO, Cockburn M, Schork NJ, Ziegler MG, O'Connor DT. Functional allelic heterogeneity and pleiotropy of a repeat polymorphism in tyrosine hydroxylase: prediction of catecholamines and response to stress in twins. Physiol Genomics. 2004;19:277–291. doi: 10.1152/physiolgenomics.00151.2004. [DOI] [PubMed] [Google Scholar]
- 29.Kumer SC, Vrana KE. Intricate regulation of tyrosine hydroxylase activity and gene expression. J Neurochem. 1996;67:443–462. doi: 10.1046/j.1471-4159.1996.67020443.x. [DOI] [PubMed] [Google Scholar]
- 30.Rao F, Zhang L, Wessel J, Zhang K, Wen G, Kennedy BP, Rana BK, Das M, Rodriguez-Flores JL, Smith DW, Cadman PE, Salem RM, Mahata SK, Schork NJ, Taupenot L, Ziegler MG, O'Connor DT. Tyrosine hydroxylase, the rate-limiting enzyme in catecholamine biosynthesis: discovery of common human genetic variants governing transcription, autonomic activity, and blood pressure in vivo. Circulation. 2007;116:993–1006. doi: 10.1161/CIRCULATIONAHA.106.682302. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Li B, Lu X, Zhao Q, Li Y, Ge D, Li H, Zhang P, Chen S, Chen R, Qiang B, Gu D. A functional intronic variant in the tyrosine hydroxylase (TH) gene confers risk of essential hypertension in the Northern Chinese Han population. Clin Sci (Lond) 2008;115:151–158. doi: 10.1042/CS20070335. [DOI] [PubMed] [Google Scholar]
- 32.Zhang K, Zhang L, Rao F, Brar B, Rodriguez-Flores JL, Taupenot L, O'Connor DT. Human tyrosine hydroxylase natural genetic variation: delineation of functional transcriptional control motifs disrupted in the proximal promoter. Circ Cardiovasc Genet. 2010;3:187–198. doi: 10.1161/CIRCGENETICS.109.904813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielsen SJ, Jeppesen J, Torp-Pedersen C, Hansen TW, Linneberg A, Fenger M. Tyrosine hydroxylase polymorphism (C-824T) and hypertension: a population-based study. Am J Hypertens. 2010;23:1306–1311. doi: 10.1038/ajh.2010.165. [DOI] [PubMed] [Google Scholar]
- 34.Chen L, Chen X, Shi R, Guo Y, Chen A, Bai Y, Chen J, Yang T, Zhang G. [Genetic polymorphism in tyrosine hydroxylase gene and essential hypertension in Hunan Han population] Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010;35:826–832. doi: 10.3969/j.issn.1672-7347.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 35.van Deventer CA, Louw R, van der Westhuizen FH, Vorster CB, Malan L. The contribution of the C-824T tyrosine hydroxylase polymorphism to the prevalence of hypertension in a South African cohort: the SABPA study. Clin Exp Hypertens. 2013;35:614–619. doi: 10.3109/10641963.2013.776569. [DOI] [PubMed] [Google Scholar]
- 36.Wachtel H, Kennedy EH, Zaheer S, Bartlett EK, Fishbein L, Roses RE, Fraker DL, Cohen DL. Preoperative metyrosine improves cardiovascular outcomes for patients undergoing surgery for oheochromocytoma and paraganglioma. Ann Surg Oncol. 2015;22(Suppl 3):S646–S654. doi: 10.1245/s10434-015-4862-z. [DOI] [PubMed] [Google Scholar]