Abstract
Background
Though peanut oral immunotherapy (OIT) is a promising investigational therapy, its potential is limited by substantial adverse events (AEs), which are relatively understudied.
Objective
To conduct a retrospective analysis pooling three pediatric peanut OIT trials, comprising the largest analysis of peanut OIT safety to date.
Methods
We pooled 104 peanut-allergic children from three peanut OIT studies. We catalogued AEs from parental report, daily symptom diaries, and dose escalations. We included events that were likely related to OIT and identified potential baseline predictors of higher AE rates using generalized linear regression models.
Results
Eighty percent of subjects experienced likely-related AEs during OIT (72% during buildup and 47% during maintenance). Of these AEs, over 90% occurred while at home. Approximately 42% of subjects experienced systemic reactions, and 49% experienced gastrointestinal symptoms. Twenty percent of subjects dropped out, with half (10% of overall group) due to persistent gastrointestinal symptoms. Baseline allergic rhinitis (AR), asthma, and peanut skin prick test (SPT) were significant predictors of higher overall AE rates. SPT predicted increased gastrointestinal AEs, and AR predicted increased systemic reactions. Over the course of OIT, 61% of subjects received treatment for likely-related AEs, 59% with antihistamines and 12% with epinephrine.
Conclusion
Peanut OIT is associated with frequent AEs, with rates declining over time, and most graded mild. However, systemic reactions and intolerable gastrointestinal AEs do occur and are significantly associated with AR and peanut SPT, respectively. Further study is needed of predictive biomarkers and the overall risks and benefits of OIT.
Keywords: Peanut allergy, Oral Immunotherapy, Safety, Adverse Events
Introduction
Food allergy is a potentially life-threatening condition affecting approximately 3–8% of U.S. children (1–3). With no approved curative therapy, management is restricted to allergen avoidance and supportive measures if symptoms occur (4, 5). A major focus of current research is the development of disease-modifying treatments that modulate the allergic immune response, protecting against accidental exposure. Oral immunotherapy (OIT) for peanut allergy has been shown to successfully desensitize a majority of peanut-allergic children, which has generated excitement about OIT for peanut allergy, but significant concerns remain regarding its safety (6).
Evaluating the safety profile, however, is complicated by the lack of detailed assessments of safety in larger sample sizes. Furthermore, OIT trials vary widely in both protocols and the methods used to present adverse events (AEs). The few studies focused on safety acknowledge that most reactions are mild or moderate, but risk of systemic reactions requiring epinephrine remains (7–9).
The goals of our study were to address this knowledge gap by: (1) characterizing the frequency of OIT-associated AEs and study withdrawals, and (2) identifying baseline characteristics that may predict subjects at higher risk for AEs. Accordingly, we pooled three trials performed by the same group, examining both AEs in the research unit (i.e. staff-observed) and home AEs, when parents must manage reactions without the support of clinical staff.
Methods
Study Design
In this retrospective analysis, we compiled data from three peanut OIT studies: the Jones et al. trial, an uncontrolled pilot study (10, 11); the Varshney et al. study, a randomized, placebo-controlled trial (12); and the DEVIL study, an ongoing randomized single-center trial. See supplemental methods and Table E1 for further details.
Safety Data Collection
Safety data was collected from three sources: records of symptoms occurring during dose escalation at the research unit, symptom diaries of home AEs, and parent reports of home AEs. All analyses primarily focus on events that were deemed likely related to therapy. See supplemental methods for details.
Statistical Methods
We computed means, standard deviations, frequencies and proportions for all clinical history and immunological variables. Statistical analyses were conducted using t-tests, chi-square tests, Fisher’s exact tests, or generalized linear regression modeling (see supplemental methods for details). For all analyses (unless specified otherwise), home and research unit AEs were grouped together to best represent the overall risk experienced by participants receiving OIT.
Ethical Considerations
All of the trials were conducted in accordance with the principles of the Declaration of Helsinki. For the clinical trials from which these data were generated, ethics approval was obtained through the Institutional Review Boards of the institutions involved. Written informed consent was obtained prior to participation, in accordance with each institution’s ethics guidelines for pediatric research.
Results
Subject demographics and participant flow
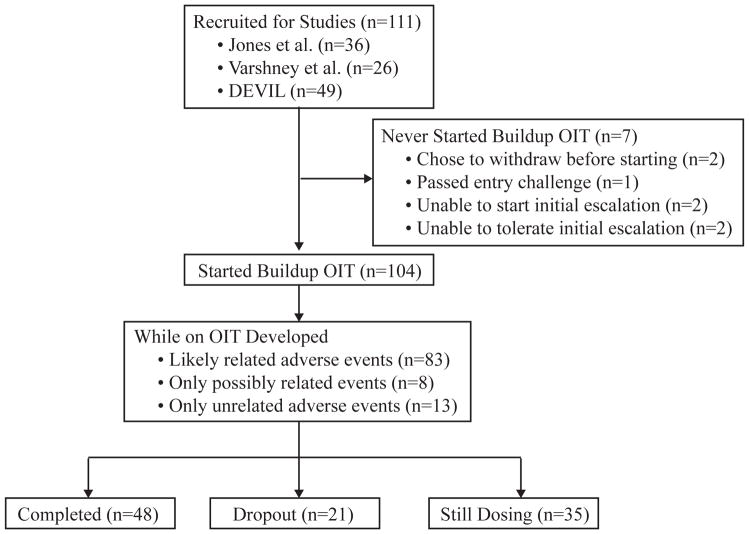
Of recruited subjects, 94% (104/111) tolerated the initial day escalation and went on to administer OIT at home (Figure 1). The remaining seven included one individual who passed the entry challenge, two who withdrew prior to initial escalation, and four who did not tolerate initial escalation. Of these four, two had difficulties establishing intravenous access in preparation for the protocol and two developed symptoms during the escalation itself (one with asthma symptoms and one with severe abdominal pain and vomiting, requiring epinephrine) (Figure 1).
Fig 1.
Consort Diagram of Study Population
The final study cohort of 104 subjects consisted of a mostly Caucasian pediatric population with a slight male predominance (Table 1). A majority of subjects had other allergic diseases including asthma (44%), atopic dermatitis (77%), and allergic rhinitis (AR) (46%). All subjects had a positive peanut SPT, and 91 subjects (88%) also had an elevated peanut-specific IgE ≥ 7 kU/L.
Table 1.
Baseline Characteristics and Duration of Therapy for all subjects (N=104). Summary statistics above are either counts (percentage) or medians (first and third quartiles).
| Females | 39 (38%) |
| Age at Starting OIT (years) | |
| 0–3 | 57 (55%) |
| 4–7 | 39 (38%) |
| 8–13 | 8 (8%) |
| Race | |
| White | 92 (88%) |
| Black | 6 (6%) |
| Other | 6 (6%) |
| History of Asthma | 46 (44%) |
| History of Atopic Dermatitis | 80 (77%) |
| History of Allergic Rhinitis | 48 (46%) |
| Peanut Skin Prick Test (mm) | 13 (9, 17) |
| Peanut IgE (kU/L) | 55 (17, 189) |
| Peanut IgG4 (kU/L) | 0.4 (0.1, 0.8) |
| Days on Therapy | 1012 (712, 1443) |
| Days in Buildup Phase | 314 (236, 351) |
| Days in Maintenance Phase* | 570 (327, 1137) |
for those who made it to the maintenance phase (n=87).
At the time of data extraction, approximately half of the study population had completed the protocol, and a third were still dosing with OIT. Twenty-one subjects (20%) withdrew from OIT, and of these, 13 did so due to new or worsening symptoms developing on OIT. The remainder withdrew because of logistical difficulty participating in the study. Of the thirteen experiencing symptoms, 10 subjects (10% of the overall sample, and 77% of symptomatic withdrawals) dropped out due to new persistent gastrointestinal symptoms (abdominal pain, emesis, and dysphagia), 1 due to worsening asthma, and 2 due to taste aversion. Of the ten who developed gastrointestinal symptoms, mean presentation time of first gastrointestinal symptom was 17 days (range 0–74), three were evaluated by esophagogastroduodenoscopy, and two had findings consistent with eosinophilic esophagitis (EoE) (13).
Characteristics and Rates of AEs
Of the 106 subjects who underwent initial dose escalation, 85 (80%) developed at least one likely-related AE, and of the 104 who began buildup OIT, 83 (80%) developed at least one AE during their time on therapy (Figure 1). A total of 1,077 likely-related AEs were documented among these 83 participants. Among all likely-related AEs, 75 events (7%), affecting 35 subjects (34%), occurred during dose escalations in the research unit, while the remainder occurred at home (93%). The mean AE rate was 1.7% of dosing days, with an annualized rate of 3.5, (Table 2) and was higher during the buildup phase than during the maintenance phase of treatment (p=0.005). The percent of subjects affected by AEs decreased from buildup to maintenance as well (p<0.001, Table 2). This decline in AE rates from buildup to maintenance occurred both among home AEs (p=0.008) and research unit events (p<0.001).
Table 2.
Rates and Severity of Likely Related AEs by Phase of Therapy.
| Overall | Buildup | Maintenance | |
|---|---|---|---|
| Subjects Affected by AEs after starting buildup OIT | 80% (83/104 subjects) | 72% (75/104 subjects) | 47% (41/87 subjects) |
| Average Rate of AEs per person* (95% CI) | 1.7% (0.8%, 2.7%) | 2.8% (1.5%, 4.0%) | 0.4% (0.2%, 0.7%) |
| Average Number of AEs per person (95% CI) | 10.4 (6.5, 14.3) | 7.6 (4.0, 11.1) | 3.3 (1.6, 5.1) |
| Total Number of AEs | 1077 | 790 | 294 |
| Severity of AEs | |||
| Mild | 918 (85%) | 687 (87%) | 231 (79%) |
| Moderate | 159 (15%) | 99 (13%) | 60 (21%) |
| Location of AEs | |||
| Home | 1002 (93%) | 714 (91%) | 288 (99%) |
| Research Unit | 75 (7%) | 72 (9%) | 3 (1%) |
Individual rates of AEs were calculated by number of AEs divided by days on OIT, which were then averaged across all participants.
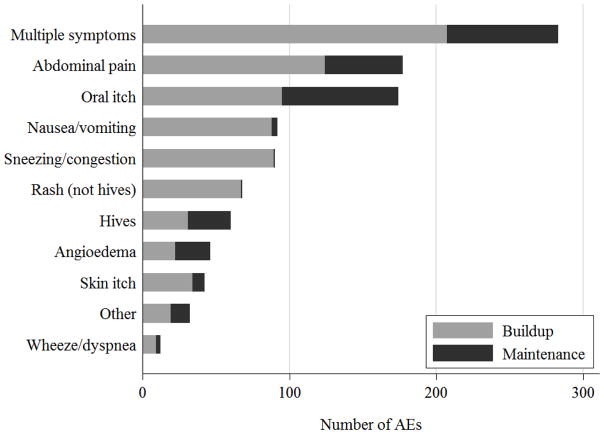
A majority of the reactions were mild (85%), with 15% moderate, and no severe AEs (Table 2). These AEs comprised a variety of symptoms; though most events involved a combination of skin, upper and lower respiratory, or gastrointestinal symptoms (26%, Figure 2). The most common isolated symptoms were abdominal pain (16%), oral pruritus (16%), nausea/vomiting (9%), and nasal symptoms (8%).
Fig 2.
Frequency of AEs by Symptoms Reported over Buildup and Maintenance
Of all AEs, 113 events (10%) included symptoms indicative of a systemic reaction (as defined in supplemental methods), with higher rates during buildup (65 events, 0.3% of dosing days) than in maintenance (48 events, 0.06% of dosing days; p<0.001). Of the 113 systemic reactions, 110 (97%) occurred at home, while only 3 occurred at the research unit. Over the course of therapy, 44 subjects (42%) experienced a systemic reaction, with rate of 0.3% of dosing days, and an annualized rate of 0.37.
Of note, 51 subjects (49%) experienced gastrointestinal events at some time during therapy. Thirty-three percent of AEs (352/1077) included gastrointestinal symptoms, and 26% (281/1077) of AEs involved isolated gastrointestinal symptoms (including abdominal pain, nausea, vomiting, dysphagia, and diarrhea). The annualized rate of gastrointestinal reactions was 1.1.
Predictors of AEs
We found that the presence of AR and the size of the peanut SPT were the only significant predictors of the overall rate of AEs, both before and after adjusting for sex, age, asthma, peanut-specific IgE, and atopic dermatitis (Table 3). After controlling for the other variables, the AE rate among subjects with AR was 2.9-fold higher than those without AR and the rates of AEs increased by 1.4-fold for every 5 mm increase in peanut SPT (Table 3). Of note, the unadjusted models for all AEs showed similar results, with both AR and peanut SPT as significant predictors of AE rate (Table E2).
Table 3.
Incidence Rate Ratios (IRR) of the Influence of Baseline Characteristics on Rates of AEs Overall and during the Buildup Phase, Maintenance Phase.
| Variable
|
Overall AEs
|
Buildup AEs
|
Maintenance AEs
|
|||
|---|---|---|---|---|---|---|
| IRR (95% CI) | P-value | IRR (95% CI) | P-value | IRR (95% CI) | P-value | |
|
|
|
|
||||
| Sex (female compared to male) | 0.73 (0.43, 1.23) | 0.24 | 0.56 (0.31, 1.01) | 0.06 | 1.23 (0.64, 2.38) | 0.54 |
| Age (per year) | 1.01 (0.90, 1.13) | 0.89 | 1.06 (0.93, 1.21) | 0.40 | 1.08 (0.96, 1.23) | 0.20* |
| Asthma | 0.86 (0.51, 1.43) | 0.55 | 0.63 (0.35, 1.11) | 0.11 | 2.30 (1.08, 4.88) | 0.03* |
| Atopic Dermatitis | 1.19 (0.64, 2.23) | 0.59 | 1.18 (0.60, 2.34) | 0.63 | 1.06 (0.47, 2.41) | 0.89 |
| Allergic Rhinitis | 2.85 (1.61, 5.04) | <0.001* | 2.12 (1.18, 3.82) | 0.01* | 6.88 (2.53, 18.7) | <0.001* |
| Peanut SPT (per 5 mm) | 1.38 (1.10, 1.72) | 0.005* | 1.40 (1.09, 1.80) | 0.01* | 1.33 (0.98, 1.81) | 0.07* |
| Log Peanut IgE (per log increase) | 0.88 (0.74, 1.04) | 0.14 | 0.87 (0.73, 1.03) | 0.10 | 0.91 (0.71, 1.16) | 0.44 |
statistically significant in the unadjusted models (see Table E2).
Splitting the models by phase, we found that AR remained significantly associated with higher AE rates during both buildup and maintenance phases, and the incidence rate ratio associated with AR increased from 2.1 during buildup to 6.9 in the maintenance phase (Table 3). SPT size was a significant predictor of AE during the buildup phase, but there was only a trend in the maintenance phase. Also, while asthma was not significant in predicting rates of AEs overall, the presence of asthma significantly increased AE rates by 2.3 times during maintenance (Table 3).
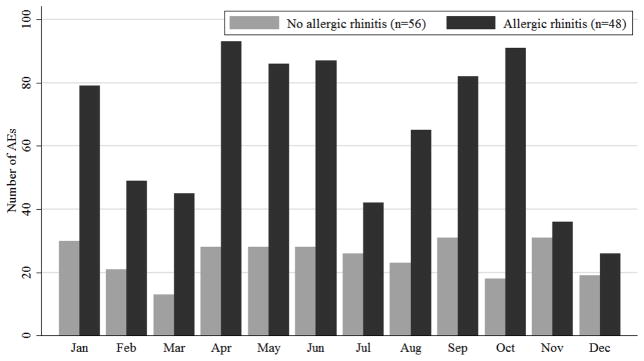
Analyzing the frequency of AEs by month revealed that among subjects without AR, AEs were highest in January and September, whereas among subjects with AR, AEs were highest in April and October (Figure 3). The counts of AEs by month were statistically significantly different between the two groups with and without AR (p=0.001). Furthermore, among participants with AR, the risk of an AE occurring during a peak pollen month compared to a non-peak month was 1.4 times as high as that of the subjects without AR (95% CI: 1.1, 1.8, p-value 0.0013). This difference is driven largely by AEs occurring in the buildup phase (buildup risk ratio 1.4, 95% CI: 1.1, 1.8, p-value 0.01).
Fig 3.
Distributions of AEs by Month By Allergic Rhinitis Status.
Isolating the likely-related events associated with systemic reactions, we determined that AR was the only significant predictor of the rate of systemic reactions, both before and after adjusting for sex, age, asthma, peanut SPT, log peanut-specific IgE, and atopic dermatitis. Rates of systemic AEs increased 2.2 times (95% CI: 1.1, 4.3; p-value: 0.03) for participants with AR, compared to those without.
Isolating the 352 events associated with gastrointestinal symptoms, we determined that the peanut SPT was the only significant predictor of the rate of gastrointestinal AEs, both before and after adjusting for sex, age, asthma, log peanut-specific IgE, atopic dermatitis, and AR. Rates of gastrointestinal AEs increased 1.8-fold (95% CI: 1.4, 2.4, p-value: <0.001) for every 5 mm increase in SPT size.
Treatment Administered for AEs
Over the course of OIT, 63 subjects (61%) reported administering treatment for 240 (22%) likely-related events (Table 4). Seventeen of these events occurred in the research unit (7%), while the remaining 93% were managed at home. All AEs occurring in the research unit were managed with antihistamines alone and epinephrine was never administered.
Table 4.
Rates of Treatment Overall and during the Buildup Phase, Maintenance Phase. Percentages reflect the the proportion of events or subjects compared to the total number listed in each column.
| Treatment | Events requiring treatment | Subjects requiring treatment | ||||
|---|---|---|---|---|---|---|
| Overall (n=1077) | Buildup (n=790) | Maintenance (n=294) | Overall (n=104) | Buildup (n=104) | Maintenance (n=87) | |
| Treated | 240 (22%) | 127 (16%) | 113 (39%) | 63 (61%) | 44 (42%) | 36 (41%) |
| Antihistamines | 228 (21%) | 118 (15%) | 110 (38%) | 61 (59%) | 43 (41%) | 34 (39%) |
| Albuterol | 46 (4%) | 26 (3%) | 20 (7%) | 22 (21%) | 11 (11%) | 15 (17%) |
| Steroids | 11 (1%) | 3 (0.4%) | 8 (3%) | 7 (7%) | 3 (3%) | 6 (7%) |
| Epinephrine | 18 (2%) | 4 (0.5%) | 14 (5%) | 12 (12%) | 4 (4%) | 10 (11%) |
| ED Visit | 18 (2%) | 5 (0.6%) | 13 (5%) | 12 (12%) | 5 (5%) | 9 (10%) |
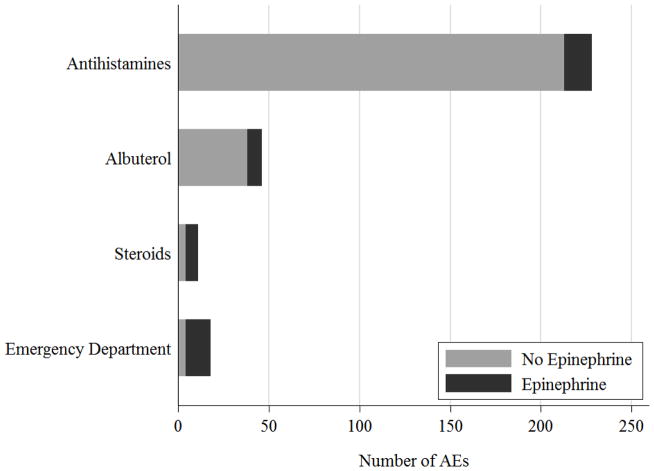
Antihistamines were administered most often, and while treatment with oral steroids, epinephrine, and ED visits occurred at similar rates, they did not always happen concurrently (Figure 4A). Twenty-two subjects (21%) experienced 40 events (4% of all AEs) where they administered albuterol, oral steroids, or visited an ED, but did not administer epinephrine. All of the 18 events that resulted in epinephrine administration occurred at home. Four events included isolated skin symptoms, one event included isolated lower respiratory symptoms, one included isolated mild gastrointestinal symptoms, and the remaining 12 involved a combination of skin, respiratory, and gastrointestinal symptoms.
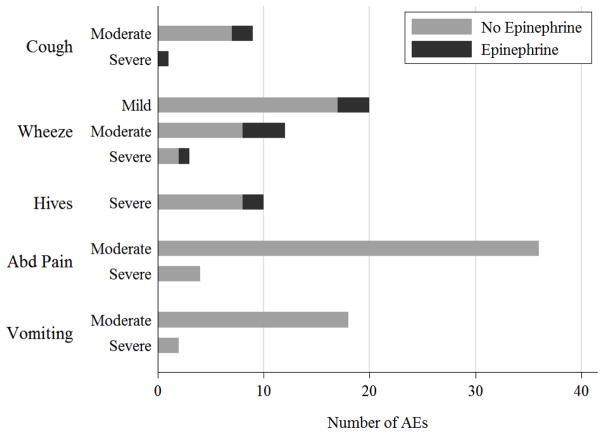
Fig 4.
Frequency of AEs resulting in Epinephrine Use. (A) Patterns of use of epinephrine concurrently with administration of antihistamines, albuterol, oral steroids, or an emergency department visit. (B) Patterns of epinephrine use in response to specific symptoms (cough, wheeze, hives, abdominal pain, or vomiting). Overlap of AEs with two or more given symptoms (ex: cough and wheeze) may be present.
Examining parental patterns of epinephrine use in response to specific symptoms, we found parents administered epinephrine for 3 of the events (43%) with moderate or severe cough, for 8 events (30%) with any severity of wheezing, and 2 of the events (25%) with whole body hives (Figure 4B). However, they did not administer epinephrine for any of the 60 events with moderate to severe abdominal pain or vomiting (Figure 4B).
Furthermore, of the previously identified 113 systemic reactions, there were 97 events (9% of all AEs), affecting 40 subjects (38%), in which epinephrine was warranted but not administered. During 38 of these events (affecting 20 subjects) either albuterol or steroids were given. Four of the subjects (4 separate events) went to the ED for their reaction. There were no hospitalizations or fatalities.
Discussion
We present the largest safety analysis of peanut OIT performed in a controlled research setting. This study revealed for the first time that AR and peanut SPT were both statistically and clinically significant predictors for the rates of AEs. Our findings predict that for two individuals with otherwise similar characteristics, one with AR might have 29 AEs for every 10 AEs in an individual without AR. AR was also predictive of higher rates of systemic reactions. The association of AEs and AR appears to be corroborated by a seasonality of AEs during the spring and fall in subjects with AR, compared to those without AR, which to our knowledge is the first demonstration of this effect. This has implications for AE surveillance and the biology of allergen co-priming.
Similarly larger SPT was also predictive of more AEs overall, as well as gastrointestinal AEs overall. Other studies have identified baseline specific IgE as a predictor of higher rates of AEs or study withdrawal, but our study did not confirm this (14). Furthermore, during maintenance, the presence of asthma at baseline was also predictive of higher rates of AEs. This may have been due to the young age of many of the participants, who may later be officially diagnosed with asthma. A common concern has been that subjects with poorly controlled asthma have difficulties even during the buildup phase of OIT, but this could not be confirmed in the model. This analysis is also limited by the fact that patients with moderate to severe asthma are generally excluded from our trials for safety reasons.
If confirmed in other settings, it is possible that presence of asthma, AR, and SPT may be influential in identifying subjects likely to experience AEs on OIT. SPT in particular may be useful in identifying the subjects with increased gastrointestinal AEs, who appear to be at greater risk of dropping out. This is especially important in generalizing to clinical settings the results of OIT performed in highly selected individuals.
Regarding the safety profile overall, despite differences in protocol and methods of reporting safety data, our rates of AEs appear to be consistent with prior studies. In our study, we found 80% of subjects reacting to OIT, while other estimates ranged from 86%–100% (9, 15, 16), and we found 47% of subjects reacting while on stable doses, compared with 54% in another study (16). Our rate of 1.7% of dosing days with AEs was also similar, with one study demonstrating 2.6% of dosing days associated with AEs (17). Our rate of study withdrawal was 20% (13% due to symptoms), which was equally high in other studies, from 5–36% (16–18).
One element in assessing the risk of OIT involves examining rates of AEs during OIT as well as those occurring during the standard of care, i.e., strict allergen avoidance. This was not a prespecified objective of this study and is best done prospectively with rigorous design features (randomization, careful AE reporting, lengthy periods of follow-up, etc.). Thus, while we acknowledge that any such observation is potentially limited by methodological differences, particularly in the way reactions are captured and reported, the illustration may be helpful to put our findings into some context. Combining several assessments of the natural course of peanut allergy with strict allergen avoidance, 39% of subjects had a post-diagnosis peanut-induced reaction over an average period of follow-up of approximately 5 years (19–21). In our study, 80% of subjects experienced an OIT-induced reaction over a median of 2.8 years; though of note, our rate of reporting reactions may also be higher due to more rigorous AE capture methods. A 3-year prospective study of accidental allergic reactions in an observational cohort of 512 young children, who were likely (but not confirmed) to have milk or egg allergy, found an annualized reaction rate of 0.81 per year to milk, egg, and peanut (22). In contrast, our annualized reaction rate was over 400% higher, at 3.5 per year. Taken together, these data suggest that OIT-induced allergic reactions to the index food may occur more commonly than accidental reactions during avoidance, which would not be unexpected given the stimulation of the immune system inherent to OIT. However, most allergic AEs during OIT are mild and self-limited, resolving without permanent sequelae even when they necessitate treatment withdrawal. Assessing the relative risks of frequent mild reactions during OIT versus long-term protective benefits is an area that requires further study with extended periods of careful surveillance, especially among adolescents and young adults, the population most at-risk of fatal anaphylaxis.
We defined systemic reactions using NIAID/FAAN definitions of anaphylaxis and found these occur at a high rate, with over 40% of participants experiencing at least one episode, and at least 38% of subjects experienced events that may have warranted epinephrine use that was not given. This is particularly concerning, given over 90% of likely-related AEs occurred at home, emphasizing the importance of anaphylaxis education. Isolating specific symptoms that might trigger the use of epinephrine, we observed that families were more likely to administer epinephrine in response to respiratory symptoms than abdominal symptoms, though use of epinephrine still fell below 50%. Furthermore, at least 20% of subjects experienced an event necessitating albuterol, steroids, or an ED visit, but in which epinephrine was not administered. Although allergic reactions are inherently complex and polymorphic, these findings suggest under-recognition or under-treatment, despite extensive education and round-the-clock provider access. While comparisons of epinephrine use are limited by differences in treatment criteria, in our study, 12% of subjects overall received epinephrine (4% during buildup and 11% during maintenance). This rate is higher than other research OIT trials, which varied from 0–2% (15, 17, 18, 23), but similar to a study of five clinical allergy practices, which described 12% of subjects requiring epinephrine during buildup and 6% during maintenance (9).
While our rates of epinephrine use are similar to some seen in other studies, our findings also suggest that epinephrine use is not an appropriate proxy for severity of reactions. Accordingly, we will need to develop new approaches for capturing the severity of reactions experienced by subjects on OIT. While reluctance to use epinephrine is well-characterized among the food-allergic population overall (22, 24), there has been relatively little study of whether and how this reluctance is affected by immunotherapy, a key area for future study.
The predominance of gastrointestinal AEs, combined with the high dropout rate from persistent gastrointestinal symptoms and the potential risk of EoE, does raise some concerns. Other studies have also reported high rates of gastrointestinal symptoms (16–18, 23), and an increasingly recognized connection between OIT and iatrogenic EoE (25, 26). Further quantifying this EoE risk is difficult because research volunteers are not commonly subjected to invasive diagnostic procedures like endoscopies, particularly prior to initiating OIT. Among symptomatic subjects receiving peanut OIT in a community practice setting, there was a substantially increased incidence of EoE (25). In our study, although ten subjects withdrew due to GI AEs, only three ultimately had an endoscopic evaluation, and two of these were diagnosed with EoE. At the time of publication, both were asymptomatic but due to incomplete gastrointestinal follow-up, it is not clear if these two patients have only temporary or longstanding EoE. Even so, our incidence rate of 648/100,000 person-years (2 subjects/308.8 person-years) is high compared with estimates of the incidence of pediatric EoE in the general population at 10/100,000 person-years (27), though this incidence among food-allergic individuals has not been well described and is potentially high as well. Taken together, these data suggest that current estimates of EoE during OIT of approximately 3–4% may be low and this requires more study (26).
We acknowledge that this study is limited by its retrospective nature and limited availability of placebo control data. While relatedness to OIT and severity of events were classified at the time of reporting, determination of which reactions were systemic and required epinephrine was made after the data was collected and may be limited by the information collected on diaries and therefore subject to bias. We also acknowledge that our methods for treating anaphylaxis may differ from other groups’ recommendations. Finally, while this analysis benefits from pooling studies conducted by the same group and largely the same clinical providers, these subjects were enrolled over 9 years. Knowledge of which symptoms may be related to OIT has evolved, with greater recognition of the importance of gastrointestinal AEs. Therefore, among the earlier events, it is likely that symptoms such as isolated gastrointestinal symptoms may have been incorrectly classified as possibly related or unrelated, and therefore excluded from analysis based on our inclusion criteria. These limitations, however, suggest that the true rates of OIT-related AEs, in particular gastrointestinal events, may be even higher than we observed.
In summary, OIT for peanut allergy, while clearly efficacious at producing robust desensitization, continues to be limited by frequent side effects, with high AE rates likely exceeding the reaction rate expected during allergen avoidance. Gastrointestinal symptoms appear to dominate, accounting for a large proportion of study withdrawal, though these symptoms are ultimately self-limited. The high proportion of subjects experiencing systemic reactions raises concerns about the apparent reluctance of our participants to administer epinephrine when warranted. This analysis suggests that larger peanut SPTs, AR (especially seasonal), and potentially asthma, may predict higher rates of AEs. Furthermore, larger SPTs are also predictive of higher rates of gastrointestinal side effects, highlighting a group that appears to be at greater risk for study withdrawal and EoE. While these results will need to be confirmed in larger prospective randomized controlled clinical trials, the implications of our data are important and hypothesis-generating. With validated predictors of risk, we could stratify individuals at high risk for side effects or treatment withdrawal, and thereby assist in the selection among multiple therapies for food allergy. Furthermore, these data support investigation into mechanistic links between OIT and gastrointestinal side effects and also aeroallergen exposure and food reactivity, methods for improving OIT safety by targeting comorbid allergic disease, as well as behavioral outcomes research of caregiver perceptions of anaphylaxis. Although OIT is a promising investigational therapy with the potential to improve food allergy associated mortality and quality of life, we have demonstrated a number of important safety variables that are both problematic and poorly understood. Additional studies beyond clinical trials (e.g. outcomes research, biomarker studies) are needed in order to weigh the substantial potential benefits of OIT against the risks, harmonize patient selection strategies, and move food allergy treatment into the realm of personalized medicine, all extremely important but beyond the scope of this paper. In the short term we would advise awaiting the results of adequately powered, placebo controlled trials before implementation into widespread practice, and suggest that for now allergen avoidance should remain the current standard of care.
Supplementary Material
Clinical Implications.
Given high rates of OIT-related adverse events, both systemic and gastrointestinal, additional safety studies are needed before implementation into widespread practice. Currently, allergen avoidance should remain the standard of care.
Acknowledgments
Funding Support: Food Allergy and Anaphylaxis Network, Gerber Foundation, National Institutes of Health - R01-AI068074, Food Allergy Project, Clinical and Translational Science Award 5M01-R000030-45, National Peanut Board, National Institutes of Health – K23-AI-099083, National Institutes of Health T32 (5T32HL098099-03), National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001117, Wallace Research Foundation, National Peanut Board; Dorothy O. Robins and Family Endowment in Peanut Allergy, Alex Orum Peanut Allergy Fund; Harvard Catalyst, The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102).
We thank P. Varshney, J. Kamilaris, C. Barnes, J. French, D. Hamilton, L. Herlihy, A. Hiegel, S. Carlisle, and L. Christie for study coordination and support; M. Kulis for laboratory support; and the families who kindly participated.
Abbreviations
- AE
adverse event
- DBPCFC
double-blinded, placebo-controlled food challenge
- OIT
oral immunotherapy
- IgE
immunoglobulin E
- SPT
skin prick test
- OFC
oral food challenge
- AR
allergic rhinitis
- EoE
eosinophilic esophagitis
Footnotes
Clinical Trial Registration Numbers: NCT01891136 (Jones et al), NCT00815035 (Varshney et al), NCT00932828 (DEVIL)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125(6):1322–6. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 2.Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128(1):e9–17. doi: 10.1542/peds.2011-0204. [DOI] [PubMed] [Google Scholar]
- 3.Keet CA, Savage JH, Seopaul S, Peng RD, Wood RA, Matsui EC. Temporal trends and racial/ethnic disparity in self-reported pediatric food allergy in the United States. Ann Allergy Asthma Immunol. 2014;112(3):222–9. e3. doi: 10.1016/j.anai.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sampson HA, Aceves S, Bock SA, James J, Jones S, Lang D, et al. Food allergy: a practice parameter update-2014. J Allergy Clin Immunol. 2014;134(5):1016–25. e43. doi: 10.1016/j.jaci.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the Diagnosis and Management of Food Allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. J Allergy Clin Immunol. 2010;126(6):1105–18. doi: 10.1016/j.jaci.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sampson HA. Peanut Oral Immunotherapy: Is It Ready for Clinical Practice? The Journal of Allergy and Clinical Immunology: In Practice. 2013;1(1):15–21. doi: 10.1016/j.jaip.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Hofmann AM, Scurlock AM, Jones SM, Palmer KP, Lokhnygina Y, Steele PH, et al. Safety of a peanut oral immunotherapy protocol in children with peanut allergy. J Allergy Clin Immunol. 2009;124(2):286–91. 91 e1–6. doi: 10.1016/j.jaci.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varshney P, Steele PH, Vickery BP, Bird JA, Thyagarajan A, Scurlock AM, et al. Adverse reactions during peanut oral immunotherapy home dosing. J Allergy Clin Immunol. 2009;124(6):1351–2. doi: 10.1016/j.jaci.2009.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wasserman RL, Factor JM, Baker JW, Mansfield LE, Katz Y, Hague AR, et al. Oral immunotherapy for peanut allergy: multipractice experience with epinephrine-treated reactions. J Allergy Clin Immunol Pract. 2014;2(1):91–6. doi: 10.1016/j.jaip.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124(2):292–300. e1–97. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vickery BP, Scurlock AM, Kulis M, Steele PH, Kamilaris J, Berglund JP, et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol. 2014;133(2):468–75. doi: 10.1016/j.jaci.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, Steele P, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011;127(3):654–60. doi: 10.1016/j.jaci.2010.12.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furuta GT, Liacouras CA, Collins MH, Gupta SK, Justinich C, Putnam PE, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133(4):1342–63. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Vazquez-Ortiz M, Alvaro-Lozano M, Alsina L, Garcia-Paba MB, Piquer-Gibert M, Giner-Munoz MT, et al. Safety and predictors of adverse events during oral immunotherapy for milk allergy: severity of reaction at oral challenge, specific IgE and prick test. Clin Exp Allergy. 2013;43(1):92–102. doi: 10.1111/cea.12012. [DOI] [PubMed] [Google Scholar]
- 15.Clark AT, Islam S, King Y, Deighton J, Anagnostou K, Ewan PW. Successful oral tolerance induction in severe peanut allergy. Allergy. 2009;64(8):1218–20. doi: 10.1111/j.1398-9995.2009.01982.x. [DOI] [PubMed] [Google Scholar]
- 16.Anagnostou K, Clark A, King Y, Islam S, Deighton J, Ewan P. Efficacy and safety of high-dose peanut oral immunotherapy with factors predicting outcome. Clin Exp Allergy. 2011;41(9):1273–81. doi: 10.1111/j.1365-2222.2011.03699.x. [DOI] [PubMed] [Google Scholar]
- 17.Blumchen K, Ulbricht H, Staden U, Dobberstein K, Beschorner J, de Oliveira LC, et al. Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol. 2010;126(1):83–91. e1. doi: 10.1016/j.jaci.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 18.Anagnostou K, Islam S, King Y, Foley L, Pasea L, Bond S, et al. Assessing the efficacy of oral immunotherapy for the desensitisation of peanut allergy in children (STOP II): a phase 2 randomised controlled trial. Lancet. 2014;383(9925):1297–304. doi: 10.1016/S0140-6736(13)62301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neuman-Sunshine DL, Eckman JA, Keet CA, Matsui EC, Peng RD, Lenehan PJ, et al. The natural history of persistent peanut allergy. Ann Allergy Asthma Immunol. 2012;108(5):326–31. e3. doi: 10.1016/j.anai.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Sicherer SH, Burks AW, Sampson HA. Clinical features of acute allergic reactions to peanut and tree nuts in children. Pediatrics. 1998;102(1):e6. doi: 10.1542/peds.102.1.e6. [DOI] [PubMed] [Google Scholar]
- 21.Vander Leek TK, Liu AH, Stefanski K, Blacker B, Bock SA. The natural history of peanut allergy in young children and its association with serum peanut-specific IgE. J Pediatr. 2000;137(6):749–55. doi: 10.1067/mpd.2000.109376. [DOI] [PubMed] [Google Scholar]
- 22.Fleischer DM, Perry TT, Atkins D, Wood RA, Burks AW, Jones SM, et al. Allergic reactions to foods in preschool-aged children in a prospective observational food allergy study. Pediatrics. 2012;130(1):e25–32. doi: 10.1542/peds.2011-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu GP, Weldon B, Neale-May S, Nadeau KC. The safety of peanut oral immunotherapy in peanut-allergic subjects in a single-center trial. Int Arch Allergy Immunol. 2012;159(2):179–82. doi: 10.1159/000336391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JS, Sinacore JM, Pongracic JA. Parental use of EpiPen for children with food allergies. J Allergy Clin Immunol. 2005;116(1):164–8. doi: 10.1016/j.jaci.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 25.Wasserman R, Sugerman R, Mireku-Akomeah A, Gallucci A, Pence D, Long N. Peanut oral immunotherapy (OIT) of food allergy (FA) carries a significant risk of eosinophilic esophagitis (EoE) [abstract] J Allergy Clin Immunol. 2011;127 Abstract 28. [Google Scholar]
- 26.Lucendo AJ, Arias A, Tenias JM. Relation between eosinophilic esophagitis and oral immunotherapy for food allergy: a systematic review with meta-analysis. Ann Allergy Asthma Immunol. 2014;113(6):624–9. doi: 10.1016/j.anai.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Soon IS, Butzner JD, Kaplan GG, deBruyn JC. Incidence and prevalence of eosinophilic esophagitis in children. J Pediatr Gastroenterol Nutr. 2013;57(1):72–80. doi: 10.1097/MPG.0b013e318291fee2. [DOI] [PubMed] [Google Scholar]
- 28.Sampson HA, Munoz-Furlong A, Campbell RL, Adkinson NF, Jr, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391–7. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.