Abstract
Objective
To examine differences in inflammation markers in sexually active vs. abstinent women, and observe changes in inflammation markers across the menstrual cycle. Cycle-related immune fluctuations may have evolved to reduce interference with conception. If so, reproductively active (i.e., sexually active) women should show the most variability in cytokine expression.
Design
Participants provided serum samples at menses and ovulation (from which cytokines were assayed) and saliva samples at menses, follicular, ovulation, and luteal phases (from which C-reactive protein was assayed). Participants self-reported intercourse frequency during the study.
Setting
Academic research laboratory.
Participants
32 healthy, naturally cycling premenopausal women (sexually active, N = 15, abstinent, N = 17).
Interventions
Observational study.
Main outcome measures
Pro-inflammatory cytokines (Interleukin-6, IL-6; Interferon γ, IFN-γ; Tumor Necrosis Factor α, TNF-α), an anti-inflammatory cytokine (Interleukin-4, IL-4), and a marker of total inflammation (C-reactive protein, CRP).
Results
Sexually active women had higher levels of all of the immune markers measured, including both pro- and anti-inflammatory cytokines, than abstinent women. Relative to sexually active women, abstinent women had less change across the menstrual cycle in levels of CRP. Among sexually active women, higher intercourse frequency predicted greater mid-cycle decreases in CRP, IL-6, and IFN-γ and mid-cycle increases in IL-4.
Conclusions
Sexual activity may stimulate a complex interaction between pro- and anti-inflammatory cytokines that subsequently drive mid-cycle declines in inflammation.
Keywords: inflammation, sexual activity, menstrual, C-reactive protein, Interleukin-6, cytokine, Interferon-γ, Tumor Necrosis Factor-α, Interleukin-4
Introduction
Inflammation is a critical process in the immune response, as it is the first-line defense against pathogens, tissue healing or remodeling, and toxin containment and removal. Variations in inflammation drive variations in symptoms such as fatigue (1), pain (2) and depression (3). In premenopausal women, inflammation varies significantly across the menstrual cycle (4). Several studies have documented mid-cycle decreases in markers of inflammation, with higher levels at menses (5, 6) and a nadir around ovulation (4, 7). Such variation has important implications for research and clinical interpretation of inflammation biomarkers. One study examined values of C-reactive protein (CRP), a marker of inflammation commonly used to index heart disease risk, and found that failure to account for menstrual cycle-related variability doubled chances of misclassification of heart disease risk in a large sample of healthy women (8).
The curvilinear pattern in C-reactive protein has been attributed to a fundamental tradeoff between reproduction and immune response: for conception to occur, the female immune system must provide a conducive environment for sperm and conceptus. Local inflammation can impair sperm motility (9) and make the uterine environment more hostile to implantation (10). More broadly, inflammation in general circulation can interfere with conception in two significant ways. Firstly, systemic inflammation can signal a potential infection or injury, which would divert energetic resources from reproduction to somatic maintenance or defense (11–13). Secondly, the female reproductive tract uses inflammatory signals such as cytokines to coordinate ovulation and implantation (14); in the presence of high systemic inflammation, however, these small, local inflammation signals fail to appropriately trigger the processes leading to ovulation or implantation (10, 15) – potentially leading to poorer rates of conception.
Thus, it is argued, a mid-cycle decrease in systemic inflammation – corresponding to peak fertility – may have evolved to reduce potential disruption of reproduction (11). If so, it would follow that these effects would be most critical (and most subject to evolutionary selective pressure) in individuals who are reproductively active, i.e., regularly engaging in sexual activity. Indeed, several studies suggest that immune parameters, including inflammatory cytokines, differ in sexually active vs. abstinent women (16–18), with sexually active women showing more variation in immune markers across the menstrual cycle than abstinent women (13, 19, 20). Thus, we would expect that inflammation would differ significantly across the menstrual cycle in sexually active, but not abstinent, women.
We examined this hypothesis by comparing changes in pro- and anti-inflammatory cytokines as well as CRP across a menstrual cycle in women who were abstinent, and women who were sexually active with a partner. We assessed four primary cytokines: Interferon-γ (IFNγ), Tumor Necrosis Factor-α (TNFα), Interleukin-6 (IL-6), and Interleukin-4 (IL-4). The first three are commonly measured as an index of inflammation signaling (21, 22), while IL-4 is considered an “anti-inflammatory” cytokine (23). We included an anti-inflammatory cytokine as there is some work suggesting sexual activity may influence inflammation through shifting T-helper cell profiles (20). In addition to their important roles in coordinating inflammation, IFNγ, TNFα and IL-6 interact with the central nervous system, supporting an suite of behavioral effects related to sickness (e.g., loss of appetite, decreased motivation for social interaction) (24). More specifically, IFNγ is predominantly produced by natural killer cells and cytotoxic T cells (25) and is a potent activator of macrophages, which in turn induce local inflammation(26). TNFα is produced primarily by activated macrophages and mast cells, and stimulates the release of histamines and other inflammation-causing agents(27). Both TNFα and IFNγ have been identified as particularly predictive of early pregnancy loss(28, 29) due to their role in stimulating natural killer cells in the uterus(28). Thus, suppressing these pro-inflammatory cytokines could improve the chances of sexual activity leading to offspring. IL-6 is widely used by a variety of immune actors such as T-cells and macrophages, and is an important mediator of inflammation and acute phase response (see, however, Schindler et al.(30) for discussion of IL-6 anti-inflammatory effects via suppression of IFNγ producing cells). Finally, CRP is an acute phase protein induced by the liver in response to cytokines such as IL-6 and has a relatively short half-life (~48 hours); as such, it is a good index of total current inflammation across the body (13).
There have been several studies investigating menstrual variations in cytokines and CRP. In healthy non-pregnant women, higher levels of IL-4 have observed in the luteal phase relative to the follicular phase (20, 31). Studies of IFNγ, TNFα and IL-6 have had more mixed findings, with some studies documenting decreases from early to mid- or late-cycle (32–34) and others increases (35, 36) or no change (37). Similarly, studies of menstrual cycle variations of CRP have been mixed, with some showing a mid-cycle nadir and others a mid-cycle peak or no change (4, 6, 7, 38, 39). The variability in findings may be due to lack of consideration of the timing (or even occurrence) of ovulation. The largest and best-controlled study to date, which accounted for differences in luteal phase progesterone (P4) in ovulatory and anovulatory cycles, found that markers of inflammation were lowest at ovulation, and rose during the luteal phase(4). Similarly, confusion across studies may occur due to lack of consideration of sexual activity. A recent study found that among women with ovulatory cycles, being sexually active (dichotomized as yes/no) was associated with a U-shaped pattern of serum CRP while sexual abstinence was associated with no significant change across the cycle (13); unfortunately, this study did not measure the frequency of sexual activity nor cytokine concentrations.
In the present study, our primary analysis compared women by sexual activity status (currently sexually active vs. abstinent). As a secondary analysis, we also considered the potential effect of frequency of sexual activity (within sexually active women only), as this may reflect how the immune system interprets sexual activity as a reflection of reproductive state and, by extension, the need for flexibility in how it interprets the “self”. One of the most puzzling questions in immunology is how the immune system adapts its responsiveness over time, maintaining vigilance while also learning tolerance of elements that are different from the original self but not harmful (e.g., commensurate microbes, the body’s own cells as it grows and changes over time). One recent theory addresses this problem by introducing the concept of the “liquid self”, a flexible state that adapts the distinction between self and non-self over time. The individuals’ life history and reproductive state are particularly important in how the immune system considers the self, increasing the dynamicity of potential responses (40). For example, a fetus is non-self and thus could potentially trigger an inflammatory response; however, these cells are tolerated by the maternal immune system due the flexible immune response associated with the reproductive state of pregnancy. Similarly, there may be different responses during the fertile window of the menstrual cycle vs. non-fertile times. Finally, immune responsivity includes regulatory processes; a stimulus can induce either a pro- or anti-inflammatory response. For example, glucocorticoids such as cortisol are thought to be anti-inflammatory; however, as predicted by the above theories, the anti-inflammatory response to glucocorticoids in one reproductive phase would be expected to differ from that experienced in other phases.
We conceptualized that sexual activity may suppress inflammation to reduce immune interference with conception. Sexual activity may provide a signal of potential to reproduce to the immune system, which responds by down-regulating inflammatory responses around ovulation. Liquid self theory would predict that the degree of immune response to sexual activity (to the extent that it occurs at all) would indicate something about how the immune system interprets that stimulus. One possibility is that the transition from sexual inactivity to activity would be interpreted as moving from one reproductive state to another. As long as a woman remains sexually active to some degree, the immune system continues provide a stable degree of response. If so, we should expect that all women who have been consistently sexually active to have similar patterns of immune response, regardless of sexual frequency. This would further suggest that the effect of sexual activity on immune response is tied to a discrete mechanism (such as changing the likelihood of ovulation). Another possibility is that each act of sexual activity is considered separately. Each act of intercourse would further amplify the immune system’s interpretation of the reproductive state as potentially conceptive, increasing its flexibility across the menstrual cycle. In this case, we should expect different immune responses among women who are frequently vs. infrequently sexually active. This would further suggest the effect of sexual activity on immune response is graded, such as endocrine changes associated with each act of intercourse. In short, testing if frequency of sexual activity changes the degree of immune response (rather than simply sexual activity status) may shed light on how the immune system interprets sexual activity and possible mechanisms for these effects.
Taking together the findings on the association between sexual activity and immunity in women, and the changes in cytokines and C-reactive protein across the menstrual cycle, we hypothesized that, in a sample of women with confirmed ovulation, sexual activity would moderate cycle-related variations in inflammation. Specifically, women reporting higher frequency of sexual activity should show greater mid-cycle decrease in pro-inflammatory cytokines and CRP, as well as greater mid-cycle increase in anti-inflammatory cytokines. In contrast, abstinent women should show less change over the menstrual cycle in all markers of inflammation.
Methods
Participants
Participants were recruited from the community via flyers and online advertisements, and screened over the phone. General inclusion criteria included: self-reported good health, regularly cycling (menses every 26–34 days with no more than one missed period in the past six months). General exclusion criteria included: use of hormonal medications (including oral contraceptives), self-reported use of anti-inflammatory or immunoactive medications, current illness or allergies, history of medical conditions known to impact immune response (e.g., cancer), cigarette use, and pregnancy or lactation within the last six months. Currently sexually active participants were restricted to women reporting regularly engaging in intercourse at least once per week with a one and only one partner. Women taking hormonal contraception were excluded, and thus sexually active participants were included only if they reported either consistent condom use or non-hormonal intrauterine devices as contraception. As further confirmation, all participants completed a commercially available pregnancy test (OneStep hCG Test, BlueCross Biomedical, Beijing, China) at intake and at the mid-study lab session; no woman was found to be pregnant at any point during the trial. Women who reported trying to conceive were excluded. Abstinent participants were restricted to participants reporting no partnered genital sexual activity in the last four months; however, individuals reporting lifetime sexual history were included. All participants provided informed consent and study procedures were approved by the Indiana University Institutional Review Board.
Data collection
General procedures
Participants (N = 32, sexually active, N = 15, abstinent, N = 17) were scheduled for two lab sessions, one within two days of menses onset and one within two days of ovulation (periovulatory). Ovulation was estimated from the participant’s onset of menses and typical cycle length via backwards counting (41) and confirmed via commercially available urine test (OneStep Urine Ovulation Test, BlueCross Biomedical, Beijing, China) performed two days prior and two days following the expected date of ovulation. At both study visits, participants were measured for body composition with a floor scale (FitScale 585F, Tanita Corporation, Illinois USA).
Blood sampling
Each woman provided two blood samples, at menses and at ovulation (i.e., during lab visits). Blood was collected via standard venipuncture techniques, drawing blood from the anterior cubital fossa (a vein in the inner elbow). Whole blood was collected into uncoated glass tubes, allowed to coagulate at room temperature for 45 minutes, and spun down. Serum was drawn and aliquotted into individual cyrovials, and stored at −80C until analysis. Serum was used for cytokine assays.
Saliva sampling
Each woman provided four saliva samples, at menses, follicular, ovulation and luteal phases. The menses and ovulation samples were collected at lab visits. In addition, participants collected two saliva samples at home during their mid-late follicular phase (7–10 days following onset of menses), and luteal phase (7–10 days following ovulation). At-home samples were frozen in the participant’s home freezer immediately following collection, and transported frozen to the laboratory in Styrofoam boxes lined with ice packs (41). Saliva samples were collected via passive drool into a polypropylene tube (42). Participants were asked not to eat, drink, or chew gum one hour prior to collection. Like serum, saliva samples were stored at −80°C until analysis; no sample was subjected to more than two freeze-thaw cycles. Saliva was used for CRP assay.
Survey data
In the first lab session, participants completed demographics questionnaires, and reported on their sexual history including typical frequency of partnered and partnered sexual activity. At home, participants completed an online survey following each partnered sexual event. From these reports, frequency of intercourse events was tallied by phase, that is, within 7 – 10 days of the menses, follicular, ovulation and luteal phase samples. No sexual event was tallied under multiple phases; activity reported on the cusp of phases was counted in the later phase. These data were used for analyses within the sexually active group only (see below).
Cytokine and CRP assay
Cytokines
Unstimulated cytokine concentrations in serum were determined by enzyme-linked immunosorbent assay (ELISA) using procedures recommended by kit manufacturers (Cytoset kits from Invitrogen Corporation, Maryland, USA). Intra-assay and inter-assay coefficient of variances were 2.1 – 11.4%, and 9.2 – 18.5%, respectively. The lower limit of detection for assays were as follows: IFNγ: 3.9 pg/mL, TNFα: 1.7 pg/mL, IL-6: 2.0 pg/mL, IL-4: 2.0 pg/mL. As is typical for unstimulated samples in young, healthy individuals (43), there was a relatively high rate of undetectably low cytokine values: IFNγ: 46%, TNFα: 82%, IL-6: 67%, IL-4: 56%. Given the rate of non-detection was high, and potentially related to the phenomenon of interest, we separately modeled predictors of whether or not the cytokine was detected (i.e., missingness analysis (44, 45)), and predictors of absolute values. This provided two types of information: first, the predictors of processes by which cytokines become stimulated (that is, detection vs. being too low to detect), and second, the predictors of processes that moderate or amplify cytokine response (that is, absolute levels of cytokine concentration).
C-reactive protein
CRP was determined from saliva. We were able to sample saliva at more timepoints than serum, giving us greater time resolution for our index of total inflammation. There is a strong association between serum and salivary CRP across multiple assay techniques (46–49). Salivary CRP reliably distinguishes healthy individuals from patients with inflammation conditions (50, 51) and between high and low serum CRP (52). Salivary CRP was determined by ELISA using procedures recommended by kit manufacturers (Salimetrics LLC, Pennsylvania, USA). Intra-assay and inter-assay coefficient of variances were low (.02 – 14.9%, and 13–15%, respectively); lower detection limit was 10 pg/mL. One CRP value was > 5 standard deviations from the mean and excluded as an outlier.
Results
Participant characteristics
The majority of participants were White (68%), with 16% Asian, and 16% mixed race or other. Participants were predominantly heterosexual (97%), with an average age of 23.57 (SD = 5.61) and an average of 15.74 years of education. The average body mass index and percent body fat was 23.61 (SD = 3.94) and 27.32% (SD = 7.78%), respectively. As both age and body composition are key predictors of inflammation generally (53) and CRP and pro-inflammatory cytokine expression specifically(54), we included age and percent body fat as covariates in the analyses below. However, groups did not differ on percent body fat (sexually active M = 27.64%, SD = 5.66; abstinent M = 26.02%, SD = 8.67, t(30) = 0.61, p = 0.546) nor age (sexually active M =24.96, SD = 7.22; abstinent active M = 22.16, SD = 2.92, t(30) = 1.47, p = 0.151). Similarly, sexually active and abstinent participants were not significantly different in terms of race/ethnicity or sexual orientation (all ps > 0.1).
Sexually active women were all in a relationship, while only three abstinent women reported a (non-sexual) dating partner; this difference was significant, χ2(30)= 27.47, p < 0.001. As relationship status was co-linear with sexual activity status, we could not control for this difference between groups; this limitation is further considered in the Discussion. There was no significant difference in frequency of masturbation between abstinent (M =1.65x/week, SD = 1.57) and sexually active women (M =1.80x/week, SD = 2.43), F(1,28) = 0.03, p = 0.856.
Mean frequency of intercourse events reported on sexual event diaries was 6.67x/cycle (range = 1–18). The effect of menstrual cycle phase on frequency of sexual events was non-significant (F(1,3) = 0.08, p = 0.96) indicating women did not report more sexual events around ovulation. During recruitment, we screened out women who reported trying to conceive; however, sexual event diaries revealed that women who had reported regular condom use during screening did not always use condoms for every act of intercourse. Six participants reported using condoms on every sexual event diary that included intercourse, five reported no condom use, and four reported condom use on some but not all intercourse events. The effect of menstrual cycle phase on condom use was non-significant (χ2(6) = 7.01, p = .320), indicating women were not selectively using condoms only around ovulation. The present sample was underpowered to detect differences between condom users and non-users. However, given the potential for exposure to ejaculate as one of the mechanisms by which women’s immune system may respond to sexual activity, we conducted exploratory analyses of condom use as a predictor of change in inflammatory markers across the menstrual cycle; these analyses are presented in Supplemental Digital Content 1.
Change in cytokines across cycle
For each cytokine, we first conducted a generalized estimating equation model of detection, which characterized factors that predicted if cytokine values were above or below the limit of detection. These analyses considered a binary outcome variable (detection vs. non-detection, coded as 0 and 1). We then conducted linear mixed models of cytokine values, which characterized factors that predicted the absolute levels of cytokines. These analyses considered a continuous outcome variable, with values below the limit of detection coded as missing. The number of data points for detection analyses were the same across models (namely, 64, corresponding to 2 timepoints in 32 participants); however, available data for continuous cytokine value analyses depended on how many values were detected (IL6: 22 data points from 13 participants; IFN-γ: 35 data points from 22 participants; TNF-α: 12 data points from 6 participants; IL4: 29 data points from 17 participants; CRP: 126 data points from 31 participants).
In both types of models (Table 1), we used time point (menses, ovulation) as a repeated measures variable, group (sexually active vs. abstinent) and the interaction of time and group as predictors, and age and percent body fat as covariates. In both sets of models we specified an unstructured repeated measures covariance. Estimates of effect sizes (ϕ and Cohen’s local f2) were calculated following standard recommendations (55, 56).
Table 1.
Parameter estimates for analyses of detection and continuous levels of inflammation markers by cycle phase (time) and group (sexually active vs abstinent).
| Detectiona | Continuous values | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| B | SE(B) | p | ϕ | Effect estimate | SE | p | f2 | Ndata (%)h | |
| IL-6 | N = 22 (33.33%) | ||||||||
|
| |||||||||
| Intercept | 0.59 | 1.14 | 0.60 | 0.04 | 963.65 | 501.88 | 0.07 | 0.20 | |
| Age | −0.03 | 0.03 | 0.42 | 0.11 | 5.60 | 10.30 | 0.60 | 0.02 | |
| Body fat % | −0.01 | 0.02 | 0.58 | 0.04 | −24.12 | 13.71 | 0.10 | 0.14 | |
| Time b | 0.59 | 0.29 | 0.04 | 0.24 | −321.41 | 230.57 | 0.19 | 0.26 | |
| Sexual activity group c | 1.33 | 0.50 | 0.01 | 0.35 | −96.50 | 51.41 | 0.09 | 0.14 | |
| Time x Sex activity group d | −0.33 | 0.38 | 0.38 | 0.10 | 139.68 | 108.78 | 0.23 | 0.14 | |
|
| |||||||||
| TNF-α | N = 12 (18.8%) | ||||||||
|
| |||||||||
| Intercept | −0.14 | 1.67 | 0.94 | 0.02 | 74.99 | 130.28 | 0.57 | 0.16 | |
| Age | −0.06 | 0.04 | 0.15 | 0.20 | 7.07 | 3.42 | 0.05 | 0.32 | |
| Body fat % | 0.07 | 0.04 | 0.10 | 0.24 | −3.47 | 3.02 | 0.26 | 0.04 | |
| Time b | 0.10 | 0.11 | 0.36 | <0.01 | −117.69 | 52.41 | 0.04 | 0.21 | |
| Sexual activity groupc | 1.49 | 0.75 | 0.05 | 0.25 | −14.93 | 18.57 | 0.44 | 0.03 | |
| Time x Sex activity groupd | −0.11 | 0.11 | 0.35 | 0.02 | 45.15 | 25.74 | 0.10 | 0.15 | |
|
| |||||||||
| IFN-γ | N = 35 (54.7%) | ||||||||
|
| |||||||||
| Intercept | 0.48 | 1.17 | 0.68 | 0.06 | 74.99 | 130.28 | 0.57 | 0.03 | |
| Age | −0.03 | 0.03 | 0.31 | 0.12 | 7.07 | 3.42 | 0.05 | 0.21 | |
| Body fat % | −0.02 | 0.03 | 0.43 | 0.12 | −3.47 | 3.02 | 0.26 | 0.07 | |
| Time b | 0.97 | 0.39 | 0.01 | 0.33 | −117.69 | 52.41 | 0.04 | 0.25 | |
| Sexual activity group c | 0.53 | 0.51 | 0.29 | 0.12 | −14.93 | 18.57 | 0.44 | 0.04 | |
| Time x Sex activity group d | −0.52 | 0.46 | 0.26 | 0.16 | 45.15 | 25.74 | 0.10 | 0.16 | |
|
| |||||||||
| IL-4 | N = 29 (45.3%) | ||||||||
|
| |||||||||
| Intercept | 0.18 | 1.26 | 0.89 | 0.01 | −161.84 | 308.77 | 0.61 | 0.01 | |
| Age | 0.00 | 0.04 | 0.99 | <0.01 | 31.35 | 8.54 | 0.00 | 0.45 | |
| Body fat % | −0.01 | 0.03 | 0.65 | 0.05 | −9.21 | 7.34 | 0.22 | 0.07 | |
| Time b | 0.09 | 0.27 | 0.73 | <0.01 | −222.50 | 119.40 | 0.08 | 0.17 | |
| Sexual activity group c | 0.23 | 0.47 | 0.62 | 0.06 | 18.93 | 29.81 | 0.54 | 0.03 | |
| Time x Sex activity group d | 0.37 | 0.36 | 0.31 | 0.16 | −40.74 | 46.75 | 0.40 | 0.06 | |
|
| |||||||||
| C-reactive protein e | N = 126 (90%) | ||||||||
|
| |||||||||
| Intercept | 7.07 | 1.37 | 0.00 | 0.40 | |||||
| Age | 0.01 | 0.02 | 0.65 | <0.01 | |||||
| Body fat % | 0.06 | 0.04 | 0.22 | 0.05 | |||||
| Time = menses f | 0.25 | 0.14 | 0.08 | 0.11 | |||||
| Time = mid-late follicular | −0.06 | 0.11 | 0.57 | 0.01 | |||||
| Time = ovulation | 0.04 | 0.17 | 0.83 | <0.01 | |||||
| Sexual activity group | −0.56 | 0.58 | 0.34 | 0.03 | |||||
| Time = menses x Sex activity group g | −0.14 | 0.18 | 0.44 | 0.02 | |||||
| Time = follicular x Sex activity group | −0.01 | 0.14 | 0.97 | <0.01 | |||||
| Time = ovulation x Sex activity group | −0.12 | 0.23 | 0.61 | 0.01 | |||||
Dichotomous variable: 0 (detected), 1 (below limit of detection)
Reference category: T1 (menses)
Reference category: T1 (menses)*sexually activity group; sexually abstinent group was referent
Reference category: sexually abstinent
No detection analyses were conducted for C-reactive protein values
Reference category: T4 (luteal)
Reference category: T4 (luteal)*sexually active; sexually abstinent group were coded as 0
Number of participants with data (% of total data available)
For models showing a significant effect of sexual activity status, we then conducted follow-up analyses to evaluate the effect of frequency of intercourse. Frequency of sexual intercourse was treated as a continuous variable. As all abstinent women would have an intercourse frequency of 0 (creating a heavily skewed distribution), we restricted frequency analyses to sexually active women only.
Interleukin-6
There was a significant increase in the number of detectable IL-6 values from menses to ovulation (χ2 (1) = 4.96, p = 0.03). However, the main effect of time on continuous values of IL-6 was not significant, F(1, 10.57) = 1.05, p = 0.33.
There was a significant effect of group (χ2 (1) = 5.63, p = 0.018) on detection such that sexually active women were significantly more likely to have detectable IL-6 values than abstinent women. Similarly, there was a significant effect of group on continuous IL-6 values (F(2, 12.81) = 4.47, p = 0.034), with sexually active women showing significantly higher IL-6 levels than abstinent women.
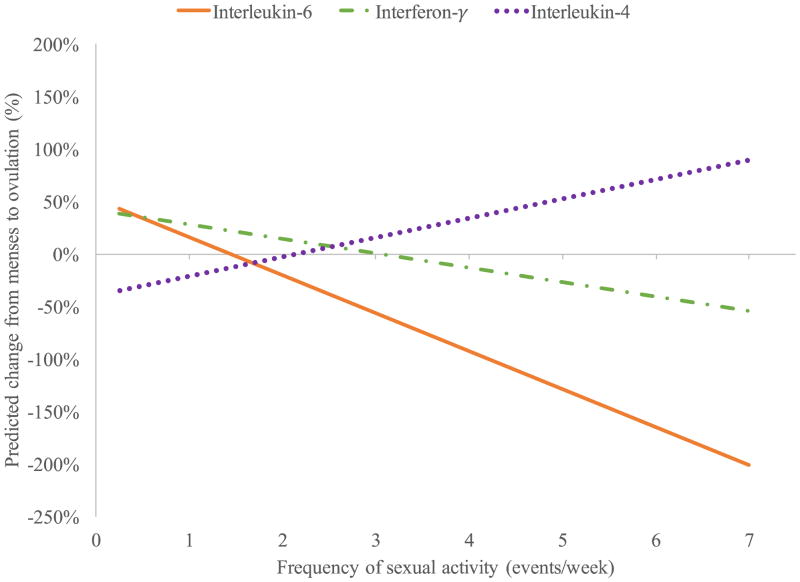
Follow-up analyses within the sexually active women only revealed a significant interaction between time and frequency of intercourse in predicting continuous IL-6 values (F(1, 6.80) = 167.13, p < 0.001). Higher frequency of intercourse was associated with greater decreases in IL6 from menses to ovulation (see Figure 1).
Figure 1.
Interaction between menstrual cycle phase and frequency of intercourse in predicting Interleukin-6, Interleukin-4, and Interferon-γ. Predicted change controls for individual differences in age and body; positive % change indicates increase from menses to ovulation while negative % change indicates decrease from menses to ovulation.
Interferon-γ
There was a significant increase in the number of detectable IFN-γ values from menses to ovulation (χ2 (1) = 9.50, p = 0.002). However, the effect of time on IFN-γ values was not significant, F(1, 14.27) = 0.002, p = 0.967.
Sexual activity group did not predict detection of IFN-γ (χ2 (1) = 9.50, p = 0.29). It did predict continuous values of IFN-γ (F(2, 19.23) = 4.82, p = 0.02), with abstinent women showing significantly lower levels of IFN-γ than sexually active women.
Among the sexually active women only, follow-up analyses revealed a significant interaction between time and frequency of intercourse in predicting continuous levels of IFN-γ (F(1, 1.89) = 29.99, p = 0.036). As with IL6, higher frequency of intercourse was associated with greater decreases in IFN-γ from menses to ovulation (Figure 1).
Tumor Necrosis Factor-α
There was no significant change over time in either detection (χ2 (1) = 0.72, p = 0.40) or continuous values of TNF-α (F(1, 5.67) = 1.35, p = 0.29). The effect of sexual activity group on detection of TNF-α was marginally significant, χ2 (1) = 3.79, p = 0.052, with sexually active women more likely to have detectable values than abstinent women. There was no significant effect of group on continuous TNF-α values F(2, 7.61) = 1.65, p = 0.253.
Interleukin-4
Finally, there was no significant main effect of time in detection of IL-4 (χ2 (1) = 2.32, p = 0.128) nor continuous values of IL-4 (F(1,12.39) = 0.06, p = 0.804).
Sexual activity group did not significantly predict detection of IL-4 (χ2 (1) = 0.95, p = 0.330). Group did significantly predict continuous values of IL-4 (F(2, 17.25) = 9.65, p = 0.002), however, with sexually active women showing higher levels of IL-4 than abstinent women.
Follow-up analyses among sexually active women only revealed a significant interaction between time and frequency of intercourse, F(1, 6.90) = 43.40, p < 0.001. Higher frequency of intercourse was associated with greater increases in IL-4 from menses to ovulation (Figure 1).
C-reactive protein
As there were few CRP values below the limit of detection, we did not conduct detection analyses for CRP. As there were four saliva samples per cycle (at menses, mid-late follicular, ovulation, and luteal phases), each woman had four CRP values. This meant that the effect of time could potentially be non-linear; indeed, given the above-cited research showing a curvilinear pattern of CRP across the menstrual cycle, we expected a non-linear effect of time. To better model potentially non-linear effects over time, we conducted a repeated measures MANCOVA, with time and sexual activity group as predictors, controlling for age and body fat percent. The outcome variable, salivary CRP levels, was natural-log transformed to reduce non-normality.
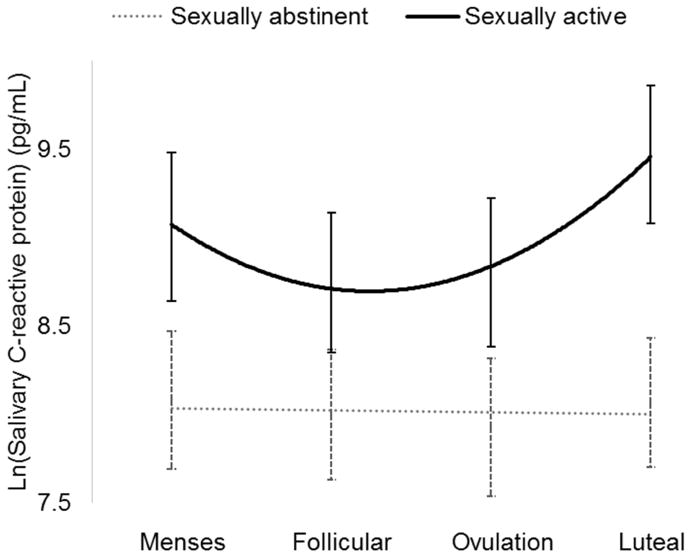
There was a significant interaction of the quadratic (i.e., curvilinear) effect of time and sexual activity group, F(1, 26) = 8.41, p = 0.008 (Figure 2). Abstinent women had generally lower CRP values than did sexually active women. Also, there was little change in CRP across the menstrual cycle in abstinent women. In sexually active women, however, there was significant variability over time, with higher levels at menses and in the luteal phase, and a nadir at mid-cycle. Finally, follow-up analyses among sexually active women only revealed no significant interaction between time and frequency of intercourse (F(1, 12) = 3.38, p = 0.096). In other words, women who engaged in intercourse once a week had similar patterns of change in CRP as women who engaged in intercourse every day.
Figure 2.
Interaction between menstrual cycle phase and frequency of intercourse in predicting C-reactive protein (CRP). CRP values are estimated marginal means from analysis.
Discussion
This was the first study to examine differences in menstrual cycle-related change in markers of inflammation between abstinent and sexually active women. In general, abstinent women had significantly lower levels of inflammation markers than did sexually active women. Among women who were sexually active, there was a significant interaction between cycle phase and frequency of intercourse, with higher frequency of intercourse associated with greater cycle-related change. That is, higher sexual frequency was associated with greater decreases in IL-6 and IFN-γ (pro-inflammatory cytokines) from menses to ovulation. Similarly, higher sexual frequency was associated with greater decreases in IL-4 (an anti-inflammatory cytokine). Finally, there was a significant group-wise difference between cycle-related change in CRP (a marker of total inflammation). Abstinent women had little change in CRP over time, but sexually active women showed a curvilinear pattern with high CRP at early and late-cycle, and lowest CRP at ovulation. Taken together, these findings suggest that sexual activity may contribute to a complex interaction between pro- and anti-inflammatory cytokines, which ultimately direct mid-cycle decline in inflammation in sexually active women.
Among sexually active women, there was an interaction between reproductive phase and frequency of sexual activity across four of the five measured markers of inflammation. The higher the frequency of intercourse, the greater the mid-cycle decline in pro-inflammatory markers (and mid-cycle increase in anti-inflammatory markers). Prior research in healthy women has shown that sexual activity is associated with lower levels of IgA (16, 19), IgE-mediated allergic responses (57), and Th1-dominant cytokine profiles (20). One common theme throughout these seemingly diverse immune factors is their potential detrimental effect on reproduction, via disruption of conception (as IgA may disrupt sperm transport and viability (58)), interference with establishment of the placenta (as when IgE is diverted from supporting the placenta towards allergic reactions(59)), or through early termination of the pregnancy (as is the case for Th1 cells(60) particularly in the context of low Th2 cells(61)). Similarly, the presence of pro-inflammatory cytokines is associated with spontaneous abortion (62), even in the absence of overt infection or wound (63, 64). Thus, suppressing these pro-inflammatory cytokines could improve the chances of sexual activity leading to offspring. Intriguingly, the down-regulation of inflammation markers appeared only when they were necessary – that is, when a woman approached the fertile phase of the menstrual cycle, and when she was sufficiently sexually active that conception could be likely. This timing likely reflects the immune system’s ongoing evaluation of the balance between defensive and reproductive priorities: favoring defense during non-fertile times but favoring reproduction during fertile times.
Relative to sexually active women, abstinent women were less likely to have detectable levels of inflammation markers and lower levels of inflammation markers overall. It should be reiterated that all of these participants were healthy and thus the relatively higher levels of inflammation markers in sexually active women were not reflective of pathology, nor significant injury or infection. Cytokines are signals; thus, the natural variation observed in these healthy women likely reflects differences in immune signaling and communication with other systems. Indeed, sexually active women had higher levels of both pro- and anti-inflammatory cytokines, further suggesting an increased need for immune system communication about inflammation rather than increased need for inflammation per se. This principle is further echoed in the differential patterns of change across the menstrual cycle in sexually active vs. abstinent women: while sexually active women had significant variation according to reproductive phase (fertile vs. non-fertile), abstinent women had less change. Taking all of these findings together, there is support for the hypothesis that in healthy women, the immune system uses information about sexual activity status to coordinate tradeoffs with reproduction.
Females must commit considerable time and energy to reproduction, and there is risk associated with pregnancy and childbirth. In response, the female reproductive system has evolved to be highly attuned to environmental factors related to current availability of physiologic and psychosocial resources. The propensity to balance tradeoffs between reproduction and somatic growth and maintenance (including immune defense) across the lifespan has been noted in both humans and non-humans (11, 65–69). Humans are notable for their relatively high level of investment of time and resources for each offspring, and the diversity of social cues that appear to influence tradeoffs in reproductive-somatic investment (11). It is possible that frequency of sexual activity is one such cue, eliciting the aspects of immune response that promote reproduction by signaling that conditions are favorable. For example, more frequent sexual activity may indicate relationship stability and satisfaction (70), health of the woman and her partner (71), or even eagerness for reproduction (72), all of which could benefit offspring.
Of course, the selection pressure to develop different inflammation profiles in sexually active vs. abstinent women would only be present if both states occurred in our evolutionary past. Indeed, there is evidence that hunter-gatherer societies may have used sexual abstinence as a means of population control (73, 74) (75). Similarly, some early societies systematically regulated the sexual availability of females after their husband’s death, or prior to marriage (76, 77). Still other societies (predominantly in African and India (73, 78–80)) practiced (and continue to practice) “terminal abstinence” in which a pre-menopausal woman commits to permanent sexual abstinence to balance family dynamics (e.g., to re-direct her caretaking energies to grandchildren rather than new children of her own). Finally, scholars have suggested that sexual abstinence was particularly common among societies in which sexually transmitted diseases were prominent (81). In sum, while widespread abstinence among healthy reproductive-age females was probably rare, it likely did occur sufficiently often to have an effect on immunity.
Much more work is needed to elucidate the mechanisms by which sexual activity influences immune response. Given significant differences between sexually active and inactive women in estradiol (E2) and P4 across the cycle (82), it is possible that endocrine factors play a role. Both E2 and P4 have immune effects, with the former thought to be generally anti-inflammatory (with notable exceptions (83)) and the latter thought to be generally pro-inflammatory, particularly within the female reproductive tract (84). Insofar as sexual activity changes E2/P4 profiles across the menstrual cycle, there should be associated changes in immune response (20), including inflammation. Another possible endocrine mediator is oxytocin, which is released during sexual activity (85–87). Oxytocin has a broad effect on inflammation, suppressing the inflammatory action of pro-inflammatory cytokines (88) and possibly increasing production of IL-4 (87). And of course, there are many other potential mechanisms, such as such as immune adaptation to repeated contact with a partner’s microbiome (89); immune redistribution to combat sexually transmitted infections or other immune challenges associated with repeated contact with the intimate partner; activation of the autonomic nervous system during sexual arousal (90); or input from the central nervous system during sexual stimulation. It is very likely, given the complexity of the associations noted here, that no one of these mechanisms will fully explain the impact of sexual activity on immune function.
Limitations
This was an exploratory study in a small sample of healthy, regularly cycling women. Most notably, our continuous variable analyses were limited by high rates of non-detects in several of the cytokines measured. It is also possible that missingness influenced effect estimates for our continuous value models: simply put, missing data cannot contribute as much to effect estimates than non-missing data. If an effect were significant in both detection and continuous value analyses, this would warrant suspicion as it would indicate that the significant effect in the continuous value analysis may be driven by non-detects. This condition applied in one case – the main effect of time on IFN-γ – and these findings should be thus treated with particular caution.
We were limited in the number of time points and cycles over which we could sample inflammation. Some work measuring cycle-related shifts in inflammation have found substantial within-woman variation across multiple cycles (4, 13, 91), which further supports the need to replicate these effects in larger samples over longer time periods. Our sample was relatively homogenous in terms of age, race/ethnicity, and parity; all three factors have strong effects on inflammation patterns (91–94). Finally, in the present study we excluded women taking hormonal medications and thus the generalizability to women using hormonal contraceptives (approximately 27.6% of women in the US (95)) is unknown. More research extending these findings into the population of women taking hormonal medications is needed to shed light both on the generalizability and the possible endocrine mechanisms underlying the observed effects.
Future directions
While the greatest difference between our sexually active and abstinent groups was the level of sexual activity, there may be other factors that co-varied with sexual activity status; most notably, while all of the sexually active women were partnered, only a few of the abstinent women were partnered. Research on mechanisms is needed to evaluate if and how sexual activity (vs. other factors such as partnership) influences women’s immune patterns. For example, relative to women who do not live with an intimate partner, women who do live with an intimate partner tend to eat a more anti-inflammatory diet with more vegetables and less meat (96). While partnership is unlikely to fully explain our findings regarding sexual frequency, it is possible that partnership moderates the effect of sexual activity on inflammation. Further study of the effect of sexual activity in women in different kinds of relationships, and/or who have sex with multiple partners, is warranted. It would be valuable to examine the effect of sexual activity within the same woman over time – for example, in an experimental manipulation of sexual activity from cycle to cycle. It would also be valuable to know the timing and duration of the effect – that is, how long after sexual activity do these immune effects peak? Finally, life history theory suggests that may expect different immune effects among nulliparous and multiparous women, and between younger and older (but still premenopausal) women. These groups would be subject to different evolutionary selection pressures, and thus potentially different thresholds for the tradeoffs between reproduction and somatic maintenance (66, 97).
Clinical implications
Our findings are consistent with prior literature documenting similar patterns in markers of inflammation(4, 5, 7, 8). Such variability in inflammation in healthy women has important clinical implications, as markers of inflammation are often used in prognosis of risk for CVD and other inflammation-related conditions (98). In our sample, women had the lowest levels of inflammation markers at midcycle, particularly if they were sexually active. In other words, there is a significant chance a sexually active woman will be differently classified (and possibly misclassified) if she presents at mid-cycle (around ovulation) vs. other points in her cycle. These findings suggest that in sexually active women of reproductive age, there is limited prognostic value of a single measurement of CRP (such as is typically conducted in epidemiologic studies). Clinicians and researchers using biomarkers of CVD risk may find it useful to take multiple measurements across the cycle or, if this is not feasible, scheduling measurements at midcycle (if possible, around the time ovulation is detected) when CRP can be expected to be at a minimum for both sexually active and sexually abstinent women.
Given the prognostic value of CRP and other inflammation markers, much research has investigated sources of intra-individual variations in the measurement of these markers (99). Sometimes overlooked in the search for stability across measurements, however, is the potential prognostic value of variability. Although some researchers have suggested that extreme variability of CRP is indicative of pathology (100), moderate variation in response to cues from the social or physical environment – such as reproductive activity – may represent adaptability and as such, potential for good health. Further research is needed to examine if cycle-related changes in biomarkers of inflammation, corresponding to responsivity of the immune system to environmental demands, are associated with other markers of health.
Conclusions
We observed a significant mid-cycle decrease in inflammatory markers in sexually active, but not abstinent, women. In addition, frequency of partnered genital sexual activity moderated this effect such that women reporting more frequent sexual activity demonstrated greater mid-cycle decreases in inflammation markers than women reporting less frequent sexual activity. The present study was limited in sample size and restricted to young, primarily White women, regularly cycling and not taking hormonal medications; thus, further research is needed to replicate and extend these findings to other populations. While several studies have previously documented menstrual cycle-related variations in inflammation, this is the first to examine the effect of sexual activity on these patterns. Our findings call for critical evaluation of inflammation biomarkers in clinical care of reproductive aged women, and offer a new avenue for research on the intersection between immune health, reproduction, and sexual behaviors in women.
Supplementary Material
Acknowledgments
This work was partially funded by the Office of the Vice Provost of Research at Indiana University-Bloomington through the Collaborative Research and Creative Activity Funding Award, and partially by the American Psychological Foundation’s Henry P. David Award for Research in Human Reproductive Behavior and Population Studies. Dr. Lorenz was supported by grant T32HD049336 from the National Institute of Child Health and Human Development.
Footnotes
Disclosures:
The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stringer EA, Baker KS, Carroll IR, Montoya JG, Chu L, Maecker HT, et al. Daily cytokine fluctuations, driven by leptin, are associated with fatigue severity in chronic fatigue syndrome: evidence of inflammatory pathology. J Transl Med. 2013;11:93–114. doi: 10.1186/1479-5876-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Affleck G, Tennen H, Urrows S, Higgins P. Neuroticism and the pain-mood relation in rheumatoid arthritis: insights from a prospective daily study. J Consult Clin Psychol. 1992;60:119–26. doi: 10.1037//0022-006x.60.1.119. [DOI] [PubMed] [Google Scholar]
- 3.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–86. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 4.Gaskins AJ, Wilchesky M, Mumford SL, Whitcomb BW, Browne RW, Wactawski-Wende J, et al. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle. Am J Epidemiol. 2012;175:423–31. doi: 10.1093/aje/kwr343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blum CA, Muller B, Huber P, Kraenzlin M, Schindler C, De Geyter C, et al. Low-grade inflammation and estimates of insulin resistance during the menstrual cycle in lean and overweight women. The Journal of Clinical Endocrinology & Metabolism. 2005;90:3230–5. doi: 10.1210/jc.2005-0231. [DOI] [PubMed] [Google Scholar]
- 6.Capobianco G, de Muro P, Cherchi GM, Formato M, Lepedda AJ, Cigliano A, et al. Plasma levels of C-reactive protein, leptin and glycosaminoglycans during spontaneous menstrual cycle: Differences between ovulatory and anovulatory cycles. Arch Gynecol Obstet. 2010;282:207–13. doi: 10.1007/s00404-010-1432-2. [DOI] [PubMed] [Google Scholar]
- 7.Wander K, Brindle E, O’Connor KA. C-reactive protein across the menstrual cycle. Am J Phys Anthropol. 2008;136:138–46. doi: 10.1002/ajpa.20785. [DOI] [PubMed] [Google Scholar]
- 8.Schisterman EF, Mumford SL, Sjaarda LA. Failure to consider the menstrual cycle phase may cause misinterpretation of clinical and research findings of cardiometabolic biomarkers in premenopausal women. Epidemiol Rev. 2014;36:71–82. doi: 10.1093/epirev/mxt007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suarez S, Pacey A. Sperm transport in the female reproductive tract. Hum Reprod Update. 2006;12:23–37. doi: 10.1093/humupd/dmi047. [DOI] [PubMed] [Google Scholar]
- 10.Weiss G, Goldsmith LT, Taylor RN, Bellet D, Taylor HS. Inflammation in Reproductive Disorders. Reprod Sci. 2009;16:216–29. doi: 10.1177/1933719108330087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abrams ET, Miller EM. The roles of the immune system in women’s reproduction: Evolutionary constraints and life history tradeoffs. Am J Phys Anthropol. 2011;146:134–54. doi: 10.1002/ajpa.21621. [DOI] [PubMed] [Google Scholar]
- 12.McDade TW. The ecologies of human immune function. Annu Rev Anthropol. 2005;34:495–521. [Google Scholar]
- 13.Lorenz T, Worthman C, Vitzthum VJ. Links between inflammation, sexual activity and ovulation: Evolutionary trade-offs and clinical implications. Evolution, Medicine, and Public Health. 2015;1:304–24. doi: 10.1093/emph/eov029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark DA, Manuel J, Lee L, Chaouat G, Gorczynski RM, Levy GA. Ecology of Danger-dependent Cytokine-boosted Spontaneous Abortion in the CBA × DBA/2 Mouse Model. I. Synergistic Effect of LPS and (TNF-α + IFN-γ) on Pregnancy Loss. Am J Reprod Immunol. 2004;52:370–8. doi: 10.1111/j.1600-0897.2004.00237.x. [DOI] [PubMed] [Google Scholar]
- 15.Garlanda C, Maina V, Martinez de la Torre Y, Nebuloni M, Locati M. Inflammatory Reaction and Implantation: the New Entries PTX3 and D6. Placenta. 2008;29(Supplement 2):129–34. doi: 10.1016/j.placenta.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Lorenz T, van Anders SM. Interactions of sexual activity, gender, and depression with immunity. J Sex Med. 2014;11:966–79. doi: 10.1111/jsm.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown SG, Morrison LA, Calibuso MJ, Christiansen TM. The menstrual cycle and sexual behavior: relationship to eating, exercise, sleep, and health patterns. Women Health. 2008;48:429–44. doi: 10.1080/03630240802575179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charnetski CJ, Brennan FX. Sexual frequency and Immunoglobulin A (IgA) Psychol Rep. 2004;94:839–44. doi: 10.2466/pr0.94.3.839-844. [DOI] [PubMed] [Google Scholar]
- 19.Lorenz T, Heiman JR, Demas GE. Interaction of menstrual phase and sexual activity predicts mucosal and systemic humoral immunity in healthy women. Physiol Behav. 2015;152:92–8. doi: 10.1016/j.physbeh.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lorenz T, Heiman JR, Demas GE. Sexual activity modulates shifts in Th1/Th2 cytokine profile across the menstrual cycle: An observational study. Fertil Steril. 2015;104:1513–21. doi: 10.1016/j.fertnstert.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mann DL. Innate Immunity and the Failing Heart The Cytokine Hypothesis Revisited. Circul Res. 2015;116:1254–68. doi: 10.1161/CIRCRESAHA.116.302317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vila N, Castillo J, Dávalos A, Esteve A, Planas AM, Chamorro Á. Levels of anti-inflammatory cytokines and neurological worsening in acute ischemic stroke. Stroke. 2003;34:671–5. doi: 10.1161/01.STR.0000057976.53301.69. [DOI] [PubMed] [Google Scholar]
- 24.Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain, Behav, Immun. 2007;21:153–60. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 26.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-γ: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–89. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 27.Walsh LJ, Trinchieri G, Waldorf HA, Whitaker D, Murphy GF. Human dermal mast cells contain and release tumor necrosis factor alpha, which induces endothelial leukocyte adhesion molecule 1. Proceedings of the National Academy of Sciences. 1991;88:4220–4. doi: 10.1073/pnas.88.10.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christiansen OB, Nielsen HS, Kolte AM. Semin Fetal Neonatal Med. Elsevier; 2006. Inflammation and miscarriage; pp. 302–8. [DOI] [PubMed] [Google Scholar]
- 29.Calleja-Agius J, Jauniaux E, Pizzey AR, Muttukrishna S. Investigation of systemic inflammatory response in first trimester pregnancy failure. Hum Reprod. 2012;27:349–57. doi: 10.1093/humrep/der402. [DOI] [PubMed] [Google Scholar]
- 30.Schindler R, Mancilla J, Endres S, Ghorbani R, Clark S, Dinarello C. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. 1990;75:40–7. [PubMed] [Google Scholar]
- 31.Faas M, Bouman A, Moesa H, Heineman MJ, de Leij L, Schuiling G. The immune response during the luteal phase of the ovarian cycle: A Th2-type response? Fertil Steril. 2000;74:1008–13. doi: 10.1016/s0015-0282(00)01553-3. [DOI] [PubMed] [Google Scholar]
- 32.Schwarz E, Schäfer C, Bode J, Bode C. Influence of the menstrual cycle on the LPS-induced cytokine response of monocytes. Cytokine. 2000;12:413–6. doi: 10.1006/cyto.1999.0570. [DOI] [PubMed] [Google Scholar]
- 33.Angstwurm MWA, Gärtner R, Ziegler-Heitbrock HWL. CYCLIC PLASMA IL-6 LEVELS DURING NORMAL MENSTRUAL CYCLE. Cytokine. 1997;9:370–4. doi: 10.1006/cyto.1996.0178. [DOI] [PubMed] [Google Scholar]
- 34.Agarwal SK, Marshall GD. Perimenstrual alterations in type-1/type-2 cytokine balance of normal women. Ann Allergy, Asthma Immunol. 1999;83:222–8. doi: 10.1016/S1081-1206(10)62644-0. [DOI] [PubMed] [Google Scholar]
- 35.Willis C, Morris JM, Danis V, Gallery EDM. Cytokine production by peripheral blood monocytes during the normal human ovulatory menstrual cycle. Hum Reprod. 2003;18:1173–8. doi: 10.1093/humrep/deg231. [DOI] [PubMed] [Google Scholar]
- 36.O’Brien SM, Fitzgerald P, Scully P, Landers AMT, Scott LV, Dinan TG. Impact of Gender and Menstrual Cycle Phase on Plasma Cytokine Concentrations. Neuroimmunomodulation. 2007;14:84–90. doi: 10.1159/000107423. [DOI] [PubMed] [Google Scholar]
- 37.Al-Harthi L, Wright DJ, Anderson D, Cohen M, Matityahu D, Cohn J, et al. The Impact of the Ovulatory Cycle on Cytokine Production: Evaluation of Systemic, Cervicovaginal, and Salivary Compartments. J Interferon Cytokine Res. 2000;20:719–24. doi: 10.1089/10799900050116426. [DOI] [PubMed] [Google Scholar]
- 38.Wiunder DM, Yared M, Bersinger NA, Widmer D, Kretschmer R, Birkhauser M. Serum leptin and C-reactive protein levels in the physiological spontaneous menstrual cycle in reproductive age women. European Journal of Endocrinology / European Federation of Endocrine Societies. 2006;155:137–42. doi: 10.1530/eje.1.02178. [DOI] [PubMed] [Google Scholar]
- 39.Jilma B, Dirnberger E, Loscher I, Rumplmayr A, Hildebrant J, Eichler HG, et al. Menstrual cycle associated changes in blood levels of interleukin-6, alpha1 acid glycoprotein, and C-reactive protein. J Lab Clin Med. 1997;130:69–75. doi: 10.1016/s0022-2143(97)90060-3. [DOI] [PubMed] [Google Scholar]
- 40.Grignolio A, Mishto M, Faria AMC, Garagnani P, Franceschi C, Tieri P. Towards a Liquid Self: How Time, Geography, and Life Experiences Reshape the Biological Identity. Front Immunol. 2014:5. doi: 10.3389/fimmu.2014.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Anders SM, Goldey KL, Bell SN. Measurement of testosterone in human sexuality research: methodological considerations. Arch Sex Behav. 2014;43:231–50. doi: 10.1007/s10508-013-0123-z. [DOI] [PubMed] [Google Scholar]
- 42.Granger DA, Kivlighan KT, Fortunato C, Harmon AG, Hibel LC, Schwartz EB, et al. Integration of salivary biomarkers into developmental and behaviorally-oriented research: Problems and solutions for collecting specimens. Physiol Behav. 2007;92:583–90. doi: 10.1016/j.physbeh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Byrne ML, O’Brien-Simpson NM, Reynolds EC, Walsh KA, Laughton K, Waloszek JM, et al. Acute phase protein and cytokine levels in serum and saliva: a comparison of detectable levels and correlations in a depressed and healthy adolescent sample. Brain, Behav, Immun. 2013;34:164–75. doi: 10.1016/j.bbi.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 44.Crawford SL, Tennstedt SL, McKinlay JB. A comparison of analytic methods for non-random missingness of outcome data. J Clin Epidemiol. 1995;48:209–19. doi: 10.1016/0895-4356(94)00124-9. [DOI] [PubMed] [Google Scholar]
- 45.Uh H-W, Hartgers FC, Yazdanbakhsh M, Houwing-Duistermaat JJ. Evaluation of regression methods when immunological measurements are constrained by detection limits. BMC Immunol. 2008;9:59. doi: 10.1186/1471-2172-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ouellet-Morin I, Danese A, Williams B, Arseneault L. Validation of a high-sensitivity assay for C-reactive protein in human saliva. Brain, Behav, Immun. 2011;25:640–6. doi: 10.1016/j.bbi.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 47.Punyadeera C, Dimeski G, Kostner K, Beyerlein P, Cooper-White J. One-step homogeneous C-reactive protein assay for saliva. J Immunol Methods. 2011;373:19–25. doi: 10.1016/j.jim.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 48.Dillon MC, Opris DC, Kopanczyk R, Lickliter J, Cornwell HN, Bridges EG, et al. Detection of homocysteine and C-reactive protein in the saliva of healthy adults: comparison with blood levels. Biomarker insights. 2010;5:57. doi: 10.4137/bmi.s5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dekker RL, Lennie TA, Moser DK, Miller CS, Ebersole JL, Chung ML, et al. Levels of Oral Inflammation--not Salivary or Serum Biomarkers--Predict Worse Functional Status in Hospitalized Patients With Heart Failure. Circulation. 2015;132:A10367-A. [Google Scholar]
- 50.Floriano PN, Christodoulides N, Miller CS, Ebersole JL, Spertus J, Rose BG, et al. Use of saliva-based nano-biochip tests for acute myocardial infarction at the point of care: a feasibility study. Clin Chem. 2009;55:1530–8. doi: 10.1373/clinchem.2008.117713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krasteva A, Perenovska P, Ivanova A, Altankova I, Bocheva T, Kisselova A. Alteration in salivary components of children with allergic asthma. Biotechnology & Biotechnological Equipment. 2010;24:1866–9. [Google Scholar]
- 52.Out D, Hall RJ, Granger DA, Page GG, Woods SJ. Assessing salivary C-reactive protein: Longitudinal associations with systemic inflammation and cardiovascular disease risk in women exposed to intimate partner violence. Brain, Behav, Immun. 2012;26:543–51. doi: 10.1016/j.bbi.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Festa A, D’Agostino R, Jr, Williams K, Karter A, Mayer-Davis E, Tracy R, et al. The relation of body fat mass and distribution to markers of chronic inflammation. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2001;25:1407–15. doi: 10.1038/sj.ijo.0801792. [DOI] [PubMed] [Google Scholar]
- 54.Pannacciulli N, Cantatore F, Minenna A, Bellacicco M, Giorgino R, De Pergola G. C-reactive protein is independently associated with total body fat, central fat, and insulin resistance in adult women. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2001;25:1416–20. doi: 10.1038/sj.ijo.0801719. [DOI] [PubMed] [Google Scholar]
- 55.Selya AS, Rose JS, Dierker LC, Hedeker D, Mermelstein RJ. A practical guide to calculating Cohen’s f2, a measure of local effect size, from PROC MIXED. Front Psychol. 2012;3:111. doi: 10.3389/fpsyg.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu M, Tilley BC. Use of odds ratio or relative risk to measure a treatment effect in clinical trials with multiple correlated binary outcomes: data from the NINDS t-PA stroke trial. Stat Med. 2001;20:1891–901. doi: 10.1002/sim.841. [DOI] [PubMed] [Google Scholar]
- 57.Kimata H. Kissing selectively decreases allergen-specific IgE production in atopic patients. J Psychosom Res. 2006;60:545–7. doi: 10.1016/j.jpsychores.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 58.Fahrbach KM, Malykhina O, Stieh DJ, Hope TJ. Differential binding of IgG and IgA to mucus of the female reproductive tract. PLoS One. 2013;8:e76176. doi: 10.1371/journal.pone.0076176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rindsjö E, Joerink M, Papadogiannakis N, Scheynius A. IgE in the human placenta: why there? Allergy. 2010;65:554–60. doi: 10.1111/j.1398-9995.2010.02345.x. [DOI] [PubMed] [Google Scholar]
- 60.Saito S, Sakai M, Sasaki Y, Tanebe K, Tsuda H, Michimata T. Quantitative analysis of peripheral blood Th0, Th1, Th2 and the Th1: Th2 cell ratio during normal human pregnancy and preeclampsia. Clin Exp Immunol. 1999;117:550. doi: 10.1046/j.1365-2249.1999.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saito S, Nakashima A, Shima T, Ito M. REVIEW ARTICLE: Th1/Th2/Th17 and Regulatory T-Cell Paradigm in Pregnancy. Am J Reprod Immunol. 2010;63:601–10. doi: 10.1111/j.1600-0897.2010.00852.x. [DOI] [PubMed] [Google Scholar]
- 62.Bansal AS. Joining the immunological dots in recurrent miscarriage. Am J Reprod Immunol. 2010;64:307–15. doi: 10.1111/j.1600-0897.2010.00864.x. [DOI] [PubMed] [Google Scholar]
- 63.Giannubilo SR, Landi B, Pozzi V, Sartini D, Cecati M, Stortoni P, et al. The involvement of inflammatory cytokines in the pathogenesis of recurrent miscarriage. Cytokine. 2012;58:50–6. doi: 10.1016/j.cyto.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 64.Hattori Y, Nakanishi T, Ozaki Y, Nozawa K, Sato T, Sugiura-Ogasawara M. Uterine Cervical Inflammatory Cytokines, Interleukin-6 and -8, as Predictors of Miscarriage in Recurrent Cases. Am J Reprod Immunol. 2007;58:350–7. doi: 10.1111/j.1600-0897.2007.00516.x. [DOI] [PubMed] [Google Scholar]
- 65.Charnov EL. Life history invariants: some explorations of symmetry in evolutionary ecology. Oxford University Press; USA: 1993. [Google Scholar]
- 66.Vitzthum VJ. Flexibility and paradox: The nature of adaptation in human reproduction. In: Galloway A, Zihman A, editors. The evolving female: A life history perspective. Princeton: Princeton University Press; 1997. pp. 242–58. [Google Scholar]
- 67.Stearns SC. Trade-offs in life-history evolution. Funct Ecol. 1989;3:259–68. [Google Scholar]
- 68.Sheldon BC, Verhulst S. Ecological immunology: Costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol. 1996;11:317–21. doi: 10.1016/0169-5347(96)10039-2. [DOI] [PubMed] [Google Scholar]
- 69.Demas GE, Grieves T, Chester E, French S. The energetics of immunity: Mechanisms mediating tradeoffs in eco-immunology. In: Demas G, Nelson R, editors. Ecoimmunology. New York, NY: Oxford University Press; 2012. pp. 259–96. [Google Scholar]
- 70.Yabiku ST, Gager CT. Sexual frequency and the stability of marital and cohabiting unions. Journal of Marriage and Family. 2009;71:983–1000. [Google Scholar]
- 71.Schneidewind-Skibbe A, Hayes RD, Koochaki PE, Meyer J, Dennerstein L. The frequency of sexual intercourse reported by women: a review of community-based studies and factors limiting their conclusions. The journal of sexual medicine. 2008;5:301–35. doi: 10.1111/j.1743-6109.2007.00685.x. [DOI] [PubMed] [Google Scholar]
- 72.Singh D, Meyer W, Zambarano RJ, Hurlbert DF. Frequency and timing of coital orgasm in women desirous of becoming pregnant. Arch Sex Behav. 1998;27:15–29. doi: 10.1023/a:1018653724159. [DOI] [PubMed] [Google Scholar]
- 73.Caldwell JC, Caldwell P. The role of marital sexual abstinence in determining fertility: a study of the Yoruba in Nigeria. Popul Stud. 1977;31:193–217. doi: 10.1080/00324728.1977.10410427. [DOI] [PubMed] [Google Scholar]
- 74.Bentley GR, Goldberg T, Jasieńska Gzy. The fertility of agricultural and non-agricultural traditional societies. Popul Stud. 1993;47:269–81. [Google Scholar]
- 75.Hayden B. Population control among hunter/gatherers. Wld Archaeol. 1972;4:205–21. doi: 10.1080/00438243.1972.9979533. [DOI] [PubMed] [Google Scholar]
- 76.Enel C, Pison G. Sexual relations in the rural area of Mlomp (Casamance Senegal) 1992. [Google Scholar]
- 77.Benagiano G, Mori M. The origins of human sexuality: procreation or recreation? Reprod Biomed Online. 2009;18:50–9. doi: 10.1016/s1472-6483(10)60116-2. [DOI] [PubMed] [Google Scholar]
- 78.Leidy LE. The practice of terminal abstinence in Nigeria and Cameroon. Am J Hum Biol. 1993;5:565–73. doi: 10.1002/ajhb.1310050508. [DOI] [PubMed] [Google Scholar]
- 79.Caldwell JC. Variations in the incidence of sexual abstinence and the duration of postnatal abstinence among the Yoruba of Nigeria. 1980. [Google Scholar]
- 80.Santow G. A simulation approach to the study of human fertility. Springer Science & Business Media; 2012. [Google Scholar]
- 81.Mackey WC, Immerman RS. Restriction of Sexual Activity as a Partial Function of Disease Avoidance: A Cultural Response to Sexually Transmitted Diseases. Cross-Cultural Research. 2001;35:400–23. [Google Scholar]
- 82.Prasad A, Mumford SL, Louis GMB, Ahrens KA, Sjaarda LA, Schliep KC, et al. Sexual activity, endogenous reproductive hormones and ovulation in premenopausal women. Horm Behav. 2014;66:330–8. doi: 10.1016/j.yhbeh.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Straub RH. The Complex Role of Estrogens in Inflammation. Endocr Rev. 2007;28:521–74. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 84.Wira CR, Rodriguez-Garcia M, Patel MV. The role of sex hormones in immune protection of the female reproductive tract. Nature Reviews Immunology. 2015;15:217–30. doi: 10.1038/nri3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Flanagan L, Pfaus J, Pfaff D, McEwen B. Induction of FOS immunoreactivity in oxytocin neurons after sexual activity in female rats. Neuroendocrinology. 1993;58:352–8. doi: 10.1159/000126562. [DOI] [PubMed] [Google Scholar]
- 86.Carmichael MS, Humbert R, Dixen J, Palmisano G, Greenleaf W, Davidson JM. Plasma Oxytocin Increases in the Human Sexual Response*. The Journal of Clinical Endocrinology & Metabolism. 1987;64:27–31. doi: 10.1210/jcem-64-1-27. [DOI] [PubMed] [Google Scholar]
- 87.Clodi M, Vila G, Geyeregger R, Riedl M, Stulnig TM, Struck J, et al. Oxytocin alleviates the neuroendocrine and cytokine response to bacterial endotoxin in healthy men. American Journal of Physiology-Endocrinology and Metabolism. 2008;295:E686–E91. doi: 10.1152/ajpendo.90263.2008. [DOI] [PubMed] [Google Scholar]
- 88.Gouin J-P, Carter CS, Pournajafi-Nazarloo H, Glaser R, Malarkey WB, Loving TJ, et al. Marital behavior, oxytocin, vasopressin, and wound healing. Psychoneuroendocrinology. 2010;35:1082–90. doi: 10.1016/j.psyneuen.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kort R, Caspers M, van de Graaf A, van Egmond W, Keijser B, Roeselers G. Shaping the oral microbiota through intimate kissing. Microbiome. 2014;2:41. doi: 10.1186/2049-2618-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lorenz T, Harte CB, Hamilton LD, Meston CM. Evidence for a curvilinear relationship between sympathetic nervous system activation and women’s physiological sexual arousal. Psychophysiology. 2012;49:111–7. doi: 10.1111/j.1469-8986.2011.01285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Clancy KBH, Klein LD, Ziomkiewicz A, Nenko I, Jasienska G, Bribiescas RG. Relationships between biomarkers of inflammation, ovarian steroids, and age at menarche in a rural Polish sample. Am J Hum Biol. 2013;25:389–98. doi: 10.1002/ajhb.22386. [DOI] [PubMed] [Google Scholar]
- 92.Picklesimer AH, Jared HL, Moss K, Offenbacher S, Beck JD, Boggess KA. Racial differences in C-reactive protein levels during normal pregnancy. Am J Obstet Gynecol. 2008;199:523e1–e6. doi: 10.1016/j.ajog.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Feairheller DL, Park JY, Sturgeon KM, Williamson ST, Diaz KM, Veerabhadrappa P, et al. Racial differences in oxidative stress and inflammation: in vitro and in vivo. Clin Transl Sci. 2011;4:32–7. doi: 10.1111/j.1752-8062.2011.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Daniels K, Daugherty J, Jones J. Current contraceptive status among women aged 15–44: United States, 2011–2013. NCHS data brief. 2014;173:1–8. [PubMed] [Google Scholar]
- 96.Lee S, Cho E, Grodstein F, Kawachi I, Hu FB, Colditz GA. Effects of marital transitions on changes in dietary and other health behaviours in US women. Int J Epidemiol. 2005;34:69–78. doi: 10.1093/ije/dyh258. [DOI] [PubMed] [Google Scholar]
- 97.Norris K, Evans MR. Ecological immunology: Life history trade-offs and immune defense in birds. Behav Ecol. 2000;11:19–26. [Google Scholar]
- 98.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, et al. Markers of inflammation and cardiovascular disease application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 99.Braga F, Panteghini M. Biologic variability of C-reactive protein: Is the available information reliable? Clin Chim Acta. 2012;413:1179–83. doi: 10.1016/j.cca.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 100.Metti AL, Yaffe K, Boudreau RM, Simonsick EM, Carnahan RM, Satterfield S, et al. Trajectories of inflammatory markers and cognitive decline over 10 years. Neurobiol Aging. 2014;35:2785–90. doi: 10.1016/j.neurobiolaging.2014.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.